Symbiosis-dependent microtubule rearrangement and processing in Medicago truncatula by DREPP occur in membrane nanodomains.
Abstract
The initiation of intracellular host cell colonization by symbiotic rhizobia in Medicago truncatula requires repolarization of root hairs, including the rearrangement of cytoskeletal filaments. The molecular players governing microtubule (MT) reorganization during rhizobial infections remain to be discovered. Here, we identified M. truncatula DEVELOPMENTALLY REGULATED PLASMA MEMBRANE POLYPEPTIDE (DREPP), a member of the MT binding DREPP/PCaP protein family, and investigated its functions during rhizobial infections. We show that rhizobial colonization of drepp mutant roots as well as transgenic roots overexpressing DREPP is impaired. DREPP relocalizes into symbiosis-specific membrane nanodomains in a stimulus-dependent manner. This subcellular segregation coincides with DREPP-dependent MT fragmentation and a partial loss of the ability to reorganize the MT cytoskeleton in response to rhizobia, which might rely on an interaction between DREPP and the MT-organizing protein SPIRAL2. Taken together, our results reveal that establishment of symbiotic associations in M. truncatula requires DREPP in order to regulate MT reorganization during initial root hair responses to rhizobia.
INTRODUCTION
The legume–Rhizobium root nodule symbiosis represents an exceptional example for an intracellular accommodation of a bacterial mutualist. In the model legume Medicago truncatula, rhizobial infection strictly depends on the molecular recognition of strain-specific rhizobial signaling molecules, called Nod factors (NFs; Oldroyd, 2013). These are secreted by its symbiont Sinorhizobium meliloti and are perceived by the host lysin motif–type receptor-like kinase (RLK) NOD FACTOR PERCEPTION (NFP; Amor et al., 2003; Arrighi et al., 2006) that possibly forms a complex with the entry receptor LYSIN MOTIF RECEPTOR KINASE3 (LYK3; Limpens et al., 2003; Smit et al., 2007). This preinfection molecular dialogue also requires the function of DOES NOT MAKE INFECTIONS2 (DMI2; Catoira et al., 2000; Stracke et al., 2002; Hogg et al., 2006), an leucine-rich repeat–RLK that acts in concert with NFP and LYK3 (Antolín-Llovera et al., 2014). All three receptors physically interact with the molecular scaffold protein SYMREM1 in membrane nanodomains (Lefebvre et al., 2010; Tóth et al., 2012; Liang et al., 2018).
Localization of RLKs to such submicrometer protein/lipid assemblies has also been described for other receptors such as the innate immune receptor FLAGELLIN SENSING2 (FLS2) and the brassinosteroid receptor BRI1 (Bücherl et al., 2017). Since membrane nanodomains also host other signaling components, they have been hypothesized to play a major role in subcompartmentalizing the plasma membrane (PM) into functionally distinct territories (reviewed by Ott, 2017 and Yu et al., 2020). While the LYK3 receptor displays a comparably high lateral mobility at the PM prior to infection (Haney et al., 2011), it gets immobilized in membrane nanodomains labeled by FLOTILLIN4 (FLOT4) following infection by rhizobia (Haney and Long, 2010; Haney et al., 2011). This immobilization is mediated by the remorin protein SYMREM1 and results in a stabilization of LYK3 during infection (Liang et al., 2018). Genetic evidence suggests that LYK3 recruitment into FLOT4/SYMREM1-positive nanodomains is required for successful rhizobial infections (Liang et al., 2018).
Ligand-dependent activation of the primary symbiotic receptors NFP, LYK3, and DMI2 ultimately results in regular nuclear and perinuclear calcium oscillations, a hallmark of symbiotic infections (Ehrhardt et al., 1996; Kosuta et al., 2008). These calcium spikes are decoded by a calcium-calmodulin–dependent kinase (Does not Make Infections 3 [DMI3]; Lévy et al., 2004), that, in turn, activates the transcription factor CYCLOPS/interacting protein of DMI3 (IPD3; among others) via phosphorylation (Messinese et al., 2007; Yano et al., 2008; Hayashi et al., 2010; Singh et al., 2014). As a consequence, two distinct genetic programs are triggered: one program controlling different steps of rhizobial infection and the other program controlling the organogenetic pathway that results in the formation of root nodules (Madsen et al., 2010).
In M. truncatula, infection requires a transient growth retardation of susceptible root hairs (RHs) that results in RH swelling and a subsequent deformation. This redirects the resumed RH growth and leads to the formation of a RH curl that entraps a single rhizobial colony within the so-called infection chamber (IC; Fournier et al., 2015). Upon rhizobial divisions inside the IC, the PM of the host invaginates, leading to the formation of a tunnel-like structure, the infection thread (IT), that continues growing into the newly formed nodule primordium (Gage, 2004; Fournier et al., 2008, 2015). Here, rhizobia will be released into infected cells, where they differentiate into nitrogen-fixing bacteroids (Gage, 2004; Montiel et al., 2017). In so-called indeterminate nodules, as formed by M. truncatula, these infected cells undergo senescence at the base of the nodule (zone IV), while newly infected cells are formed close to the nodule apex (zone II; Vasse et al., 1990).
The initial RH responses are probably mediated by F‐actin that undergoes significant rearrangements enabling the induction of RH deformation, RH curling, and IT formation (Crdenas et al., 1998; Yokota et al., 2009; Hossain et al., 2012; Zepeda et al., 2014). Later, a polarity switch is required to redirect polar secretion toward the IC (Fournier et al., 2015) and for IT growth (Fournier et al., 2008). Among other genetic components, the Lotus japonicus SCAR‐Nodulation (LjSCARN), an activator of the ARP2/3 actin nucleation complex, is required during RH polar growth (Qiu et al., 2015). Similar to mutations in other members of the SCAR/WAVE complex, such as NAP1 and PIR1 (Yokota et al., 2009), scarn mutants display short RHs and mostly transverse or web-like actin filaments compared to the wild type with a predominantly longitudinally oriented actin cytoskeleton (Qiu et al., 2015). Further involvement of the ARP2/3 complex at later stages of the symbiotic interaction was presented in M. truncatula, where MtARP3 controls symbiosome development (Gavrin et al., 2015).
By contrast, our knowledge of the molecular regulation of microtubule (MT) arrangements during symbiotic interactions is comparably sparse. Only those RHs that recently stopped elongating are able to respond to NFs and rhizobia by RH curling (Heidstra et al., 1994). Here, MT growth rates are decreased by ∼45%, suggesting an active role in this process (Vassileva et al., 2005). Additionally, imaging of Rhizobium-treated RHs revealed that the reorganization of MTs is tightly linked to nuclear repositioning during RH curling and IT initiation (Timmers et al., 1999). Despite the unequivocal importance of MT patterning during rhizobial infections, these processes have not been genetically dissected. Interestingly, plant-specific DEVELOPMENTALLY REGULATED PLASMA MEMBRANE POLYPEPTIDE (DREPP) proteins were shown in Arabidopsis (Arabidopsis thaliana) to regulate the actin and MT cytoskeleton in a calcium-dependent manner (Li et al., 2011; Zhu et al., 2013; Qin et al., 2014). The DREPP protein family comprises only two members in Arabidopsis: the PLASMA MEMBRANE-ASSOCIATED CATION BINDING PROTEINs PCaP1 (AtMDP25) and PCaP2 (AtMAP18; Li et al., 2011; Zhu et al., 2013; Qin et al., 2014). PCaP1 is sufficient to sever MTs and actin filaments in vitro, while this function has not been confirmed in vivo. The process is enhanced upon calcium treatment and additionally results in the dissociation of PCaP1 from the membrane to the cytosol. In the presence of calcium, PCaP2 interacts with calmodulin and phosphatidylinositol phosphates (PtdInsPs; Nagasaki et al., 2008).
Here, we demonstrate that M. truncatula DREPP is required for successful infection by rhizobia. The application of calcium and NFs resulted in a relocalization of DREPP into nanodomains that colocalize with MTs. This accumulation coincides with fragmentation of MTs in RHs. Given the ability of DREPP to interact with MTs and MT-associated proteins, we propose that DREPP processes MTs in functional membrane nanodomains.
RESULTS
drepp Mutants Are Affected in Rhizobial Infections and Nodulation
Rhizobial infections in legumes are accompanied by significant cytoskeleton rearrangements prior to and during IT progression. To understand this phenomenon in more detail, we studied the single member (Medtr2g437530) of the DREPP protein family in M. truncatula. Large-scale phylogenetic analysis using 104 plant genomes revealed an unambiguous presence of DREPP putative paralogs in 91 species, indicating that DREPP is conserved throughout the angiosperms, that is, in species that are able to nodulate and also in species from lineages that have lost, or never evolved nodulation (Supplemental Data Set 1).
In M. truncatula, DREPP transcripts can be continuously detected and are transiently but mildly induced ∼3 d after inoculation (DAI) of roots with rhizobia (Supplemental Figure 1A). Fusing a 1.5-kb-long fragment of the putative DREPP promoter to β-glucuronidase (GUS) revealed promoter activity in root cortical cells and RHs at 7 DAI (Supplemental Figure 1B). Residual promoter activity of DREPP was also observed in the two receptor mutants nfp-1 and dmi2 (5P) as well as in the dmi3-1 mutant background (Supplemental Figure 1B), indicating that basal expression is, as expected, independent of the induction of symbiotic signaling.
