Abstract
Background
Human papillomavirus (HPV) testing has been employed by several European countries to augment cytology-based cervical screening programs. A number of research groups have demonstrated potential utility of next-generation sequencing (NGS) for HPV genotyping, with comparable performance and broader detection spectrum than current gold standards. Nevertheless, most of these NGS platforms may not be the best choice for medium sample throughput and laboratories with less resources and space. In light of this, we developed a Nanopore sequencing assay for HPV genotyping and compared its performance with cobas HPV Test and Roche Linear Array HPV Genotyping Test (LA).
Methods
Two hundred and one cervicovaginal swabs were routinely tested for Papanicolaou smear, cobas HPV Test and LA. Residual DNA was used for Nanopore protocol after routine testing. Briefly, HPV L1 region was amplified using PGMY and MGP primers, and PCR-positive specimens were sequenced on MinION flow cells (R9.4.1). Data generated in first 2 h were aligned with reference sequences from Papillomavirus Episteme database for genotyping.
Results
Nanopore detected 96 HPV-positive (47.76%) and 95 HPV-negative (47.26%) specimens, with 10 lacking β-globin band and not further analyzed (4.98%). Substantial agreement was achieved with cobas HPV Test and LA (κ: 0.83–0.93). In particular, Nanopore appeared to be more sensitive than cobas HPV Test for HPV 52 (n = 7). For LA, Nanopore revealed higher concordance for high-risk (κ: 0.93) than non-high risk types (κ: 0.83), and with similar high-risk positivity in each cytology grading. Nanopore also provided better resolution for HPV 52 in 3 specimens co-infected with HPV 33 or 58, and for HPV 87 which was identified as HPV 84 by LA. Interestingly, Nanopore identified 5 additional HPV types, with an unexpected high incidence of HPV 90 (n = 12) which was reported in North America and Belgium but not in Hong Kong.
Conclusions
We developed a Nanopore workflow for HPV genotyping which was economical (about USD 50.77 per patient specimen for 24-plex runs), and with comparable or better performance than 2 reference methods in the market. Future prospective study with larger sample size is warranted to further evaluate test performance and streamline the protocol.
Keywords: Cervical cancer, HPV, Nanopore, NGS
Introduction
Human papillomavirus (HPV) is generally accepted as the causative agent of cervical cancer (CC) [1], which was first unmasked by the landmark studies of Meisels and Fortin [2] and Purola and Savia [3]. Currently, there are 198 reference HPV types listed on Papillomavirus Episteme (PaVE) database, and at least 12 were classified as high-risk by World Health Organization (WHO) International Agency for Research on Cancer (IARC) Monographs Working Group [4–6]. HPV testing has been adopted by several European countries for primary CC screening, to augment cytology-based screening programs [7, 8]. A number of HPV assays are available commercially, which are mainly based on direct HPV genome detection, HPV DNA amplification and E6/ E7 mRNA detection [9]. Recent advent of next-generation sequencing (NGS) technologies has facilitated high throughput tools for infectious disease diagnostics and epidemiological research. Several research groups have explored utility of Illumina MiSeq and Ion Torrent platforms for HPV genotyping, with comparable sensitivity to well-established line blot assays and broader detection spectrum [10–12]. While the reagent cost is comparable to existing commercial assays for large sample batches, these NGS platforms may not be the best choice for medium sample throughput and laboratories with less resources and space. In this regard, portable Nanopore sequencers may allow more flexibility with shorter sequencing time and lower reagent cost. In light of this, we developed a Nanopore HPV genotyping protocol using 2 published primer sets, and compared its performance with 2 commercial HPV assays: cobas HPV Test and Roche Linear Array HPV Genotyping Test (LA).
Methods
Specimens
Two hundred and one cervicovaginal swabs were collected from March to July, 2019 in Hong Kong Sanatorium & Hospital. The swabs were preserved in SurePath preservative fluid (Becton, Dickson and Company, Sparks, MD, USA) and routinely tested for Papanicolaou smear (Pap smear, following The Bethesda System for reporting), cobas HPV Test and LA (Roche Diagnostics, Mannheim, Germany). Routine test results are shown in Table 1.
Table 1.
Results of Pap smear, cobas HPV Test, Roche Linear Array HPV Genotyping Test, and Nanopore sequencing
| Patient | Pap smear | Roche Linear Array | Cobas HPV | Nanopore (PGMY) | Nanopore (MGP) | Total HPV reads | |||
|---|---|---|---|---|---|---|---|---|---|
| HR | Non-HR | HR | Non-HR | HR | Non-HR | ||||
| 1 | AGUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 2 | ASCH | 52, 59 | 62 | Other HR | 59 | Neg | 59 | 90 | 4956 |
| 3 | ASCUS | 52 | 55 | Neg | 52 | 55 | Neg | Neg | 4262 |
| 4 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 5 | ASCUS | 31, 33 | 54 | Other HR | 31, 33, 52 | Neg | Neg | 90 | 8973 |
| 6 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 7 | ASCUS | 31 | Neg | Other HR | Neg | Neg | 31 | Neg | 1430 |
| 8 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 9 | ASCUS | Neg | 81 | Neg | Neg | 81 | Neg | 81 | 48,477 |
| 10 | ASCUS | 18 | Neg | 18 | 18 | Neg | 18 | Neg | 16,206 |
| 11 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 12 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 13 | ASCUS | 52 | 53, 54 | Other HR | 52 | 44, 53, 74 | 52 | 74, 90 | 15,419 |
| 14 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 15 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 16 | ASCUS | 52 | 81 | Neg | 52 | 81 | Neg | 81 | 8873 |
| 17 | ASCUS | 52 | 54 | Other HR | 52 | 54 | 52 | 54 | 36,258 |
| 18 | ASCUS | 52, 59 | 11 | Other HR | 52, 59 | 11 | 52, 59 | 11 | 44,702 |
| 19 | ASCUS | Neg | Neg | Neg | PCR inhibition | ||||
| 20 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 7 |
| 21 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 22 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 23 | ASCUS | 39 | 61, 72 | Other HR | 39 | 61, 72 | 39 | 87 | 1624 |
| 24 | ASCUS | 66 | Neg | Other HR | 66 | Neg | 66 | Neg | 10,383 |
| 25 | ASCUS | 68 | 61 | Other HR | Neg | 61 | Neg | 61 | 10,644 |
| 26 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | 90 | 541 |
| 27 | ASCUS | 52 | Neg | Neg | 52 | Neg | Neg | 87 | 3614 |
| 28 | ASCUS | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 45 |
| 29 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 30 | ASCUS | 35 | Neg | Other HR | 35 | Neg | 35 | Neg | 1641 |
| 31 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 32 | ASCUS | 52 | Neg | Other HR | 52 | Neg | 52 | Neg | 399 |
