Studies of P. patens, a powerful non-seed plant model system, have made fundamental contributions to diverse fields, including evolutionary developmental and cell biology.
Abstract
Since the discovery two decades ago that transgenes are efficiently integrated into the genome of Physcomitrella patens by homologous recombination, this moss has been a premier model system to study evolutionary developmental biology questions, stem cell reprogramming, and the biology of nonvascular plants. P. patens was the first non-seed plant to have its genome sequenced. With this level of genomic information, together with increasing molecular genetic tools, a large number of reverse genetic studies have propelled the use of this model system. A number of technological advances have recently opened the door to forward genetics as well as extremely efficient and precise genome editing in P. patens. Additionally, careful phylogenetic studies with increased resolution have suggested that P. patens emerged from within Physcomitrium. Thus, rather than Physcomitrella patens, the species should be named Physcomitrium patens. Here we review these advances and describe the areas where P. patens has had the most impact on plant biology.
Introduction: Development of P. patens as a Model Species
P. patens Gransden was established as a model species based on cultures derived from a single spore of a sample collected by H.L.K. Whitehouse from a site in Gransden Wood (Huntingdonshire, UK) in 1962. From the 1960s to the 1980s, this moss was primarily used as a genetic system to isolate and study mutants in a variety of processes including plant morphology, hormone biology, nutrition, and responses to gravity (Engel, 1968; Ashton and Cove, 1977; Ashton et al., 1979; Cove, 1983). Early studies on the effects of phytohormones (Reski and Abel, 1985; Knight et al., 1995; Machuka et al., 1999) revealed that the action of abscisic acid (ABA) was conserved throughout the evolutionary history of land plants (Khandelwal et al., 2010; Richardt et al., 2010). In 1997 (Schaefer and Zrÿd, 1997) reported that gene targeting via homologous recombination was feasible in P. patens, triggering an immediate generation of mutants (Girke et al., 1998), which, following the development of a method to induce sexual reproduction (Hohe et al., 2002) and initial transcriptomic characterization (Rensing et al., 2002), raised this moss to functional genomics model status (Reski and Reg, 2004; Cove, 2005; Frank et al., 2005). Due to its phylogenetic position as a member of the sister linage to vascular plants and thus other established model species, P. patens was soon targeted for genome sequencing (see Genetics and Genomics of P. patens) and destined to become a model for evolutionary developmental and cell biological studies (see Hot Topics in P. patens Research).
Systematics, Ecology, and Geography of P. patens
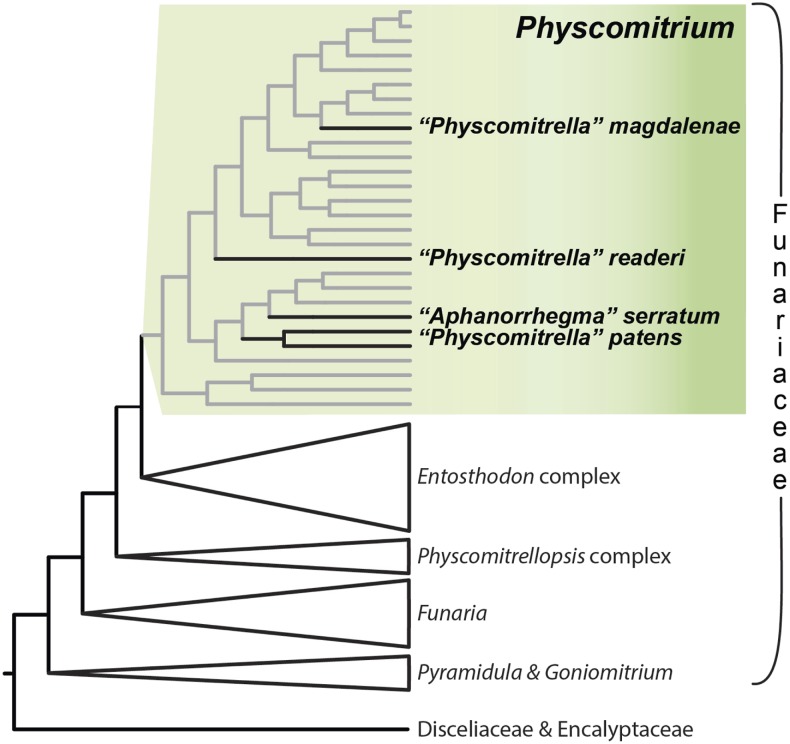
Physcomitrella belongs to the Funariaceae within the Funariales, a diverse lineage of small, typically once subapically branched plants with terminal sex organs, possessing a sporophyte with a capsule dehiscing via the loss of a lid, and two opposite sets of peristome teeth lining the capsule mouth (Vitt, 1984). Physcomitrella is, however, somewhat atypical for the family, as it lacks both a peristome and a differentiated line of sporangial dehiscence. Physcomitrella is a rather derived member of the family (Figure 1), having arisen ∼10 million years ago (Beike et al., 2014; Medina et al., 2019), and may be sister to a clade comprising Aphanorrhegma serratum (now Physcomitrium serratum), Physcomitrium immersum (both with immersed capsules), and Physcomitrium collenchymatum and Physcomitrium sphaericum (both with capsules emerging on a stalk from among the leaves; Medina et al., 2019).
Figure 1.
Summarized Phylogeny of the Funariaceae.
The phylogeny was inferred from variation in 648 target capture nuclear exons, highlighting the polyphyletic distribution of Physcomitrella s. lat. (including Aphanorrhegma) within Physcomitrium (Medina et al., 2019). All relationships between main lineages represented (i.e., genera), except Entosthodon (i.e., bootstrap between 90 and 100%) are maximally supported under maximum likelihood analyses of a concatenated alignment of the nuclear loci, concordance among a majority of gene trees inferred from exons and their flanking regions, and a coalescence-based gene tree summary analysis (see Medina et al. [2019] for details).
Physcomitrella patens was originally recognized by Hedwig (1801) in the genus Phascum (now Tortula, Pottiaceae) due to its small and immersed capsules lacking a peristome. Subsequently, the species was transferred to the genus Ephemerum (Hampe, 1837), a phenotypically similar genus long considered to be allied to the Funariaceae (Vitt, 1984) but subsequently recognized as resulting from convergent evolution in the Pottiaceae (Goffinet and Cox, 2000). In 1849, Physcomitrella was established as a single species, P. patens (Bruch and Schimper, 1849). Subsequently, in 1864, the species was moved to Aphanorrhegma (Lindberg, 1864), a then monospecific genus established by (Sullivant, 1848) as an eastern North American endemic species resembling P. patens except for its equatorial line of dehiscence of the sporangium. In 1851, an argument was made for accommodating P. patens in Physcomitrium (Mitten, 1851), a hypothesis congruent with phylogenetic (Liu et al., 2012; Beike et al., 2014) and phylogenomic inferences (Medina et al., 2018, 2019), whereby the species (Beike et al., 2014) or subspecies of Physcomitrella sensu (Tan, 1979) do not share a unique common ancestor (Liu et al., 2012) but arose independently from within Physcomitrium (Figure 1; Beike et al., 2014; Medina et al., 2018, 2019). Consequently, all taxa of Physcomitrella were transferred to Physcomitrium, and the correct name for Physcomitrella patens is thus Physcomitrium patens Mitten (Medina et al., 2019). The species is also known by its common name, the spreading earthmoss (Edwards, 2012).
Distribution
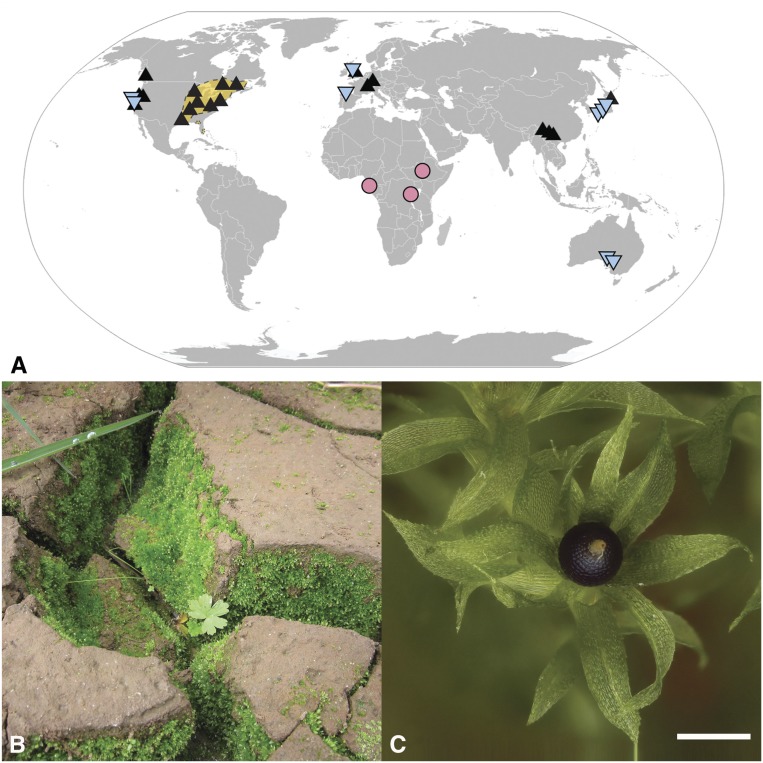
Physcomitrium (previously Physcomitrella) patens occurs in Europe, North America, and East Asia (Medina et al., 2015; Higuchi and Takahashi, 2012), whereas Physcomitrium readeri (including Physcomitrium californica) is disjunct among Southern Australia, Western North America, Japan, and Western Europe (Figure 2; Medina et al., 2015). Such ranges are likely the result of a rather recent expansion (Beike et al., 2014). By contrast, P. magdalenae is endemic to Africa, where it is known to occur in three localities at equatorial latitudes (Figure 2; Beike et al., 2014). Physcomitrium serratum (formerly Aphanorrhegma serratum) is endemic to Eastern North America (Goffinet, 2007a). All these species grow in moist open soil along paths or in fields, or in seasonally wet areas such as flood plains of lakes and edges of rice fields, at low to moderate elevations.
Figure 2.
P. patens.
(A) Geographic distribution (black triangle), contrasted to the distribution of P. readeri including P. californica (blue triangles), P. magdalenae (pink circles), and P. serratum (orange area). Map adapted from Medina et al. (2015) based on specimens examined by Goffinet (2007a, 2007b) complemented by reports by Faubert (2013) and Higuchi and Takahashi (2012).
(B) Example of an ecological habitat of Physcomitrium patens (i.e., lake floodplain in Yunnan China).
(C) A gametophore with a single terminal mature sporophyte. Scale bar = 1 mm.
Anatomy and Morphology of P. patens
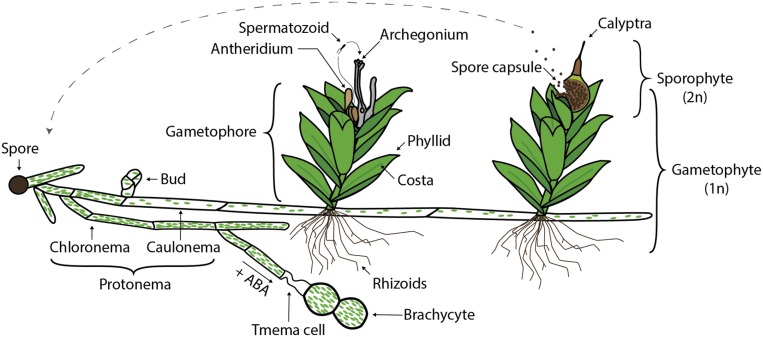
Like all land plants, mosses possess a haplodiplontic life cycle in which both generations, the haploid gametophyte and the diploid sporophyte, are multicellular (reviewed in Rensing, 2016). However, unlike all extant polysporangiophytes (e.g., seed plants), the dominant moss generation is the haploid phase, and the sporophytic phase is monosporangiate (i.e., the sporophyte bears a single terminal capsule). Starting from the germination of the spore, i.e., the juvenile phase of the gametophyte, the protonema develops (Figures 3 and 4). Protonemata are tip-growing filaments that emerge and spread by branching and apical extension and are composed of two major cell types: chloronema and caulonema. Chloronema, the most basal cell type to emerge from the germinating spore, are rich in chloroplasts and have cell plates that are transverse to the long axis of the cell. The apical cell continues to divide, and several days after germination it transitions into a caulonemal cell, which has obliquely positioned cell plates, initially has fewer chloroplasts, and is typically thinner and grows faster than the chloronema. In the absence of light but in the presence of a carbon source, only caulonemal cells grow against the gravity vector (Cove et al., 1978) and are then also referred to as skotonema. Caulonemata are reminiscent of fungal runner hyphae and may serve to cover long distances. Under the action of ABA, vegetative diaspores (brachycytes or brood cells) develop (Arif et al., 2019), while the formation of specialized side branch initials (so-called buds) represent the transition to the three-dimensional growth phase leading to the hormone-controlled development of gametophores, the leafy shoots of the moss plant (Reski and Abel, 1985; Harrison et al., 2009; Coudert et al., 2015).