To genetically assess the relevance of DREPP during root nodule symbiosis, we isolated and analyzed two independent drepp mutant alleles, drepp-1 (NF1952) and drepp-2 (NF8505), carrying TNT1 transposon insertions at positions 1038 bp and 988 bp downstream of the translational start codon of DREPP, respectively (Figure 1A). RT-qPCR revealed that endogenous DREPP transcripts were significantly decreased by ∼10-fold in homozygous drepp-1 and drepp-2 mutant plants, confirming that they can be considered as transcriptional knockdown alleles (Figure 1B). Since DREPP proteins have been reported to function during RHdevelopment and nuclear movement in RHs (Zhang et al., 2015), which would indirectly interfere with symbiotic infections, we inspected RH development and additionally scored the distance between the nucleus and the tip of elongating RHs in the susceptible zone ∼10 mm above the root cap. No significant differences were observed between the genotypes (Supplemental Figure 2). To confirm, however, that DREPP functions in MT rearrangements as described for the putative orthologous protein from Arabidopsis (Wang et al., 2007), we performed immunofluorescence using an anti-tubulin antibody to visualize cortical MT orientation in the wild type (R108), drepp mutants, and transgenic roots constitutively overexpressing DREPP. Parallel arrays of transversely oriented MTs were predominantly observed in wild-type root epidermal cells (Supplemental Figure 3). While drepp mutants displayed mostly transversely oriented MTs and only few with random MT arrays (Supplemental Figure 3), ectopic expression of DREPP resulted in a significant reorientation of MTs with random, oblique, or longitudinal MT alignments in root epidermal cells (Supplemental Figure 3). This indicates that the M. truncatula DREPP protein retained conserved roles but may have lost generic functions with respect to RH development.
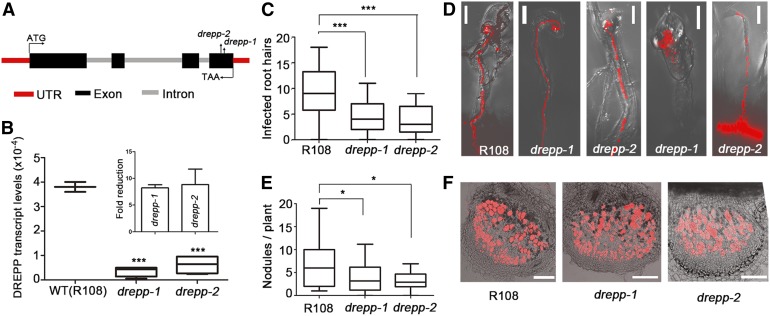
Figure 1.
DREPP Regulates Rhizobial Infections.
(A) Schematic representation of the DREPP gene structure and mapped Tnt1 transposon insertion sites in two independent drepp mutant alleles. UTR, untranslated region.
(B) to (F) Endogenous transcript levels were quantified by RT-qPCR (B). Values are normalized to the housekeeping gene Ubiquitin. Inset shows fold reduction of DREPP transcripts in both mutant alleles; n = 6 root systems for each genotype. Infected RHs (curled RH; see [C]) and nodules (E) were scored at 7 DAI, with n ≥ 19 root systems for each genotype and condition. (D) While several RHs developed wild-type (WT)–like ITs in drepp-1 and drepp-2 mutants, a great proportion was prematurely aborted or bacteria were released into trichoblasts (two rightmost panels). Bars = 20 µm. (F) Semithin (70-μm) longitudinal sections of 7 DAI whole nodules from the wild type and drepp mutants. Red fluorescence is derived from mCherry-labeled S. meliloti hosted in infected nodule cells. Bars = 100 µm. Data are means ± se. Statistics was performed using an unpaired two-tailed t test: *P < 0.05, ***P < 0.001.
DREPP Regulates Rhizobial Infections
To investigate whether DREPP is important for rhizobial infections, we phenotypically characterized drepp mutants by assessing primary infection success of rhizobia at 7 DAI. For this, we scored responsive RHs (curled RHs with or without ITs) and nodule numbers. The number of responsive RHs was significantly reduced in both drepp mutants compared to wild-type plants (Figure 1C), even though several mature ITs were observed (Figure 1D). As a consequence, drepp mutants developed fewer nodule primordia at 7 DAI (Figure 1E), which were fully colonized by rhizobia and did not exhibit any differences compared to the wild type (Figure 1F). Interestingly, the infection patterns of roots overexpressing DREPP resembled the mutant phenotype (Supplemental Figure 4). Furthermore, we systematically found young elongated mutant nodules with impaired bacterial release. In addition, 4-week-old drepp mutant nodules showed an extended nodular senescence zone (zone IV) at their base (Supplemental Figure 5).
DREPP Localizes to Membrane Nanodomains in Response to Symbiotic Stimuli
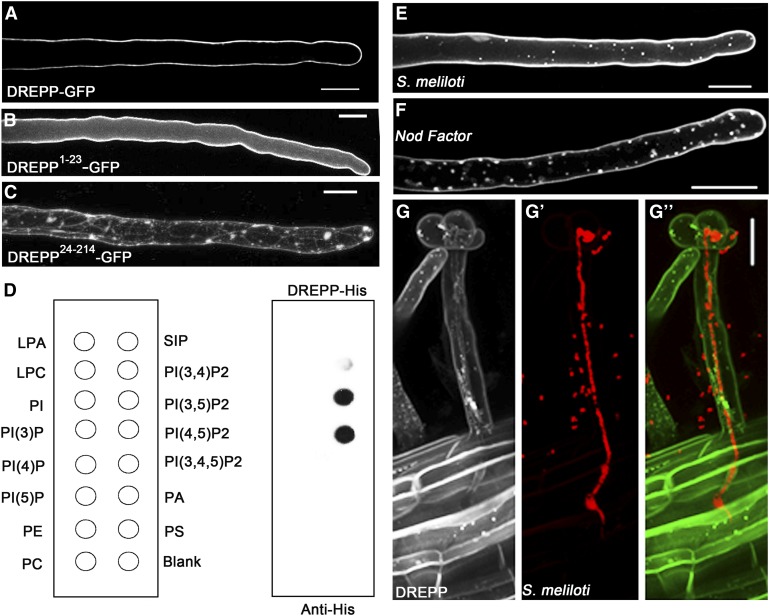
To elucidate the molecular function of DREPP during symbiotic infections, we first examined the localization of a DREPP-GFP fusion protein. Ectopic expression of DREPP-GFP resulted in a peripheral fluorescent signal in young RHs, indicating an association of the protein with the PM (Figure 2A). PM localization is mediated by the N-terminal region of the protein since the N-terminal 23 amino acids (DREPP1–23) of the DREPP polypeptide were required and sufficient to immobilize GFP at the PM (Figure 2B). Reciprocally, deletion of the N-terminal 23 amino acids (DREPP24–214) resulted in a cytosolic accumulation of the protein (Figure 2C).
Figure 2.
DREPP Binds to the PM and Relocalizes to Membrane Nanodomains in a Symbiosis-Dependent Manner.
(A) A DREPP-GFP fusion protein localizes to the PM in uninoculated M. truncatula RHs.
(B) and (C) The N-terminal 23 amino acids (1 to 23) are required (C) and sufficient (B) to associate soluble GFP with the PM.
(D) to (F) Hybridization of recombinant His-tagged DREPP to PIP-Strips revealed a specific association with PI(3,4)P2, PI(3,5)P2 and PI(4,5)P2. This assay was repeated twice with independently isolated recombinant DREPP, of which one replicate is shown. Open circles indicate position of the corresponding lipids immobilized on the membrane ([E] and [F]) DREPP-GFP–expressing RHs were inoculated with S. meliloti for 7 d (E) and incubated in the presence of isolated NFs for 12 h (F) before inspection. These treatments resulted in a specific accumulation of DREPP in membrane nanodomains as indicated by punctate structures. LPA, Lysophosphatidic acid; LPC, Lysophosphocholine; PtdIns, Phosphatidylinositol; PtdIns(3)P, Phosphatidylinositol (3)-phosphate; PtdIns(4)P, Phosphatidylinositol (4)-phosphate; PtdIns(5)P, Phosphatidylinositol (5)-phosphate; PE, Phosphatidylethanolamine; PC, Phosphatidylcholine; S1P, Sphingosine 1-Phosphate; PtdIns(3,4)P2, Phosphatidylinositol (3,4)-bisphosphate; PtdIns(3,5)P2, Phosphatidylinositol (3,5)-bisphosphate; PtdIns(4,5)P2, Phosphatidylinositol (4,5)-bisphosphate; PtdIns(3,4,5)P3, Phosphatidylinositol (3,4,5)-trisphosphate; PA, Phosphatidic acid; PS, Phosphatidylserine.
(G) to (G’’) Nanodomain localization of DREPP is abolished in IT-containing root hairs (harboring mCherry-labelled rhizobia) but present in cortical cells prior to infection. (G) indicates DREPP-GFP, (G’) indicates mCherry-labelled rhizobia and (G’’) shows the merged channels. Scale bars = 20 µm.
As the DREPP polypeptide harbors a Gly residue after the N-terminal Met, we tested whether this residue serves as a myristoylation site by expressing a mutant variant where we exchanged the Gly by an Ala (DREPPG2A) in Nicotiana benthamiana leaf epidermal cells. While the wild-type DREPP protein localized to the PM, the DREPPG2A mutant showed an increased cytosolic fraction, although the majority of the protein remained at the PM (Supplemental Figure 6A). This suggests that myristoylation at least contributes to PM association of DREPP, as shown for several other proteins (Konrad and Ott, 2015). However, an even stronger effect was observed when generating an N-terminal GFP fusion with DREPP (GFP-DREPP), where the presence of GFP abolishes the recognition of the Gly residue as a myristoylation site in the DREPP polypeptide (Supplemental Figure 6A).
