Abstract
Introduction
Metabolic abnormalities are one of the most important risk factors for urinary stone disease. Our objective was to determine the prevalence of metabolic abnormalities in patients referred to the urolithiasis outpatient clinic of a tertiary centre.
Material and methods
We performed a cross-sectional study evaluating 67 patients referred to the urolithiasis outpatient clinic. Metabolic evaluation was performed, including one 24-hour urine sample.
Results
Metabolic abnormalities could be identified in 92.5% patients. Almost a quarter of the patients had only one metabolic abnormality and 67.6% had more than one abnormality. The most prevalent metabolic abnormalities were hypercalciuria (54.5%), hyperoxaluria (34.7%) and hyperuricosuria (32.3%). Patients with hypercalciuria were older (54.7 vs. 47.8 years, p = 0.018) and family history of stone disease was significantly more frequent among patients with hyperoxaluria (71.4% vs. 28.6%, p = 0.013). There was a positive linear relationship between body mass index (BMI) and urinary calcium (r = 0.247, p = 0.048) and a negative linear relationship between BMI and urinary pH (r = −0.254, p = 0.046).
Conclusions
Given the high prevalence of metabolic abnormalities, metabolic evaluation should be performed in every patient with urolithiasis evaluated in a tertiary setting.
Keywords: urolithiasis, urinary stone disease, metabolic evaluation
INTRODUCTION
Urinary stone disease or urolithiasis is a common, painful and costly condition [1]. The prevalence of urolithiasis is growing worldwide, irrespective of age, sex and race [2–5], with rates ranging from 7 to 13% in North America, 5–9% in Europe, and 1–5% in Asia [4].
This increase may be explained by changes in diet and lifestyle, higher prevalence of obesity and diabetes, global warming, with rising temperature resulting in dehydration and high urinary concentration of calcium and other stone-forming salts [4, 6].
The metabolic environment of the urine influences stone formation, with crystal production depending on the interplay between the saturation of each salt (calcium, oxalate, phosphate, uric acid) and the urinary concentration of inhibitors (citrate, magnesium, sulphate) and promoters (sodium) [7].
Lifestyle and dietary habits (such as an high salt and protein consumption and a low fluid intake) can induce urinary metabolic abnormalities, undoubtedly playing an important role in the risk of stone disease [3, 4, 8, 9, 10]. Hypertension, metabolic syndrome, diabetes and gout are diseases of affluent societies also associated with a higher prevalence of urolithiasis. Although a genetic component is often considered part of the stone formers’ diagnostic work-up, patients with known genetic causes appear to be less frequent [4]. Age, sex, race, climate, seasonal and geographic variation are also recognised predictors [4].
In general, 50% of patients experience recurrent urinary stones within five years without prophylactic intervention and about ten percent of patients even experience three or more recurrences during their lifetime [2, 11, 12], especially if the metabolic causes remain untreated.
In order to apply effective recurrence prevention (metaphylaxis), understanding the metabolic environment of each patient is required, so that a personalized treatment can be addressed.
To our knowledge, there are scarce epidemiological data concerning the prevalence of metabolic abnormalities among patients diagnosed with urolithiasis.
For that reason, we conducted a cross-sectional study in order to determine the prevalence of metabolic abnormalities in patients with urolithiasis referred to the urolithiasis outpatient clinic of a tertiary centre. A second objective was to evaluate potential risk factors associated with several metabolic abnormalities.
MATERIAL AND METHODS
We performed a cross-sectional study including 108 patients over 18 years old admitted in the urolithiasis outpatient clinic of a tertiary centre. These patients were referred to the study centre mainly by general practitioners. The recruitment process took place during a period of 6 months, between January 2017 and June 2017.