| 33 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 34 | ASCUS | 51 | 84 | Other HR | 51 | Neg | Neg | Neg | 1853 |
| 35 | ASCUS | Neg | Neg | Neg | Neg | 74 | Neg | 74 | 11,499 |
| 36 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 93 |
| 37 | ASCUS | 51 | Neg | Other HR | 51 | Neg | 51 | Neg | 2897 |
| 38 | ASCUS | Neg | 40, 55, 83 | Neg | Neg | 40, 55, 83 | Neg | 40, 55, 83 | 47,736 |
| 39 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 40 | ASCUS | 58 | 53, 55, 62 | Other HR | 52, 58 | 53, 55, 62, 74 | 52 | 53, 62, 74 | 42,106 |
| 41 | ASCUS | 52 | 42, 73 | Other HR | 52 | 42, 73 | 52 | 42, 73 | 15,778 |
| 42 | ASCUS | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 116 |
| 43 | HSIL | 16 | Neg | 16 | 16 | Neg | 16 | Neg | 15,918 |
| 44 | HSIL | 16 | Neg | 16 | 16 | Neg | 16 | Neg | 34,654 |
| 45 | HSIL | 59 | Neg | Other HR | 59 | Neg | 59 | Neg | 15,381 |
| 46 | HSIL | 31, 58 | Neg | Other HR | 31, 58 | Neg | 31, 58 | Neg | 3367 |
| 47 | LSIL | 52, 68 | 84 | Other HR | 52, 68 | 84 | 52, 68 | 84, 90 | 24,366 |
| 48 | LSIL | 66 | 84 | Other HR | 66 | 44, 84 | 66 | 44 | 57,206 |
| 49 | LSIL | 52 | Neg | Neg | 52 | Neg | 52 | Neg | 14,516 |
| 50 | LSIL | Neg | 40, 53 | Neg | Neg | 40, 53 | Neg | 40, 53 | 9265 |
| 51 | LSIL | 52 | 11, 81 | Other HR | 52 | 11, 81 | 52 | 11, 43, 81 | 29,748 |
| 52 | LSIL | 66 | Neg | Other HR | 66 | Neg | 66 | Neg | 40,328 |
| 53 | LSIL | 51 | Neg | Other HR | 51 | Neg | 51 | 43, 90 | 4454 |
| 54 | LSIL | 16, 51, 56 | 54, 62, 81 | 16, other HR | 16, 51, 56 | 54, 62, 81 | 16, 51 | 40, 62, 81 | 20,455 |
| 55 | LSIL | 56 | 53 | Other HR | 56 | 53 | 56 | 53 | 28,377 |
| 56 | LSIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 57 | LSIL | 66 | 54, 55, 81 | Other HR | 66 | 54, 55, 81 | 66 | 55, 81, 90 | 25,606 |
| 58 | LSIL | 52 | Neg | Neg | 52 | 42 | 52 | 90 | 15,103 |
| 59 | LSIL | 59 | Neg | Other HR | 59 | Neg | Neg | Neg | 11,235 |
| 60 | LSIL | 59 | 89 | Neg | 59 | 89 | Neg | 89 | 67,220 |
| 61 | LSIL | 56 | 82 | Other HR | 56 | 82 | 56 | 43, 82 | 42,160 |
| 62 | LSIL | 52 | Neg | Other HR | 52 | Neg | 52 | Neg | 39,323 |
| 63 | LSIL | 33, 51 | Neg | Other HR | 33, 51 | 44 | 51 | 44 | 19,704 |
| 64 | LSIL+ ASCH | 51 | Neg | Other HR | 51 | Neg | 51 | Neg | 4621 |
| 65 | NIL | 16 | Neg | 16 | 16 | Neg | 16 | Neg | 1958 |
| 66 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 67 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 68 | NIL | Neg | Neg | Neg | 59 | Neg | 59 | Neg | 2455 |
| 69 | NIL | Neg | Neg | Neg | Neg | 87 | Neg | 87 | 8775 |
| 70 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 71 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 72 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 73 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 74 | NIL | 58 | Neg | Other HR | 58 | Neg | 52, 58 | 62 | 8619 |
| 75 | NIL | 58 | Neg | Other HR | 58 | Neg | 58 | Neg | 13,149 |
| 76 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 77 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 78 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | 90 | 2289 |
| 79 | NIL | 56 | 70 | Other HR | Neg | 44, 70 | 56 | 44, 70 | 7855 |
| 80 | NIL | Neg | Neg | Neg | PCR inhibition | ||||
| 81 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 74 |
| 82 | NIL | Neg | 42 | Neg | Neg | Neg | Neg | 42 | 1406 |
| 83 | NIL | Neg | Neg | Neg | Neg | 74 | Neg | 74 | 7441 |
| 84 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 85 | NIL | Neg | 82 | Neg | Neg | 82 | Neg | 82 | 1162 |
| 86 | NIL | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 65,368 |
| 87 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 88 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 89 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 90 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 142 |
| 91 | NIL | 39, 52 | Neg | Other HR | 52 | Neg | 52 | 90 | 15,703 |
| 92 | NIL | 68 | Neg | Other HR | 68 | 42 | 68 | Neg | 19,777 |
| 93 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 94 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 95 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 96 | NIL | 52 | Neg | Neg | 52 | Neg | 52 | Neg | 5242 |
| 97 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 98 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 99 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 41 |
| 100 | NIL | 52 | Neg | Other HR | 52 | Neg | 52 | Neg | 24,478 |
| 101 | NIL | Neg | 61 | Neg | PCR inhibition | ||||
| 102 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 72 |
| 103 | NIL | 39 | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 104 | NIL | Neg | 62, 84 | Neg | Neg | 62 | Neg | 62 | 3589 |
| 105 | NIL | Neg | 71 | Neg | Neg | Neg | Neg | Neg | ND |
| 106 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 107 | NIL | 52 | 62 | Other HR | 52 | 44, 53, 62 | 52 | 44 | 18,086 |
| 108 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 109 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 110 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 111 | NIL | Neg | 84 | Neg | Neg | Neg | Neg | Neg | ND |
| 112 | NIL | 16, 52 | Neg | 16 | 16, 52 | Neg | 16 | Neg | 72,357 |
| 113 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 114 | NIL | Neg | 55, 89 | Neg | Neg | 26, 55, 89 | 59 | 26, 55, 62, 89 | 8926 |
| 115 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | 74 | 1586 |
| 116 | NIL | Neg | 81 | Neg | Neg | Neg | Neg | Neg | ND |
| 117 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 118 | NIL | Neg | 6, 62 | Neg | Neg | 6, 62 | Neg | 6, 62 | 9414 |
| 119 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 120 | NIL | Neg | 54 | Neg | Neg | Neg | Neg | Neg | ND |
| 121 | NIL | Neg | Neg | Neg | PCR inhibition | ||||
| 122 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 8 |
| 123 | NIL | 68 | Neg | Other HR | Neg | Neg | Neg | Neg | ND |
| 124 | NIL | Neg | 81 | Neg | Neg | 81 | Neg | 81 | 8735 |
| 125 | NIL | Neg | 84 | Neg | Neg | Neg | Neg | 87 | 1025 |
| 126 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | 90 | 1719 |
| 127 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 128 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 129 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 10 |
| 130 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 131 | NIL | Neg | 84 | Neg | Neg | Neg | Neg | Neg | ND |
| 132 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 133 | NIL | 59 | 62, 71 | Other HR | Neg | Neg | Neg | Neg | 30 |
| 134 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 135 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 522 |
| 136 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 137 | NIL | 51 | 84 | Other HR | PCR inhibition | ||||
| 138 | NIL | 39 | Neg | Other HR | 39 | Neg | 39 | Neg | 19,305 |
| 139 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 195 |
| 140 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 141 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 23 |
