Abstract
Objective:
We conducted meta-analyses and meta-analytic structural equation modeling of longitudinal studies among cancer survivors to (a) quantify associations between psychosocial predictors and physical activity, (b) test how psychosocial predictors combine to influence physical activity, and (c) identify study, demographic, and clinical characteristics that moderate associations.
Method:
Eligible studies used a longitudinal, observational design, included a sample of cancer survivors, and measured both a psychosocial predictor at baseline and physical activity at a later time-point. Of 2,431 records located through computerized searches, 25 independent tests (N = 5,897) met the inclusion criteria for the review. Random effects meta-analyses and meta-analytic structural equation modeling were conducted.
Results:
Eight psychosocial predictors of physical activity were identified. Self-efficacy (r+ = 0.26) and intentions (r+ = 0.33) were the strongest predictors in bivariate analyses. The structural equation models included attitudes, injunctive norms, self-efficacy, intentions, and physical activity (k = 22, N = 4,385). The model with the best fit (X2[2] = 0.11, p = .95, RMSEA = .00, CFI = 1.00, TLI = 1.00) indicated that all specified paths were significant. Intentions were the strongest predictor of physical activity (β = 0.27, p < .001), and attitudes and self-efficacy were strong predictors of intentions (both βs = 0.29, ps < .001). Few significant moderators were observed.
Conclusion:
This review indicates that self-efficacy and intentions are direct predictors of physical activity in cancer survivors. Further, attitudes and norms predict physical activity through intentions. Findings inform intervention development to increase physical activity engagement among cancer survivors.
Keywords: cancer, physical activity, exercise, meta-analysis, predictor
Cancer survivors are persons living with a cancer diagnosis, including those who have been newly diagnosed, are undergoing treatment, or have completed treatment (Denlinger et al., 2014). There are currently more than 15.5 million cancer survivors in the United States and this number is projected to increase to 20.3 million by 2026 (National Cancer Institute, 2018). Cancer survivors live with increased mortality and recurrence risk, as well as decreased quality of life, due to disease- and treatment-related long-term and late effects (van Leeuwen et al., 2018).
Physical activity is associated with up to 50% lower cancer mortality and recurrence risk (Cormie, Zopf, Zhang, & Schmitz, 2017). Physical activity also improves quality of life for cancer survivors through mechanisms such as decreased pain and fatigue (Loprinzi & Lee, 2014). Engagement in physical activity is safe and feasible for cancer survivors before, during, and after treatment (Balogh, 2018). Thus, the American College of Sports Medicine and American Cancer Society recommend that cancer survivors adhere to general guidelines of 150 weekly aerobic minutes of moderate-intensity physical activity or 75 weekly aerobic minutes of vigorous-intensity physical activity and strength training two times per week (Rock et al., 2012). Despite numerous efforts to promote physical activity in this population, up to 70% of survivors do not achieve these recommendations (Blanchard, Courneya, & Stein, 2008). Understanding what psychosocial factors predict physical activity among cancer survivors is a key step towards designing effective interventions to promote physical activity (Sheeran, Klein, & Rothman, 2017). Accordingly, we undertook a meta-analysis of longitudinal, observational studies to quantify associations between predictors and physical activity in research to date.
Theories of Physical Activity Change
Health behavior theories are often used to guide physical activity interventions because they contain constructs that can be manipulated and measured to assess whether and why interventions produce behavior change (Rhodes, McEwan, & Rebar, 2018). Key theories that have been used in physical activity interventions for cancer survivors include the theory of planned behavior (Ajzen, 1991), social cognitive theory (Bandura, 2004), self-determination theory (Ryan & Deci, 2017), and the transtheoretical model (Prochaska & Velicer, 1997). These theories specify a series of overlapping predictors of behavior (Sheeran et al., 2017). The most extensively researched factors are attitudes, norms, self-efficacy, social support, past behavior, and intentions. Attitudes are people’s overall evaluation of the outcomes of performing a behavior (e.g., “For me, engaging in 150 mins of physical activity per week would be good/bad”) and are conceptually equivalent to the constructs, pros-and-cons, and outcome expectancies. Factor analysis and memory paradigms have shown that attitudes have two components – affective and instrumental (Trafimow & Sheeran, 1998). Affective attitudes refer to people’s feelings about performing the behavior (e.g., “For me, engaging in 150 mins of physical activity per week would be enjoyable”) whereas instrumental attitudes refer to utilitarian consequence of acting (e.g., “For me, engaging in 150 mins of physical activity per week would benefit my health”). Norms come in two varieties – injunctive and descriptive – that both predict health behaviors (Rivis & Sheeran, 2003). Injunctive norms refer to beliefs about what behaviors significant others think that one should perform (e.g., “Most people who are important to me think that I should engage in 150 mins of physical activity per week”), whereas descriptive norms refer to beliefs about how others themselves behave (Cialdini, Kallgren, & Reno, 1991). Self-efficacy refers to confidence in one’s ability to perform a behavior and is conceptually equivalent to perceived behavioral control from the theory of planned behavior (Ajzen, 2002; Bandura, 2004). Social support refers to people’s perceptions of how others enable and assist them to strive for goals (e.g., “My loved ones encourage me to stick to my physical activity plans”) (Sallis, Grossman, Pinski, Patterson, & Nader, 1987). Finally, intentions refer to people’s decisions and determination to act; intentions summarize people’s motivation to perform a behavior (e.g., “I intend to engage in 150 mins of physical activity per week” (Ajzen, 2002). Other constructs, such as perceived success (Courneya et al., 2004), affect (Brunet, Burke, & Sabiston, 2013; Mack, Meldrum, Wilson, & Sabiston, 2013), and planning (Karvinen et al., 2009) have also been tested as potential predictors of physical activity in one or two studies. However, there were too few tests to warrant inclusion in the present meta-analysis.