Because of a strongly increased cytosolic fraction of the GFP-DREPP protein (Supplemental Figure 6B) compared to the DREPPG2A mutant, we assumed an additional mechanism to contribute to PM binding. Since PM association of Nicotiana tabacum DREPP2 is mediated by a strong polybasic cluster in its N-terminal domain (Vosolsobě et al., 2017), we predicted surface-exposed residues in the M. truncatula DREPP protein. Indeed, four Lys residues (K7, K11, K14, and K18) were found to form a putative polybasic cluster (Supplemental Figure 6C). To test the impact of this poly-Lys stretch on PM association experimentally, we generated three mutant variants in which we replaced one (DREPPK14E, DREPPK18E) or two (DREPPK14/18E) lysines with acidic glutamates. While expressing DREPPK14E and DREPPK18E resulted in more visible cytosolic strands (Supplemental Figure 6A) and an increased cytosolic fraction (Supplemental Figure 6B), this effect was further pronounced in the DREPPK14/18E double mutant (Supplemental Figure 6). As polybasic clusters have been reported to support the association of proteins with phosphoinositolphosphates (PIPs; Heo et al., 2006), we recombinantly expressed and purified full-length Medicago DREPP from Escherichia coli and hybridized it to PIP-Strips containing a series of different PIPs. Indeed, DREPP strongly bound to PI(3,5)P2 and PI(4,5)P2 (Figure 2D). Taken together, these data suggest a bifunctional mechanism of PM association for DREPP.
Since the subcellular localization and the actin-severing function of Arabidopsis PCaP1 were shown to change in the presence of calcium (Li et al., 2011; Zhu et al., 2013; Qin et al., 2014), we tested whether the application of calcium alters the PM localization of the DREPP-GFP fusion protein. Expression of DREPP-GFP under the control of the native DREPP promoter (1.5 kb) resulted in a weak but clear signal at the PM of RHs (Supplemental Figure 7A). Interestingly, application of 1 mM CaCl2 for 2 h induced a recruitment of the protein into punctate structures at the PM (Supplemental Figure 7A). The same effect was observed when ectopically expressing DREPP using the L. japonicus Ubiquitin10 promoter (Supplemental Figure 7A). Because of the low fluorescence of the ProDREPP:DREPP-GFP construct, we continued using the ubiquitin promoter-driven construct.
To study the dynamics of DREPP during nodulation, we inoculated roots constitutively expressing DREPP-GFP with symbiotic S. meliloti (Figure 2E) or isolated S. meliloti NFs (Figure 2F). Here, we again observed a relocalization of DREPP into punctate membrane structures at 7 DAI and 12 hours post inoculation, respectively. However, these structures almost fully disappeared in IT-containing RHs but were present in cortical cells prior to their penetration by the IT (Figure 2G).
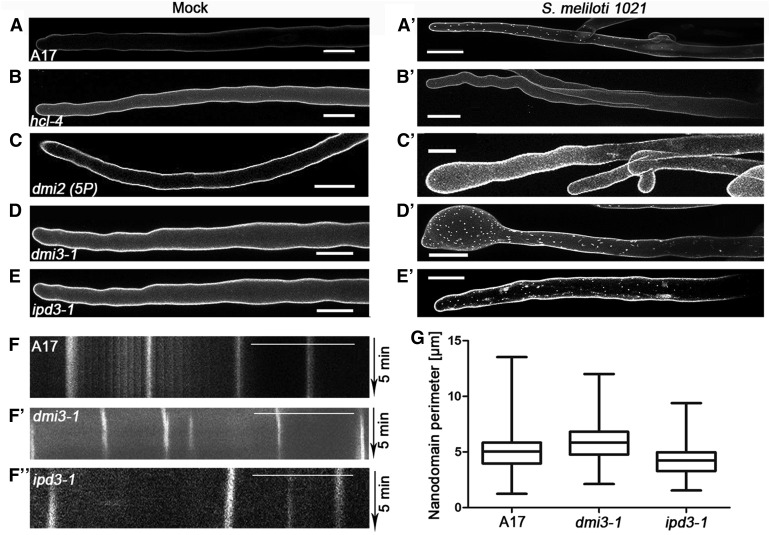
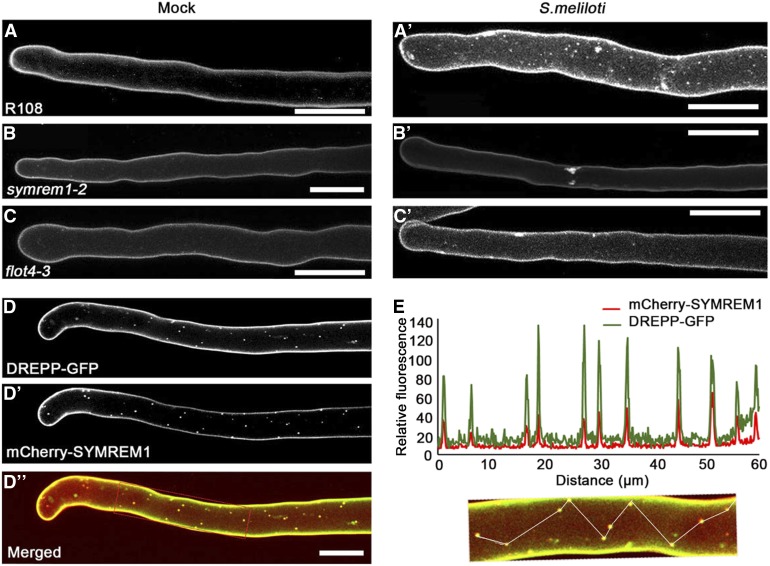
To genetically position the recruitment of DREPP into these potential membrane nanodomains, we ectopically expressed DREPP-GFP in different mutant backgrounds that show symbiotic phenotypes. In the absence of rhizobia, a peripheral GFP signal was observed, indicating that DREPP resided in the PM in all tested genotypes (Figures 3A to 3E). Interestingly and different to the wild type (A17; Figure 3A’), the lateral segregation of DREPP into nanodomains was abolished in the LYK3 and DMI2 receptor mutant alleles hcl-4 and dmi2 (5P), respectively (Figures 3B’ to 3C’). By contrast, DREPP localization was unaffected in dmi3-1 and ipd3-1 mutants (Figures 3D to 3E’). However, nanodomain recruitment of DREPP was rescued in dmi2 (5P), hcl-1, and hcl-4 mutants by exogenous calcium application (Supplemental Figure 7B). As in the A17 wild-type RHs, membrane nanodomains were laterally stable over 5 min (Figures 3F to F’’), which is a hallmark of these membrane subcompartments in plants (Jarsch et al., 2014; Liang et al., 2018). To address whether DREPP is recruited into a recently described symbiosis-induced nanodomain (Haney et al., 2011; Liang et al., 2018), we tested whether DREPP localization depends on the two nanodomain scaffolds SYMREM1 and FLOT4 by expressing DREPP in the symrem1-2 and flot4-3 mutant backgrounds. While DREPP uniformly localized to the PM in the absence of rhizobia (Figures 4A to 4C), nanodomain recruitment was impaired in both mutant alleles (Figures 4B’ to 4C’), but not in the corresponding R108 wild type (Figure 4A’). Furthermore, calcium treatment was not sufficient to induce nanodomain recruitment of DREPP in flot4-3 and symrem1-2 mutants (Supplemental Figure 7B, ). These data implied that FLOT4 and/or SYMREM1 are essential to recruit DREPP into membrane nanodomains. Since FLOT4 and SYMREM1 colocalize in the same nanodomains upon rhizobial inoculation (Liang et al., 2018), we hypothesized that DREPP is recruited into the same SYMREM1-positive structures. To test this, we constitutively coexpressed DREPP and SYMREM1 in M. truncatula roots and inoculated these transgenic roots for 7 d with S. meliloti. Both proteins colocalized in immobile nanodomains under these conditions (Figures 4D to 4E), demonstrating that DREPP is recruited to this symbiosis-induced nanodomain in a stimulus-dependent manner. To test whether both scaffolds are able to associate with DREPP, we pairwise coexpressed DREPP with FLOT4 or SYMREM1 in N. benthamiana leaf epidermal cells and performed coimmunoprecipitation (co-IP) experiments by pulling GFP-tagged DREPP using anti-GFP nanotraps. Indeed, DREPP copurified with both FLOT4 and SYMREM1, indicating that these proteins reside in the same complex (Figure 5A).
Figure 3.
Genetic Requirements for Nanodomain Recruitment of DREPP.
(A) to (E) Ubiquitously expressed DREPP-GFP localizes homogenously to the PM of RHs in different M. truncatula mutants (see [A] to [E]) in the absence of rhizobia.
(A’) to (E’) Relocalization of DREPP to membrane nanodomains occurred in a symbiosis-dependent manner at 7 DAI in all genotypes (see [A’], [D’], and [E’]) except in the receptor mutants hcl-4 (B’) and dmi2 (5P) (C’).
(F) to (F’’) Kymograph analysis of an ∼80 frame time-lapse (5-min) image series to determine spatio-temporal mobility of nanodomain-localized DREPP in the wild type (A17; see [F]), dmi3-1 (F’), and ipd3-1 (F’’). Vertical lines indicate full immobility of the protein. Scale bars = 20 µm.
(G) ImageJ-based analysis of nanodomain perimeters did not reveal any significant difference between the genotypes. n = 10 RHs for each genotype and measurement. Data are means ± se. Statistical analysis was performed using an unpaired, two-tailed t test. Bars = 20 µm.
Figure 4.
DREPP Localizes to Membrane Nanodomains in a FLOT4- and SYMREM1-Dependent Manner.
(A) to (C’) M. truncatula RHs transformed with pUBI:DREPP:GFP in the wild type (R108; see [A] and [A’]), symrem1-2 ([B] and [B’]), and flot4-3 ([C] and [C’]) under noninoculated (mock) conditions ([A] to [C]) and after 7 DAI with S. meliloti ([A’] to [C’]). Images are maximum intensity z-projections of ∼70 optical sections acquired at 0.5- to 1-µm increments.
(D) to (D’’’) Coexpression and localization of DREPP-GFP (D) and mCherry-SYMREM1 (D’) in transgenic M. truncatula A17 RHs at 7 DAI with S. meliloti. Colocalization analysis using a transect as indicated by the line within the image panel ([D] to [E]). Images are maximum intensity z-projections of around 40 optical sections at 0.5- to 1-µm increments. Bars = 20 µm.