| 142 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 143 | NIL | Neg | 42, 81 | Neg | Neg | 40, 74, 81 | Neg | 40, 74, 81, 87 | 19,118 |
| 144 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 145 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 146 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 147 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 40 |
| 148 | NIL | 59 | Neg | Neg | 59 | Neg | Neg | Neg | 12,681 |
| 149 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 14 |
| 150 | NIL | Neg | Neg | Neg | PCR inhibition | ||||
| 151 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 79 |
| 152 | NIL | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 14,353 |
| 153 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 154 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 155 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 156 | NIL | 52 | 54 | Neg | 52 | 54 | 52 | 54 | 18,397 |
| 157 | NIL | 39, 52 | 53, 61 | Other HR | 39 | 53, 61 | 39 | 53, 61 | 20,332 |
| 158 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 159 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 60 |
| 160 | NIL | Neg | Neg | Neg | PCR inhibition | ||||
| 161 | NIL | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 13,545 |
| 162 | NIL | Neg | Neg | Neg | Neg | 74 | Neg | 74 | 4514 |
| 163 | NIL | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 11,894 |
| 164 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 165 | NIL | 59 | Neg | Neg | PCR inhibition | ||||
| 166 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 167 | NIL | 39 | Neg | Other HR | 39 | Neg | 39 | Neg | 52,831 |
| 168 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 169 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 170 | NIL | 66 | Neg | Other HR | 66 | Neg | 66 | Neg | 54,943 |
| 171 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 172 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 173 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 174 | NIL | 66 | Neg | Other HR | 66 | Neg | 66 | Neg | 57,791 |
| 175 | NIL | Neg | 54 | Neg | Neg | 54 | Neg | 54 | 23,583 |
| 176 | NIL | Neg | Neg | Neg | PCR inhibition | ||||
| 177 | NIL | 16 | 62 | 16 | Neg | 53, 62 | 16 | 62 | 28,181 |
| 178 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 206 |
| 179 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 180 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 181 | NIL | 51, 66 | Neg | Other HR | 51, 66, 68 | Neg | 51, 66, 68 | Neg | 6952 |
| 182 | NIL | 16, 51, 58 | 61 | Other HR | 58 | 61 | Neg | 61 | 5737 |
| 183 | NIL | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 184 | NIL | 58 | Neg | Other HR | 58 | Neg | 58 | Neg | 43,034 |
| 185 | NIL | 58 | 70, 89 | Other HR | 58 | 70, 89 | 58 | 89 | 33,842 |
| 186 | ND | Neg | Neg | Neg | Neg | Neg | Neg | Neg | 414 |
| 187 | ND | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 188 | ND | 16 | Neg | 16 | 16 | Neg | 16 | Neg | 96,549 |
| 189 | ND | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 190 | ND | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 191 | ND | 56 | Neg | Other HR | 56 | Neg | 56 | Neg | 18,782 |
| 192 | ND | 51 | Neg | Other HR | 51 | Neg | 51 | Neg | 6020 |
| 193 | ND | Neg | 62 | Neg | Neg | 62 | Neg | 62 | 20,373 |
| 194 | ND | Neg | Neg | Neg | Neg | Neg | Neg | Neg | ND |
| 195 | ND | 52, 59 | Neg | Other HR | 52, 59 | Neg | 59 | Neg | 11,926 |
| 196 | ND | 59 | Neg | Other HR | 59 | Neg | 59 | Neg | 24,045 |
| 197 | ND | 52, 59 | 54, 70 | Other HR | 52, 59 | 70 | 52, 59 | 70, 90 | 46,523 |
| 198 | ND | 56, 66 | 53, 61, 84 | Other HR | 66 | 32, 53, 61, 84 | 56 | 32, 53, 61, 84 | 62,600 |
| 199 | ND | Neg | 62 | Neg | Neg | Neg | Neg | Neg | ND |
| 200 | ND | Neg | 53, 54, 81, 83 | Neg | Neg | 53, 54, 83 | Neg | 53, 81, 83 | 32,868 |
| 201 | ND | Neg | Neg | Neg | PCR inhibition | ||||
AGUS Atypical glandular cells of undetermined significance, ASCH Atypical squamous cells – cannot exclude HSIL, ASCUS Atypical squamous cells of undetermined significance, HR High-risk, HSIL High-grade squamous intraepithelial lesion, LSIL Low-grade squamous intraepithelial lesion, ND Pap smear/ MinION sequencing not done, Neg Negative, NIL normal cytology
DNA extraction
DNA extraction and cobas HPV Test were performed using cobas 4800 system (Roche Diagnostics, Rotkreuz, Switzerland). Briefly, 500 μL of cervicovaginal specimen was added to 500 μL of sample preparation buffer and heated at 120 °C for 20 min. The mixture was brought to ambient temperature for 10 min and processed on cobas × 480 using ‘high-risk HPV DNA PCR’ protocol. Real-time polymerase chain reaction (PCR) was performed on cobas z 480. Fifty microliter of DNA extract was used for LA according to manufacturer’s recommendations. Residual DNA was used for Nanopore protocol after routine testing.
HPV PCR
For each specimen, L1 region of HPV genome was amplified in 2 separate PCRs using PGMY and MGP primer sets [13, 14]. Primer sequences and cycling conditions are shown in Tables 2 and 3. Human β-globin gene was used as inhibition control and contamination was monitored by negative extraction control. Five microliter of each PCR amplicon was electrophoresized in 2% agarose gel (Invitrogen, Carlsbad, CA, USA) and analyzed. PCR-positive specimens were sequenced using Nanopore MinION.
Table 2.
Primer sequences
| Primer | 5′ to 3′ sequence | References |
|---|---|---|
| PGMY PCR | ||
| PGMY11-A | GCA CAG GGA CAT AAC AAT GG | [13] |
| PGMY11-B | GCG CAG GGC CAC AAT AAT GG | |
| PGMY11-C | GCA CAG GGA CAT AAT AAT GG | |
| PGMY11-D | GCC CAG GGC CAC AAC AAT GG | |
| PGMY11-E | GCT CAG GGT TTA AAC AAT GG | |
| PGMY09-F | CGT CCC AAA GGA AAC TGA TC | |
| PGMY09-G | CGA CCT AAA GGA AAC TGA TC | |
| PGMY09-H | CGT CCA AAA GGA AAC TGA TC | |
| PGMY09-I | G CCA AGG GGA AAC TGA TC | |
| PGMY09-J | CGT CCC AAA GGA TAC TGA TC | |
| PGMY09-K | CGT CCA AGG GGA TAC TGA TC | |
| PGMY09-L | CGA CCT AAA GGG AAT TGA TC | |
| PGMY09-M | CGA CCT AGT GGA AAT TGA TC | |
| PGMY09-N | CGA CCA AGG GGA TAT TGA TC | |
| PGMY09-P | G CCC AAC GGA AAC TGA TC | |
| PGMY09-Q | CGA CCC AAG GGA AAC TGG TC | |
| PGMY09-R | CGT CCT AAA GGA AAC TGG TC | |
| HMB01 | GCG ACC CAA TGC AAA TTG GT | |
| Human β-globin forward | GAAGAGCCAAGGACAGGTAC | [15] |
| Human β-globin reverse | GGAAAATAGACCAATAGGCAG | |
| MGP PCR | ||
| MGPA | ACGTTGGATGTTTGTTACTGTGGTGGATACTAC | [16] |
| MGPB | ACGTTGGATGTTTGTTACCGTTGTTGATACTAC | |
| MGPC | ACGTTGGATGTTTGTTACTAAGGTAGATACCACTC | |
| MGPD | ACGTTGGATGTTTGTTACTGTTGTGGATACAAC | |
| MGP31 | ACGTTGGATGTTTGTTACTATGGTAGATACCACAC | |
| MGPG | ACGTTGGATGGAAAAATAAACTGTAAATCATATTCCT | |
| MGPH | ACGTTGGATGGAAAAATAAATTGTAAATCATACTC | |
| MGPI | ACGTTGGATGGAAATATAAATTGTAAATCAAATTC | |
| MGPJ | ACGTTGGATGGAAAAATAAACTGTAAATCATATTC | |
| MGP18 | ACGTTGGATGGAAAAATAAACTGCAAATCATATTC | |
Table 3.