Figure 3.
Life Cycle of P. patens.
Modified and reproduced from Strotbek et al. (2013), doi.org/10.1387/ijdb.130189wf with the permission of UPV/EHU Press.
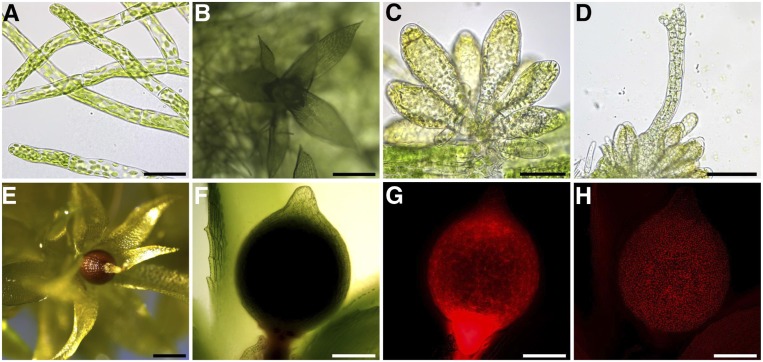
Figure 4.
Anatomy of P. patens during Different Developmental Stages.
(A) and (B) Tip-growing filamentous protonemata eventually develop buds (A), which grow into leafy gametophores (B).
(C) and (D) Under cold- and short day conditions, sexual reproductive organs (gametangia) develop on the top (apex) of the gametophore.
(C) The male antheridia, which release motile biflagellate spermatozoids upon maturity, emerge in bundles comprising different developmental stages of antheridia.
(D) The female archegonia arise next to the male organs and are flask-shaped.
(E) to (H) The egg is located in the archegonial venter and after fertilization, a sporophyte develops.
(E) Upon maturity, the brown sporophyte releases spores of the next generation.
(F) and (H) A heterozygote sporophyte of the fluorescent Reute-mCherry strain and the nonfluorescent Gransden strain.
(G) mCherry-fluorescent sporophyte is shown on top of the nonfluorescent gametophore, indicating a successful cross of both strains.
(H) Chloroplast autofluorescence is visible in the sporophyte as well as the leaflets.
Scale bars (A) and (C) = 50 μm; (B), (F), (G), and (H) 200 μm; (D) 100 μm; and (E) 500 μm.
Buds develop into erect, foliate gametophores. Gametophores are structured into a stem and phyllids (also called leaflets; nonvascular leaves). Both structures may possess specialized cell types for water and nutrient transport whose formation is controlled by NAC transcription factors, a process similar to sporophytic vasculature development in vascular plants (Xu et al., 2014). The stem is short and considered to be unbranched (see Coudert et al., 2017), and its inner cells are differentiated in an axial or central strand of long, narrow, thin-walled cells. The leaves are few, spirally inserted, and spreading when moist. They are typically ovate lanceolate to obovate, with a short, slender apex. The blade is unistratose (comprising a single cell layer) except for the median region, which is differentiated into a costa or midrib extending two-thirds up the leaf. The laminal cells vary from rectangular to rhombic in shape, and are chlorophyllose throughout.
Under short day conditions (Hohe et al., 2002), gametangiogenesis occurs, yielding the male and female gametangia mixed in a single cluster at the apex of now adult gametophores (Landberg et al., 2013; Hiss et al., 2017). Individuals are bisexual, with male (antheridia) and female (archegonia) gametangia split between apical clusters (Goffinet, 2007b). In culture, mixed sex organs have rarely been observed (Nakosteen and Hughes, 1978). Instead, antheridia initially develop at the apex of the stem, which subsequently resume growth to develop archegonia, relegating the antheridia to an axillary position (Figure 4). Thus, under optimal growth conditions, male and female sex organs are terminal, but when vegetative growth resumes after male gametangiogenesis, female sex organs may appear to be present in a distinct cluster. Moss male gametes are biflagellate and require liquid water to swim into the archegonia (Renzaglia and Garbary, 2001). After passing the archegonial neck canal cells and reaching the archegonial venter (base), the sperm cells (also known as spermatozoids or antherozoids) fertilize the egg cell to form the diploid zygote. The zygote develops into an embryo and then into a sporophyte, which possesses a single apical capsule in which meiosis eventually occurs, yielding haploid spores (Landberg et al., 2013; Hiss et al., 2017). A single sporophyte typically develops on a gametophore (Figure 4). The sporophyte is composed of a short stalk terminated by a spherical capsule covered by a small fleeting calyptra (thin hood) and bearing stomata defined by a single guard cell in its lower portion. A differentiated line of dehiscence is lacking, and the mature capsule opens irregularly to free the small unicellular spores. The sporophyte is anchored to the gametophore by a foot, with transfer cells at the intersection of the two generations insuring the movement of nutrients (Regmi et al., 2017). The spores are covered in part by sporopollenin, a compound also found in male gametophytic pollen of seed plants (Daku et al., 2016).
Genetics and Genomics of P. patens
As outlined above, the phylogenetic position of the mosses between the (then already sequenced) genomes of Arabidopsis (Arabidopsis thaliana)/rice (Oryza sativa)/poplar (Populus trichocarpa) and Chlamydomonas reinhardtii prompted the idea to sequence the P. patens genome. At the Seventh Annual Moss International Conference, MOSS 2004, in Freiburg, Germany, a genome consortium was formed, and the P. patens genome was subsequently sequenced by the U.S. Department of Energy Joint Genome Institute. The nuclear genome was published in 2008 (Rensing et al., 2008) and lived up to the expectations that its comparison with the other available plant genomes would allow key events in land plant evolution to be traced. The plastid genome was published by Sugiura et al. (2003) and the mitochondrial genome by Terasawa et al. (2007), completing the draft genomic resources.
Since 2010, P. patens has been one of the U.S. Department of Energy flagship genomes (https://jgi.doe.gov/our-science/science-programs/plant-genomics/plant-flagship-genomes/), and more genomic and transcriptomic resources continue to be developed. The genome assembly, previously consisting of ∼2,000 scaffolds and containing ∼5% gap regions, was recently brought to the pseudo-chromosomal level, with most of the sequence data represented in 27 pseudochromosomes (with 1% remaining gaps), as well as updated plastid and mitochondrial genomes (Lang et al., 2018). Moreover, substantial transcriptomic evidence was generated using RNA sequencing (RNA-seq; Perroud et al., 2018; Meyberg et al., 2020), complementing microarray-based expression profiling datasets (Hiss et al., 2014; Ortiz-Ramírez et al., 2016) and epigenetic data (Lang et al., 2018; Widiez et al., 2014). The most recent gene annotation (v3.3) used large-scale RNA-seq data from the P. patens gene atlas project as expression evidence. This release contains 32,458 gene models and 86,669 protein-coding transcripts and is available from different sources (Table 1), with CoGe and Phytozome being the primary repositories.
Table 1. Advances in Genome Editing.
| Technique | Consequences | References |
|---|---|---|
| Transient expression of Cas9 and guide RNA | Nonhomologous end joining (NHEJ) repair leads to indels and frame shifts | (Collonnier et al., 2017) |
| Transient expression of Cas9 and guide RNAs targeting multiple genomic sites | NHEJ repair leads to indels and frame shifts | (Lopez-Obando et al., 2016; Mallett et al., 2019) |
| Transient expression of Cas9 and guide RNA together with a homology plasmid | HDR enables the insertion of sequences encoding fluorescent proteins and small cassette containing stop codons in all possible frames to ensure that the stop codon is close to the protospacer site | (Mallett et al., 2019) |
| Transient expression of Cas9 and guide RNA together with homology oligos | HDR enables the insertion of stop codons from the oligos to ensure that the stop codon is close to the protospacer site | (Yi and Goshima, 2019) |
| Transient expression of LbCas12A and multiple CRISPR RNAs | Efficient method to multiplex target sites use the CRISPR RNAs; NHEJ results in diverse deletions | (Pu et al., 2019) |
| Transient expression of SpCas9-NG and guide RNA | Makes it possible to perform base editing by relaxing the stringency of the Protospacer Adjacent Motif sequence requirement | (Veillet et al., 2020) |
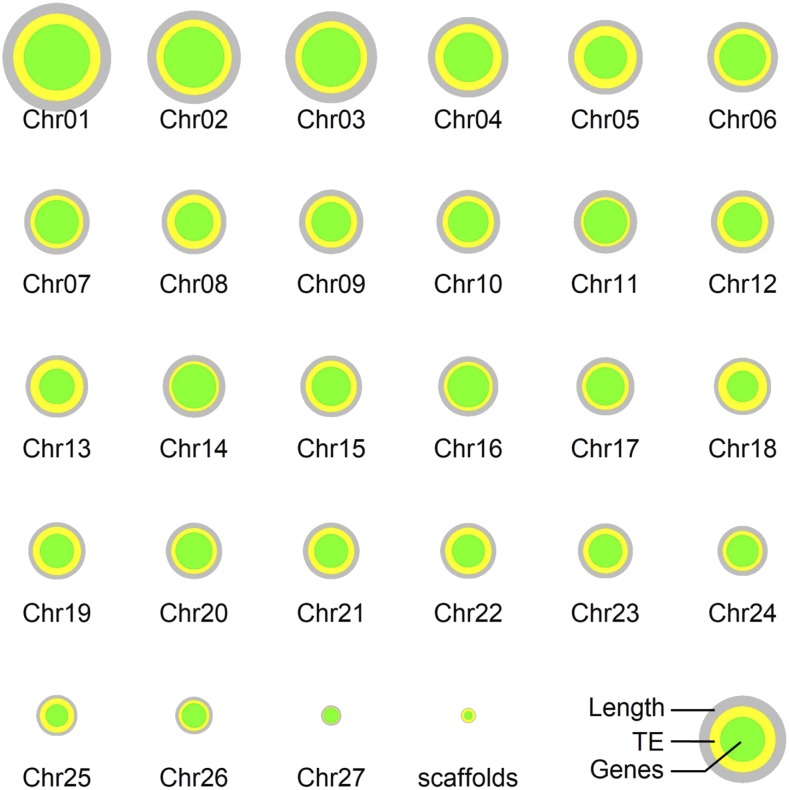
The analysis of the most recent genome assembly (Figure 5) revealed that, contrary to most flowering plants, the distribution of genes and transposable elements (TE) is homogeneous along the P. patens chromosomes (Lang et al., 2018). This is mirrored by a homogeneous distribution of recombination rates, which is potentially rooted in the observation that P. patens is predominantly selfing. Interestingly however, the purging of deleterious mutations works efficiently in this moss (Szövényi et al., 2013). An analysis of the pseudochromosomal assembly also suggested that giant viruses (nucleocytoplasmic large DNA viruses [NCLDV]) embedded into the genome become transcriptionally active during gametogenesis and protect the gametes from viral infection via siRNA-mediated silencing. In terms of epigenetics, an analysis of histone modifications suggested that protonemal tissue is epigenetically primed for the drought stress response (a condition that likely affects the gametophore stage), potentially representing an adaptation to the P. patens lifestyle (Widiez et al., 2014). In contrast to flowering plants, where genes carrying methylation in the gene body are expressed, most genes showing DNA methylation within their gene bodies in P. patens are not expressed (Lang et al., 2018).