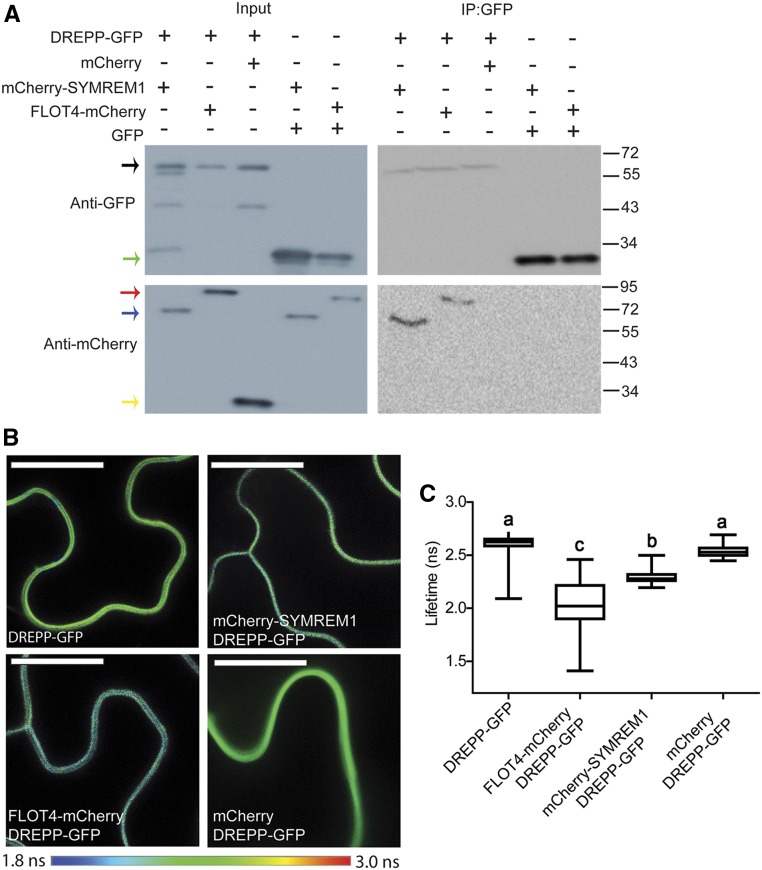
Figure 5.
DREPP Interact with FLOT4 and SYMREM1.
(A) Immunoprecipitation experiment after pairwise coexpression of DREPP with SYMREM1 or FLOT4 in N. benthamiana leaves using anti-GFP nanotraps revealed DREPP to be a component of the FLOT4-SYMREM1 complex. Black arrow indicates DREPP-GFP, green arrow indicates free GFP, red arrow indicates FLOT4-mCherry, blue arrow indicates mCherry-SYMREM1, and yellow arrow indicates free mCherry.
(B) and (C) FLIM analysis in transformed N. benthamiana leaves. Data in (C) are means ± se, n = 50. Statistical analysis was performed using a one-way analysis of variance (Tukey) test. Different letters indicate significant difference in GFP lifetime between the respective donor alone and the respective combination. Free mCherry was used as the negative control. Bars = 10 µm.
To further validate this finding and to additionally test whether the interaction between DREPP and both scaffolds is rather direct, we performed fluorescence lifetime imaging microscopy (FLIM). This method relies on a Foerster resonance energy transfer between a donor fluorophore (here GFP) and an acceptor (here mCherry) when in close proximity (∼1 nm) to each other. Expressing DREPP-GFP alone in N. benthamiana leaf epidermal cells resulted in a GFP lifetime of 2.61 ns (donor only), which served as a baseline (Figures 5B and 5C). In agreement with our co-IP data (Figure 5A), coexpression of mCherry-SYMREM1 or FLOT4-mCherry with DREPP-GFP resulted in significantly decreased donor lifetimes with 2.03 and 2.30 ns, respectively (Figures 5B and 5C). By contrast, no lifetime reduction was observed when expressing free mCherry as a negative control in the presence of DREPP-GFP, suggesting that DREPP specifically and directly interacts with SYMREM1 and FLOT4.
DREPP Nanodomains Are Linked with MT Fragmentation
Since few DREPP proteins have been shown to function in MT processing in vitro, we first tested a possible association of DREPP with MTs using an in vitro cosedimentation assay. For this, we used purified dimers of α- and β-tubulin and made use of their self-assembling capacity at 30°C in the presence of GTP and paclitaxel, an MT-stabilizing agent. When adding recombinantly expressed and purified His-tagged DREPP protein, the DREPP associated with polymerized MTs directly as indicated by the presence of DREPP in the MT pellet (Supplemental Figure 8).
Next, we asked whether MT association and possible processing occur in membrane nanodomains. For this, we coexpressed DREPP-mCherry and the MT marker YFP-MAP4 under the control of the Ubiquitin-promoter in M. truncatula RHs. As shown before in the root epidermis, ectopic expression of DREPP resulted in a reorientation of MTs, but the filaments appeared largely intact (Supplemental Figure 3). This pattern was also observed in RHs (Figure 6). However, when scoring DREPP localization and MT fragmentation 7 d after inoculating roots with S. meliloti, that is, after DREPP relocalization into nanodomains, MTs were found to be occasionally fragmented in the wild-type roots, but not in the drepp mutants (Figure 6). However, MT fragmentation was largely pronounced when overexpressing DREPP (Figure 6) and DREPP-labeled nanodomains aligned with fragmented MTs (Supplemental Figure 9). However, treating uninoculated RHs with the MT-depolymerizing drug oryzalin did not prevent recruitment of DREPP to the PM (Supplemental Figure 10), indicating that DREPP processes MTs at the PM rather than requires them for its localization.
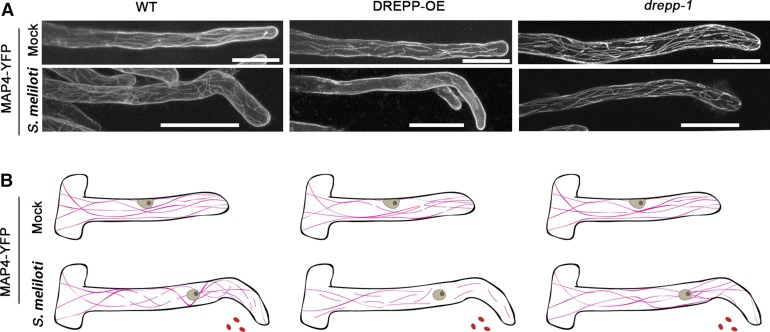
Figure 6.
Recruitment of DREPP to Nanodomains Coincides with MT Fragmentation.
(A) M. truncatula RHs of the wild type (A17) ubiquitously expressing MAP4-YFP (WT, left panel) or DREPP-GFP together with MAP4-YFP (DREPP-OE, middle panel) and MAP4-YFP (drepp-1, right panel). Plants were inspected under uninoculated (control) conditions or at 7 DAI with S. meliloti. Images are maximum intensity z-projections of ∼70 optical sections acquired at 0.5- to 1-µm increments. Bars = 20 µm.
(B) Graphical model of observed MT (magenta) patterns in trichoblasts. Rhizobia and nuclei are indicated in red and gray, respectively.
Since DREPP does not uniformly label MTs, we asked whether it associates predominantly with the plus or minus end of the MT filaments and may interfere with the corresponding protein machinery there. For this, we used N. benthamiana and constitutively coexpressed DREPP together with the plus-end markers MICROTUBULE-END BINDING1b (EB1b) and CLIP-ASSOCIATED PROTEIN (CLASP); and the minus-end markers GCP3-INTERACTING PROTEIN1A (GIP1a)/GAMMA-TUBULIN COMPLEX PROTEIN2 (GCP2) and KATANIN1 (KTN1), a marker for MT branch points, or with SPIRAL2 (SPR2), which labels both ends and branch points. When performing coimmunoprecipitation experiments using anti-GFP nanobody traps on protein extracts from N. benthamiana leaves, we only obtained signals for SPR2 and EB1b (Supplemental Figure 11), indicating that DREPP may predominantly associate with the plus end of the MTs and interferes with their elongation.
Taken together, these results show that DREPP controls MT reorganization by interfering with their plus-end elongation, predominantly upon localizing to membrane nanodomains after inoculation with rhizobia.
DISCUSSION
Rhizobial infections of legume RHs require a tight spatio-temporal coordination of molecular and morphological changes in order to provide a suitable environment for the initial bacterial entry and for maintaining a fast-growing IT. Earlier work demonstrated that prolonged exposure of RHs to isolated NFs or application of rhizobia results in a reorganization of membrane-resident signaling proteins such as the entry receptor LYK3 and the molecular scaffolds FLOT4 and SYMREM1 into membrane nanodomains (Haney et al., 2011; Liang et al., 2018). While the precise molecular composition of this symbiosis-induced nanodomain remains elusive, FLOT4 and actin are ultimately required for the subsequent recruitment of SYMREM1 and LYK3, which has been hypothesized to mark the transition between initial signaling events and morphological changes to prepare the cell for infection (Liang et al., 2018). This process is accompanied by a reorganization of the RH cytoskeleton (Sieberer et al., 2005). Here, we demonstrate that DREPP is a novel component of this symbiosis-related nanocluster at the PM that is recruited into these domains in an NF-dependent manner (Figure 2) and by the association with SYMREM1 and FLOT4 (Figures 4 and 5).