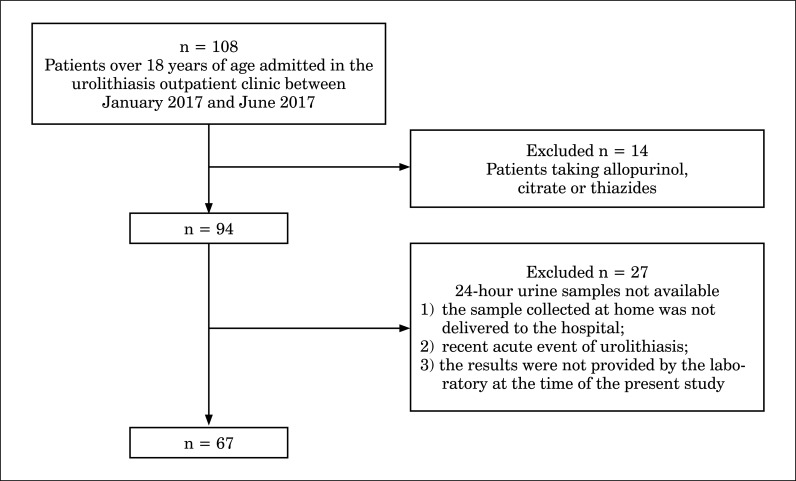
Forty-one patients were excluded due to their medication chart, concerning allopurinol, citrate or thiazides, which could change the urinary metabolic profile, or due to the unavailability of assessment of the 24-hour urine samples (Figure 1).
Figure 1.
Patient selection process.
Patient demographic data were obtained from medical records and during the medical appointment, including age, sex, tobacco (non-smoker, former or present smoker; pack-years) and alcohol (yes/no) consumption, physical exercise (no exercise, 1–3 days/ week, 4–5 days/week, 6–7 days/week), weight and height to calculate the body mass index (BMI), comorbidities such as hypertension, diabetes mellitus type 2 and dyslipidaemia, medical or surgical history (including past history of urologic interventions such as extracorporeal shock wave lithotripsy, ureteroscopy laser stone fragmentation or nephrolithotomy), medication chart, personal history and family stone history.
Initial evaluation of every patient also included: urinalysis, 24-hour urine sample (with analysis of urinary calcium, oxalate, phosphate, uric acid, sodium and magnesium), bacteriologic examination of urine, serum analysis of creatinine, urea, ionised calcium and parathyroid hormone (PTH) and non-contrast computerized tomography (CT). Hydrochloric acid (HCl) was added into the container for oxalate measurement in the 24-hour urine sample. For the other measurements, patients received an empty container and were instructed to keep it in a cool place during the collection.
We considered the following metabolic abnormalities: hyperoxaluria, hypercalciuria, raised PTH, hypercalcemia, hyperuricosuria, hypomagnesuria and hyperphosphaturia.
Reference laboratory values for urine and serum biochemical parameters in adults were made according to the European Association of Urology (EAU) Guidelines for Urolithiasis [13]. Since the reference values for serum PTH and urinary sodium are not given in these guidelines, we used those from our hospital laboratory (high levels for serum PTH was 65 pg/ml and 220 mmol/day for urinary sodium).
Statistical analysis was performed using the software Statistical Package for the Social Sciences (SPSS), version 24.0. Descriptive statistics were calculated for the sociodemographic, clinical and metabolic abnormalities. We used t-test for independent samples and nonparametric Mann-Whitney U test for comparisons between categorical and continuous variables, Chi-square for categorical variables and linear correlation for assessment between continuous variables. We considered statistically significant results for a p value <0.05.
Ethical approval was granted by the local Ethical Committee. Participants were informed about the study and methods and confidentiality was ensured. All participants signed a written informed consent according to the Declaration of Helsinki for human research ethics.
RESULTS
Participant characterization – demographic and clinical characterization
The majority of the population were women, making a female/male ratio of 1.39:1. The median age at the time of the consultation was 52 years old, with most patients between 43 and 60 years of age. Frequencies of smoking and alcohol consumption and physical activity are shown in Table 1.
Table 1.