Figure 5.
P. patens Chromosomes.
Visualization of the V3 pseudochromosomal nuclear genome assembly and v3.3 annotation (Lang et al., 2018). Each chromosome is represented by a gray circle, the area of which corresponds to the length of the chromosome. Concentric circles reflect the relative amounts of TEs (yellow) and genes (green), with the areas of each circle corresponding to the total length. The 330 unordered scaffolds are represented as an extra green and yellow circle. Figure courtesy of Fabian Haas.
Resources and Databases
Plant material
For a long time, the P. patens Gransden accession going back to the 1962 Whitehouse isolate was exclusively used for analysis. This accession was mainly distributed vegetatively and spread to laboratories around the world. However, many laboratories observed a severe loss of fertility in their Gransden strains over time, whereas others are still fertile. To overcome the problem of low fertility, a new ecotype, Reute, was recently introduced (Hiss et al., 2017). The use of Reute allows sexual reproduction to be analyzed with ease. A multi-omic comparison of Reute versus Gransden revealed the accumulation of (epi-)mutations that affect the fertility of the male germline in Gransden (Meyberg et al., 2020). Moreover, fluorescent mutant strains are available (Perroud et al., 2011, 2019) that allow for routine crossing and make it easy to analyze 1:1 segregation of the F1 generation. Another ecotype, Villersexel, is genetically more divergent and has been used to generate the genetic map used for the V3 genome assembly (Kamisugi et al., 2008; McDaniel et al., 2010; Lang et al., 2018). The genetic variability of Gransden, Reute, Villersexel and another accession, Kaskaskia, was revealed using deep sequencing. Many more accessions have also been used in several studies (von Stackelberg et al., 2006; McDaniel et al., 2010; Liu et al., 2012; Szövényi et al., 2013; Beike et al., 2014; Medina et al., 2015, 2018, 2019). These accessions are available from the authors, as well as the International Moss Stock Center (http://www.moss-stock-center.org/).
Databases
The main repository for the most recent P. patens genome assembly is CoGe (Table 2). In addition to the genome sequence (nuclear, plastid, and mitochondrion), this assembly contains annotation (genes, TEs), genetic variability (single nucleotide polymorphisms), and processed evidence from deep sequencing (transcript evidence, DNA methylation, and chromatin modification). In terms of comparative genomics, Phytozome allows cross-species gene families to be identified, PLAZA allows synteny (gene order) analysis to be performed, and TAPscan lists transcription factors and transcriptional regulators. Several tools make use of expression profiling data, namely Genevestigator (Hiss et al., 2014), the P. patens eFP browser (Ortiz-Ramírez et al., 2016), and most recently Phytozome (Perroud et al., 2018). The data present in these tools, as well as novel data, were recently integrated into a common web-based tool, PEATmoss (https://peatmoss.online.uni-marburg.de/ppatens_db/contact.php), which includes a gene model lookup database that allows different versions of gene annotations to be compared (Fernandez-Pozo et al., 2019).
Table 2. Key Features of P. patens and Online Resources.
| Category | Feature/Resource |
|---|---|
| Lifestyle | Haploid, gametophyte-dominant |
| Annual | |
| Grows axenically in simple mineral medium | |
| Self-fertile (predominantly selfing) | |
| Stress-tolerant | |
| Genetics and genomics | Can be crossed for genetic studies |
| Gene targeting by homologous recombination | |
| Genome editing by CRISPR/Cas9 | |
| Transfection of protoplasts and Agrobacterium-mediated transformation | |
| Complete genomes, relatively small nuclear genome (500 Mbp, 27 chromosomes) | |
| Transcriptome (expression profiling) data | |
| Many mutants published | |
| Genomes and primary annotation | Nuclear genome assembly V3 for browsing at CoGe (including gene models v3.3, single nucleotide polymorphisms, RNA-seq expression evidence, TE and noncoding RNA annotation, microarray probes, DNA methylation, and histone modification) |
| https://genomevolution.org/coge/GenomeInfo.pl?gid=33928 | |
| Nuclear, plastid, and mitochondrial genomes at CoGe | |
| https://genomevolution.org/coge/OrganismView.pl?gid=33928 | |
| Nuclear genome and gene models at Phytozome (including gene family assignment and RNA-seq expression evidence) | |
| https://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Ppatens | |
| P. patens gene model lookup database (for converting between different annotation versions) | |
| https://peatmoss.online.uni-marburg.de/ppatens_db/pp_search_input.php | |
| Online tools | P. patens transcription factors and transcriptional regulators in TAPscan |
| https://plantcode.online.uni-marburg.de/tapscan/TAPs_for_species.php?species=Physcomitrella%20patens&id=98 | |
| P. patens expression atlas PEATmoss (including microarray and RNA-seq dataset) | |
| https://peatmoss.online.uni-marburg.de/expression_viewer/input | |
| Older expression atlas tools include Genevestigator (https://genevestigator.com/gv/) and an eFP browser (http://www.bar.utoronto.ca/efp_physcomitrella/cgi-bin/efpWeb.cgi) | |
| P. patens in the PLAZA comparative genomics environment | |
| https://bioinformatics.psb.ugent.be/plaza/versions/plaza_v4_dicots/organism/view/Physcomitrella+patens | |
| https://bioinformatics.psb.ugent.be/plaza/versions/plaza_v4_monocots/organism/view/Physcomitrella+patens | |
| P. patens in ENSEMBL plants | |
| http://plants.ensembl.org/Physcomitrella_patens/Info/Index | |
| Protocols and stocks | Cold Spring Harbor protocols on cultivation of P. patens |
| http://cshprotocols.cshlp.org/content/2009/2/pdb.prot5136.abstract | |
| Protonema cultivation at Bio-protocol | |
| https://bio-protocol.org/e1556 | |
| PHYSCObase protocols | |
| http://moss.nibb.ac.jp/protocol.html | |
| International Moss Stock Center | |
| http://www.moss-stock-center.org/ | |
| Protocols on the Bezanilla Laboratory website | |
| https://sites.dartmouth.edu/bezanillalab/moss-methods/ |
Available Tools and Technologies
Transformation
To perform genetic manipulation, it is critical to be able to transfer DNA molecules of choice into the organism. P. patens protonemata can be transformed with DNA by particle bombardment. This method allows for the stable incorporation of DNA (Cho et al., 1999) as well as transient expression assays (Bezanilla et al., 2003; Marella et al., 2006). However, early on in the development of molecular genetics tools for P. patens, gene targeting events were achieved using polyethylene-glycol (PEG)-mediated transformation of protoplasts (Kammerer and Cove, 1996; Schaefer and Zrÿd, 1997; Strepp et al., 1998). Given the proper osmoticum, P. patens protoplasts can be efficiently regenerated into whole plants, allowing plants derived from a single protoplast harboring DNA molecules of interest to be isolated. Thus, PEG-mediated transformation has become the most widely used transformation method.
Reverse genetic approaches
For over two decades, researchers have taken advantage of the innately high frequencies of homologous recombination in P. patens to insert DNA molecules into specific sites in the genome. The insertion of sequences encoding fluorescent proteins has opened the door to analyzing the localizations of fusion proteins expressed from the native genomic context, which is critically important, as the overexpression of fusion proteins often leads to aberrant localization. Furthermore, the generation of gene deletions in P. patens has allowed gene functional analysis to be performed throughout development.
However, because of gene family expansion due to recent genome duplications (Lang et al., 2018), which is common in plants, the functions of medium to large gene families have been difficult to study using traditional knock-out approaches due to a large degree of functional redundancy. Furthermore, due to the predominant haploid state of P. patens, it is impossible to isolate knock-outs of essential genes by transforming haploid protoplasts. To overcome these limitations, researchers have employed gene silencing mediated by RNA interference (RNAi; Bezanilla et al., 2003, 2005; Nakaoka et al., 2012). The most common approach is to transiently express a DNA construct containing sequences targeting specific genes arranged in inverted repeats. Using this approach, multiple genes can be targeted simultaneously (Vidali et al., 2007). Transient expression of RNAi constructs in 7-d–old protonemata is a powerful approach to studying members of gene families (Vidali et al., 2007, 2009a) and essential genes (Augustine et al., 2008) involved in protonemal tissue development, particularly genes required for polarized growth. However, the stable integration of inverted repeat sequences does not occur at high frequency and thus, it can be challenging to obtain stable transformants containing functional silencing constructs. Thus, to analyze later stages of development, the expression of artificial microRNA constructs (Khraiwesh et al., 2010) and inducible expression using the estradiol-inducible system (Zuo et al., 2000; Kubo et al., 2013) for stably integrated RNAi constructs (Nakaoka et al., 2012) have been employed. However, while gene silencing approaches are often very effective, they can suffer from variable levels of silencing.
Recent advances in genome editing technologies (Table 1) capitalizing on CRISPR-Cas9–mediated double-stranded break formation are quickly overcoming the limitations of traditional homologous recombination and gene silencing approaches. The transient expression of a guide RNA and the Cas9 enzyme readily produces double-stranded breaks in P. patens, which are repaired by nonhomologous end-joining, invariably incorporating mutations that generate frame-shift mutations (Collonnier et al., 2017). Thus, by transforming P. patens with multiple guide RNAs and Cas9, multiple genes can be targeted simultaneously to generate higher-order knockouts in a single transformation (Lopez-Obando et al., 2016). To ensure that regenerating plants have been transformed with the plasmid containing Cas9 and/or the guide RNA, the plants can be subjected to selection for ∼7 d (Mallett et al., 2019). The selection pressure is then removed, and the plasmid is lost. After the plants have grown to a sufficient size, DNA is isolated from the plants to identify plants that have been edited. Editing efficiencies can be as high as 90% depending on the efficiency of the protospacer and the expression levels of the guide RNA and Cas9 (Mallett et al., 2019). Various techniques, such as T7 endonuclease assays (Mashal et al., 1995) and competition PCR (Harayama and Riezman, 2017), can be used to rapidly identify the edited sites.
When a homologous template is provided in addition to the guide RNA and Cas9, the double-stranded breaks are repaired from the homologous template, a process known as homology-directed repair (HDR), allowing precise modifications to be incorporated at the cut site. In P. patens, the homologous template can be delivered as super-coiled plasmid (Collonnier et al., 2017; Mallett et al., 2019) or as double-stranded oligos (Yi and Goshima, 2019). Using HDR, it is possible to seamlessly integrate any number of modifications, from single bp changes (Yi and Goshima, 2019) to the integration of sequences encoding fluorescent proteins (Mallett et al., 2019). The overexpression or inducible expression of a particular gene could be accomplished easily by changing the promoter sequence.
CRISPR-Cas9-mediated genome manipulation adds a powerful set of genome editing tools to an already genetically tractable system. In particular, CRISPR-Cas9 genome editing occurs without incorporating a selectable marker into the genome. The selection is only used to ensure that the plants have taken up the super-coiled plasmid enabling expression of the guide RNA and Cas9. After a week on selection, untransformed plants have died, and the selection pressure is no longer needed. Using HDR coupled to CRISPR-Cas9, the genome can be altered seamlessly, making tagging a gene with a fluorescent protein at the 5′ end trivial. In addition, super-coiled plasmids, which are easier to generate than linear DNA templates, act as efficient HDR templates. Finally, CRISPR-Cas9 editing provides an unbiased approach to uncovering hypomorphs of essential genes. With high rates of editing, null mutations in essential genes will result in very few transformants. However, by targeting the gene at multiple sites with several protospacers, it may be possible to identify viable edited plants with lesions that reduce the function of the protein. In addition to Cas9, a recent study demonstrated that CRISPR using the programmable DNA endonuclease LbCas12a efficiently edited the genome, expanding the tools available for gene editing in P. patens (Pu et al., 2019).