In general, RH growth is characterized by longitudinal cortical microtubule (CMT) arrays within the shanks of the RH while the most apical region is devoid of CMTs (Sieberer et al., 2002) in M. truncatula but comprises rather organelle-depleted cytoplasm with highly enriched secretory vesicles (reviewed by Sieberer et al., 2005). Growth arrest of RHs results in decreased but longitudinally arranged CMTs in M. truncatula (Sieberer et al., 2002). In addition to CMTs, legumes display dense arrays of endoplasmic microtubules (EMTs) that populate the subapical region between the nucleus and the RH tip (Sieberer et al., 2002, 2005; Weerasinghe et al., 2003). The fact that abundant EMTs have not been observed in other model plants such as Arabidopsis indicates differences in MT organization in between plant families. This is also supported by an additional and interesting difference that has been observed when studying the effect of paclitaxel (Taxol), which inhibits MT dynamics by stabilizing and bundling MT polymers (Sieberer et al., 2002). In contrast to Arabidopsis (Ketelaar et al., 2002), paclitaxel application to M. truncatula RHs does not inhibit RH tip growth but only reduces the growth rates (Sieberer et al., 2002), raising the hypothesis that these EMT arrays serve specialized functions during rhizobial infections of legume RHs. Given the fragmentation pattern of MTs in RHs overexpressing DREPP (Figure 6) and its NF-induced localization into membrane nanodomains (Figure 2F), we tend to speculate that DREPP serves specialized rather than general functions in M. truncatula. This would explain the absence of a generic RH phenotype in our M. truncatula drepp mutants (Supplemental Figure 2), which is different from phenotypes observed for the paralogs in Arabidopsis (Kato et al., 2013). Interestingly, DREPP is conserved in legumes that have lost nodulation such as Cercis canadensis or Nissolia schottii (Griesmann et al., 2018), indicative of additional functions and likely being context dependent. Mechanistically, this functional specialization could be achieved by temporally controlled association to the MT end tracking proteins SPR2 and EB1b (Supplemental Figure 11).
Interestingly, the application of calcium resulted in the same nanodomain localization pattern of DREPP (Supplemental Figure 7). A calcium-dependent interplay between MT association and nanodomain localization has already been reported for other protein-like members of the IQ67 DOMAIN (IQD) family such as IQD24 and IQD25 (Bürstenbinder et al., 2017). Although the precise function of these proteins remains unclear, it has been hypothesized that they play roles in MT patterning (Abel et al., 2013). However, DREPP recruitment into nanodomains may not be absolutely essential for rhizobial infections as these structures were not observed in the LYK3 mutant hcl-4 (Figure 3B’), which still forms initial ITs. Whether NF-induced calcium spiking is an ultimate prerequisite for nanodomain accumulation of DREPP remains open, since the presence of calcium spiking in single locus lyk3 mutants (hcl-4 and hcl-1; Wais et al., 2000) might be due to functional redundancy of the receptor. Furthermore, exogenous application of calcium to dmi2 (5P) and hcl-1/hcl-4 mutants restored DREPP localization to nanodomains, whereas this was not observed in symrem1-2 and flot4-3 mutants (Supplemental Figure 7). These results led us to conclude that calcium-dependent recruitment to nanodomains is an integral response to active symbiotic signaling that fails to occur in hcl-4 and dmi2 (5P) mutants, while SYMREM1 and FLOT4 provide rather structural components for the recruitment. In addition, FLOT4- and SYMREM1-positive nanodomains have been shown to form independently of a disrupted MT network, while interfering with the actin cytoskeleton resulted in a loss of these structures (Liang et al., 2018). By contrast, nanodomain recruitment of several other membrane-resident proteins outside of the symbiosis-specific nanodomain such as HIR1 (Lv et al., 2017), groups 1 and 6 remorins, and other proteins (Jarsch et al., 2014; Szymanski et al., 2015) have be shown to largely depend on intact MT arrays and/or be restricted in their lateral mobility by them. Our finding that overexpression of DREPP results in altered MT orientation patterns (Supplemental Figure 3) and that MT fragmentation coincides with the FLOT4- and SYMREM1-dependent nanodomain recruitment of DREPP (Figure 4; Supplemental Figure 9) allows the additional hypothesis that this catalytic activity of DREPP may be restricted to membrane nanodomains providing a unique physiological environment. As a consequence, DREPP may serve specialized functions in active MT rearrangement during RH curling in response to rhizobial inoculation as general RH length was not significantly different between our drepp mutant alleles and the wild type (Supplemental Figure 2A; Supplemental Data Set 2). Whether DREPP is also involved in MT fragmentation during bacterial release, a process that has been recently described (Kitaeva et al., 2016), remains to be studied in detail, but altered or delayed release patterns in 2-week-old nodules (Supplemental Figure 5) indicates such a role.
METHODS
Plant Growth and Phenotypic Analysis
Medicago truncatula seeds were activated by incubating them in pure sulfuric acid for 10 to 15 min followed by six successive washing steps in sterile water. Seeds were surface sterilized using a 12% (v/v) sodium hypochlorite solution containing 0.1% (w/v) SDS for 1 min followed by extensive washing in sterile water. Seeds were then placed on 1% water agar plates and vernalized at 4°C for 3 d in darkness. Germination was allowed for up to 24 h at 24°C in darkness before transferring the seedlings for growth on vertical plates containing Fahraeus medium.
For phenotyping experiments, seeds were germinated accordingly. After 1 d of germination, the seeds were placed onto solid Fahraeus medium (Medicago handbook, https://www.noble.org/medicago-handbook) containing 0.5 mM ammonium nitrate. One week later, the seedlings were transferred onto new Fahraeus medium without nitrate containing 0.1 µM aminoethoxyvinylglycine. M. truncatula roots were kept for 4 d under nitrogen starvation conditions prior to rhizobial inoculation. Sinorhizobium meliloti (Sm2011) liquid cultures were sedimented (for 3 min at 800g), washed, and resuspended in liquid Fahräeus medium to a final OD600 = 0.05 to 0.07. Each root was covered with 1 mL of rhizobia suspension that was removed after 6 min. Afterwards, plants were grown at 24°C in white light (150 µmol s−1 m−2) with the roots being kept in darkness. For growth in pots, the seedlings were directly transferred to a mixture of equal volume of quartz sand and vermiculite and watered with Fahraeus liquid medium (without nitrate) twice a week. After 1 week of growth, the seedlings were inoculated with S. meliloti (Sm2011) at a final OD600 = 0.005.
Hairy Root Transformation
M. truncatula hairy root transformation was performed as described previously (Boisson-Dernier et al., 2001). Transgenic Agrobacterium rhizogenes (ARqua1) strains, carrying the plasmids of choice, were grown in Luria-Bertani (LB) liquid medium for 1 d and subsequently plated on solid LB agar plates with corresponding antibiotics (100 µg/mL spectinomycin for BB20/BB25, 50 µg/mL kanamycin for BB53, and 100 µg/mL ampicillin for pDEST17) for another 2 d before transformation. M. truncatula seeds were surface sterilized and vernalized as described above. Emerging roots were removed from the seedlings prior to their shoots being dipped into the A. rhizogenes culture and placed on solid Fahraeus medium containing 0.5 mM ammonium nitrate. Transformed seedlings were incubated for 3 d at 22°C in darkness, followed by 4 d at 22°C in white light with roots covered. One week after transformation, seedlings were transferred onto fresh Fahraeus medium and grown for another 7 to 9 d at 24°C in white light. Afterwards, the seedlings were screened for transformation efficiency using a stereomicroscope scoring the presence of a fluorescent nuclear marker. Untransformed roots were removed and seedlings were transferred onto nitrogen-free solid FP medium containing 0.1 µM aminoethoxyvinylglycine. After 3 to 5 d at 24°C, the seedlings were inoculated with S. meliloti for further studies. All seedling preparation steps were performed under sterile conditions.
Promoter Isolation and Analysis
For promoter studies, a 1.5-kb-long fragment of the putative DREPP promoter was amplified from the Medicago A17 genome and used to drive expression of GUS in transgenic M. truncatula roots. Roots were harvested at 7 d after inoculation with rhizobia and incubated at 37°C in GUS solution (10 mM Na-EDTA, 1 mM potassium ferricyanide, 1 mM potassium ferrocyanide, 100 mM sodium phosphate, pH 7.0, 0.1% [v/v] Triton X-100, and 0.3% [w/v] x-Gluc) in darkness. Afterwards, the roots were washed once with double-distilled water and stored in 70% (v/v) ethanol. Analysis of GUS expression was done using stereomicroscopy.
Construct Design
For expression and purification of the recombinant M. truncatula DREPP protein, the coding sequence (GenBank no. XM_013607856.1) was recombined into the Gateway-compatible pDEST17 vector using LR clonase. All other constructs were cloned in a Golden Gate–compatible cloning system as previously described by Binder et al. (2014). The DREPP coding and genomic sequences were amplified from mRNA and genomic DNA from the A17 wild-type plants, respectively. All the MT markers used in co-IP assays were synthesized by Thermo Fisher Scientific. BpiI and BsaI restriction sites were removed from all sequences prior to cloning them into level II or level III expression vectors. All designed constructs and cloning primers are listed in Supplemental Tables 1 and 2, respectively. Identifiers for genes used in this study are list in Supplemental Table 3.
Genotyping of Tnt1 Insertion Lines and Expression Analysis
M. truncatula (R108 background) Tnt1 transposon insertion lines were obtained from the Noble Research Institute in Oklahoma. Insertions were verified and precisely mapped using primers listed in Supplemental Table 2. RNA isolation from at least six individuals, cDNA synthesis, and RT-qPCR were conducted as described previously (Liang et al., 2018) using a SYBR Green assay (Applied Biosystems). All data were normalized to cycle threshold values of the housekeeping gene Ubiquitin (Satgé et al., 2016). For the time-course experiments, material from at least two independently grown and inoculated plants was harvested and processed as described above.
DREPP Protein Expression and Purification
The His-tagged DREPP fusion protein was purified from Escherichia coli strain BL21 (DE3). Bacteria were grown overnight and then refreshed in 100 mL of LB medium containing the respective antibiotics at 37°C. Gene expression was induced at an OD600 of 0.6 by adding β-d-1-thiogalactopyranoside (final concentration, 1 mM) in the culture and further culturing at 16°C overnight. Recombinant DREPP was purified using nickel-nitrilotriacetic acid beads (Qiagen) as described previously (Tao et al., 2010).