The Present Review
Even though a large literature has accumulated on psychosocial predictors of physical activity among cancer survivors, a quantitative synthesis of this research has yet to be undertaken. The present meta-analysis provides this synthesis. As longitudinal studies that measure predictors at one time-point and measure physical activity at a later time-point afford stronger inferences about the direction of effects than cross-sectional studies (Webb & Sheeran, 2006), our review focused on longitudinal research. The meta-analysis had three aims: First, to quantify the associations between constructs from behavioral theories (psychosocial predictors) and physical activity among adult cancer survivors. Second, to model how these different constructs combine to predict physical activity over time using meta-analytic structural equation modeling. And third, to identify moderating effects of methodological (e.g., measurement intervals, study rigor), demographic (e.g., race/ethnicity, age), and clinical (e.g., type of cancer, treatment phase) characteristics on the relationship between proximal psychosocial predictors and physical activity.
Methods
This meta-analysis was registered in Prospero (# CRD42018085268) and conducted according to PRISMA guidelines (Moher, Liberati, Tetzlaff, Altman, & Group, 2009).
Search Strategy
Computerized searches were conducted by a medical librarian on November 6, 2017. Databases searched include PubMed, Web of Science, Embase, SportDiscus, PsycINfo, and CINAHL. Search terms were optimized for each database and included terms for (a) psychosocial constructs (e.g., determinants, self-efficacy, theory of planned behavior), (b) physical activity (e.g. exercise, walk*, strength*), (c) cancer (e.g. neoplasm, tumor, leukemia), and (d) longitudinal design (e.g. prospective, predict*). The precise search terms are detailed in Supplementary Materials Table S1. A call for unpublished data was posted through listservs of the Society of Behavioral Medicine and American Psychosocial Oncology Society.
There were four criteria for inclusion. First, participants were cancer survivors according to the definition of a cancer survivor as someone living with a cancer diagnosis, regardless of treatment status (Denlinger et al., 2014). Thus, studies of participants who had not yet begun, were undergoing, and who had completed adjuvant treatment, met the criteria. Studies with adult survivors of all types of cancer except childhood cancer were included. Second, studies had to have a primary outcome of physical activity that was quantitatively measured objectively and/or subjectively. Light, moderate, or vigorous intensity aerobic or strength training physical activity (e.g., yoga, dancing, Tai Chi, resistance bands, and weight lifting) were permissible, though meditation and mindfulness outcomes were excluded. Physical activity could be unsupervised or supervised, including intervention adherence or attendance. Third, studies had to report one or more psychosocial constructs that predict physical activity. Finally, the study had to have a longitudinal, observational design with predictors measured at baseline and physical activity measured at a later time-point. Experimental and cross-sectional studies were excluded.
The initial search yielded 2,431 articles. De-duplication removed ten articles, leaving 2,421 titles and abstracts for screening. Two researchers independently reviewed each title and abstract for eligibility, resulting in 59 articles for full text review. Full-text reviews were conducted independently by two researchers. Discrepancies were discussed and, when necessary, resolved with a third researcher. Reasons for exclusion at this stage included (a) not a longitudinal, observational design (n = 10); (b) was a conference abstract (n = 6); (c) did not include a psychosocial predictor of physical activity (n = 5); (d) effect size was not reported and data transformations were not possible (n = 8); (e) physical activity was not measured (n = 4); and (f) the study population did not include cancer survivors (n = 1). Ultimately, 25 studies met the criteria for inclusion in this meta-analysis, as detailed in Figure 1. The references for all included studies are presented in the Supplemental Materials.
Figure 1.