Sociodemographic and clinical characterization. Absolute and relative frequency of sociodemographic and clinical variables
| Variables | |
|---|---|
| Age (years)* | 52 (43–60)† |
| Sex* Male Female |
28 (41.8)‡ 39 (58.2)‡ |
| Smoker (including former-smoker)* | 30 (44.8)‡ |
| Smoking load§ 1–10 pack-years 11–20 pack-years >20 pack-years |
14 (46.7)‡ 5 (16.7)‡ 11 (37.7)‡ |
| Alcohol consumer (Yes)* | 23 (34.3)‡ |
| Physical exercise|| No 1–3 days per week 4–5 days per week 6–7 days per week |
30 (45.5)‡ 20 (30.3)‡ 4 (6.3)‡ 12 (17.9)‡ |
| BMI (kg/m2)¶ Underweight** Normal†† Overweight‡‡ Obesity§§ |
1 (1.5)‡ 25 (37.9)‡ 26 (39.4)‡ 14 (21.2)‡ |
| Hypertension (yes)* | 14 (20.9)‡ |
| Diabetes mellitus (yes)* | 8 (11.9)‡ |
| Hyperlipidemia (yes)* | 18 (26.9)‡ |
| Personal history of lithiasis (yes)¶ | 45 (72.6)‡ |
| Age of first episode (median; P25-P75) (years)|||| | 45 (36-52)† |
| Past lithiasic intervention¶¶ None SWL URS SWL + URS SWL + PNL URS + PNL |
53 (79.1)‡ 6 (8.9)‡ 5 (7.5)‡ 1 (1.5)‡ 1 (1.5)‡ 1 (1.5)‡ |
| Family history of urolithiasis (yes)¶¶ | 22 (42.3)‡ |
BMI – body mass index; SWL – shock wave lithotripsy; URS – ureteroscopy; PNL – percutaneous nephrolithotomy
67 patients were evaluated in this variable;
The results represent the median and P25-P75, respectively;
The results represent the frequency, n (%);
30 patients were evaluated in this variable;
66 patients were evaluated in this variable;
62 patients were evaluated in this variable;
48 patients were evaluated in this variable;
52 patients were evaluated in this variable;
Underweight – BMI <18 kg/m2;
Normal – BMI between 18 and 24.9 kg/m2;
Overweight – BMI between 25 and 29.9 kg/m2;
Obesity – BMI >30 kg/m2
As depicted in Table 1 for clinical characterization, 1.5% of patients were underweight, 37.9% presented normal BMI and 60.6% were overweight or obese. Taking other comorbidities into account, 20.9% had hypertension, 11.9% had diabetes mellitus and 26.9% presented with dyslipidaemia. Almost 73% of patients admitted had personal history of lithiasis, with a median age of 45 years at first episode. Regarding past lithiasic intervention, 20.9% of patients presented with positive history. According to family history of urolithiasis, 32.7% of patients only referred one first degree relative while 9.6% mentioned two or more first degree relatives.
Frequency and type of metabolic abnormality in patients with urolithiasis
Metabolic abnormalities (considering any of these – hyperoxaluria, hypercalciuria, raised PTH, hypercalcemia, hyperuricosuria, hypomagnesuria or hyperphosphaturia) were found in 92.5% patients (Confidence Interval 95%: 86.2–98.8%). Almost a quarter (24.9% [CI95%: 15.0–35.8%]) only had one metabolic abnormality, and 67.6% patients (CI95%: 55.9–78.4%) had multiple metabolic abnormalities. Hypercalciuria was the most commonly observed metabolic abnormality and was found in 54.5% (CI95%: 42.5–66.6%) of patients. Other significant metabolic abnormalities were hyperoxaluria (34.7% [CI95%: 21.4–48.8%]), hyperuricosuria (32.3% [CI95%: 20.9–43.7%]) and hypomagnesuria (31.7% [CI95%: 20.3–43.2%]), as shown in Table 2.
Table 2.