Transformation of Nicotiana benthamiana Leaves, Microsomal Fractionation, and Immunoblot Analysis
Transgenic Agrobacterium tumefaciens (Agl1), carrying the plasmids of interest, were grown in LB liquid culture overnight at 28°C, using the appropriate antibiotics. The culture was sedimented at 1000g for 2 min and subsequently resuspended in Agromix (10 mM MgCl2, 10 mM MES/KOH, pH 5.6, and 150 μM acetosyringone) to a final OD600 = 0.3. Bacteria were incubated for 2 h at 25°C in darkness prior to syringe infiltration into the abaxial site of N. benthamiana leaves. Microsomal fractions protocol was followed as in Abas and Luschnig (2010), with some modifications. Extraction buffer was added to frozen root-pulverized samples (generally 1.0 to 1.5 μL extraction buffer/mg material; minimum volume, 300 μL). The homogenate was transferred to prepared polyvinyl polipyrrolidone pellets to adsorb phenolic compounds, vortexed for 30 s, and left for 5 min. Samples were centrifuged twice (at 600g for 3 min at 4°C), and the pellet (debris and nuclear fraction) was kept aside. Supernatants were centrifuged (at 2000g for 2 min at 4°C) to obtain a cleared homogenate, and the pellet containing organelles was kept aside. Cleared homogenates were diluted with water to 0.37 to 0.40 M Suc (usually an equal volume of water was added), vortexed for 10 s, and centrifuged (at 21,000g for 2 h at 4°C) to obtain the microsomal fraction (pellet) and cytosolic fraction (supernatant). For co-IP assays, DREPP-GFP was coexpressed with the different mCherry-tagged MTs markers in N. benthamiana leaves for 2 to 3 d. Extracted proteins were immunoprecipitated using anti-GFP nanobody traps (no. 90722001MA, Chromotek). Proteins were separated on a 12% (w/v) SDS gel and transferred overnight at 30 V to a polyvinylidene difluoride membrane. Blots were then blocked with 5% (w/v) milk for 1 h at room temperature (RT) before being hybridized with the corresponding anti-GFP (no. 632381, Takara) or anti-mCherry (no. 632496, Takara) antibodies at dilutions of 1:5000 and 1:3000, respectively. All experiments have been repeated at least three times using independently grown plants and bacterial suspensions.
Treatment of M. truncatula Roots for Microscopy Analysis
We solubilized 10 nM S. meliloti NF and 1 mM CaCl2 in sterile water and applied to excised roots for 12 and 2 h, respectively, prior to their microscopy analysis. For MT depolymerization experiments, excised roots were emerged in a 10 µM oryzalin solution for 14 h at RT. In all cases, the control (mock) samples were treated in the same way using sterile water for incubation. For nodule sections, the material was harvested 2 or 4 weeks after being inoculated with S. meliloti in open pots. Nodules were directly embedded in 6% (w/v) low melting agarose before being sectioned into semithin (70-μm) longitudinal sections using a vibratome.
Whole Mount Staining of MT Cytoskeleton
The MT cytoskeleton was stained according to Ditengou et al. (2003), with minor modifications. Roots were fixed in 2% (v/v) paraformaldehyde and 0.25% (v/v) glutaraldehyde in microtubule-stabilizing buffer (MTSB: 50 mM Pipes, 5 mM EGTA, 5 mM MgSO4, and 0.01% [v/v] Triton X-100, pH 7.0) for 30 min at RT. Fixed roots were washed twice and alternating for 5 min in MTSB and 5 min in water, respectively. After partial digestion of cell walls with 0.2% (w/v) macerozyme and 0.2% (w/v) driselase in 10 mM MES, pH 5.0, for 30 min at 37°C, roots were incubated in permeabilization solution (10% [v/v] DMSO/3% [v/v] Nonidet P-40 in MTSB) at RT for 1 h and washed five times for 5 min in MTSB at RT. Next, roots were incubated with a monoclonal anti–α-tubulin (bovine), mouse IgG1 antibody (A11126, Molecular Probes), and diluted 1:50 in MTSB and supplemented with 2% (w/v) BSA overnight at 4°C. After two washes in MTSB (10 min each time at RT), the primary antibody was recognized by an anti-mouse Alexa Fluor 488 antibody (A-11001, Thermo Fisher Scientific) at the final concentration of 1:1000 in MTSB with 2% (w/v) BSA at RT, in the dark. Roots were successively washed five times in MTSB and water (10 min each time) and mounted in ProLong Gold Antifade solution containing 4′,6-diamidino-2-phenylindole.
Confocal Laser Scanning Microscopy and Data Analysis
Confocal laser scanning microscopy was performed using a Leica TCS SP8 confocal microscope. Images of cells expressing single fluorophores were acquired using an HC PL APO 20×/0.75 IMM CORR CS2 water immersion objective and the following settings: GFP (excitation [Ex]: 488 nm; emission [Em]: 500 to 550 nm); YFP (Ex: 514 nm; Em: 520 to 552 nm); mCherry (Ex: 561 nm; Em: 575 to 630 nm). Samples coexpressing two fluorophores were imaged in sequential mode between frames. Because of the low signal intensities of the MAP4-YFP reporter, the corresponding fluorescence was detected using Leica HyD detectors. Images were taken with a Leica DFC350FX digital camera. MT orientation analysis was done using CytoSpectre (Kartasalo et al., 2015), and all other image analyses and projections were performed with ImageJ/(Fiji; Schindelin et al., 2012). For kymograph analysis, images were acquired over 5 min. Regions of interest were manually selected and analyzed using the kymograph plugin in Fiji. For nanodomain perimeter analysis, images were subjected to background subtraction with a rolling ball radius of 20 pixels prior to analysis. All material used for imaging was inspected without fixation. For all images, at least three independent rounds of transformation were conducted, and 5 to 10 transformed roots/root segments per round were imaged.
FLIM Analysis
Transgenic A. tumefaciens strains (ARqua1) carrying either an expression plasmid containing the donor (GFP) fusion protein only or both the donor (GFP) and acceptor (mCherry) fusion proteins were used to transform N. benthamiana leaf epidermal cells, following the same procedure as described above (final OD600 = 0.2). FLIM was performed with a SP8 Falcon confocal microscope (Leica) equipped with a pulsed white light laser using an 80-mHz pulse rate, an image resolution of 512 × 512, and a pixel dwell time of 3.16 µs. Images of cells expressing the different constructs were acquired with a 20×/0.75 IMM CORR CS2 water immersion objective using a 488-nm laser line for GFP excitation and 561-nm laser line for mCherry excitation, activated in sequential mode; emissions were detected sequentially using the HyD SMD detector, collecting photons emitted from 500 to 550 nm and from 600 to 650 nm, respectively. For donor fluorescence lifetime measurements, 20 repetitions of each frame were acquired. Three to five regions of interest located on the membrane were selected on each image and fitted with biexponential fitting functions in both donor only and donor plus acceptor samples. For each condition, an average of 10 to 15 different cells per leaf were imaged in at least three biological replicates (independent transformations).
Lipid Binding Assays
Lipid binding assays were performed as suggested by the supplier. In brief, PIP-Strips (Echelon Biosciences) were blocked using PBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.1% [w/v] Tween 20) supplemented with 3% (w/v) fatty acid–free BSA for 1 h at 25°C. The strips were then incubated with recombinant DREPP protein in 7 mL of PBST (final concentration: 100 ng/mL) at 4°C overnight before being washed with PBST five times (5 min per wash), blotted, and detected using an anti-His antibody (Sigma-Aldrich) for 2 h at 25°C. The blots were subsequently washed five times in PBST prior to visualization.
MT Binding Assays
The cosedimentation of the recombinant DREPP protein and MTs was performed as previously described by Li et al. (2011), with the following modifications. Purified protein preparations were sedimented at 200,000g and 4°C for 20 min before use. Prepolymerized and paclitaxel-stabilized MTs were obtained using brain tubulin (2 mg/mL; stored at –80°C), which was incubated in PEM buffer containing paclitaxel (PEMT buffer: 1 mM MgCl2, 1 mM EGTA, 100 mM Pipes-KOH, 1 mM GTP, 150 mM KCl, and 10 mM paclitaxel, pH 6.9) at 30°C for 20 min. Different amounts of recombinant DREPP fusion protein were added, and the mixtures were incubated at 30°C for an additional 20 min. After sedimentation at 100,000g for 20 min, supernatant and pellets were subjected to SDS-PAGE, and DREPP was detected using anti-His antibodies (A7058-1VL, Sigma-Aldrich) at a 1:2000 dilution. The experiment was repeated twice, of which one replicate is shown.
Phylogenetic Analysis
DREPP orthologs were searched within 104 plant genomes covering all main orders from gymnosperms to angiosperms (Supplemental Data Set 1). Searches were performed using tBLASTn v2.8.1+ (Camacho et al., 2009), an e-value of 1e–10, and the DREPP protein from M. truncatula as query. From blast results, protein sequences of all putative orthologs were retrieved and aligned using MUSCLE v3.8.31 (Edgar, 2004) with default parameters. Next, the alignment was cleaned by removing all positions containing more than 20% of gaps using trimAl v1.5rev57 (Capella-Gutiérrez et al., 2009). Resulting alignments were subjected to phylogenetic analysis using the maximum likelihood approach and IQ-TREE v1.6.7 (Nguyen et al., 2015). Prior to the phylogenetic analysis, the evolutionary best-fitting model was tested using ModelFinder (Kalyaanamoorthy et al., 2017) and retained according to the Bayesian Information Criteria. Branch support was tested with 10,000 replicates of UltrFast Bootstraps (Hoang et al., 2018). The final tree was visualized and annotated using the iTOL platform v4.3 (Letunic and Bork, 2016).
DREPP functional domains (PF05558.12) were retrieved for each putative ortholog using the Pfam database (Finn et al., 2016), an e-value of 0.01, and default parameters. Domains were then mapped on the phylogenetic tree using iTOL.
Statistical Analysis
ANOVA analyses or Student’s t tests were conducted according to the experimental setups. Full results of the analyses and parameters used are deposited in Supplemental Data Set 2.
Accession Numbers
Accession numbers for all genes used in this study can be found in Supplemental Table 3.