Master mix constituents and PCR conditions
| PGMY PCR | ||
| Master mix constituents (for single reaction) | ||
| Reagent | Volume/μL | |
| 10X PCR buffer II (Applied Biosystems) | 5 | |
| 25 mM MgCl2 (Applied Biosystems) | 3 | |
| PGMY primer mix (10 μM) | 1 | |
| Human β-globin primer mix (5 μM) | 1 | |
| 10 mM dNTPs (Roche) | 1 | |
| 5 M betaine (Sigma) | 10 | |
| AmpliTaq Gold DNA Polymerase (Applied Biosystems) | 0.25 | |
| Molecular grade water (Sigma) | 23.75 | |
| DNA | 5 | |
| PCR conditions | ||
| Temperature/oC | Time | No. of cycles |
| 95 | 9 min | 1 |
| 95 | 1 min | 40 (50% ramp) |
| 55 | 1 min | |
| 72 | 1 min | |
| 72 | 5 min | 1 |
| 15 | Hold | / |
| MGP PCR | ||
| Master mix constituents (for single reaction) | ||
| Reagent | Volume/μL | |
| 10X PCR buffer II (Applied Biosystems) | 2.5 | |
| 25 mM MgCl2 (Applied Biosystems) | 1.5 | |
| MGP primer mix (10 μM) | 0.5 | |
| 10 mM dNTPs (Roche) | 0.5 | |
| AmpliTaq Gold DNA Polymerase (Applied Biosystems) | 0.1 | |
| Molecular grade water (Sigma) | 14.9 | |
| DNA | 5 | |
| PCR conditions | ||
| Temperature/oC | Time | No. of cycles |
| 95 | 10 min | 1 |
| 95 | 30 s | 5 |
| 42 | 30 s | |
| 72 | 30 s | |
| 95 | 30 s | 45 |
| 64 | 30 s | |
| 72 | 30 s | |
| 72 | 5 min | 1 |
| 15 | Hold | / |
Nanopore sequencing library preparation
PGMY and MGP PCR amplicons of each positive specimen were pooled and purified using AMPure XP beads (Beckman-Coulter, Brea, CA, USA). Nanopore sequencing libraries were prepared from purified amplicons using Ligation Sequencing Kit 1D (SQK-LSK109) and PCR-free Native Barcoding Expansion Kit (EXP-NBD104/114) (Oxford Nanopore Technologies, Oxford, England). The barcoded libraries were loaded and sequenced on MinION flow cells (FLO-MIN106D R9.4.1, Oxford Nanopore Technologies, Oxford, England) after quality control runs.
Data analysis
Data from first 2 h of sequencing runs was analyzed. FASTQ files generated by live basecalling (MinKNOW version 2.0) were demultiplexed using ‘FASTQ Barcoding’ workflow on EPI2ME (Oxford Nanopore Technologies, Oxford, England) with default minimum qscore of 7, ‘auto’ and ‘split by barcode’ options. FASTQ files of each specimen were concatenated into a single file and analyzed using a 2-step custom workflow on Galaxy bioinformatics platform. Briefly, FASTQ files were converted into FASTA format, followed by aligning sequences against HPV reference genomes from PaVE database using NCBI BLAST+ blastn (Galaxy version 1.1.1). PGMY and MGP reads were sorted based on sequence length and analyzed individually. Threshold of each run was derived from average number of background reads plus 10 standard deviations, which were calculated using interquartile rule, excluding first and last quartiles. A positive HPV call was based on either (1) the number of reads for a particular HPV type was above threshold, or (2) the specimen had the highest number of reads for a particular HPV type. All positive calls were further assessed by aligning FASTQ sequences against HPV reference genomes using minimap2 (Galaxy version 2.17 + galaxy0), and consensus sequences were built from BAM files using Unipro UGENE (version 1.29.0) for determining their percentage of identity to reference genomes.
Results
As HPV 66 is categorized as ‘other high-risk’ by cobas HPV Test, all calculations were based on this grouping, albeit HPV 66 was found as a single infection in cancers with extreme rarity and re-classified as possible carcinogen (Group 2B) by IARC Monographs Working Group [6].
The results are summarized in Table 1. PCR was successful for 191 specimens (191/201, 95.02%), with 10 specimens (10/201, 4.98%) lacking β-globin band and therefore regarded as inappropriate for further analysis. Seventy-six specimens (76/201, 37.81%) were negative for both PGMY and MGP PCRs, and 115 (115/201, 57.21%) were positive for either of the two. PCR-positive specimens were sequenced on 10 MinION flow cells with 145–890 active pores, generating 31,748–525,880 HPV reads in first 2 h (Table 4). For the 115 specimens sequenced, 19 were negative (7–522 reads, 113 in average) and 96 were positive (45–96,549 reads, 20,158 in average) for HPV. Taken together, there were 95 HPV-negative (95/201, 47.26%) and 96 HPV-positive (96/201, 47.76%) specimens by Nanopore workflow.
Table 4.
Details of Nanopore sequencing runs
| Run | No. of active pores | Elapsed sequencing time | No. of HPV reads |
|---|---|---|---|
| 1 | 611 | 2 h 11 min | 60,976 |
| 2 | 458 | 1 h 59 min | 246,521 |
| 3 | 690 | 2 h 1 min | 279,520 |
| 4 | 467 | 2 h 5 min | 111,885 |
| 5 | 462 | 2 h 5 min | 31,748 |
| 6 | 247 | 2 h 3 min | 113,521 |
| 7 | 330 | 2 h 5 min | 111,702 |
| 8 | 753 | 2 h 1 min | 478,711 |
| 9 | 145 | 1 h 59 min | 207,094 |
| 10 | 890 | 1 h 59 min | 525,880 |
Table 5 shows concordance of Nanopore workflow with cobas HPV Test and LA, which was based on the 37 HPV types detectable by LA. For cobas HPV Test, our workflow achieved 93.19, 93.19 and 81.94% for perfect, total and positive agreement, respectively, with Cohen’s kappa of 0.85. For LA, Nanopore achieved a perfect agreement of 83.77% for both high-risk and non-high risk HPVs. For high-risk types, total and positive agreement were 96.86 and 91.78%, respectively, with Cohen’s kappa of 0.93. For non-high risk types, total and positive agreement were 93.19 and 77.59%, respectively, with Cohen’s kappa of 0.83.
Table 5.
Agreement between cobas HPV Test, Roche Linear Array HPV Genotyping Test (LA) and Nanopore
| Nanopore | Perfect agreement | Total agreement | Positive agreement | Cohen’s κ | ||||
|---|---|---|---|---|---|---|---|---|
| + | – | |||||||
| cobas HPV Test | + | 59 | 2 | 93.19% | 93.19% | 81.94% | 0.85 | |
| – | 11 | 119 | ||||||
| LA | HR | + | 67 | 4 | 83.77% | 96.86% | 91.78% | 0.93 |
| – | 2 | 118 | ||||||
| Non-HR | + | 45 | 10 | 93.19% | 77.59% | 0.83 | ||
| – | 3 | 133 | ||||||
Table 6 shows per-type concordance of Nanopore and LA. A total of 13 high-risk and 19 non-high risk HPV types were evaluated. Positive agreement for HPV 16 (n = 8) and 18 (n = 1) were 87.5 and 100%, respectively. Positive agreement was 75–100% for high-risk HPV 31, 33, 35, 39, 51, 52, 56, 58, 59 and 66, and 20% for HPV 68 (n = 5). For non-high risk HPVs, positive agreement was 37.5–100% for HPV 6, 11, 40, 42, 53, 54, 55, 61, 62, 70, 72, 73, 81, 82, 83, 84 and 89. There were 2 non-high risk types with 0% positive agreement (HPV 26 and 71). HPV 26 (n = 1) was only detected by Nanopore workflow, whereas HPV 71 (n = 2) was only detected by LA.
Table 6.