Blood and urine parameters. Absolute and relative frequency of blood and urine parameters for metabolic abnormalities
| Metabolic abnormality | Frequency (%) | CI 95% (%) |
|---|---|---|
| Hypercalciuria (>5 mmol/day)* | 36 (54.5) | 42.5–66.6 |
| Hyperoxaluria (oxalate >0.5 mmol/day)† | 17 (34.7) | 21.4–48.8 |
| Hyperuricosuria (>4 mmol/day in women and >5 mmol/day in men)‡ | 21 (32.3) | 20.9–43.7 |
| Hypomagnesuria (<3 mmol/day)§ | 20 (31.7) | 20.3–43.2 |
| Hyperphosphaturia (phosphate level >1.3 g/dl)|| | 10 (17.5) | 7.7–27.4 |
| Elevated PTH (>65 pg/ml)¶ | 9 (14.5) | 5.7–23.3 |
| Hypercalcemia (ionised calcium >1.32 mmol/L)** | 5 (8.3) | 1.3–15.3 |
CI – confidence interval; PTH – parathyroid hormone
66 patients were evaluated in this variable;
49 patients were evaluated in this variable;
65 patients were evaluated in this variable;
63 patients were evaluated in this variable;
57 patients were evaluated in this variable;
62 patients were evaluated in this variable;
60 patients were evaluated in this variable
As for other urinary parameters, the median of the 24-hour urinary volume was 1650 ml, with 62.7% of patients presenting a 24-hour urinary volume below 2000 ml. The majority of urinary samples had a pH between 5.5 and 6.5 – almost a third (29%) of patients with a urinary pH less than 5.5 and 16.1% higher than 6.5. Approximately one-sixth (15.3%) of patients presented a positive urinary bacteriologic exam, with Escherichia coli as the most frequent isolated agent (5 in 9).
Comparison between clinical and metabolic abnormalities
Table 3 compares several sociodemographic and clinical variables with the most frequent metabolic abnormalities found in our study (hypercalciuria, hyperoxaluria, hyperuricosuria and hypomagnesuria). Patients with hypercalciuria were older (54.8 ±13.9 years vs. 47.8 ±7.8 years, p = 0.018), family history of stone disease was significantly more frequent among patients with hyperoxaluria (71.4% vs. 28.6%, p = 0.013) and there was a higher prevalence of present and former smokers among patients with hyperoxaluria (p = 0.016). Urinary volume was higher in patients with hypercalciuria (1899.4 ±627.9 ml vs. 1467.3 ±736.1 ml, p = 0.012) and hyperuricosuria (1946.2 ±675.2 ml vs. 1551.8 ±709.7, p = 0.037).
Table 3.
Comparison between clinical variables and metabolic abnormalities
| Hypercalciuria | Hyperoxaluria | Hyperuricosuria | Hypomagnesuria | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-value | Yes | No | p-value | Yes | No | p-value | |
| Age (years)* | 54.7 (±13.7) | 47.8 (±7.8) | 0.018† | 52.2 (±10.0) | 51.6 (±12.4) | 0.875† | 52.2 (±9.2) | 51.1 (±12.2) | 0.716† | 52.7 (±12.2) | 51.5 (±11.2) | 0.698† |
| Sex (Male) | 17 (47.2%) | 10 (33.3%) | 0.253‡ | 8 (47.1%) | 9 (28.1%) | 0.185‡ | 8 (38.1%) | 19 (43.2%) | 0.697‡ | 7 (35.0%) | 19 (44.2%) | 0.491‡ |
| Smoker (Yes) | 15 (41.7%) | 14 (46.7%) | 0.684‡ | 12 (70.6%) | 11 (34.4%) | 0.016‡ | 10 (47.6%) | 19 (43.2%) | 0.736‡ | 8 (40.0%) | 20 (46.5%) | 0.628‡ |
| BMI (Kg/m2)* | 27.2 (±3.9) | 25.6 (±3.7) | 0.116† | 27.1 (±2.8) | 25.8 (±4.2) | 0.257† | 26.8 (±3.7) | 26.1 (±3.8) | 0.488† | 27.5 (±4.1) | 25.8 (±3.7) | 0.113† |
| Hypertension (Yes) | 8 (22.2%) | 6 (20.0%) | 0.826‡ | 5 (29.9%) | 6 (18.8%) | 0.480‡ | 6 (28.6%) | 8 (18.2%) | 0.353‡ | 5 (25.0%) | 8 (18.6%) | 0.739‡ |
| Diabetes mellitus (Yes) | 5 (13.9%) | 3 (10.0%) | 0.719‡ | 3 (17.6%) | 2 (6.3%) | 0.326‡ | 2 (9.5%) | 5 (11.4%) | 1.000‡ | 4 (20.0%) | 3 (7.0%) | 0.195‡ |