Supplemental Data
Supplemental Figure 1. Transcriptional regulation of DREPP.
Supplemental Figure 2. Medicago drepp mutants do not display a generic nucleus positioning phenotype in RHs.
Supplemental Figure 3. Cortical microtubule patterns in root epidermal cells of different genotypes.
Supplemental Figure 4. DREPP overexpression (DREPP-OE) reduces rhizobial infections.
Supplemental Figure 5. Patterning within Medicago drepp mutant nodules is altered.
Supplemental Figure 6. A bifunctional mechanism of PM association for DREPP.
Supplemental Figure 7. Calcium treatment induces DREPP-GFP relocalization into membrane nanodomains.
Supplemental Figure 8. DREPP directly associates with microtubules.
Supplemental Figure 9. DREPP nanodomains are linked with microtubule fragmentation in RHs.
Supplemental Figure 10. Depolymerization of microtubule filaments does not affect DREPP-GFP localization.
Supplemental Figure 11. Identifying DREPP-interacting proteins by co-immunoprecipitation (co-IP).
Supplemental Table 1. Constructs used in this study.
Supplemental Table 2. List of primers used for this study.
Supplemental Table 3. Identifiers for genes used in this study.
Supplemental Data Set 1. Phylogenetic tree. Members of the DREPP protein family can be found throughout the angiosperms.
Supplemental Data Set 2. ANOVA/t test results and raw data of RH length measurements.
DIVE Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank the staff of the Life Imaging Center in the Center for Biological Systems Analysis (Zentrale Servicestelle Berufsanerkennung) of the Albert Ludwigs University of Freiburg for help with their confocal microscopy resources and for the excellent support in image recording. Special thanks also to Giles Oldroyd (Sainsbury Laboratory Cambridge University, Cambridge, United Kingdom) for providing the S. meliloti NFs and to Magda Magiera (Carsten Janke group, Institut Curie, Université Paris Sciences et Lettres Research University, France) for providing tubulin for the cosedimentation assay. Furthermore, we thank Eija Schulze (Cell Biology, Faculty of Biology, University of Freiburg) for her excellent technical support. Many thanks to all the members of our team for fruitful discussions and providing their individual expertise throughout the course of the project. The M. truncatula plants utilized in this research project, which are jointly owned by the Centre National de la Recherche Scientifique, were obtained from Noble Research Institute and were created through research funded, in part, by the National Science Foundation (grant NSF-0703285). This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft) under Germany’s Excellence Strategy (CIBSS – EXC-2189 – Project ID 39093984); by the University of Cambridge (subgrant to the University of Freiburg to T.O.); by the Bill and Melinda Gates Foundation (through the Engineering Nitrogen Symbiosis for Africa project, grant OPP1172165); and by the China Scholarship Council (grant 201708080016 to C.S.). J.K. and P.-M.D. belong to the Laboratoire de Recherche en Sciences Végétales, which is part of the TULIP LABEX, Agence Nationale de la Recherche(grant ANR-10-LABX-41).
Author Contributions
C.S., M.-L.K., and T.O. designed the research; C.S., M.-L.K., C.H.-R., M.B., F.A.D., J.K., and B.L. performed the research; J.K. and P.-M.D. contributed new computational tools; C.S., P.-M.D., B.L., and T.O. analyzed the data; and C.S. and T.O. wrote the article.
Footnotes
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Abas L., Luschnig C.(2010). Maximum yields of microsomal-type membranes from small amounts of plant material without requiring ultracentrifugation. Anal. Biochem. 401: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S., Bürstenbinder K., Müller J.(2013). The emerging function of IQD proteins as scaffolds in cellular signaling and trafficking. Plant Signal. Behav. 8: e24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor B.B., Shaw S.L., Oldroyd G.E., Maillet F., Penmetsa R.V., Cook D., Long S.R., Dénarié J., Gough C.(2003). The NFP locus of Medicago truncatula controls an early step of Nod factor signal transduction upstream of a rapid calcium flux and root hair deformation. Plant J. 34: 495–506. [DOI] [PubMed] [Google Scholar]
- Antolín-Llovera M., Ried M.K., Parniske M.(2014). Cleavage of the SYMBIOSIS RECEPTOR-LIKE KINASE ectodomain promotes complex formation with Nod factor receptor 5. Curr. Biol. 24: 422–427. [DOI] [PubMed] [Google Scholar]
- Arrighi J.F., et al. (2006). The Medicago truncatula lysin [corrected] motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 142: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A., Lambert J., Morbitzer R., Popp C., Ott T., Lahaye T., Parniske M.(2014). A modular plasmid assembly kit for multigene expression, gene silencing and silencing rescue in plants. PLoS One 9: e88218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Chabaud M., Garcia F., Bécard G., Rosenberg C., Barker D.G.(2001). Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol. Plant Microbe Interact. 14: 695–700. [DOI] [PubMed] [Google Scholar]
- Bücherl C.A., Jarsch I.K., Schudoma C., Segonzac C., Mbengue M., Robatzek S., MacLean D., Ott T., Zipfel C.(2017). Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürstenbinder K., Möller B., Plötner R., Stamm G., Hause G., Mitra D., Abel S.(2017). The IQD Family of calmodulin-binding proteins links calcium signaling to microtubules, membrane subdomains, and the nucleus. Plant Physiol. 173: 1692–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L.(2009). BLAST+: Architecture and applications. BMC Bioinformatics 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T.(2009). trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoira R., Galera C., de Billy F., Penmetsa R.V., Journet E.P., Maillet F., Rosenberg C., Cook D., Gough C., Dénarié J.(2000). Four genes of Medicago truncatula controlling components of a nod factor transduction pathway. Plant Cell 12: 1647–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crdenas L., Vidali L., Domnguez J., Prez H., Snchez F., Hepler P.K., Quinto C.(1998). Rearrangement of actin microfilaments in plant root hairs responding to Rhizobium etli nodulation signals. Plant Physiol. 116: 871–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditengou F.A., Raudaskoski M., Lapeyrie F.(2003). Hypaphorine, an indole-3-acetic acid antagonist delivered by the ectomycorrhizal fungus Pisolithus tinctorius, induces reorganisation of actin and the microtubule cytoskeleton in Eucalyptus globulus ssp bicostata root hairs. Planta 218: 217–225. [DOI] [PubMed] [Google Scholar]
- Edgar R.C.(2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt D.W., Wais R., Long S.R.(1996). Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell 85: 673–681. [DOI] [PubMed] [Google Scholar]
- Finn R.D., et al. (2016). The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 44 (D1): D279–D285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J., Teillet A., Chabaud M., Ivanov S., Genre A., Limpens E., de Carvalho-Niebel F., Barker D.G.(2015). Remodeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol. 167: 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J., Timmers A.C., Sieberer B.J., Jauneau A., Chabaud M., Barker D.G.(2008). Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol. 148: 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage D.J.(2004). Infection and invasion of roots by symbiotic, nitrogen-fixing rhizobia during nodulation of temperate legumes. Microbiol. Mol. Biol. Rev. 68: 280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrin A., Jansen V., Ivanov S., Bisseling T., Fedorova E.(2015). ARP2/3-mediated actin nucleation associated with symbiosome membrane is essential for the development of symbiosomes in infected cells of Medicago truncatula root nodules. Mol. Plant Microbe Interact. 28: 605–614. [DOI] [PubMed] [Google Scholar]
- Griesmann M., et al. (2018). Phylogenomics reveals multiple losses of nitrogen-fixing root nodule symbiosis. Science 361: eaat1743. [DOI] [PubMed] [Google Scholar]
- Haney C.H., Long S.R.(2010). Plant flotillins are required for infection by nitrogen-fixing bacteria. Proc. Natl. Acad. Sci. USA 107: 478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney C.H., Riely B.K., Tricoli D.M., Cook D.R., Ehrhardt D.W., Long S.R.(2011). Symbiotic rhizobia bacteria trigger a change in localization and dynamics of the Medicago truncatula receptor kinase LYK3. Plant Cell 23: 2774–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Banba M., Shimoda Y., Kouchi H., Hayashi M., Imaizumi-Anraku H.(2010). A dominant function of CCaMK in intracellular accommodation of bacterial and fungal endosymbionts. Plant J. 63: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra R., Geurts R., Franssen H., Spaink H.P., Van Kammen A., Bisseling T.(1994). Root hair deformation activity of nodulation factors and their fate on Vicia sativa. Plant Physiol. 105: 787–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo W.D., Inoue T., Park W.S., Kim M.L., Park B.O., Wandless T.J., Meyer T.(2006). PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science 314: 1458–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang D.T., Chernomor O., von Haeseler A., Minh B.Q., Vinh L.S.(2018). UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35: 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg B.V., Cullimore J.V., Ranjeva R., Bono J.J.(2006). The DMI1 and DMI2 early symbiotic genes of Medicago truncatula are required for a high-affinity nodulation factor-binding site associated to a particulate fraction of roots. Plant Physiol. 140: 365–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M.S., Liao J., James E.K., Sato S., Tabata S., Jurkiewicz A., Madsen L.H., Stougaard J., Ross L., Szczyglowski K.(2012). Lotus japonicus ARPC1 is required for rhizobial infection. Plant Physiol. 160: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsch I.K., Konrad S.S., Stratil T.F., Urbanus S.L., Szymanski W., Braun P., Braun K.H., Ott T.(2014). Plasma membranes are subcompartmentalized into a plethora of coexisting and diverse microdomains in Arabidopsis and Nicotiana benthamiana. Plant Cell 26: 1698–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S.(2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartasalo K., Pölönen R.-P., Ojala M., Rasku J., Lekkala J., Aalto-Setälä K., Kallio P.(2015). CytoSpectre: A tool for spectral analysis of oriented structures on cellular and subcellular levels. BMC Bioinformatics 16: 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Aoyama T., Maeshima M.(2013). The Ca(2+)-binding protein PCaP2 located on the plasma membrane is involved in root hair development as a possible signal transducer. Plant J. 74: 690–700. [DOI] [PubMed] [Google Scholar]