Forward genetic approaches
In 1968, Engel used treatment with X-rays, as well as chemical mutagens such as ethyl methanesulfonate, to isolate auxotrophic and morphological mutants in P. patens (Engel, 1968). Subsequent studies identified P. patens auxotrophs (Ashton and Cove, 1977), hormone-resistant mutants (Ashton et al., 1979), and mutants with altered responses to gravity (Cove et al., 1978). However, due to a lack of genetic mapping resources and the inability to clone by complementation, the causal lesions for many of these mutants were never identified. Prigge et al. (2010) took a candidate gene approach and identified the causal lesions in a number of mutants resistant to the synthetic auxin, 1-Naphthaleneacetic acid (NAA). More recently, the availability of a well-annotated genome coupled with marker lines in ecotypes that enable rapid identification of crossed sporophytes (Figure 4; Perroud et al., 2011, 2019) has paved the way for new genetic screens to identify causal mutations (Tables 3 and 4; Stevenson et al., 2016). In one such screen, Ding et al. (2018) mutagenized protoplasts with UV light and isolated conditional loss of growth mutants. Moody et al. (2018b) also used UV light mutagenesis to identify mutants no longer able to form gametophores. Without gametophores, however, it would not be possible to cross the mutant strain to a different ecotype to obtain a mapping population. To overcome this problem, Moody et al. (2018b) took advantage of somatic diploidization and performed a cross between two somatic diploids. Both of these groups then successfully identified the causal mutations using whole-genome sequencing approaches on plants derived from their mapping population (Ding et al., 2018; Moody et al., 2018b). In addition to mutagenic agents, the tobacco (Nicotiana tabacum) Tnt1 retrotransposon can be introduced into P. patens using PEG-mediated protoplast transformation (Vives et al., 2016) or Agrobacterium-)mediated mutagenesis (Mohanasundaram et al., 2019), which produced insertional mutations with a preference for genes and GC-rich regions in the genome (Vives et al., 2016; Mohanasundaram et al., 2019). These studies have laid the foundation for robust forward genetic approaches by greatly expanding the available tools and resources, making it possible to readily perform a variety of screens, such as enhancer and suppressor screens.
Table 3. Forward Genetic Approaches Using UV Light as a Mutagen.
| Screen | Identification of Lesion | References |
|---|---|---|
| Isolated ABA mutants | Crossed mutant plants to a distinct ecotype and performed whole-genome sequencing on pooled mutants derived from the cross | (Stevenson et al., 2016) |
| Temperature-sensitive mutant screen identifying mutants that are unable to grow protonemata at the restrictive temperature | Crossed mutant plants to a distinct ecotype and performed whole-genome sequencing on pooled mutants derived from the cross | (Ding et al., 2018) |
| Isolated mutants that were unable to form gametophores in an effort to find genes involved in the transition from two-dimensional to three-dimensional growth | Took advantage of the ability of moss to form somatic hybrids; crossed two somatic hybrids to generate a mapping population and performed whole-genome sequencing on mutants derived from the cross | (Moody et al., 2018a) |
Table 4. Forward Genetic Approaches Using the Tobacco Tnt1 Retrotransposon as a Mutagen.
| Transformation Method | Genomic Regions Showing Insertions | References |
|---|---|---|
| PEG-mediated transformation of protoplasts | Generated mutations in genic regions | (Vives et al., 2016) |
| Agrobacterium-mediated transformation | Generated mutations in genic regions and GC-rich regions | (Mohanasundaram et al., 2019) |
Imaging
Due to its small stature and anatomical simplicity, most P. patens tissues are amenable to microscopic observation (Figures 4 and 6). Protonemal tissue is a two-dimensional network of filaments comprising single cells arranged in linear branching arrays. The developing gametophore emerges from a protonemal filament as a single cell that undergoes a series of stereotypic cell divisions switching from polarized expansion to three-dimensional diffuse expansion (Harrison et al., 2009). Expanded gametophore phyllids (or leaflets) are only a single-cell-layer–thick, except for their midribs. Given the simplicity of these tissues, microscopic observation readily occurs without the need for dissection or sectioning. In fact, with the recent advent of microfluidic imaging devices, it is now possible to continuously observe development from juvenile to adult tissues under a microscope at high temporal and spatial resolution (Bascom et al., 2016). These microfluidic imaging devices are constructed from polydimethylsiloxane, which is highly permeable to gases, allowing for continuous plant growth in a small dish filled with liquid medium in the presence of light. With designs that allow for protoplast trapping (Sakai et al., 2019) in addition to careful control of osmolarity (Bascom et al., 2016), it is also possible to observe protoplast regeneration.
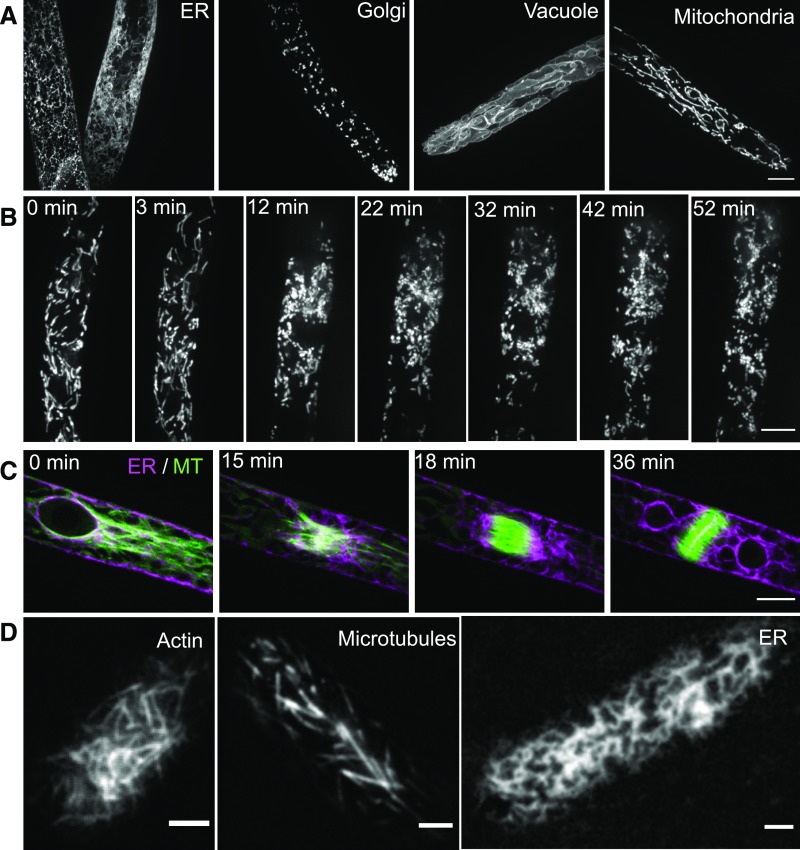
Figure 6.
Fluorescence Imaging of P. patens.
(A) Confocal images of cells expressing fluorescent proteins targeted to different organelles. From left to right: Endoplasmic reticulum labeled with signal peptide-mEGFP-KDEL, Golgi bodies labeled with the first 49 amino acids of the soybean α-1,2-mannosidase fused to YFP, vacuolar membrane (tonoplast) labeled with mEGFP fused to PpVam3 (Pp3c2_35310), and the mitochondria labeled with the first 76 amino acids of the protein encoded by Pp3c18_16000V3.1 fused to mCherry.
(B) Time-lapse confocal images of a cell expressing a mitochondria protein fused to mCherry. Mitochondria became fragmented when the cell enters cell division (12 min). Scale bar = 10 μm.
(C) Time-lapse confocal images of the endoplasmic reticulum (ER) and microtubules (MT) during cell division. The ER is labeled with signal peptide-mCherry-KDEL and microtubules are labeled with mEGFP-tubulin.
(A), (B), and (C) Images are maximum projections of Z-stacks. Scale bars = 10 μm.
(D) Images of Lifeact-mEGFP labeling the actin cytoskeleton, mEGFP-tubulin labeling microtubules, and endoplasmic reticulum acquired by variable angle epifluorescence microscopy, which produces images of low background and high contrast, allowing fine structures to be observed at high temporal resolution. Scale bars = 2 μm.
Using these systems, coupled with versatile molecular manipulation, numerous subcellular processes can be observed at high resolution. For example, by imaging the P. patens cytoskeleton using a variety of imaging modalities, detailed studies of polarized growth and cell division have been performed (Figure 6; Vidali et al., 2009b, 2010; Furt et al., 2013; Wu and Bezanilla, 2014; Kosetsu et al., 2017; van Gisbergen et al., 2018; Wu and Bezanilla, 2018; Yamada and Goshima, 2018; Kozgunova et al., 2019). Imaging fluorescent proteins in subcellular compartments has provided insights into their dynamics (Figure 6; Furt et al., 2012). Imaging using a recently developed P. patens ratiometric auxin-sensing reporter revealed subtle differences between individual cells (Thelander et al., 2019). A ratio-metric calcium reporter was used to monitor subcellular calcium levels during polarized growth (Bascom et al., 2018) and plant-wide responses to dehydration in gametophore tissue (Storti et al., 2018). With the numerous approaches available to image P. patens, this model system is poised to yield fundamental advances in our understanding of detailed cellular and subcellular processes at high temporal and spatial resolution in a phylogenetically informative lineage of plants.
Missing Tools and Resources
Furthering our understanding of genetic processes in P. patens would benefit from a larger collection of accessions/ecotypes encompassing broader genetic variation. Currently, we are considering Gransden, Reute, and Villersexel to be ecotypes, because they show different phenotypes and genetic variability, and can be crossed (Lang et al., 2018). However, studies of genetic variability within populations are underway and will be needed to answer questions about genetic and epigenetic variability. Also, while P. patens is comparatively well represented in terms of available accessions, this is not the case for P. magdalenae and P. readeri, for which additional accessions would be helpful to study convergent trait evolution (see Hot Topics in P. patens Research). While the above-mentioned International Moss Stock Center makes it possible to store mutants and have them available to the community, there is no resource yet like the Arabidopsis Salk lines, i.e., no collection of mutants that the community can draw upon.
In terms of databases, more unity of web-based tools and more cross links would aid in finding and comparing information. It would be useful to develop a unified expression profile interface and gene model lookup database, as outlined above.
Hot Topics in P. patens Research
Polyploidy and hybridization
Polyploidy and hybridization have long been studied in mosses, including Physcomitrella/Physcomitrium (von Wettstein, 1924; Pettet, 1964; Fritsch, 1991; reviewed by Rensing et al., 2012). Engel (1968) described 14 chromosomes for P. patens Gransden. Approximately 26 chromosomes were subsequently identified in this moss (Fritsch, 1991), while most recently, 27 chromosomes were identified (Reski et al., 1994). Due to their small size, it is difficult to observe mitotic chromosomes in P. patens. Even before the genome was sequenced, an ancestral whole genome duplication was evident in this species (Rensing et al., 2007). The genetic map used for the recent V3 assembly (Lang et al., 2018) is ordered into 27 linkage groups thought to represent the chromosomes. Indeed, the most recent genome assembly suggests that at least two rounds of genome duplication might have brought the ancestral chromosome number of seven to 14 and then to 27 (potentially by the fusion of two chromosomes).