Per HPV type positive agreement between Roche Linear Array Genotyping Test (LA) and Nanopore
| HPV Genotypes | Number of specimens | Positive agreement | |||||
|---|---|---|---|---|---|---|---|
| Nanopore−/LA−/LA- | Nanopore +/LA- | Nanopore−/LA+ | Nanopore+/LA+ | Total | |||
| High-risk | 16 | 183 | 0 | 1 | 7 | 191 | 87.5% |
| 18 | 190 | 0 | 0 | 1 | 191 | 100% | |
| 31 | 188 | 0 | 0 | 3 | 191 | 100% | |
| 33 | 189 | 0 | 0 | 2 | 191 | 100% | |
| 35 | 190 | 0 | 0 | 1 | 191 | 100% | |
| 39 | 185 | 0 | 1 | 5 | 191 | 83.33% | |
| 51 | 182 | 0 | 1 | 8 | 191 | 88.89% | |
| 52 | 165 | 3 | 2 | 21 | 191 | 80.77% | |
| 56 | 185 | 0 | 0 | 6 | 191 | 100% | |
| 58 | 184 | 0 | 0 | 7 | 191 | 100% | |
| 59 | 179 | 2 | 1 | 9 | 191 | 75% | |
| 66 | 182 | 1 | 0 | 8 | 191 | 88.89% | |
| 68 | 186 | 2 | 2 | 1 | 191 | 20% | |
| Non-high risk | 6 | 190 | 0 | 0 | 1 | 191 | 100% |
| 11 | 189 | 0 | 0 | 2 | 191 | 100% | |
| 26 | 190 | 1 | 0 | 0 | 191 | 0% | |
| 40 | 187 | 2 | 0 | 2 | 191 | 50% | |
| 42 | 186 | 2 | 1 | 2 | 191 | 40% | |
| 53 | 181 | 3 | 0 | 7 | 191 | 70% | |
| 54 | 181 | 0 | 4 | 6 | 191 | 60% | |
| 55 | 186 | 0 | 0 | 5 | 191 | 100% | |
| 61 | 186 | 0 | 0 | 5 | 191 | 100% | |
| 62 | 174 | 2 | 2 | 13 | 191 | 76.47% | |
| 70 | 188 | 0 | 0 | 3 | 191 | 100% | |
| 71 | 189 | 0 | 2 | 0 | 191 | 0% | |
| 72 | 190 | 0 | 0 | 1 | 191 | 100% | |
| 73 | 190 | 0 | 0 | 1 | 191 | 100% | |
| 81 | 182 | 0 | 1 | 8 | 191 | 88.89% | |
| 82 | 189 | 0 | 0 | 2 | 191 | 100% | |
| 83 | 189 | 0 | 0 | 2 | 191 | 100% | |
| 84 | 183 | 0 | 5 | 3 | 191 | 37.5% | |
| 89 | 188 | 0 | 0 | 3 | 191 | 100% | |
Table 7 reveals the percentage of identity of Nanopore consensus sequences to HPV reference genomes. In general, Nanopore consensus sequences showed an average identity of 98% to the best matches, with an average difference of 15% from second BLAST hits.
Table 7.
Percentage of identity of Nanopore consensus sequences to HPV reference genomes
| Patient | Nanopore results | Best BLAST hit | Second BLAST hit | Difference | ||
|---|---|---|---|---|---|---|
| HPV type | % identity | HPV type | % identity | |||
| 2 | 59 | 59 | 99% | 18 | 77% | 22% |
| a90 | 90 | 97% | 106 | 84% | 15% | |
| 3 | 52 | 52 | 99% | 58 | 80% | 19% |
| 55 | 55 | 100% | 44 | 93% | 7% | |
| 5 | 31 | 31 | 98% | 35 | 80% | 18% |
| 33 | 33 | 99% | 58 | 86% | 13% | |
| a52 | 52 | 99% | 58 | 80% | 19% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 7 | 31 | 31 | 95% | 35 | 79% | 16% |
| 9 | 81 | 81 | 99% | 62 | 85% | 14% |
| 10 | 18 | 18 | 99% | 45 | 85% | 14% |
| 13 | a44 | 44 | 99% | 55 | 92% | 7% |
| 52 | 52 | 99% | 58 | 80% | 19% | |
| 53 | 53 | 99% | 30 | 85% | 14% | |
| a74 | 74 | 99% | 55 | 83% | 16% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 16 | 52 | 52 | 99% | 58 | 81% | 18% |
| 81 | 81 | 99% | 62 | 85% | 14% | |
| 17 | 52 | 52 | 99% | 58 | 80% | 19% |
| 54 | 54 | 99% | 45 | 74% | 25% | |
| 18 | 11 | 11 | 99% | 6 | 87% | 12% |
| 52 | 52 | 99% | 58 | 80% | 19% | |
| 59 | 59 | 99% | 18 | 77% | 22% | |
| 23 | 39 | 39 | 99% | 70 | 81% | 18% |
| 61 | 61 | 99% | mEV06c12b | 83% | 16% | |
| 72 | 72 | 92% | mEV06c12b | 89% | 3% | |
| a87 | 87 | 98% | 86 | 85% | 13% | |
| 24 | 66 | 66 | 98% | 56 | 84% | 14% |
| 25 | 61 | 61 | 99% | mEV06c12b | 83% | 16% |
| 26 | a90 | 90 | 97% | 106 | 85% | 12% |
| 27 | 52 | 52 | 99% | 58 | 80% | 19% |
| a87 | 87 | 98% | 86 | 84% | 14% | |
| 28 | 62 | 62 | 99% | 81 | 84% | 15% |
| 30 | 35 | 35 | 98% | 31 | 80% | 18% |
| 32 | 52 | 52 | 99% | 58 | 81% | 18% |
| 34 | 51 | 51 | 99% | 82 | 85% | 14% |
| 35 | a74 | 74 | 99% | 55 | 84% | 15% |
| 37 | 51 | 51 | 99% | 82 | 85% | 14% |
| 38 | 40 | 40 | 99% | 7 | 88% | 11% |
| 55 | 55 | 99% | 44 | 93% | 6% | |
| 83 | 83 | 99% | 102 | 84% | 15% | |
| 40 | a52 | 52 | 99% | 58 | 80% | 19% |
| 53 | 53 | 98% | 30 | 85% | 13% | |
| 55 | 55 | 100% | 44 | 93% | 7% | |
| 58 | 58 | 99% | 33 | 86% | 13% | |
| 62 | 62 | 99% | 81 | 85% | 14% | |
| a74 | 74 | 98% | 55 | 84% | 14% | |
| 41 | 42 | 42 | 98% | 32 | 83% | 15% |
| 52 | 52 | 100% | 58 | 81% | 19% | |
| 73 | 73 | 99% | 34 | 85% | 14% | |
| 43 | 16 | 16 | 100% | 35 | 78% | 22% |
| 44 | 16 | 16 | 99% | 35 | 78% | 21% |
| 45 | 59 | 59 | 99% | 18 | 76% | 23% |
| 46 | 31 | 31 | 98% | 35 | 80% | 18% |
| 58 | 58 | 99% | 33 | 86% | 13% | |
| 47 | 52 | 52 | 98% | 58 | 80% | 18% |
| 68 | 68 | 93% | 39 | 81% | 12% | |
| 84 | 84 | 98% | 87 | 84% | 14% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 48 | a44 | 44 | 99% | 55 | 93% | 6% |
| 66 | 66 | 98% | 56 | 84% | 14% | |
| 84 | 84 | 99% | 87 | 84% | 15% | |
| 49 | 52 | 52 | 99% | 58 | 80% | 19% |
| 50 | 40 | 40 | 98% | 7 | 87% | 11% |
| 53 | 53 | 98% | 30 | 85% | 13% | |
| 51 | 11 | 11 | 100% | 6 | 87% | 13% |
| a43 | 43 | 95% | 45 | 77% | 18% | |
| 52 | 52 | 99% | 58 | 80% | 19% | |
| 81 | 81 | 99% | 62 | 84% | 15% | |
| 52 | 66 | 66 | 98% | 56 | 83% | 15% |
| 53 | a43 | 43 | 95% | 45 | 78% | 17% |
| 51 | 51 | 99% | 82 | 84% | 15% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 54 | 16 | 16 | 100% | 35 | 78% | 22% |
| a40 | 40 | 93% | 7 | 85% | 8% | |
| 51 | 51 | 99% | 82 | 84% | 15% | |
| 54 | 54 | 99% | 45 | 73% | 26% | |
| 56 | 56 | 90% | 66 | 76% | 14% | |