| Hyperlipidemia (Yes) | 13 (36.1%) | 5 (16.7%) | 0.077‡ | 5 (29.4%) | 7 (21.9%) | 0.729‡ | 6 (28.6%) | 11 (25.0%) | 0.759‡ | 5 (25.0%) | 12 (27.9%) | 0.809‡ |
| Family history of urolithiasis (Yes) | 12 (44.4%) | 9 (39.1%) | 0.704‡ | 10 (71.4%) | 6 (28.6%) | 0.013‡ | 8 (50.0%) | 13 (38.2%) | 0.432‡ | 7 (41.2%) | 15 (45.5%) | 0.773‡ |
| Water intake (litres) | 1.5 (±0.9) | 1.5 (±0.7) | 0.868§ | 1.6 (±0.8) | 1.3 (±0.7) | 0.196§ | 1.5 (±0.7) | 1.4 (±0.8) | 0.233§ | 1.4 (±0.6) | 1.5 (±0.8) | 0.988§ |
| Urinary volume (ml)* | 1899.4 (±627.9) | 1467.3 (±736.1) | 0.012† | 1902.9 (±603.2) | 1560.9 (±729.6) | 0.105† | 1946.2 (±675.2) | 1551.8 (±709.7) | 0.037† | 1488.5 (±690.6) | 1807.7 (±708.4) | 0.099† |
BMI – body mass index
Level of significance <0.05. p-values denoting statistical significance are highlighted in bold
Results represent mean and the respective standard deviation;
t-test for independent samples;
Chi-square;
Non-parametric test used: Mann-Whitney U test
Body mass index was associated with urinary calcium and urinary pH. There was a positive linear relationship between BMI and urinary calcium (r = 0.247, p = 0.048) and a negative linear relationship between BMI and urinary pH (r = −0.254, p = 0.046). There was also a positive linear relationship between urinary calcium compared with urinary sodium (r = 0.509, p < 0.0001), uricosuria (r = 0.514, p <0.0001), magnesuria (r = 0.532, p <0.0001) and phosphaturia (r = 0.649, p <0.0001).
DISCUSSION
In our series, the most striking finding was the very high prevalence of metabolic abnormalities (92.5%), resembling the findings of several other studies, which considered metabolic abnormalities as one of the most important factors for stone formation [10, 14, 15].
Hypercalciuria (detected in 54.5% of patients) was the most frequent metabolic abnormality, followed by hyperolaxuria (34.7%) and hyperuricosuria (32.3%), a finding also observed in larger studies, most of them with patients with recurrent stone formation history [10, 14, 16, 17]. We included 17 patients with first self-reported episode of stone disease and, interestingly, all of them presented at least one metabolic abnormality.
Nevertheless, in some studies, a predominance of other abnormalities such as hyperoxaluria [10] or hypomagnesuria [18] was found. These disparate results may be explained by climate, seasonal, dietary or lifestyle variations, which highlights the importance of studying the prevalence of different metabolic changes in each population. This matter assumes a preponderant role, given the ability to modify these metabolic abnormalities with preventive (metaphylatic) measures, including diet and lifestyle changes along with the introduction of some pharmacological options (i.e. allopurinol, citrate or thiazides) that might prevent urinary stone formation [10].
There is great variability in the literature regarding the prevalence of urolithiasis according to gender. Our sample revealed a predominance of women (1.39:1), the same ratio pointed by Amaro et al. [14], but different from other studies, with a gender ratio male/female of 1.5–2.5 across the world. The gender ratio discussion of this study is precluded by the methods of patients’ referral which influences our outpatient clinic representativeness of the general population.