Inferences from transcriptomic data reveal signatures of past whole-genome duplications across the phylogeny of mosses (Devos et al., 2016; Johnson et al., 2016; Lang et al., 2018) unlike the other two bryophyte groups (Lang et al., 2018), and karyological data from extant populations suggest the widespread occurrence of neopolyploidization within many species (Fritsch, 1991). In mosses, as in other plants, polyploidy results from either hybridization or autopolyploidy. The former mechanism is not uncommon in mosses (Natcheva and Cronberg, 2004; Shaw, 2009), although its significance during the diversification of mosses remains ambiguous. Hybridization may account for the origin of several species in the Funariaceae, which might have involved a particular species of Physcomitrium as one of the parents (McDaniel et al., 2010; Beike et al., 2014; Medina et al., 2018). Physcomitrium patens itself served as one parent for hybridization with species of Physcomitrium (Britton, 1895) and even with the more distantly related Funaria hygrometrica (von Wettstein, 1924). The ability to trigger somatic hybridization (Moody et al., 2018a) and to visualize hybridization events involving P. patens (Perroud et al., 2011) provide unique tools to explore the genomic consequences of hybridization in the Funariaceae. Autopolyploidy may be more common than allopolyploidy considering the large number of species, such as P. patens, known to harbor more than one karyotype (Fritsch, 1991). How autopolyploidy triggers speciation in plants is a wide-open area of research and remains completely unexplored in bryophytes. Yet, artificial autopolyploids are easily created in mosses through apospory, i.e., the development of a diploid gametophyte from (injured) sporophytic tissue. Furthermore, intragametophytic selfing, which may be preponderant in the Funariaceae, would result in a perfectly homozygous sporophyte. Consequently, the aposporous, diploid gametophyte would in theory bear two identical copies of the genome of the original haploid gametophyte, providing a unique system among land plants for investigating the genomic or epigenetic changes and their transcriptomic consequences in the first generation after autopolyploidy. Ongoing studies in F. hygrometrica, for which aposporous gametophyte are more readily developed, reveal that autopolyploidy is immediately characterized by significant shifts in gene expression (Rahmatpour, 2019).
Evolutionary developmental approaches
As outlined above, P. patens is an excellent moss for evolutionary developmental biology (evo-devo) studies due to its phylogenetic position, its propensity for gene targeting via homologous recombination, its relatively simple morphology, and the dominant haploid phase that is the hallmark of all bryophytes. The latter allows for the analysis of various mutants such as those affected in sexual reproduction: in flowering plants, such mutants are often embryo-lethal. In such cases, the P. patens gametophytic generation can nevertheless be grown and propagated. On top of that, both generations can easily be tracked and accessed. Prominent evo-devo studies over the past decade include studies revealing the conservation of the polycomb group complex in repressing the sporophytic body plan (Mosquna et al., 2009; Okano et al., 2009) and of HD-TALE transcription factors in repressing the gametophytic or promoting the sporophytic developmental program (Sakakibara et al., 2003; Horst et al., 2016; Ortiz-Ramírez et al., 2017). Another hot topic is identifying and analyzing the conservation of regulators of plant three-dimensional and tip growth (Spinner et al., 2010; Pires et al., 2013; Perroud et al., 2014, 2020; Proust et al., 2016; Moody et al., 2018b; Tang et al., 2020). The relatively simple body plan of protonema and the observation that they undergo tip growth and cell division enable them to be analyzed using approaches not feasible in many other plants. These and other approaches have greatly increased our understanding of early land plant evolution. P. patens is in an informative phylogenetic position, facilitating trait inference during plant terrestrialization (Puttick et al., 2018; Rensing, 2018; Delaux et al., 2019). The analysis of P. patens, together with other model non-seed plants and streptophyte algae (Bowman et al., 2017; Rensing, 2017; Nishiyama et al., 2018), furthers our understanding of how plants conquered land via the analysis of gene presence (comparative genomics) and conservation (evo-devo).
Reprogramming
Early on, P. patens was recognized as a unique system for studying the molecular basis of stem cell reprogramming due to the ability of differentiated cells to re-enter the cell cycle (Kofuji and Hasebe, 2014), de-differentiate, and form new protonemal cells in response to wounding and in the absence of any exogenously applied phytohormones. In fact, P. patens protoplasts can regenerate into whole plants without exogenous hormones, which is an extreme example of reprogramming in response to wounding. These attributes have been used to study the regulatory networks involved in cellular reprogramming. An analysis of gene expression during reprogramming revealed that initially, genes involved in stress responses and proteolysis were upregulated while genes involved in metabolic processes, particularly photosynthesis, were downregulated. As reprogramming progressed, metabolic gene expression including genes involved in photosynthesis and biosynthetic processes recovered. Auxin and cytokinin signaling pathways were also activated (Nishiyama et al., 2012). Quantitative proteomic analyses of protoplast regeneration yielded similar results (Wang et al., 2017). Stemming from gene expression studies (Nishiyama et al., 2012), cyclin-dependent kinase A was found to be essential for coordinating cell cycle re-entry and protonemal gene expression required to establish a new stem cell (Ishikawa et al., 2011). In addition, two WUSCHEL-related homeobox13-like genes were shown to be required for cell growth during reprogramming and the establishment of stem cell fate, as genes for cell wall loosening factors were no longer upregulated in WUSCHEL-related homeobox13-like gene knockout lines (Sakakibara et al., 2014). Intriguingly, Cold-Shock Domain Protein1 (PpCsp1), which shows sequence similarity and shared domains with Lin28, a gene required for induced pluripotent stem cell generation in humans, is upregulated in P. patens cells during reprogramming. The upregulation of PpCsp1 promoted reprogramming, while deleting PpCsp1 and its closely related genes inhibited reprogramming. This study identified a potentially conserved stem cell factor functioning in both plants and animals (Li et al., 2017). Finally, by screening for factors involved in stem cell reprogramming, Ishikawa et al. (2019) discovered a transcription factor from the AP2/ERF subgroup that, when overexpressed, altered the epigenetic landscape, decreasing repressive chromatin marks to a level sufficient to induce reprogramming in undamaged differentiated cells. By isolating and examining the reprogramming ability of one, two, or three cells from gametophores, Sato et al. (2017) discovered that there is an inhibitory signal that diffuses from the cell that alters the stem cell fate of neighboring cells, such that if two cells are isolated, 80% of the time only one of the cells successfully reprograms. These studies have provided unprecedented insights into cellular reprogramming. Future studies are sure to mechanistically dissect the signaling pathways involved in stem cell fate specification and reprogramming.
Cell biology
In the past 10 years, P. patens has become a key model system for studying plant cell biology. Due to the ease of genetically transforming plants and the ability to tag genes at their loci with DNA encoding fluorescent proteins, it has become standard to analyze the localization of proteins expressed from their native genomic context. It is also routine to demonstrate that the tagged protein is functional. With these tools in hand, a number of actin-associated proteins have been identified that are required to carry out tip growth (Vidali et al., 2007, 2009a, 2010; Augustine et al., 2008, 2011; Wu et al., 2011; van Gisbergen et al., 2012, 2018). A tour-de-force study tagged every single kinesin gene (encoding microtubule-based motors) in the moss genome and analyzed their localization, discovering that some kinesins have conserved functions, while others have surprisingly divergent functions (Miki et al., 2014). Careful quantitative analysis of organellar dynamics in moss protonemata demonstrated that most organelles in P. patens move significantly more slowly in protonemata than in pollen tubes or root hairs (Furt et al., 2012). Analysis of organellar dynamics also demonstrated that moss protonemata do not exhibit cytoplasmic streaming (Furt et al., 2012). Thus, protonemata can be used to analyze the intracellular motility of organelles without the complexity of actively streaming cytoplasm. Taking advantage of this feature, cell biological observations coupled with loss-of-function studies have identified cargos for kinesin motors, such as the nucleus and chloroplasts (Suetsugu et al., 2012; Miki et al., 2015; Yamada et al., 2017; Yamada and Goshima, 2018). These studies suggest that microtubules might play important roles in organelle transport in plants, potentially shifting the long-standing paradigm that plants predominantly use actin, not microtubules, for long-distance intracellular transport.
Protonemal cells are an excellent system for studying both cell division and polarized growth. Using these cells, the actin-based molecular motor myosin VIII was found to couple the actin cytoskeleton to microtubules, effectively guiding phragmoplast expansion. This study answered a long-standing question regarding the role of actin in cell division (Wu and Bezanilla, 2014). Subsequently, myosin VIII was also shown to link actin to microtubules at the apex of protonemal cells, serving to ensure persistent polarized growth (Wu and Bezanilla, 2018). A number of studies have begun to piece together the role of interdigitating microtubules at the phragmoplast equator in P. patens. The overlap zone, which requires the function of the microtubule cross-linking protein Map65, is spatially confined by the microtubule motor Kinesin-4 and recruits components of the exocyst complex, thereby defining the region where vesicles are delivered to build the new cell plate (Kosetsu et al., 2013; de Keijzer et al., 2017; Tang et al., 2019). Relatively few studies have focused on mitosis in P. patens. However, a recent study (Kozgunova et al., 2019) demonstrated that the depletion of kinetochore proteins leads to cytokinesis failures, resulting in polyploidy, which is in contrast to animal cells, where depletion leads to aneuploidy. This study raises the intriguing possibility that cytokinesis failure may be a route to polyploidy in plants.
With respect to polarized growth, key factors essential for cell polarity have been identified in P. patens, including the small GTPase ROP (Burkart et al., 2015) and actin-associated proteins such as actin depolymerizing factor (Augustine et al., 2008), profilin (Vidali et al., 2007), and myosin XI (Vidali et al., 2010). Using rapid RNAi approaches, the functions of whole gene families in polarized growth have been analyzed. The actin filament-promoting factors formins have class-specific functions, with class-II rather than class-I formins essential for polarity (Vidali et al., 2009a). The regulators of ROP function comprise a network of GTPase-activating proteins, exchange factors, and dissociation inhibitors, all of which are encoded by small gene families. A systematic RNAi approach was used to identify regulators involved in tip growth (Bascom et al., 2019). Together, these studies have elucidated the framework for the molecular regulation of the polarized secretion of cell wall materials in P. patens, with many factors conserved across plant lineages.
Outlook
P. patens represents only one lineage out of the many in mosses (e.g., orders in Liu et al., 2019). While more flowering plant models are being developed, the non-seed (or flagellated) plants are lagging behind. To determine such things as whether the unusual genome structure of P. patens (Lang et al., 2018) is peculiar or typical for mosses or bryophytes, we need more model organisms (Kenrick, 2017; Rensing, 2017). The liverwort Marchantia polymorpha has been established as a model system for similar types of analysis as P. patens (Bowman et al., 2017). Together, they represent the monophyletic Setaphyta (Renzaglia et al., 2018), and it is highly probable that while the Marchantia lineage subsequently lost genes and regulatory complexity, the Physcomitrium lineage gained them (Puttick et al., 2018). Hence, we need to study both organisms, as well as additional species. The hornwort Anthoceros is currently being established as a model system, representing the third lineage of bryophytes (Szövényi et al., 2015). Finally, a recent analysis of the genome of Chara, representing one of the sister lineages of land plants, revealed many typical land plant genes that were evolutionarily gained before the water-to-land-transition (Nishiyama et al., 2018).
Acknowledgments
We thank Fabian Haas for contributing to Figure 5, and we thank members of the Bezanilla lab for carefully reading the article. This work was supported by the National Science Foundation (grant DEB-1753811 to B.G.).
Footnotes
Articles can be viewed without a subscription.