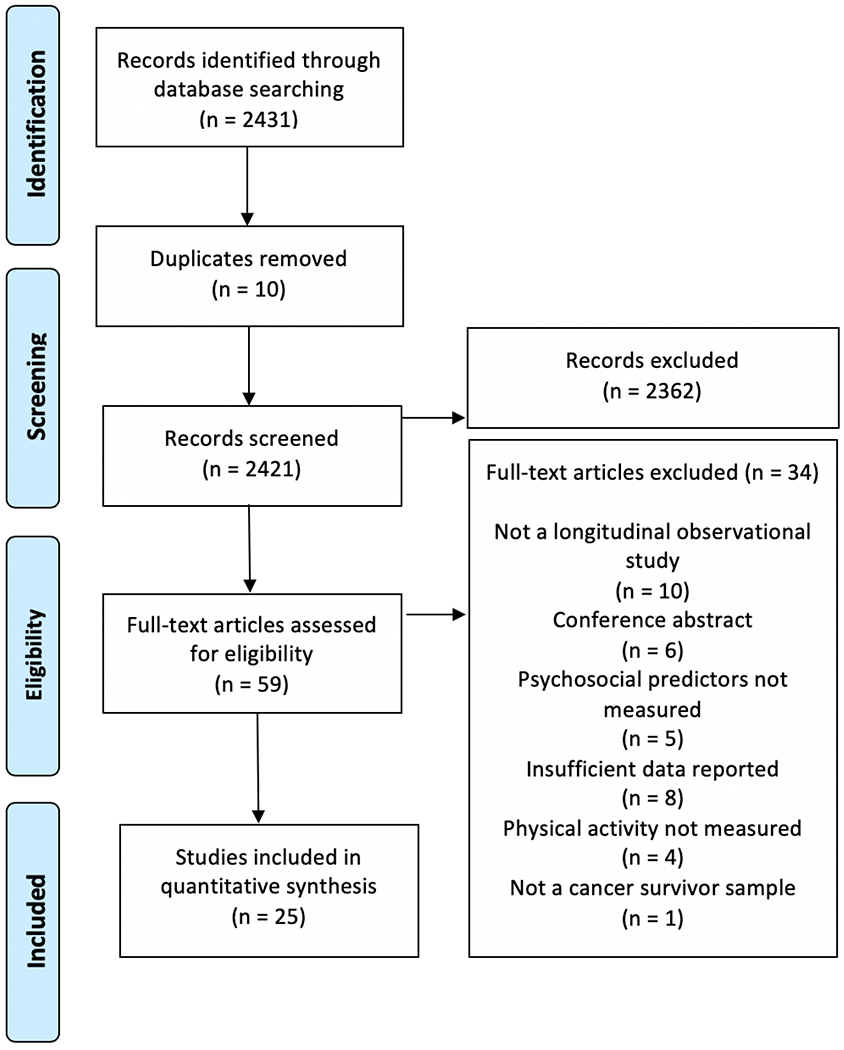
PRISMA Flow Diagram
Data Abstraction
Two researchers independently reviewed each article and extracted data into a database. Thirteen study authors were contacted for data needed to conduct the meta-analysis. Of the thirteen contacted authors, seven provided all requested data and six did not. Of the six authors who did not respond, we were able to include two studies using only the published data. We extracted data on study characteristics, sample demographics, cancer condition, treatment type, and correlations between psychosocial predictor variables and physical activity from each paper. Theoretical and operational definitions of each predictor variable were examined. Those with similar conceptual and/or operational definitions (e.g., self-efficacy and perceived behavioral control) were grouped together and assigned the title most commonly used in behavioral research. This process resulted in eight psychosocial predictor variables: instrumental attitudes, affective attitudes, overall attitudes (i.e., attitude scales that combined instrumental and affective content), injunctive norms, descriptive norms, self-efficacy, intentions, and past behavior. In cases where there were multiple measures of the same construct, the average within-study correlation of the relevant measures was calculated and used in analyses.
Analyses
Random-effects meta-analysis was used to compute averaged sample-weighted correlations (r+) between psychosocial variables and physical activity using R. The weighted correlations (r+) were calculated by transforming each correlation into Fisher’s Z score and weighting the value by the sample size. The average Z was then back-transformed to r. The 95% confidence intervals (95% CI) were calculated in similar fashion. Cochran’s Q and the I2 statistics were conducted to test for heterogeneity. Statistically significant Q values and I2 values exceeding 25% are indicative of heterogeneity in the correlations (Higgins, Thompson, Deeks, & Altman, 2003).