It is known that obesity and weight gain increase the risk of stone formation [8]. In our study, the majority (60.6%) of patients were overweight or obese and the increase of BMI was associated with a higher urinary calcium excretion. These patients may benefit from nutritional counseling for weight loss, aiming for the reduction of stone recurrence and decreasing cardiovascular risk. We also observed that BMI was inversely related to urinary pH, explained by the hypothesis that obesity may induce excessive production of acidic urine secondary to insulin resistance [19]. Almost a third (29%) of patients presented a urinary pH less than 5.5, a documented risk for uric acid nephrolithiasis. Patients with persistently low urinary pH benefit from urine alkalization [7, 19].
Patients with hypercalciuria were older than those without this metabolic abnormality, with a median age around 55 years. Otto et al. also observed that middle age patients are more likely to be hypercalciuric [20].
The higher prevalence of present and former smokers among patients with hyperoxaluria sustains the studies of Hamano et al. The authors suggested an interplay between cigarette smoking and arginine vasopressin, resulting in a further decrease of urinary output and enhancing stone formation. Therefore, smoking cessation can be effective in reducing calcium stone recurrence [21].
According to the European Association of Urology Guidelines for Urolithiasis [13], one preventative risk for stone formation relies on a higher fluid intake, 2.5–3.0 l/day, in order to maintain a urinary volume between 2 and 2.5 l/day. We observed that 62.7% of patients presented a lower 24-hour urine output (<2 l in 24 h) and recommending an increased fluid intake should be considered the first option as a general treatment of these patients.
Despite our findings, in clinical practice the implementation of an extensive metabolic evaluation is still controversial. In our opinion, given the high prevalence of metabolic abnormalities in our population, it seems to be worth performing an extended metabolic evaluation in all patients admitted to the urolithiasis clinics in a tertiary hospital. In fact, this work-up has prompted the prescription of preventive measures and drugs in a significant number of patients. We believe that this work-up is also cost effective, with a reduction of new stone-related events, such as admission to the Emergency Department (ER) and possible need for urgent surgical intervention, follow-up consultations and future non-invasive or minimally invasive procedures. Whether or not this proactive attitude should be extended to primary health care level remains to be elucidated.
Despite being an important ionic molecular inhibitor, our laboratory was unable to measure urinary citrate which is an important limitation of this study. Also, some urine samples did not present oxalate measurement due to unexpected laboratory technical problems. We decided to perform just one 24-hour urine collection for the sake of the patient’s convenience and adherence, and in fact the need for one versus two 24-hour urine collections during the initial metabolic evaluation is still an unsolved issue [22].
Although this is a cross-sectional descriptive study, the present patient database represents a cohort of patients that will allow further investigation. Ongoing studies intend to evaluate initial metabolic profiles as predictors of therapeutic success and recurrence outcomes. The identification of metabolic abnormalities and the implementation of specific diet changes and pharmacological treatment, when clinically indicated, should be complemented with a close follow-up of all patients in order to estimate future recurrence rates, clinical outcomes for ER admission and the need for surgical procedures.