References
- Arif M.A., Hiss M., Tomek M., Busch H., Meyberg R., Tintelnot S., Reski R., Rensing S.A., Frank W.(2019). ABA-induced vegetative diaspore formation in Physcomitrella patens. Front Plant Sci 10: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton N.W., Cove D.J.(1977). The isolation and preliminary characterisation of auxotrophic and analogue resistant mutants of the moss, Physcomitrella patens. Mol. Gen. Genet. 145: 87–95. [Google Scholar]
- Ashton N.W., Grimsley N.H., Cove D.J.(1979). Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144: 427–435. [DOI] [PubMed] [Google Scholar]
- Augustine R.C., Vidali L., Kleinman K.P., Bezanilla M.(2008). Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J. 54: 863–875. [DOI] [PubMed] [Google Scholar]
- Augustine R.C., Pattavina K.A., Tüzel E., Vidali L., Bezanilla M.(2011). Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 23: 3696–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C.S. Jr., Wu S.-Z., Nelson K., Oakey J., Bezanilla M.(2016). Long-term growth of moss in microfluidic devices enables subcellular studies in development. Plant Physiol. 172: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C.S. Jr., Winship L.J., Bezanilla M.(2018). Simultaneous imaging and functional studies reveal a tight correlation between calcium and actin networks. Proc. Natl. Acad. Sci. USA 115: E2869–E2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C. Jr., Burkart G.M., Mallett D.R., O’Sullivan J.E., Tomaszewski A.J., Walsh K., Bezanilla M.(2019). Systematic survey of the function of ROP regulators and effectors during tip growth in the moss Physcomitrella patens. J. Exp. Bot. 70: 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beike A.K., von Stackelberg M., Schallenberg-Rüdinger M., Hanke S.T., Follo M., Quandt D., McDaniel S.F., Reski R., Tan B.C., Rensing S.A.(2014). Molecular evidence for convergent evolution and allopolyploid speciation within the Physcomitrium-Physcomitrella species complex. BMC Evol. Biol. 14: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Pan A., Quatrano R.S.(2003). RNA interference in the moss Physcomitrella patens. Plant Physiol. 133: 470–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla M., Perroud P.-F., Pan A., Klueh P., Quatrano R.S.(2005). An RNAi system in Physcomitrella patens with an internal marker for silencing allows for rapid identification of loss of function phenotypes. Plant Biol (Stuttg) 7: 251–257. [DOI] [PubMed] [Google Scholar]
- Bowman J.L., et al. (2017). Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.e15. [DOI] [PubMed] [Google Scholar]
- Britton E.(1895). Contributions to American Bryology—IX. Bull. Torrey Bot. Club 22: 62–68. [Google Scholar]
- Bruch P., Schimper W.P.(1849). Ephemerum, Acaulon, Physcomitrella, Eustichium, Pottia suppl., Orthotrichum suppl., Fissidens suppl., Grimmia suppl., Hymenostomum suppl In Bryologia Europaea Fasc. 42. (Stuttgart: E. Schweizerbart; ). [Google Scholar]
- Burkart G.M., Baskin T.I., Bezanilla M.(2015). A family of ROP proteins that suppresses actin dynamics, and is essential for polarized growth and cell adhesion. J. Cell Sci. 128: 2553–2564. [DOI] [PubMed] [Google Scholar]
- Cho S.H., Chung Y.S., Cho S.K., Rim Y.W., Shin J.S.(1999). Particle bombardment mediated transformation and GFP expression in the moss Physcomitrella patens. Mol. Cells 9: 14–19. [PubMed] [Google Scholar]
- Collonnier C., Epert A., Mara K., Maclot F., Guyon-Debast A., Charlot F., White C., Schaefer D.G., Nogué F.(2017). CRISPR-Cas9-mediated efficient directed mutagenesis and RAD51-dependent and RAD51-independent gene targeting in the moss Physcomitrella patens. Plant Biotechnol. J. 15: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y., Palubicki W., Ljung K., Novak O., Leyser O., Harrison C.J.(2015). Three ancient hormonal cues co-ordinate shoot branching in a moss. eLife 4: e06808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudert Y., Bell N.E., Edelin C., Harrison C.J.(2017). Multiple innovations underpinned branching form diversification in mosses. New Phytol. 215: 840–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D.J., Schild A., Ashton N.W., Hartmann E.(1978). Genetic and physiological studies of the effect of light on the development of the moss, Physcomitrella patens. Photochem. Photobiol. 27: 249–254. [Google Scholar]
- Cove D.J.(1983). Genetics of Bryophyta In New Manual of Bryology, Schuster R.M., ed (Tokyo: Hatt Botanical Laboratory; ), pp. 221–231. [Google Scholar]
- Cove D.J.(2005). The moss Physcomitrella patens. Annu. Rev. Genet. 39: 339–358. [DOI] [PubMed] [Google Scholar]
- Daku R.M., Rabbi F., Buttigieg J., Coulson I.M., Horne D., Martens G., Ashton N.W., Suh D.-Y.(2016). PpASCL, the Physcomitrella patens anther-specific chalcone synthase-like enzyme implicated in sporopollenin biosynthesis, is needed for integrity of the moss spore wall and spore viability. PLoS One 11: e0146817–e0146820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keijzer J., Kieft H., Ketelaar T., Goshima G., Janson M.E.(2017). Shortening of microtubule overlap regions defines membrane delivery sites during plant cytokinesis. Curr. Biol. 27: 514–520. [DOI] [PubMed] [Google Scholar]
- Delaux P.-M., et al. (2019). Reconstructing trait evolution in plant evo-devo studies. Curr. Biol. 29: R1110–R1118. [DOI] [PubMed] [Google Scholar]
- Devos N., Szövényi P., Weston D.J., Rothfels C.J., Johnson M.G., Shaw A.J.(2016). Analyses of transcriptome sequences reveal multiple ancient large-scale duplication events in the ancestor of Sphagnopsida (Bryophyta). New Phytol. 211: 300–318. [DOI] [PubMed] [Google Scholar]
- Ding X., Pervere L.M., Bascom C. Jr., Bibeau J.P., Khurana S., Butt A.M., Orr R.G., Flaherty P.J., Bezanilla M., Vidali L.(2018). Conditional genetic screen in Physcomitrella patens reveals a novel microtubule depolymerizing-end-tracking protein. PLoS Genet. 14: e1007221–e1007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S.R.(2012). English Names for British Bryophytes. British Bryological Society Special Volume No. 5. (Cardiff, UK: British Bryological Society; ), pp. 1–89. [Google Scholar]
- Engel P.P.(1968). The induction of biochemical and morphological mutants in the moss Physcomitrella patens. Am. J. Bot. 55: 438–446. [Google Scholar]
- Faubert J.(2013). Bryophyte Flora of Quebec Labrador, Volume 2: Mosses, Part 1, Volume Xiv (Saint Valérien, Québec: Société Québecoise de Bryologie; ), p. 402. [Google Scholar]
- Fernandez-Pozo N., et al. (2019). PEATmoss (Physcomitrella Expression Atlas Tool): a unified gene expression atlas for the model plant Physcomitrella patens. Plant J. 10.1111/tpj.14607. [DOI] [PubMed] [Google Scholar]
- Frank W., Decker E.L., Reski R.(2005). Molecular tools to study Physcomitrella patens. Plant Biol (Stuttg) 7: 220–227. [DOI] [PubMed] [Google Scholar]
- Fritsch R.(1991). Index to Bryophytorum Bibliotheca. (Berlin, Stuttgart: J. Cramer; ). [Google Scholar]
- Furt F., Lemoi K., Tüzel E., Vidali L.(2012). Quantitative analysis of organelle distribution and dynamics in Physcomitrella patens protonemal cells. BMC Plant Biol. 12: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F., Liu Y.-C., Bibeau J.P., Tüzel E., Vidali L.(2013). Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J. 73: 417–428. [DOI] [PubMed] [Google Scholar]
- Girke T., Schmidt H., Zähringer U., Reski R., Heinz E.(1998). Identification of a novel delta 6-acyl-group desaturase by targeted gene disruption in Physcomitrella patens. Plant J. 15: 39–48. [DOI] [PubMed] [Google Scholar]
- Goffinet B., Cox C.J.(2000). Phylogenetic relationships among basal-most arthrodontous mosses with special emphasis on the evolutionary significance of the Funariineae. Bryologist 103: 212–223. [Google Scholar]
- Goffinet B.(2007a). Aphanorrhegma Sull. Flora of North America Editorial Committee 27: 181–182. [Google Scholar]
- Goffinet B.(2007b). Physcomitrella Bruch & Schimper. Flora of North America Editorial Committee 27: 194–195. [Google Scholar]
- Hampe E.(1837). Musci frondosi Germaniae ad methodum naturalem dispositi In Allgemeine Botanische Zeitung (Regensburg), Volume 18, pp. 273–287. [Google Scholar]
- Harayama T., Riezman H.(2017). Detection of genome-edited mutant clones by a simple competition-based PCR method. PLoS One 12: e0179165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C.J., Roeder A.H.K., Meyerowitz E.M., Langdale J.A.(2009). Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr. Biol. 19: 461–471. [DOI] [PubMed] [Google Scholar]
- Hedwig J.(1801). Species Muscorum Frondosorum: Descriptae et Tabulis Aeneis lxxvii Coloratis Illustratae.. (Leipzig: J.A. Barth; ). [Google Scholar]
- Higuchi M., Takahashi Y.(2012). Physcomitrella patens newly found in Japan. Shokubutsu Kenkyu Zasshi 87: 402–404. [Google Scholar]
- Hiss M., et al. (2014). Large-scale gene expression profiling data for the model moss Physcomitrella patens aid understanding of developmental progression, culture and stress conditions. Plant J. 79: 530–539. [DOI] [PubMed] [Google Scholar]
- Hiss M., Meyberg R., Westermann J., Haas F.B., Schneider L., Schallenberg-Rüdinger M., Ullrich K.K., Rensing S.A.(2017). Sexual reproduction, sporophyte development and molecular variation in the model moss Physcomitrella patens: Introducing the ecotype Reute. Plant J. 90: 606–620. [DOI] [PubMed] [Google Scholar]
- Hohe A., Rensing S.A., Mildner M., Lang D., Reski R.(2002). Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-Box gene in the moss Physcomitrella patens (vol 4, pg 595, 2002). Plant Biol (Stuttg) 4: 762. [Google Scholar]
- Horst N.A., Katz A., Pereman I., Decker E.L., Ohad N., Reski R.(2016). A single homeobox gene triggers phase transition, embryogenesis and asexual reproduction. Nature Plants 2: 15209. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., et al. (2011). Physcomitrella cyclin-dependent kinase A links cell cycle reactivation to other cellular changes during reprogramming of leaf cells. Plant Cell 23: 2924–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., et al. (2019). Physcomitrella STEMIN transcription factor induces stem cell formation with epigenetic reprogramming. Nat. Plants 5: 681–690. [DOI] [PubMed] [Google Scholar]
- Johnson M.G., Malley C., Goffinet B., Shaw A.J., Wickett N.J.(2016). A phylotranscriptomic analysis of gene family expansion and evolution in the largest order of pleurocarpous mosses (Hypnales, Bryophyta). Mol. Phylogenet. Evol. 98: 29–40. [DOI] [PubMed] [Google Scholar]
- Kamisugi Y., von Stackelberg M., Lang D., Care M., Reski R., Rensing S.A., Cuming A.C.(2008). A sequence-anchored genetic linkage map for the moss, Physcomitrella patens. Plant J. 56: 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerer W., Cove D.J.(1996). Genetic analysis of the effects of re-transformation of transgenic lines of the moss Physcomitrella patens. Mol. Gen. Genet. 250: 380–382. [DOI] [PubMed] [Google Scholar]
- Kenrick P.(2017). How land plant life cycles first evolved. Science 358: 1538–1539. [DOI] [PubMed] [Google Scholar]
- Khandelwal A., Cho S.H., Marella H., Sakata Y., Perroud P.-F., Pan A., Quatrano R.S.(2010). Role of ABA and ABI3 in desiccation tolerance. Science 327: 546. [DOI] [PubMed] [Google Scholar]
- Khraiwesh B., Arif M.A., Seumel G.I., Ossowski S., Weigel D., Reski R., Frank W.(2010). Transcriptional control of gene expression by microRNAs. Cell 140: 111–122. [DOI] [PubMed] [Google Scholar]