Meta-analytic structural equation modeling was conducted using the metaSEM package version 1.2.0 (Cheung, 2018) in R version 3.5.1 to examine how the psychosocial constructs collectively influence physical activity. A modified version of the theory of planned behavior was used to model relationships between the variables, as this theory specifies relations among the constructs that were available for analysis. A two-stage random effects approach was used to fit the path models (Jak, 2015). With the metaSEM package, a correlation matrix was created for each study, and the diagonal element in the matrix was omitted for missing variables. Additionally, for each missing correlation, one variable – the one that had the fewest remaining correlations with other variables – was treated as missing and omitted on the diagonal element. During the first stage of the two-stage random effects analysis, correlation matrices were pooled; in the second stage, a structural model was fit to the pooled correlation matrix using Weighted Least Squares (WLS) estimation (Cheung, 2014; Cheung & Chan, 2005). For studies that reported different sample sizes for different correlations, we adopted the conservative strategy of using the minimum sample size to represent the study correlations (Sheeran, Abraham, & Orbell, 1999).
Bias Assessment
Each study was assessed for methodological quality using a modified version of the Observational Longitudinal Research Tool (Tooth, Ware, Bain, Purdie, & Dobson, 2005). Discrepancies were resolved through discussion by the research team. Small-study bias was evaluated by computing statistics based on plots of the correlations from each study against study precision (the reciprocal of the study sample size). Asymmetry in the predicted ‘funnel’ shape of the plots was considered evidence of small study bias, that is, the tendency for studies included in the analysis to exhibit large effects relative to their sample size. Publication bias was evaluated using rank-order correlation to test for the interdependence of variance and effect size, with a significant correlation indicative of bias (Begg & Mazumdar, 1994)
Results
Study Characteristics
This meta-analysis includes 25 studies. The average sample size was 236 (SD = 360). The time interval between measurement of predictors and physical activity behavior ranged between 5 and 260 weeks, with a mean of 29 weeks (SD = 50). Most studies were guided by the theory of planned behavior (k = 11) or social cognitive theory (k = 5). The average methodological quality score was 8.64 (SD = 1.30) out of a possible 0 - 11 range; higher scores indicate greater methodological quality. Most participants were white (91%), female (79%), and an average of 56 years old (SD = 6). Most studies included only survivors of breast cancer (k = 14). Most included only those who had completed curative treatment (k = 13), or a mixed sample of participants who had completed and were receiving treatment (k = 9). About half of the studies measured physical activity via self-reports only (k = 14), several used both objective and subjective measures (k = 7) and few used only objective physical activity measures (k = 4). Table 1 presents a summary of study characteristics. Supplementary Materials Table S1 presents demographic information of participants for each individual study.
Table 1.
Study Characteristics
| Author (Year) | Cancer diagnosis | PA measure | Time interval | Psychosocial predictors coded for meta-analysis | n | Methodological quality |
|---|---|---|---|---|---|---|
| Basen-Engquis et al. (2013) | Endometrial | Accelerometer, CHAMPS, EMA | 8 | Attitudes, self-efficacy | 86 | 9 |
| Brunet et al. (2013) | Breast | Accelerometer | 24 | Attitudes | 110 | 10 |
| Cadmus-Bertram et al. (2013) | Breast | Session attendance, home activity log | 26 | Self-efficacy, past behavior | 32 | 7 |
| Castonguay et al. (2017) | Breast | Prochaska, Sallis & Long tool | 24 | Affective attitudes, past behavior | 149 | 8 |
| Courneya et al. (1999) | Colon/rectal | GLETQ | 16 | Attitudes, injunctive norms, self-efficacy, intentions, past behavior | 66 | 8 |
| Courneya et al. (2001) | Breast | Session attendance | 12 | Attitudes, injunctive norms, self-efficacy, intentions, past behavior | 24 | 5 |
| Courneya et al. (2004) | Mixed | GLETQ | 5 | Past behavior | 30 | 8 |
| Courneya et al. (2004) | Colon/rectal | GLETQ | 16 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, self-efficacy, intentions, past behavior | 102 | 9 |
| Courneya et al. (2004) | Prostate | Session attendance | 12 | Attitudes, injunctive norms, self-efficacy, intentions, past behavior | 82 | 10 |
| Courneya et al. (2008) | Breast | Session attendance | 17 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, self-efficacy, intentions | 78 | 9 |
| Courneya et al. (2009) | Breast | GLETQ | 24 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, self-efficacy, intentions, past behavior | 201 | 9 |
| Courneya et al. (2012) | Blood | GLETQ | 24 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, descriptive norms, self-efficacy, intentions | 108 | 8 |