CONCLUSIONS
Given the significant burden of urinary stone disease in our clinical practice along with the significantly high prevalence of metabolic abnormalities, we support the metabolic evaluation, irrespective of the presence or absence of recurrence, as an invaluable step in the clinical work-up of these patients. This immediate metabolic evaluation is the key for a better and individualized management, guiding the selection of proper pharmacological and dietary measures to prevent recurrent stone formation and to relieve all clinical and economic burden behind this condition.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed 2015. [Google Scholar]
- 2.Hesse A, Brändle E, Wilbert D, Köhrmann KU, Alken P. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 3.Scales CD, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin I, Mamoulakis C, Miyazawa K, Rodgers A, Talati J, Lotan Y. Epidemiology of stone disease across the world. World J Urol. 2017:1301–1320. doi: 10.1007/s00345-017-2008-6. [DOI] [PubMed] [Google Scholar]
- 5.Roth B, Bonny O. The Swiss Kidney Stone Cohort: An Observational Study to Unravel the Cause of Renal Stone Formation. Eur Urol Focus. 2017;3:7–9. doi: 10.1016/j.euf.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Romero V, Akpinar H, Assimos DG. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev Urol. 2010;12:e86–96. [PMC free article] [PubMed] [Google Scholar]
- 7.McAninch JW, Lue TF. Smith & Tanagho ’ S General Urology. 18th ed. McGraw Hill; 2013. [Google Scholar]
- 8.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 9.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad I, Pansota MS, Tariq M, Tabassum SA. Frequency of metabolic abnormalities in urinary stones patients. Pak J Med Sci. 2013;29:1363–1369. doi: 10.12669/pjms.296.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strohmaier WL. Course of calcium stone disease without treatment. What can we expect? Eur Urol. 2000;37:339–344. doi: 10.1159/000052367. [DOI] [PubMed] [Google Scholar]
- 12.Keoghane S, Walmsley B, Hodgson D. The natural history of untreated renal tract calculi. BJU Int. 2010;105:1627–1629. doi: 10.1111/j.1464-410X.2010.09389.x. [DOI] [PubMed] [Google Scholar]
- 13.Türk C, Neisius A, Petrik A, et al. EAU Guidelines on Urolithiasis 2017. The Netherlands: 2017. ISBN 978-90-79754-91-5. EAU Guidelines Office, Arnhem. [Google Scholar]
- 14.Amaro CR, Goldberg J, Damasio PC, et al. An update on metabolic assessment in patients with urinary lithiasis. World J Urol. 2015;33:125–129. doi: 10.1007/s00345-014-1271-z. [DOI] [PubMed] [Google Scholar]
- 15.Parvin M, Shakhssalim N, Basiri A, et al. The most important metabolic risk factors in recurrent urinary stone formers. Urol J. 2011;8:99–106. [PubMed] [Google Scholar]
- 16.Karabacak OR, Ipek B, Ozturk U, Demirel F, Saltas H, Altug U. Metabolic evaluation in stone disease metabolic differences between the pediatric and adult patients with stone disease. Urology. 2010;76:238–241. doi: 10.1016/j.urology.2010.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Shah S, Calle JC. Dietary and medical management of recurrent nephrolithiasis. Cleve Clin J Med. 2016;83:463–471. doi: 10.3949/ccjm.83a.15089. [DOI] [PubMed] [Google Scholar]
- 18.Abdel Goad EH, Bereczky ZB. Metabolic risk factors in patients with renal stones in KwaZulu Natal: an inter-racial study (Asian and Whites) BJU Int. 2004;93:120–123. doi: 10.1111/j.1464-410x.2004.04569.x. [DOI] [PubMed] [Google Scholar]
- 19.Maalouf NM, Sakhaee K, Parks JH, Coe FL, Adams-Huet B, Pak CYC. Association of urinary pH with body weight in nephrolithiasis. Kidney Int. 2004;65:1422–1425. doi: 10.1111/j.1523-1755.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 20.Otto BJ, Bozorgmehri S, Kuo J, Canales M, Bird V, Canales BK. Age, BMI, and Gender Predict 24 Hour Urine Parameters in Recurrent Idiopathic Calcium Oxalate Stone Formers. J Endourol. 2017;31:1335–1341. doi: 10.1089/end.2017.0352. [DOI] [PubMed] [Google Scholar]
- 21.Hamano S, Nakatsu H, Suzuki N, Tomioka S, Tanaka M, Murakami S. Kidney stone disease and risk factors for coronary heart disease. Int J Urol. 2005;12:859–863. doi: 10.1111/j.1442-2042.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- 22.Alruwaily AF, Dauw CA, Bierlein MJ, et al. How Much Information is Lost When You Only Collect One 24-Hour Urine Sample during the Initial Metabolic Evaluation? J Urol. 2016;196:1143–1148. doi: 10.1016/j.juro.2016.04.074. [DOI] [PubMed] [Google Scholar]