- Knight C.D., Sehgal A., Atwal K., Wallace J.C., Cove D.J., Coates D., Quatrano R.S., Bahadur S., Stockley P.G., Cuming A.C.(1995). Molecular responses to abscisic acid and stress are conserved between moss and cereals. Plant Cell 7: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji R., Hasebe M.(2014). Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr. Opin. Plant Biol. 17: 13–21. [DOI] [PubMed] [Google Scholar]
- Kosetsu K., de Keijzer J., Janson M.E., Goshima G.(2013). MICROTUBULE-ASSOCIATED PROTEIN65 is essential for maintenance of phragmoplast bipolarity and formation of the cell plate in Physcomitrella patens. Plant Cell 25: 4479–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosetsu K., Murata T., Yamada M., Nishina M., Boruc J., Hasebe M., van Damme D., Goshima G.(2017). Cytoplasmic MTOCs control spindle orientation for asymmetric cell division in plants. Proc. Natl. Acad. Sci. USA 114: E8847–E8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozgunova E., Nishima M., Goshima G.(2019). Kinetochore protein depletion underlies cytokinesis failure and somatic polyploidization in the moss Physcomitrella patens. eLife 8: e43652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo M., Imai A., Nishiyama T., Ishikawa M., Sato Y., Kurata T., Hiwatashi Y., Reski R., Hasebe M.(2013). System for stable β-estradiol-inducible gene expression in the moss Physcomitrella patens. PLoS One 8: e77356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K., Pederson E.R.A., Viaene T., Bozorg B., Friml J., Jönsson H., Thelander M., Sundberg E.(2013). The MOSS Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol. 162: 1406–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D., et al. (2018). The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J. 93: 515–533. [DOI] [PubMed] [Google Scholar]
- Li C., et al. (2017). A Lin28 homologue reprograms differentiated cells to stem cells in the moss Physcomitrella patens. Nat. Commun. 8: 14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg S.O.(1864). List of the family Funariineae. Öfversigt af Förhandlingar: Kongl. Svenska Vetenskaps‐Akademien 10: 589–608. [Google Scholar]
- Liu Y., Budke J.M., Goffinet B.(2012). Phylogenetic inference rejects sporophyte based classification of the Funarineae (Bryophyta): Rapid radiation suggests rampant homoplasy in sporophyte evolution. Mol. Phylogenet. Evol. 62: 130–145. [DOI] [PubMed] [Google Scholar]
- Liu Y., et al. (2019). Resolution of the ordinal phylogeny of mosses using targeted exons from organellar and nuclear genomes. Nat. Commun. 10: 1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Obando M., Hoffmann B., Géry C., Guyon-Debast A., Téoulé E., Rameau C., Bonhomme S., Nogué F.(2016). Simple and efficient targeting of multiple genes through CRISPR-Cas9 in Physcomitrella patens. G3 (Bethesda) 6: 3647–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machuka J., Bashiardes S., Ruben E., Spooner K., Cuming A., Knight C., Cove D.(1999). Sequence analysis of expressed sequence tags from an ABA-treated cDNA library identifies stress response genes in the moss Physcomitrella patens. Plant Cell Physiol. 40: 378–387. [DOI] [PubMed] [Google Scholar]
- Mallett D.R., Chang M., Cheng X., Bezanilla M.(2019). Efficient and modular CRISPR-Cas9 vector system for Physcomitrella patens. Plant Direct 3: e00168-e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella H.H., Sakata Y., Quatrano R.S.(2006). Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J. 46: 1032–1044. [DOI] [PubMed] [Google Scholar]
- Mashal R.D., Koontz J., Sklar J.(1995). Detection of mutations by cleavage of DNA heteroduplexes with bacteriophage resolvases. Nat. Genet. 9: 177–183. [DOI] [PubMed] [Google Scholar]
- McDaniel S.F., von Stackelberg M., Richardt S., Quatrano R.S., Reski R., Rensing S.A.(2010). The speciation history of the Physcomitrium–Physcomitrella species complex. Evolution 64: 217–231. [DOI] [PubMed] [Google Scholar]
- Medina R., Liu Y., Li-Song W., Shuiliang G., Hylander K., Goffinet B.(2015). DNA based revised geographic circumscription of species of Physcomitrella s.l. (Funarineae): P. patens new to East Asia and P. magdalenae new to East Africa. Bryologist 118: 22–31. [Google Scholar]
- Medina R., Johnson M.G., Liu Y., Wilding N., Hedderson T.A., Wickett N., Goffinet B.(2018). Evolutionary dynamism in bryophytes: Phylogenomic inferences confirm rapid radiation in the moss family Funarineae. Mol. Phylogenet. Evol. 120: 240–247. [DOI] [PubMed] [Google Scholar]
- Medina R., Johnson M.G., Liu Y., Wickett N.J., Shaw A.J., Goffinet B.(2019). Phylogenomic delineation of Physcomitrium (Bryophyta: Funarineae) based on targeted sequencing of nuclear exons and their flanking regions rejects the retention of Physcomitrella, Physcomitridium and Aphanorrhegma. J Systematics Evolution 57: 404–417. [Google Scholar]
- Meyberg R., Perroud P., Haas F.B., Schneider L., Heimerl T., Renzaglia K.S., Rensing S.A.(2020). Characterization of evolutionarily conserved key players affecting eukaryotic flagellar motility and fertility using a moss model. New Phytol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Naito H., Nishina M., Goshima G.(2014). Endogenous localizome identifies 43 mitotic kinesins in a plant cell. Proc. Natl. Acad. Sci. USA 111: E1053–E1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Nishina M., Goshima G.(2015). RNAi screening identifies the armadillo repeat-containing kinesins responsible for microtubule-dependent nuclear positioning in Physcomitrella patens. Plant Cell Physiol. 56: 737–749. [DOI] [PubMed] [Google Scholar]
- Mitten W.(1851). A list of all the mosses and hepaticae hitherto observed in Sussex. Ann. Mag. Nat. History 2: 362–370. [Google Scholar]
- Mohanasundaram B., Rajmane V.B., Jogdand S.V., Bhide A.J., Banerjee A.K.(2019). Agrobacterium-mediated Tnt1 mutagenesis of moss protonemal filaments and generation of stable mutants with impaired gametophyte. Mol. Genet. Genomics 294: 583–596. [DOI] [PubMed] [Google Scholar]
- Moody L.A., Kelly S., Coudert Y., Nimchuk Z.L., Harrison C.J., Langdale J.A.(2018a). Somatic hybridization provides segregating populations for the identification of causative mutations in sterile mutants of the moss Physcomitrella patens. New Phytol. 218: 1270–1277. [DOI] [PubMed] [Google Scholar]
- Moody L.A., Kelly S., Rabbinowitsch E., Langdale J.A.(2018b). Genetic regulation of the 2D to 3D growth transition in the moss Physcomitrella patens. Curr. Biol. 28: 473–478.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosquna A., Katz A., Decker E.L., Rensing S.A., Reski R., Ohad N.(2009). Regulation of stem cell maintenance by the Polycomb protein FIE has been conserved during land plant evolution. Development 136: 2433–2444. [DOI] [PubMed] [Google Scholar]
- Nakaoka Y., Miki T., Fujioka R., Uehara R., Tomioka A., Obuse C., Kubo M., Hiwatashi Y., Goshima G.(2012). An inducible RNA interference system in Physcomitrella patens reveals a dominant role of augmin in phragmoplast microtubule generation. Plant Cell 24: 1478–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakosteen P.C., Hughes K.W.(1978). Sexual life cycle of three species of Funarineae in culture. Bryologist 81: 307–314. [Google Scholar]
- Natcheva R., Cronberg N.(2004). What do we know about hybridization among bryophytes in nature? Can. J. Bot. 82: 1687–1704. [Google Scholar]
- Nishiyama T., Miyawaki K., Ohshima M., Thompson K., Nagashima A., Hasebe M., Kurata T.(2012). Digital gene expression profiling by 5′-end sequencing of cDNAs during reprogramming in the moss Physcomitrella patens. PLoS One 7: e36471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama T., et al. (2018). The Chara genome: Secondary complexity and implications for plant terrestrialization. Cell 174: 448–464.e24. [DOI] [PubMed] [Google Scholar]
- Okano Y., Aono N., Hiwatashi Y., Murata T., Nishiyama T., Ishikawa T., Kubo M., Hasebe M.(2009). A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proc. Natl. Acad. Sci. USA 106: 16321–16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Ramírez C., Hernandez-Coronado M., Thamm A., Catarino B., Wang M., Dolan L., Feijó J.A., Becker J.D.(2016). A transcriptome atlas of Physcomitrella patens provides insights into the evolution and development of land plants. Mol. Plant 9: 205–220. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ramírez C., Michard E., Simon A.A., Damineli D.S.C., Hernández-Coronado M., Becker J.D., Feijó J.A.(2017). GLUTAMATE RECEPTOR-LIKE channels are essential for chemotaxis and reproduction in mosses. Nature 549: 91–95. [DOI] [PubMed] [Google Scholar]
- Perroud P.-F., Cove D.J., Quatrano R.S., McDaniel S.F.(2011). An experimental method to facilitate the identification of hybrid sporophytes in the moss Physcomitrella patens using fluorescent tagged lines. New Phytol. 191: 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P.-F., Demko V., Johansen W., Wilson R.C., Olsen O.-A., Quatrano R.S.(2014). Defective Kernel 1 (DEK1) is required for three-dimensional growth in Physcomitrella patens. New Phytol. 203: 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroud P.-F., et al. (2018). The Physcomitrella patens gene atlas project: Large-scale RNA-seq based expression data. Plant J. 95: 168–182. [DOI] [PubMed] [Google Scholar]
- Perroud P.-F., Meyberg R., Rensing S.A.(2019). Physcomitrella patens Reute mCherry as a tool for efficient crossing within and between ecotypes. Plant Biol (Stuttg) 21: 143–149. [DOI] [PubMed] [Google Scholar]
- Perroud P., Meyberg R., Demko V., Quatrano R.S., Olsen O., Rensing S.A.(2020). DEK1 displays a strong subcellular polarity during Physcomitrella patens 3D growth. New Phytol.. [DOI] [PubMed] [Google Scholar]
- Pettet A.(1964). Hybrid sporophytes in Funariaceae. I. Hybrid sporophytes on Physcomitrella patens (Hedw.) B. & S., and Physcomitrium sphaericum (Schkuhr) Brid. Britain. Trans. Br. Bryol. Soc 4: 642–648. [Google Scholar]
- Pires N.D., Yi K., Breuninger H., Catarino B., Menand B., Dolan L.(2013). Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proc. Natl. Acad. Sci. USA 110: 9571–9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M.J., Lavy M., Ashton N.W., Estelle M.(2010). Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr. Biol. 20: 1907–1912. [DOI] [PubMed] [Google Scholar]
- Proust H., Honkanen S., Jones V.A.S., Morieri G., Prescott H., Kelly S., Ishizaki K., Kohchi T., Dolan L.(2016). RSL class I genes controlled the development of epidermal structures in the common ancestor of land plants. Curr. Biol. 26: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu X., Liu L., Li P., Huo H., Dong X., Xie K., Yang H., Liu L.(2019). A CRISPR/LbCas12a-based method for highly efficient multiplex gene editing in Physcomitrella patens. Plant J. 100: 863–872. [DOI] [PubMed] [Google Scholar]
- Puttick M.N., Morris J.L., Williams T.A., Cox C.J., Edwards D., Kenrick P., Pressel S., Wellman C.H., Schneider H., Pisani D., et al. (2018). The interrelationships of land plants and the nature of the ancestral embryophyte. Curr. Biol. 28: 733–745.e2. [DOI] [PubMed] [Google Scholar]
- Rahmatpour N. (2019). Patterns of gene expression during shifts in ploidy and function in the moss Funaria hygrometrica. Doctoral dissertation, (University of Connecticut, Storrs, CT). https://opencommons.uconn.edu/dissertations/2375.