| Emery et al. (2009) | Breast | 7DPAR | 260 | Social support | 217 | 9 |
| Endrighi et al. (2016) | Endometrial | Accelerometer | 24 | Instrumental attitudes, affective attitudes, attitudes | 99 | 7 |
| Fallon et al. (2018) | Mixed | ACS scale | 53 | Attitudes, self-efficacy | 679 | 10 |
| Karvinen et al. (2009) | Bladder | GLETQ | 12 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, descriptive norms, self-efficacy, intentions | 397 | 11 |
| Loprinzi et al. (2012) | Breast | CHAMPS | 24 | Attitudes, self-efficacy | 69 | 10 |
| Mack et al. (2013) | Breast | GLETQ | 24 | Self-efficacy | 119 | 10 |
| Mama et al. (2017) | Breast | IPAQ | 16 | Descriptive norms, social support, self-efficacy | 79 | 8 |
| Morielli et al. (2017) | Rectal | GLETQ | 13 | Instrumental attitudes, affective attitudes, attitudes, social support, self-efficacy, intentions | 13 | 8 |
| Peddle et al. (2009) | Lung | Session attendance | Variable (mean 8) | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, self-efficacy, intentions | 19 | 8 |
| Phillips et al. (2013) | Breast | GLETQ | 24 | Attitudes, social support, intentions | 1527 | 9 |
| Pinto et al. (2009) | Breast | Pedometer, exercise log | 12 | Intentions, self-efficacy | 43 | 8 |
| Rabin et al. (2006) | Breast | Paffenbarger activity questionnaire | 12 | Attitudes | 53 | 9 |
| Vallance et al. (2010) | Breast | GLETQ | 24 | Instrumental attitudes, affective attitudes, attitudes, injunctive norms, descriptive norms, self-efficacy, intentions | 377 | 9 |
Note. CHAMPS = Community Healthy Activities Model Program for Seniors activity questionnaire, n = sample size used in analyses, EMA = ecological momentary assessment, GLETQ = Godin leisure time exercise questionnaire, 7DPAR = 7 day physical activity recall, IPAQ = international physical activity questionnaire, ACS scale = American Cancer Society’s Cancer Prevention Study-3 scale LPA = light physical activity, MVPA = moderate to vigorous physical activity, METS = metabolic equivalents,
time interval indicates weeks between psychosocial predictor measure and PA measure.
Sample-Weighted Average Correlations Between Psychosocial Predictors and Physical Activity
Table 2 presents the sample-weighted average correlations between the psychosocial predictors and physical activity. Self-efficacy and intentions were the strongest predictors of physical activity, with medium-sized correlations (r+ = 0.26 and 0.33, respectively). Instrumental attitudes, affective attitudes, and overall attitudes were each similarly predictive of physical activity (r+ = 0.15, 0.18, and 0.18, respectively). There were small average correlations between physical activity and injunctive norms (r+ = 0.14), descriptive norms (r+ = 0.07), and social support (r+ = 0.13). Not surprisingly, physical activity was stable over time; the average correlation between past and future behavior was r+ = 0.40. The Q and I2 statistics showed significant heterogeneity in the relationships between physical activity and all of the predictors except norms and past behavior. Funnel plots revealed little evidence of small-study bias, and Begg and Mazumdar’s (1994) rank correlation tests were not significant for publication bias (Zs < 1.03, ps > .30)
Table 2.
Sample-Weighted Average Correlations Between Psychosocial Predictors and Physical Activity
| Predictor variable | k | N | r+ | 95% CI | Q | I2 |
|---|---|---|---|---|---|---|
| Intentions | 12 | 2,993 | 0.33 | 0.25 to 0.40 | 31.31** | 70% |
| Self-efficacy | 18 | 2,597 | 0.26 | 0.20 to 0.33 | 43.19*** | 58% |
| Overall attitude | 19 | 4,158 | 0.18 | 0.12 to 0.24 | 40.85** | 65% |
| Affective attitude | 10 | 1,542 | 0.18 | 0.09 to 0.27 | 23.19** | 60% |
| Instrumental attitude | 9 | 1,393 | 0.15 | 0.05 to 0.26 | 24.27** | 68% |
| Injunctive norms | 10 | 1,453 | 0.14 | 0.07 to 0.21 | 13.15 | 30% |
| Descriptive norms | 4 | 960 | 0.07 | 0.01 to 0.14 | 2.89 | ~0% |
| Social support | 8 | 685 | 0.13 | −0.01 to 0.27 | 9.51* | 69% |
| Past behavior | 8 | 685 | 0.40 | 0.33 to 0.46 | 6.07 | 0% |
Note. k = number of studies, N = sample size, r+ = sample-weighted average correlation, 95% CI = 95% confidence interval. Q and I2 = tests of heterogeneity,
p < 0.05,
p < 0.01,
p < 0.001.
Meta-Analytic Structural Equation Model
Sample-weighted average intercorrelations for all predictors and physical activity were calculated (see Table S2 in the Supplemental Materials), and submitted to meta-analytic structural equation modeling. Because instrumental attitudes and affective attitudes were so highly correlated with overall attitudes (r+ = 0.87 and 0.93, respectively), and the correlations between physical activity and instrumental attitudes, affective attitudes, and overall attitudes were similar (r+ = 0.15, 0.18, and 0.18, respectively), only overall attitude was included in the model. Past behavior and descriptive norms could not be included in the model because there were too few correlations with the other predictors to permit analyses. Thus, the final models included five variables – attitudes, injunctive norms, self-efficacy, intentions, and physical activity (k = 22, N = 4,385).