| 62 | 62 | 99% | 81 | 84% | 15% | |
| 81 | 81 | 99% | 62 | 85% | 14% | |
| 55 | 53 | 53 | 99% | 56 | 79% | 20% |
| 56 | 56 | 99% | 66 | 84% | 15% | |
| 57 | 54 | 54 | 87% | 31 | 74% | 13% |
| 55 | 55 | 100% | 44 | 93% | 7% | |
| 66 | 66 | 98% | 56 | 84% | 14% | |
| 81 | 81 | 99% | 62 | 84% | 15% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 58 | a42 | 42 | 99% | 32 | 84% | 15% |
| 52 | 52 | 98% | 58 | 80% | 18% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 59 | 59 | 59 | 99% | 18 | 77% | 22% |
| 60 | 59 | 59 | 99% | 18 | 76% | 23% |
| 89 | 89 | 99% | 81 | 78% | 21% | |
| 61 | a43 | 43 | 96% | 45 | 79% | 17% |
| 56 | 56 | 97% | 66 | 83% | 14% | |
| 82 | 82 | 99% | 51 | 84% | 15% | |
| 62 | 52 | 52 | 99% | 58 | 80% | 19% |
| 63 | 33 | 33 | 99% | 58 | 86% | 13% |
| a44 | 44 | 99% | 55 | 93% | 6% | |
| 51 | 51 | 99% | 82 | 83% | 16% | |
| 64 | 51 | 51 | 99% | 82 | 84% | 15% |
| 65 | 16 | 16 | 100% | 35 | 78% | 22% |
| 68 | a59 | 59 | 99% | 18 | 77% | 22% |
| 69 | a87 | 87 | 99% | 86 | 86% | 13% |
| 74 | a52 | 52 | 99% | 58 | 81% | 18% |
| 58 | 58 | 99% | 33 | 86% | 13% | |
| a62 | 62 | 99% | 81 | 85% | 14% | |
| 75 | 58 | 58 | 99% | 33 | 85% | 14% |
| 78 | a90 | 90 | 97% | 106 | 85% | 12% |
| 79 | a44 | 44 | 99% | 55 | 92% | 7% |
| 56 | 56 | 96% | 66 | 84% | 12% | |
| 70 | 70 | 99% | 39 | 81% | 18% | |
| 81 | a74 | 74 | 93% | 55 | 81% | 12% |
| 82 | 42 | 42 | 95% | 32 | 83% | 12% |
| 83 | a74 | 74 | 97% | 55 | 83% | 14% |
| 85 | 82 | 82 | 99% | 51 | 84% | 15% |
| 86 | 62 | 62 | 99% | 81 | 85% | 14% |
| 91 | 52 | 52 | 99% | 58 | 80% | 19% |
| a90 | 90 | 97% | 106 | 84% | 13% | |
| 92 | a42 | 42 | 93% | 32 | 78% | 15% |
| 68 | 68 | 92% | 39 | 80% | 12% | |
| 96 | 52 | 52 | 99% | 58 | 80% | 19% |
| 100 | 52 | 52 | 99% | 58 | 80% | 19% |
| 104 | 62 | 62 | 98% | 81 | 85% | 13% |
| 107 | a44 | 44 | 99% | 55 | 93% | 6% |
| 52 | 52 | 99% | 58 | 81% | 18% | |
| a53 | 53 | 100% | 30 | 86% | 14% | |
| 62 | 62 | 99% | 81 | 85% | 14% | |
| 112 | 16 | 16 | 98% | 58 | 78% | 20% |
| 52 | 52 | 99% | 58 | 81% | 18% | |
| 114 | a26 | 26 | 100% | 69 | 83% | 17% |
| 55 | 55 | 100% | 44 | 93% | 7% | |
| a59 | 59 | 99% | 18 | 77% | 22% | |
| a62 | 62 | 99% | 81 | 85% | 14% | |
| 89 | 89 | 99% | 81 | 77% | 22% | |
| 115 | a74 | 74 | 95% | 55 | 83% | 12% |
| 118 | 6 | 6 | 99% | 11 | 87% | 12% |
| 62 | 62 | 99% | 81 | 84% | 15% | |
| 124 | 81 | 81 | 99% | 62 | 85% | 14% |
| 125 | a87 | 87 | 98% | 86 | 85% | 13% |
| 126 | a90 | 90 | 97% | 106 | 85% | 12% |
| 138 | 39 | 39 | 99% | 68 | 81% | 18% |
| 143 | a40 | 40 | 99% | 7 | 88% | 11% |
| a74 | 74 | 98% | 55 | 84% | 14% | |
| 81 | 81 | 99% | 62 | 84% | 15% | |
| a87 | 87 | 97% | 86 | 84% | 13% | |
| 148 | 59 | 59 | 99% | 18 | 77% | 22% |
| 152 | 62 | 62 | 98% | 81 | 85% | 13% |
| 156 | 52 | 52 | 99% | 58 | 81% | 18% |
| 54 | 54 | 95% | 6 | 74% | 21% | |
| 157 | 39 | 39 | 94% | 70 | 81% | 13% |
| 53 | 53 | 96% | 30 | 84% | 12% | |
| 61 | 61 | 99% | mEV06c12b | 83% | 16% | |
| 161 | 62 | 62 | 98% | 81 | 83% | 15% |
| 162 | a74 | 74 | 94% | 55 | 85% | 9% |
| 163 | 62 | 62 | 99% | 81 | 85% | 14% |
| 167 | 39 | 39 | 99% | 70 | 81% | 18% |
| 170 | 66 | 66 | 98% | 56 | 83% | 15% |
| 174 | 66 | 66 | 98% | 56 | 83% | 15% |
| 175 | 54 | 54 | 99% | 45 | 73% | 26% |
| 177 | 16 | 16 | 99% | 35 | 80% | 19% |
| a53 | 53 | 99% | 30 | 84% | 15% | |
| 62 | 62 | 99% | 81 | 85% | 14% | |
| 181 | 51 | 51 | 99% | 82 | 85% | 14% |
| 66 | 66 | 98% | 56 | 83% | 15% | |
| a68 | 68 | 98% | 39 | 81% | 17% | |
| 182 | 58 | 58 | 98% | 33 | 87% | 11% |
| 61 | 61 | 100% | mEV06c12b | 83% | 17% | |
| 184 | 58 | 58 | 99% | 33 | 85% | 14% |
| 185 | 58 | 58 | 99% | 33 | 85% | 14% |
| 70 | 70 | 99% | 39 | 81% | 18% | |
| 89 | 89 | 99% | 81 | 78% | 21% | |
| 188 | 16 | 16 | 100% | 35 | 78% | 22% |
| 191 | 56 | 56 | 99% | 66 | 83% | 16% |
| 192 | 51 | 51 | 98% | 82 | 84% | 14% |
| 193 | 62 | 62 | 99% | 81 | 85% | 14% |
| 195 | 52 | 52 | 99% | 58 | 81% | 18% |
| 59 | 59 | 99% | 18 | 76% | 23% | |
| 196 | 59 | 59 | 99% | 18 | 77% | 22% |
| 197 | 52 | 52 | 100% | 58 | 81% | 19% |
| 59 | 59 | 99% | 18 | 76% | 23% | |
| 70 | 70 | 99% | 39 | 81% | 18% | |
| a90 | 90 | 97% | 106 | 85% | 12% | |
| 198 | a32 | 32 | 99% | 42 | 84% | 15% |
| 53 | 53 | 99% | 30 | 86% | 13% | |
| 56 | 56 | 99% | 66 | 84% | 15% | |
| 61 | 61 | 100% | mEV06c12b | 83% | 17% | |
| 66 | 66 | 98% | 56 | 83% | 15% | |
| 84 | 84 | 99% | 87 | 84% | 15% | |
| 200 | 53 | 53 | 98% | 30 | 85% | 13% |
| 54 | 54 | 99% | 45 | 74% | 25% | |
| 81 | 81 | 99% | 62 | 84% | 15% | |
| 83 | 83 | 95% | 102 | 82% | 13% | |
| Average % identity of the best hit | 98% | Average difference | 15% | |||
a HPV types not detected by LA
Table 8 summarizes HPV status of each cytology grading. For high-grade and low-grade squamous intraepithelial lesion (HSIL and LSIL), nearly all specimens were positive for high-risk HPV (HSIL: 4/4, 100%; LSIL: 16/18, 88.89%). For atypical squamous/ glandular cells, about half of the specimens were positive for high-risk HPV (by LA: 19/41, 46.34%; by Nanopore: 18/41, 43.90%). For cases without observable abnormalities, 22.12% (25/113) and 21.24% (24/113) were positive for high-risk HPV by LA and Nanopore, respectively.