- Regmi K.C., Li L., Gaxiola R.A.(2017). Alternate modes of photosynthate transport in the alternating generations of Physcomitrella patens. Front Plant Sci 8: 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing S.A., Rombauts S., van de Peer Y., Reski R.(2002). Moss transcriptome and beyond. Trends Plant Sci. 7: 535–538. [DOI] [PubMed] [Google Scholar]
- Rensing S.A., Ick J., Fawcett J.A., Lang D., Zimmer A., van de Peer Y., Reski R.(2007). An ancient genome duplication contributed to the abundance of metabolic genes in the moss Physcomitrella patens. BMC Evol. Biol. 7: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing S.A., et al. (2008). The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. [DOI] [PubMed] [Google Scholar]
- Rensing S.A., Beike A.K., Lang D.(2012). Evolutionary importance of generative polyploidy for genome evolution of haploid-dominant land plants In Plant Genome Diversity, Volume 2: Physical Structure, Behaviour and Evolution of Plant Genomes, Leitch I.J., Greilhuber J., Jaroslav D., and Jonathan W., eds (Vienna: Springer; ), pp. 295–305. [Google Scholar]
- Rensing S.A.(2016). (Why) does evolution favour embryogenesis? Trends Plant Sci. 21: 562–573. [DOI] [PubMed] [Google Scholar]
- Rensing S.A.(2017). Why we need more non-seed plant models. New Phytol. 216: 355–360. [DOI] [PubMed] [Google Scholar]
- Rensing S.A.(2018). Great moments in evolution: The conquest of land by plants. Curr. Opin. Plant Biol. 42: 49–54. [DOI] [PubMed] [Google Scholar]
- Renzaglia K.S., Garbary D.J.(2001). Motile gametes of land plants: Diversity, development, and evolution. Crit. Rev. Plant Sci. 20: 107–213. [Google Scholar]
- Renzaglia K.S., Villarreal Aguilar J.C., Garbary D.J.(2018). Morphology supports the setaphyte hypothesis: Mosses plus liverworts form a natural group. Bry. Div. Evo. 40: 11–17. [Google Scholar]
- Reski R., Abel W.O.(1985). Induction of budding on chloronemata and caulonemata of the moss, Physcomitrella patens, using isopentenyladenine. Planta 165: 354–358. [DOI] [PubMed] [Google Scholar]
- Reski R., Faust M., Wang X.-H., Wehe M., Abel W.O.(1994). Genome analysis of the moss Physcomitrella patens (Hedw.) B.S.G. Mol Gen 244: 352–359. [DOI] [PubMed] [Google Scholar]
- Reski R., Reg D.C.G.(2004). Quick guide: Physcomitrella patens. plant-biotech.net 14: R1–R2. [Google Scholar]
- Richardt S., Timmerhaus G., Lang D., Qudeimat E., Corrêa L.G.G., Reski R., Rensing S.A., Frank W.(2010). Microarray analysis of the moss Physcomitrella patens reveals evolutionarily conserved transcriptional regulation of salt stress and abscisic acid signalling. Plant Mol. Biol. 72: 27–45. [DOI] [PubMed] [Google Scholar]
- Sakai K., Charlot F., Le Saux T., Bonhomme S., Nogué F., Palauqui J.-C., Fattaccioli J.(2019). Design of a comprehensive microfluidic and microscopic toolbox for the ultra-wide spatio-temporal study of plant protoplasts development and physiology. Plant Methods 15: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara K., Nishiyama T., Sumikawa N., Kofuji R., Murata T., Hasebe M.(2003). Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 130: 4835–4846. [DOI] [PubMed] [Google Scholar]
- Sakakibara K., et al. (2014). WOX13-like genes are required for reprogramming of leaf and protoplast cells into stem cells in the moss Physcomitrella patens. Development 141: 1660–1670. [DOI] [PubMed] [Google Scholar]
- Sato Y., Sugimoto N., Hirai T., Imai A., Kubo M., Hiwatashi Y., Nishiyama T., Hasebe M.(2017). Cells reprogramming to stem cells inhibit the reprogramming of adjacent cells in the moss Physcomitrella patens. Sci. Rep. 7: 1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer D.G., Zrÿd J.P.(1997). Efficient gene targeting in the moss Physcomitrella patens. Plant J. 11: 1195–1206. [DOI] [PubMed] [Google Scholar]
- Shaw A.J.(2009). Bryophyte species and speciation In Bryophyte Biology, Goffinet B., ed (Cambridge: Cambridge University Press; ), pp. 445–485. [Google Scholar]
- Spinner L., Pastuglia M., Belcram K., Pegoraro M., Goussot M., Bouchez D., Schaefer D.G.(2010). The function of TONNEAU1 in moss reveals ancient mechanisms of division plane specification and cell elongation in land plants. Development 137: 2733–2742. [DOI] [PubMed] [Google Scholar]
- Stevenson S.R., et al. (2016). Genetic analysis of Physcomitrella patens identifies ABSCISIC ACID NON-RESPONSIVE, a regulator of ABA responses unique to basal land plants and required for desiccation tolerance. Plant Cell 28: 1310–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti M., Costa A., Golin S., Zottini M., Morosinotto T., Alboresi A.(2018). (2018). Systemic calcium wave propagation in Physcomitrella patens. Plant Cell Physiol. 59: 1377–1384. [DOI] [PubMed] [Google Scholar]
- Strepp R., Scholz S., Kruse S., Speth V., Reski R.(1998). Plant nuclear gene knockout reveals a role in plastid division for the homolog of the bacterial cell division protein FtsZ, an ancestral tubulin. Proc. Natl. Acad. Sci. USA 95: 4368–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotbek C., Krinninger S., Frank W.(2013). The moss Physcomitrella patens: Methods and tools from cultivation to targeted analysis of gene function. Int. J. Dev. Biol. 57: 553–564. [DOI] [PubMed] [Google Scholar]
- Suetsugu N., Sato Y., Tsuboi H., Kasahara M., Imaizumi T., Kagawa T., Hiwatashi Y., Hasebe M., Wada M.(2012). The KAC family of kinesin-like proteins is essential for the association of chloroplasts with the plasma membrane in land plants. Plant Cell Physiol. 53: 1854–1865. [DOI] [PubMed] [Google Scholar]
- Sugiura C., Kobayashi Y., Aoki S., Sugita C., Sugita M.(2003). Complete chloroplast DNA sequence of the moss Physcomitrella patens: Evidence for the loss and relocation of rpoA from the chloroplast to the nucleus. Nucleic Acids Res. 31: 5324–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivant W.(1848). Musci and hepaticae In A Manual of the Botany of the Northern United States, Gray A., ed (Boston: Monroe; ), pp. 641–696. [Google Scholar]
- Szövényi P., Ricca M., Hock Z., Shaw J.A., Shimizu K.K., Wagner A.(2013). Selection is no more efficient in haploid than in diploid life stages of an angiosperm and a moss. Mol. Biol. Evol. 30: 1929–1939. [DOI] [PubMed] [Google Scholar]
- Szövényi P., Frangedakis E., Ricca M., Quandt D., Wicke S., Langdale J.A.(2015). Establishment of Anthoceros agrestis as a model species for studying the biology of hornworts. BMC Plant Biol. 15: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.C.(1979). A new classification for the genus Physcomitrella B.S.G. J. Hattori Bot. Lab. 46: 327–336. [Google Scholar]
- Tang H., de Keijzer J., Overdijk E.J.R., Sweep E., Steentjes M., Vermeer J.E.M., Janson M.E., Ketelaar T.(2019). Exocyst subunit Sec6 is positioned by microtubule overlaps in the moss phragmoplast prior to cell plate membrane arrival. J. Cell Sci. 132: jcs222430. [DOI] [PubMed] [Google Scholar]
- Tang H., Duijts K., Bezanilla M., Scheres B., Vermeer J.E.M., Willemsen V.(2020). Geometric cues forecast the switch from two- to three-dimensional growth in Physcomitrella patens. New Phytol. 225: 1945–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa K., Odahara M., Kabeya Y., Kikugawa T., Sekine Y., Fujiwara M., Sato N.(2007). The mitochondrial genome of the moss Physcomitrella patens sheds new light on mitochondrial evolution in land plants. Mol. Biol. Evol. 24: 699–709. [DOI] [PubMed] [Google Scholar]
- Thelander M., Landberg K., Sundberg E.(2019). Minimal auxin sensing levels in vegetative moss stem cells revealed by a ratiometric reporter. New Phytol. 224: 775–788. [DOI] [PubMed] [Google Scholar]
- van Gisbergen P.A.C., Li M., Wu S.-Z., Bezanilla M.(2012). Class II formin targeting to the cell cortex by binding PI(3,5)P(2) is essential for polarized growth. J. Cell Biol. 198: 235–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gisbergen P.A.C., Wu S.-Z., Chang M., Pattavina K.A., Bartlett M.E., Bezanilla M.(2018). An ancient Sec10-formin fusion provides insights into actin-mediated regulation of exocytosis. J. Cell Biol. 217: 945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillet F., Perrot L., Guyon-Debast A., Kermarrec M.-P., Chauvin L., Chauvin J.-E., Gallois J.-L., Mazier M., Nogué F.(2020). Expanding the CRISPR toolbox in P. patens using SpCas9-NG variant and application for gene and base editing in Solanaceae crops. Int. J. Mol. Sci. 21: 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Augustine R.C., Kleinman K.P., Bezanilla M.(2007). Profilin is essential for tip growth in the moss Physcomitrella patens. Plant Cell 19: 3705–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., van Gisbergen P.A.C., Guérin C., Franco P., Li M., Burkart G.M., Augustine R.C., Blanchoin L., Bezanilla M.(2009a). Rapid formin-mediated actin-filament elongation is essential for polarized plant cell growth. Proc. Natl. Acad. Sci. USA 106: 13341–13346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Rounds C.M., Hepler P.K., Bezanilla M.(2009b). Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS One 4: e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L., Burkart G.M., Augustine R.C., Kerdavid E., Tüzel E., Bezanilla M.(2010). Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22: 1868–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitt D.H.(1984). Classification of the Bryopsida In New Manual of Bryology, Schuster R.M., ed (Nichinan: Hattori Botanical Laboratory; ), pp. 696–759. [Google Scholar]
- Vives C., Charlot F., Mhiri C., Contreras B., Daniel J., Epert A., Voytas D.F., Grandbastien M.-A., Nogué F., Casacuberta J.M.(2016). Highly efficient gene tagging in the bryophyte Physcomitrella patens using the tobacco (Nicotiana tabacum) Tnt1 retrotransposon. New Phytol. 212: 759–769. [DOI] [PubMed] [Google Scholar]
- von Stackelberg M., Rensing S.A., Reski R.(2006). Identification of genic moss SSR markers and a comparative analysis of twenty-four algal and plant gene indices reveal species-specific rather than group-specific characteristics of microsatellites. BMC Plant Biol. 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wettstein F.(1924). Morphologie und physiologie des formwechsels der moose auf genetischer grundlage I. [in German] Z. Indukt. Abstamm. Vererbungsl. 33: 1–236. [Google Scholar]
- Wang X., Chen L., Yang A., Bu C., He Y.(2017). Quantitative proteomics analysis of developmental reprogramming in protoplasts of the moss Physcomitrella patens. Plant Cell Physiol. 58: 946–961. [DOI] [PubMed] [Google Scholar]
- Widiez T., Symeonidi A., Luo C., Lam E., Lawton M., Rensing S.A.(2014). The chromatin landscape of the moss Physcomitrella patens and its dynamics during development and drought stress. Plant J. 79: 67–81. [DOI] [PubMed] [Google Scholar]
- Wu S.-Z., Ritchie J.A., Pan A.-H., Quatrano R.S., Bezanilla M.(2011). Myosin VIII regulates protonemal patterning and developmental timing in the moss Physcomitrella patens. Mol. Plant 4: 909–921. [DOI] [PubMed] [Google Scholar]
- Wu S.-Z., Bezanilla M.(2014). Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. eLife 3: e03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.-Z., Bezanilla M.(2018). Actin and microtubule cross talk mediates persistent polarized growth. J. Cell Biol. 217: 3531–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., et al. (2014). Contribution of NAC transcription factors to plant adaptation to land. Science 343: 1505–1508. [DOI] [PubMed] [Google Scholar]
- Yamada M., Tanaka-Takiguchi Y., Hayashi M., Nishina M., Goshima G.(2017). Multiple kinesin-14 family members drive microtubule minus end-directed transport in plant cells. J. Cell Biol. 216: 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Goshima G.(2018). The KCH kinesin drives nuclear transport and cytoskeletal coalescence to promote tip cell growth in Physcomitrella patens. Plant Cell 30: 1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi P., Goshima G.(2019). Transient cotransformation of CRISPR/Cas9 and oligonucleotide templates enables efficient editing of target loci in Physcomitrella patens. Plant Biotechnol. J. 15: 122–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J., Niu Q.-W., Chua N.-H.(2000). Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24: 265–273. [DOI] [PubMed] [Google Scholar]