- Ketelaar T., Faivre-Moskalenko C., Esseling J.J., de Ruijter N.C., Grierson C.S., Dogterom M., Emons A.M.(2002). Positioning of nuclei in Arabidopsis root hairs: An actin-regulated process of tip growth. Plant Cell 14: 2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaeva A.B., Demchenko K.N., Tikhonovich I.A., Timmers A.C., Tsyganov V.E.(2016). Comparative analysis of the tubulin cytoskeleton organization in nodules of Medicago truncatula and Pisum sativum: Bacterial release and bacteroid positioning correlate with characteristic microtubule rearrangements. New Phytol. 210: 168–183. [DOI] [PubMed] [Google Scholar]
- Konrad S.S.A., Ott T.(2015). Molecular principles of membrane microdomain targeting in plants. Trends Plant Sci. 20: 351–361. [DOI] [PubMed] [Google Scholar]
- Kosuta S., Hazledine S., Sun J., Miwa H., Morris R.J., Downie J.A., Oldroyd G.E.(2008). Differential and chaotic calcium signatures in the symbiosis signaling pathway of legumes. Proc. Natl. Acad. Sci. USA 105: 9823–9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B., et al. (2010). A remorin protein interacts with symbiotic receptors and regulates bacterial infection. Proc. Natl. Acad. Sci. USA 107: 2343–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., Bork P.(2016). Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44 (W1): W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy J., et al. (2004). A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303: 1361–1364. [DOI] [PubMed] [Google Scholar]
- Li J., Wang X., Qin T., Zhang Y., Liu X., Sun J., Zhou Y., Zhu L., Zhang Z., Yuan M., Mao T.(2011). MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23: 4411–4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Stratil T.F., Popp C., Marín M., Folgmann J., Mysore K.S., Wen J., Ott T.(2018). Symbiotic root infections in Medicago truncatula require remorin-mediated receptor stabilization in membrane nanodomains. Proc. Natl. Acad. Sci. USA 115: 5289–5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limpens E., Franken C., Smit P., Willemse J., Bisseling T., Geurts R.(2003). LysM domain receptor kinases regulating rhizobial Nod factor-induced infection. Science 302: 630–633. [DOI] [PubMed] [Google Scholar]
- Lv X., Jing Y., Xiao J., Zhang Y., Zhu Y., Julian R., Lin J.(2017). Membrane microdomains and the cytoskeleton constrain AtHIR1 dynamics and facilitate the formation of an AtHIR1-associated immune complex. Plant J. 90: 3–16. [DOI] [PubMed] [Google Scholar]
- Madsen L.H., Tirichine L., Jurkiewicz A., Sullivan J.T., Heckmann A.B., Bek A.S., Ronson C.W., James E.K., Stougaard J.(2010). The molecular network governing nodule organogenesis and infection in the model legume Lotus japonicus. Nat. Commun. 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinese E., Mun J.H., Yeun L.H., Jayaraman D., Rougé P., Barre A., Lougnon G., Schornack S., Bono J.J., Cook D.R., Ané J.M.(2007). A novel nuclear protein interacts with the symbiotic DMI3 calcium- and calmodulin-dependent protein kinase of Medicago truncatula. Mol. Plant Microbe Interact. 20: 912–921. [DOI] [PubMed] [Google Scholar]
- Montiel J., Downie J.A., Farkas A., Bihari P., Herczeg R., Bálint B., Mergaert P., Kereszt A., Kondorosi É.(2017). Morphotype of bacteroids in different legumes correlates with the number and type of symbiotic NCR peptides. Proc. Natl. Acad. Sci. USA 114: 5041–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki N., Tomioka R., Maeshima M.(2008). A hydrophilic cation-binding protein of Arabidopsis thaliana, AtPCaP1, is localized to plasma membrane via N-myristoylation and interacts with calmodulin and the phosphatidylinositol phosphates PtdIns(3,4,5)P(3) and PtdIns(3,5)P(2). FEBS J. 275: 2267–2282. [DOI] [PubMed] [Google Scholar]
- Nguyen L.T., Schmidt H.A., von Haeseler A., Minh B.Q.(2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldroyd G.E.(2013). Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 11: 252–263. [DOI] [PubMed] [Google Scholar]
- Ott T.(2017). Membrane nanodomains and microdomains in plant-microbe interactions. Curr. Opin. Plant Biol. 40: 82–88. [DOI] [PubMed] [Google Scholar]
- Qin T., Liu X., Li J., Sun J., Song L., Mao T.(2014). Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments. Plant Cell 26: 325–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Lin J.S., Xu J., Sato S., Parniske M., Wang T.L., Downie J.A., Xie F.(2015). SCARN a novel class of SCAR protein that is required for root-hair infection during legume nodulation. PLoS Genet. 11: e1005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satgé C., Moreau S., Sallet E., Lefort G., Auriac M.C., Remblière C., Cottret L., Gallardo K., Noirot C., Jardinaud M.F., Gamas P.(2016). Reprogramming of DNA methylation is critical for nodule development in Medicago truncatula. Nat. Plants 2: 16166. [DOI] [PubMed] [Google Scholar]
- Schindelin J., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nat. Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberer B.J., Timmers A.C., Emons A.M.(2005). Nod factors alter the microtubule cytoskeleton in Medicago truncatula root hairs to allow root hair reorientation. Mol. Plant Microbe Interact. 18: 1195–1204. [DOI] [PubMed] [Google Scholar]
- Sieberer B.J., Timmers A.C., Lhuissier F.G., Emons A.M.(2002). Endoplasmic microtubules configure the subapical cytoplasm and are required for fast growth of Medicago truncatula root hairs. Plant Physiol. 130: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Katzer K., Lambert J., Cerri M., Parniske M.(2014). CYCLOPS, a DNA-binding transcriptional activator, orchestrates symbiotic root nodule development. Cell Host Microbe 15: 139–152. [DOI] [PubMed] [Google Scholar]
- Smit P., Limpens E., Geurts R., Fedorova E., Dolgikh E., Gough C., Bisseling T.(2007). Medicago LYK3, an entry receptor in rhizobial nodulation factor signaling. Plant Physiol. 145: 183–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke S., Kistner C., Yoshida S., Mulder L., Sato S., Kaneko T., Tabata S., Sandal N., Stougaard J., Szczyglowski K., Parniske M.(2002). A plant receptor-like kinase required for both bacterial and fungal symbiosis. Nature 417: 959–962. [DOI] [PubMed] [Google Scholar]
- Szymanski W.G., Zauber H., Erban A., Gorka M., Wu X.N., Schulze W.X.(2015). Cytoskeletal components define protein location to membrane microdomains. Mol. Cell. Proteomics 14: 2493–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao H., Liu W., Simmons B.N., Harris H.K., Cox T.C., Massiah M.A.(2010). Purifying natively folded proteins from inclusion bodies using sarkosyl, Triton X-100, and CHAPS. Biotechniques 48: 61–64. [DOI] [PubMed] [Google Scholar]
- Timmers A.C., Auriac M.C., Truchet G.(1999). Refined analysis of early symbiotic steps of the Rhizobium-Medicago interaction in relationship with microtubular cytoskeleton rearrangements. Development 126: 3617–3628. [DOI] [PubMed] [Google Scholar]
- Tóth K., Stratil T.F., Madsen E.B., Ye J., Popp C., Antolín-Llovera M., Grossmann C., Jensen O.N., Schüssler A., Parniske M., Ott T.(2012). Functional domain analysis of the Remorin protein LjSYMREM1 in Lotus japonicus. PLoS One 7: e30817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasse J., de Billy F., Camut S., Truchet G.(1990). Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J. Bacteriol. 172: 4295–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva V.N., Kouchi H., Ridge R.W.(2005). Microtubule dynamics in living root hairs: Transient slowing by lipochitin oligosaccharide nodulation signals. Plant Cell 17: 1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosolsobě S., Petrášek J., Schwarzerová K.(2017). Evolutionary plasticity of plasma membrane interaction in DREPP family proteins. Biochim. Biophys. Acta Biomembr. 1859: 686–697. [DOI] [PubMed] [Google Scholar]
- Wais R.J., Galera C., Oldroyd G., Catoira R., Penmetsa R.V., Cook D., Gough C., Denarié J., Long S.R.(2000). Genetic analysis of calcium spiking responses in nodulation mutants of Medicago truncatula. Proc. Natl. Acad. Sci. USA 97: 13407–13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhu L., Liu B., Wang C., Jin L., Zhao Q., Yuan M.(2007). Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 19: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerasinghe R.R., Collings D.A., Johannes E., Allen N.S.(2003). The distributional changes and role of microtubules in Nod factor-challenged Medicago sativa root hairs. Planta 218: 276–287. [DOI] [PubMed] [Google Scholar]
- Yano K., et al. (2008). CYCLOPS, a mediator of symbiotic intracellular accommodation. Proc. Natl. Acad. Sci. USA 105: 20540–20545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota K., et al. (2009). Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Cui Y., Zhang X., Li R., Lin J.(2020). Organization and dynamics of functional plant membrane microdomains. Cell. Mol. Life Sci. 77: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepeda I., Sánchez-López R., Kunkel J.G., Bañuelos L.A., Hernández-Barrera A., Sánchez F., Quinto C., Cárdenas L.(2014). Visualization of highly dynamic F-actin plus ends in growing Phaseolus vulgaris root hair cells and their responses to Rhizobium etli nod factors. Plant Cell Physiol. 55: 580–592. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Kang E., Yuan M., Fu Y., Zhu L.(2015). PCaP2 regulates nuclear positioning in growing Arabidopsis thaliana root hairs by modulating filamentous actin organization. Plant Cell Rep. 34: 1317–1330. [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhang Y., Kang E., Xu Q., Wang M., Rui Y., Liu B., Yuan M., Fu Y.(2013). MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]