In the first model, we specified attitudes, injunctive norms, and self-efficacy as exogenous variables, with paths to intentions, leading to physical activity (see Figure 2). The model fit well, X2(3) = 12.37, p = .006, RMSEA = .03, CFI = .99, TLI = .95, and all of the specified paths were significant (ps < .001). In our second model (Figure 3), we added a direct path between self-efficacy and physical activity. This path significantly improved the model fit, ΔX2(1) = 12.26, p < .001. Model fit was excellent, X2(2) = 0.11, p = .95, RMSEA = .00, CFI = 1.00, TLI = 1.00, and all of the specified paths were significant (ps < .001). Findings indicated that intentions are the strongest predictor of physical activity (β = 0.21, p < .001), and that both attitudes and self-efficacy are strong predictors of intentions, (both βs = 0.29, ps < .001). We also tested a saturated model that specified all possible paths including direct paths from attitudes and injunctive norms to behavior. However, neither of these paths were significant (β = 0.02 and −0.004, respectively, ps > .70). Thus, Model 2 offers the best fit to the data and would seem to follow the structure of the theory of planned behavior. That is, intention is the best predictor of cancer survivors’ physical activity; there are indirect effects of attitudes, injunctive norms, and self-efficacy on physical activity through intentions; and self-efficacy also has a direct path to physical activity.
Figure 2.
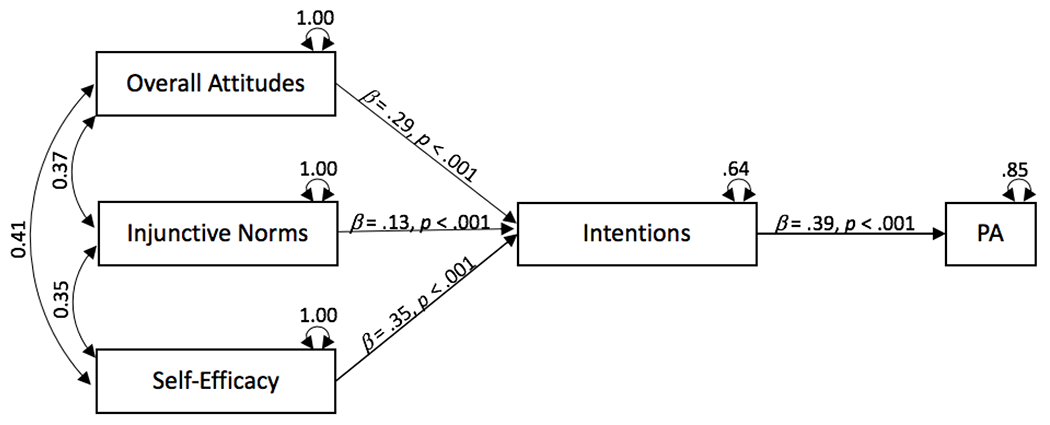
Meta-Analytic Structural Equation Model (Model 1)
Figure 3.
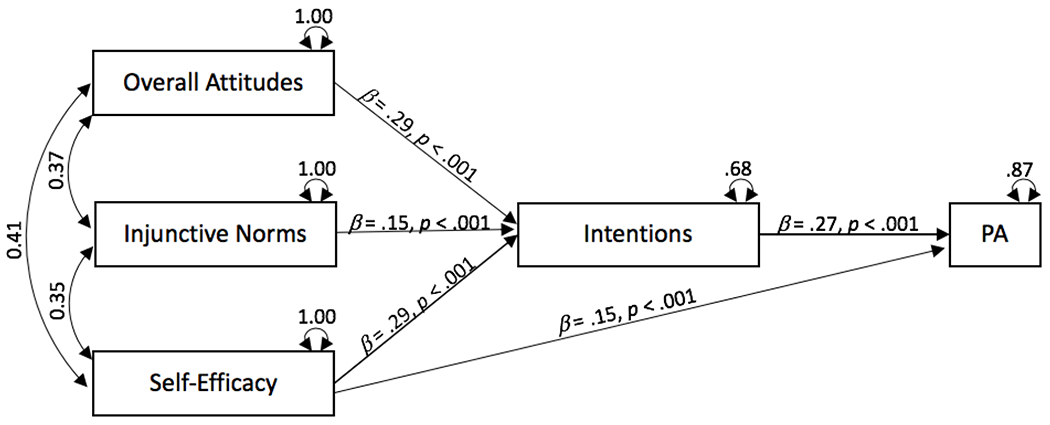
Meta-Analytic Structural Equation Model Specifying Direct Path from Self-Efficacy to Physical Activity (Model 2)
Although there were too few tests to include past behavior in the model alongside attitudes, injunctive norms, self-efficacy, and intentions, it is important to ensure that the associations observed for intentions and self-efficacy remained significant when past behavior was controlled. We therefore tested a new model that specified direct paths from past behavior, self-efficacy, and intentions to subsequent physical activity. Findings indicated that past behavior predicted behavior (β = 0.32, p < .001). Importantly, however, intentions (β = 0.21, p < .001) and self-efficacy (β = 0.15, p φ .001) retained significant paths to subsequent physical activity. These findings are consistent with the idea that intentions and self-efficacy predict changes in behavior (Sheeran et al., 2017).