Table 8.
Results of Pap smear, LA and Nanopore workflow. The calculations were based 176 quality control-valid specimens with Pap smear results available
| Pap smear interpretation | HPV status | No. of specimens | |
|---|---|---|---|
| LA | Nanopore | ||
| HSIL (n = 4) | HR/ HR + non-HR | 4 | 4 |
| Non-HR only | 0 | 0 | |
| Negative | 0 | 0 | |
| LSIL/ LSIL + ASCH (n = 18) | HR/ HR + non-HR | 16 | 16 |
| Non-HR only | 1 | 1 | |
| Negative | 1 | 1 | |
| AGUS/ ASCH/ ASCUS (n = 41) | HR/ HR + non-HR | 19 | 18 |
| Non-HR only | 3 | 6 | |
| Negative | 19 | 17 | |
| NIL (n = 113) | HR/ HR + non-HR | 25 | 24 |
| Non-HR only | 18 | 18 | |
| Negative | 70 | 71 | |
Discussion
Hong Kong has been one of the Asian regions with the lowest incidence and mortality rate of CC [16]. This might be attributable to the territory-wide cervical screening program implemented by Department of Health since 2004. The program is well-organized, which involves public education, regular cervical smear and follow-up service for eligible women, and a quality assurance mechanism on key components of the program [17]. Cytology is the mainstay of primary screening, and high-risk HPV testing may be performed for triage to colposcopy.
Cytology and HPV testing have their own value for CC screening. High quality cytology has high specificity for CC, but with lower sensitivity ranging from 50% suggested by cross-sectional studies to 75% estimated longitudinally [18]. For HPV testing, the sensitivity was reported to be about 10% higher than cytology, yet with lower specificity [18]. Complementary use of both tests could enhance the sensitivity approaching 100% with high specificity (92.5%) [19]. In fact, this combined approach has been adopted by several European countries and may become the future trend of primary CC screening in developed countries.
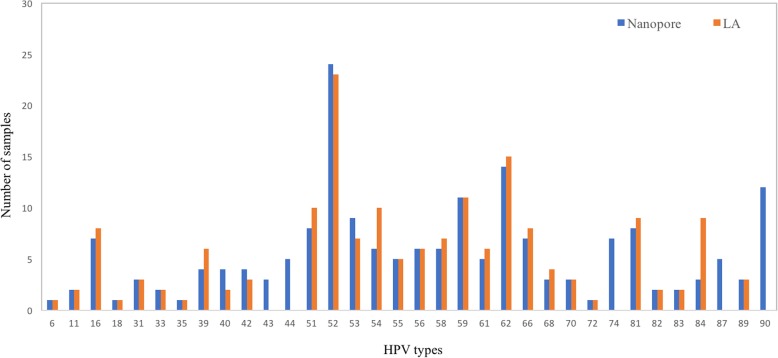
Compared with HPV assays in the market, HPV genotyping by NGS offers a broader detection spectrum which, despite minimal benefit of non-high risk HPV information for CC screening, may provide important etiologic clues for other HPV-associated infections and a more complete picture of HPV epidemiology. For the latter, Nanopore identified more HPV types per sample (Fig. 1) and 5 extra HPV types (HPV 43, 44, 74, 87 and 90, n = 34) not detectable by LA (Fig. 2), with an unexpected high incidence of HPV 90 (n = 12) which was reported in North America and Belgium but not in Hong Kong [20, 21]. Another advantage offered by NGS is its potential utility for simultaneous characterization of cervicovaginal microbiome, with its possible role in dysplasia and carcinogenesis revealed by accumulating research evidence [22–25]. These merits may facilitate a multifaceted approach for evaluation of woman health in near feature.
Fig. 1.

Number of HPV types detected per sample by Nanopore workflow and LA
Fig. 2.
Diversity of HPV types detected by Nanopore workflow and LA
In general, Nanopore had substantial agreement with cobas HPV Test and LA. Compared with cobas HPV Test, Nanopore appeared to be more sensitive for HPV 52 (n = 7) and 59 (n = 4), with 81.82% (9/11) of these discrepant results matched with LA. Compared with LA, concordance for high-risk HPV was higher than non-high risk types. Among the 37 discrepant results, 22 were false negatives by Nanopore and 15 were not detected by LA.
For the false negatives by Nanopore, more than half (12/22, 54.55%) were mixed infections, and similar finding was reported by other research groups using HPV consensus primers for NGS-based genotyping [10, 11]. Other possible causes of false negatives included (1) low viral load, as evident by Specimen 182, from which HPV 16 was missed by both Nanopore and cobas HPV Test; (2) substantial difference in DNA input (50 μL for LA versus 5 μL for PGMY/ MGP PCR), as well as (3) lower sensitivity due to reduced magnesium chloride concentration of PGMY PCR (from 4 mM to 1.5 mM), which was fine-tuned for minimal non-specific amplification.
For the 15 HPV types missed by LA, the average identity of Nanopore consensus sequences was 98.27% with an average difference of 16% from second BLAST hits (Table 7). As distinct HPV types generally have more than 10% difference in L1 sequence [26, 27], it appeared that the discrepant positive calls were less likely caused by high sequencing error rate of Nanopore. More specifically, 5 of these positive calls were identified solely by MGP PCR (5/15, 33.33%), 5 detected by PGMY PCR only (5/15, 33.33%), and 5 by both PCRs (5/15, 33.33%). These revealed differential sensitivities of PGMY and MGP PCR primers, which might complement with each other and enhance overall performance of the Nanopore assay. On the other hand, Nanopore sequencing might improve the resolution of genotyping, which might not be attained by line blot method due to cross-hybridization of certain probes. For instance, Nanopore identified HPV 52 in Specimen 5, 40 and 74, which could not be confirmed by LA due to cross-hybridization with HPV 33 and 58, respectively. Another example was Specimen 125, which was HPV 84-positive by LA and HPV 87-positive by Nanopore. From literature, Artaza-Irigaray and colleagues reported cross-hybridization between these 2 HPV types by LA, with 11.5% of HPV 84-positive cervical specimens by LA were actually HPV 87-positive by NGS [28].
The Nanopore method and LA revealed very similar high-risk HPV positivity in each cytology grading. The goal of combined cytology-HPV testing approach is to enhance cost effectiveness of CC screening. While minimizing unnecessary referral for colposcopy, HPV genotyping may identify high-risk individuals before observable cytological abnormalities, for instance, the 4 HPV 16-positive patients without abnormal cytology findings in this study. This may facilitate an early detection approach for cancer prevention.
Our study had several limitations. First, the sample size of certain HPV types, for example, HPV 18 (n = 1), was less satisfactory for evaluating type-specific performance. Second, as residual DNA was used after routine testing, DNA input for PGMY and MGP PCRs was constrained which might lower the sensitivity. In addition, as flow cells with suboptimal number of active pores were used, sequencing time and depth might be further improved if new flow cells were used.
Conclusions
We developed a Nanopore workflow for HPV genotyping, with performance comparable to or better than 2 reference methods in the market. Our method was economical, with a reagent cost of about USD 50.77 per patient specimen for 24-plex runs, which was competitive when compared to an average price of USD 106.14 (from 4 randomly-selected laboratories) for HPV genotyping referral service in our region (Table 9). The protocol was also straightforward with reasonable turnaround time of about 12 h from samples to answers. The small size and portability of MinION sequencers may well suit remote or resource-limited laboratories with constraints in space. Future prospective study with larger sample size is warranted to further evaluate test performance and streamline the protocol. As LA was discontinued in Hong Kong, the Nanopore workflow described here may provide an economical option for broad-range HPV genotyping.
Table 9.
Comparison of estimated reagent cost of Nanopore workflow (24-plex) and randomly-selected prices of HPV genotyping referral service in Hong Kong
| This study | ||
|---|---|---|
| Procedure | Number of specimens | Cost |
| DNA extraction and PCRs | 201 patients +20 controls = 221 | USD 20.02 × 221 reactions = USD 4424.42 |
| Nanopore sequencing |
115 patients / 24 = at least 5 runs N = 120 for 1 positive control per run |
USD 1155.94 × 5 runs = USD 5779.70 |
| Cost per patient specimen | (4424.42 + 5779.70) / 201 = USD 50.77 | |
| Referral service (transportation cost not included) | ||
| Lab A | USD 77.19 | |
| Lab B | USD 124.79 | |
| Lab C | USD 101.63 | |
| Lab D | USD 120.93 | |
| Average | USD 106.14 | |
Acknowledgements
We thank the colleagues of Department of Pathology, Hong Kong Sanatorium & Hospital for their dedicated and professional work on routine laboratory diagnostics.
Abbreviations
- CC
Cervical cancer
- HPV
Human papillomavirus
- HSIL
High-grade squamous intraepithelial lesion
- IARC
International Agency for Research on Cancer (IARC)
- LA
Roche Linear Array HPV Genotyping Test
- LSIL
Low-grade squamous intraepithelial lesion
- NGS
Next-generation sequencing
- Pap smear
Papanicolaou smear
- PCR
Polymerase chain reaction
- WHO
World Health Organization
Authors’ contributions
BSFT, TLC and WSC conceived and designed the study. BSFT, ESKM, MKMC, TLC, CPL, CHA, MYT, MKN, SML and WSC were involved in data collection and analysis. WSC wrote the first draft. All authors critically reviewed and approved the manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by Research Ethics Committee (REC) of Hong Kong Sanatorium & Hospital under the reference number RC-2019-18. No patient-identifying data was collected throughout the whole study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers – a brief historical account. Virology. 2009;384:260–5. [DOI] [PubMed]
- 2.Meisels A, Fortin R. Condylomatous lesions of the cervix and vagina. I Cytologic patterns Acta Cytol. 1976;20:505–509. [PubMed] [Google Scholar]
- 3.Purola E, Savia E. Cytology of gynecologic condyloma acuminatum. Acta Cytol. 1977;21:26–31. [PubMed] [Google Scholar]
- 4.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 5.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Human papillomaviruses. IARC Monogr Eval Carinog Risks Hum. 2007;90:1–636. [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffman M, Clifford G, Buonaguro FM. Classification of weakly carcinogenic human papillomavirus types: addressing the limits of epidemiology at the borderline. Infect Agent Cancer. 2009;4:8. doi: 10.1186/1750-9378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. Cervical cancer screening programs in Europe: the transition towards HPV vaccination and population-based HPV testing. Viruses. 2018;10:729. [DOI] [PMC free article] [PubMed]
- 8.Petry KU, Barth C, Wasem J, Neumann A. A model to evaluate the costs and clinical effectiveness of human papilloma virus screening compared with annual Papanicolaou cytology in Germany. Eur J Obstet Gynecol Reprod Biol. 2017;212:132–139. doi: 10.1016/j.ejogrb.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Pan American Health Organization. Section 2: Summary of commercially available HPV tests. https://www.paho.org/hq/dmdocuments/2016/manual-VPH-English-02.pdf (2016). Accessed 3 Jan 2020.
- 10.Nilyanimit P, Chansaenroj J, Poomipak W, Praianantathavorn K, Payungporn S, Poovorawan Y. Comparison of four human papillomavirus genotyping methods: next-generation sequencing, INNO-LiPA, electrochemical DNA Chip, and nested-PCR. Ann Lab Med. 2018;38:139–146. doi: 10.3343/alm.2018.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak RG, Ambulos NP, Schumaker LM, Mathias TJ, White RA, Troyer J, et al. Genotyping of high-risk anal human papillomavirus (HPV): ion torrent-next generation sequencing vs. linear array. Virol J. 2017;14:112. doi: 10.1186/s12985-017-0771-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner S, Roberson D, Boland J, Yeager M, Cullen M, Mirabello L, et al. Development of the TypeSeq assay for detection of 51 human papillomavirus genotypes by next-generation sequencing. J Clin Microbiol. 2019;57:e01794–18. [DOI] [PMC free article] [PubMed]
- 13.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Söderlund-Strand A, Carlson J, Dillner J. Modified general primer PCR system for sensitive detection of multiple types of oncogenic human papillomavirus. J Clin Microbiol. 2009;47:541–546. doi: 10.1128/JCM.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marín M, Garcia-Lechuz JM, Alonso P, Villanueva M, Alcalá L, Gimeno M, et al. Role of universal 16S rRNA gene PCR and sequencing in diagnosis of prosthetic joint infection. J Clin Microbiol. 2012;50:583–589. doi: 10.1128/JCM.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Department of Health, the Government of the Hong Kong Special Administrative Region. Evidence for organized screening programme. https://www.cervicalscreening.gov.hk/english/about/abt_evidence.html (2013). Accessed 15 Jan 2020.
- 17.Department of Health, the Government of the Hong Kong Special Administrative Region. About Cervical Screening Programme. https://www.cervicalscreening.gov.hk/english/about/about.html (2013). Accessed 15 Jan 2020.
- 18.World Health Organization: Cervical cancer screening in developing countries. Report of a WHO consultation. https://apps.who.int/iris/bitstream/handle/10665/42544/9241545720.pdf;jsessionid=2599A27FFB141B755D015B645FB889D9?sequence=1 (2002). Accessed 15 Jan 2020.
- 19.Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357:1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 20.Quiroga-Garza G, Zhou H, Mody DR, Schwartz MR, Ge Y. Unexpected high prevalence of HPV 90 infection in an underserved population: is it really a low-risk genotype? Arch Pathol Lab Med. 2013;137:1569–1573. doi: 10.5858/arpa.2012-0640-OA. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt M, Depuydt C, Benoy I, Bogers J, Antoine J, Arbyn M, et al. Prevalence and viral load of 51 genital human papillomavirus types and three subtypes. Int J Cancer. 2013;132:2395–2403. doi: 10.1002/ijc.27891. [DOI] [PubMed] [Google Scholar]
- 22.Mitra A, Maclntyre DA, Lee YS, Smith A, Marchesi JR, Lehne B, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, Park SY, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21:674. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Klein C, Gonzalez D, Samwel K, Kahesa C, Mwaiselage J, Aluthge N, et al. Relationship between the cervical microbiome, HIV status, and precancerous lesions. MBio. 2019;10:e02785–e02718. doi: 10.1128/mBio.02785-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nené NR, Reisel D, Leimbach A, Franchi D, Jones A, Evans I, et al. Association between the cervicovaginal microbiome, BRCA1 mutation status, and risk of ovarian cancer: a case-control study. Lancet Oncol. 2019;20:1171–1182. doi: 10.1016/S1470-2045(19)30340-7. [DOI] [PubMed] [Google Scholar]
- 26.King AJ, Sonsma JA, Vriend HJ, van der Sande MA, Feltkamp MC, Boot HJ, et al. Genetic diversity in the major capsid L1 protein of HPV-16 and HPV-18 in the Netherlands. PLoS One. 2016;11:e0152782. doi: 10.1371/journal.pone.0152782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burk RD, Harari A, Chen Z. Human papillomavirus genome variants. Virology. 2013;445:232–243. doi: 10.1016/j.virol.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artaza-Irigaray C, Flores-Miramontes MG, Olszewski D, Vallejo-Ruiz V, Limón-Toledo LP, Sánchez-Roque C, et al. Cross-hybridization between HPV genotypes in the linear Array genotyping test confirmed by next-generation sequencing. Diagn Pathol. 2019;14:31. doi: 10.1186/s13000-019-0808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.