Moderators of Relationships between Physical Activity and Intentions and Self-Efficacy
As intentions and self-efficacy were the only two direct predictors of physical activity among cancer survivors, we tested moderators of intention-behavior and self-efficacy-behavior relations. The Q and I2 statistics indicated that there was significant heterogeneity in these relationships, which encourages the search for moderators. We tested moderating effects of study characteristics (i.e., time interval between measurement of cognitions and behavior, rigor score), demographic factors (i.e., age, marital status, and BMI), and clinical features of the samples of cancer survivors (i.e., cancer type and treatment status). Too few tests were available to assess moderation by other variables (e.g., race).
Findings showed that only two factors moderated relationships between physical activity and intentions or self-efficacy (see Table 3). Sample age moderated the self-efficacy-physical activity relation such that older samples exhibited stronger associations between self-efficacy and behavior relative to younger samples (B = 0.02, p = 0.01). Body mass index (BMI) moderated the intention-behavior relation (B = −0.07, p = 0.03). Samples with lower BMI demonstrated improved translation of behavioral intentions into physical activity, as compared to samples with higher BMI.
Table 3.
Moderation of Self-efficacy-Physical Activity and Intention-Physical Activity Associations
| Moderator | Self-efficacy | Intentions | ||||
|---|---|---|---|---|---|---|
| B | SE | p | B | SE | p | |
| Time interval | 0.00 | 0.00 | 0.07 | −0.01 | 0.01 | 0.39 |
| Study rigor | 0.05 | 0.03 | 0.10 | −0.02 | 0.04 | 0.61 |
| Gender | 0.00 | 0.00 | 0.29 | 0.00 | 0.00 | 0.77 |
| Age | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.17 |
| Marital status | 0.00 | 0.01 | 0.64 | 0.01 | 0.01 | 0.40 |
| Body mass index | 0.00 | 0.02 | 0.97 | −0.07 | 0.03 | 0.03 |
| Cancer type | −0.12 | 0.06 | 0.06 | −0.03 | 0.09 | 0.73 |
| Treatment status | −0.01 | 0.07 | 0.36 | −0.14 | 0.13 | 0.29 |
| PA measurement | −0.11 | 0.08 | 0.14 | 0.01 | 0.10 | 0.91 |
Note. Time interval = number of weeks between first and last measurement point in the studies; Rigor = score for each study using a modified version of the Observational Longitudinal Research Tool, Gender = percent of sample reported as male, Marital status = percent of sample married legally or by common law, Cancer type = sample of exclusively breast cancer survivors (k = 14) compared to a sample with another (k = 9) or a variety of cancer diagnoses (k = 2); Treatment status = whether samples had competed curative treatment (k = 13), compared to samples who were either awaiting (k = 1) or undergoing (k = 2) treatment or a samples with mixed treatment status (k = 9); PA measurement = whether PA was only measured subjectively (k = 14), as compared to either objectively (k = 4) or objectively and subjectively (k = 7).
Discussion
Engaging in physical activity reduces cancer survivors’ risk of mortality and cancer recurrence and improves well-being. This review makes three key contributions to understanding physical activity engagement among cancer survivors. First, we quantified the relationships between eight psychosocial predictors and subsequent physical activity using a database of 25 longitudinal studies that comprised ~6,000 cancer survivors. Second, we used meta-analytic structural equation modeling to explore how the psychosocial predictors combine to influence physical activity and tested direct and indirect effects of respective predictors. And third, we evaluated the moderating effects of study, demographic, and clinical characteristics on the proximal predictors of physical activity.
Findings showed that the sample-weighted average correlations between physical activity and intentions and self-efficacy were of medium magnitude according to Cohen’s criteria (Cohen, 1992). Average correlations for the other predictors (overall attitudes, affective attitudes, instrumental attitudes, injunctive and descriptive norms, social support) were small- or small-to-medium-sized. These findings are in line with Ajzen’s (1991) theory of planned behavior (TPB) that posits intentions as the proximal predictor of behavior and attitudes, norms, self-efficacy, and other variables as indirect predictors mediated by intentions. The TPB also suggests that, to the extent that perceptions of control are accurate, self-efficacy also directly predicts behavior alongside intentions (Sheeran, Trafimow, & Armitage 2003).
Sufficient data were available to compute sample-weighted intercorrelations for physical activity, intentions, self-efficacy, overall attitudes, and injunctive norms, but not for the other predictors. Accordingly, we undertook meta-analytic structural equation modeling of these data. Our first model tested a direct path from intention to physical activity and indirect paths from overall attitudes, injunctive norms, and self-efficacy via intentions. The model exhibited good fit and all of the specified paths were significant. Next, we tested whether including direct paths from self-efficacy, overall attitudes, and injunctive norms to behavior would improve model fit. We found support for including a direct path from self-efficacy to behavior; model fit improved significantly and the fit of the overall model was excellent. Direct paths from attitudes and injunctive norms to physical activity were not empirically supported. Thus, the structure of the TPB finds strong support in meta-analytic structural equation modeling of longitudinal studies of cancer survivors’ physical activity: Intentions and self-efficacy directly predict behavior; intention mediates the attitude-behavior and injunctive norms-behavior relation, and partially mediates the self-efficacy-behavior relation.
Past behavior can function as a surrogate measure of factors that have influenced previous behavioral decisions (Albarracin & Wyer, 2000). Additionally, past behavior can reflect the extent to which behavior is under volitional control (Gardner, 2015). Thus, when testing a model’s ability to predict behavior, it is critical to control for past behavior (Hagger, Polet, & Lintunen, 2018). Significant associations between both intentions and physical activity, and self-efficacy and physical activity, held up when we tested direct paths between past behavior, self-efficacy, intentions, and subsequent physical activity. These results indicate that intentions and self-efficacy predict changes in physical activity among cancer survivors, in line with the predictions of leading theories of physical activity (Rhodes et al., 2018).
The relative size of correlations between TPB constructs and physical activity in our review are similar to those observed other meta-analyses in other populations, though the magnitude of the sample-weighted average correlations was smaller here. For example, a meta-analysis of 72 studies that included a variety of samples (e.g., college students, children, healthy adults) found that intentions most strongly predict physical activity (r+ = 0.51), followed by self-efficacy, (r+ = 0.40), and then attitudes (r+ = 0.35) (Hagger, Chatzisarantis, & Biddle, 2002; see also McEachan et al., 2016). The correlations observed in the present review follow the same order of magnitude for intention, self-efficacy, and attitudes (r+ = 0.33, 0.26, and 0.18, respectively) but are substantially smaller than those observed in Hagger et al.’s (2002) meta-analysis.
Why are smaller associations observed between psychosocial predictors and physical activity for cancer survivors compared to people with no diagnosis of cancer? The American Cancer Society Guidelines (Rock et al., 2012) pointed out that, “fewer than 10% of cancer survivors will be active during their primary treatments and only about 20% to 30% will be active after they recover from treatments” (p. 250). It therefore seems possible that health concerns or sedentary habits (Rhodes, Mark, & Temmel, 2012) could militate against cancer survivors effectively translating intentions and self-efficacy into physical activity. Difficulties in realizing intentions and self-efficacy may not be unique to cancer survivors, however. Rich, Brandes, Mullan, and Hagger (2015) meta-analyzed relationships between TPB variables and physical activity in people with chronic illness (e.g., diabetes, heart disease, hypertension). Average correlations between physical activity and intention (r+ = 0.29, k = 8) and self-efficacy (r+ = 0.16, k = 7) were considerably smaller than the associations observed by Hagger et al. (2002) and McEachan et al. (2016) for general population samples, but were similar to those observed here for cancer survivors. The implication is that while cognitions specified by the TPB predict physical activity for both patient and non-patient samples, predictive validity is weaker for cancer survivors and patient samples.
Rebar and colleagues (2019) pointed out that the strength of intention-behavior correlations greatly depends upon statistical assumptions about linearity and homoscedasticity, and recommended that researchers examine profiles of intention-behavior consistency. Profile analyses indicates that people who intend to act but subsequently do not (as opposed to non-intenders who subsequently behave contrary to their intentions) are mainly responsible for the intention-behavior ‘gap’ (Sheeran, 2002; Sheeran & Webb, 2016). This is also the case for physical activity intentions and behavior (Rhodes & de Bruijn, 2013a). Vallerand et al. (2016a) observed that intentions were effectively translated into exercise in only about 40% - 50% of hematological cancer survivors suggesting that the intention-behavior ‘gap’ is substantial in this population. Further research with cancer survivors is needed to test theoretical approaches such as the action control framework (Rhodes & de Bruijn, 2013b; Sniehotta et al., 2011) that offers traction in understanding discrepancies between intentions and physical activity in other populations.
It is also notable that the strength of relationships between physical activity and instrumental vs. affective attitudes differed for cancer survivors compared to participants in previous reviews. In McEachan et al.’s (2016) meta-analysis, affective attitudes better predicted behavior than instrumental attitudes for general population samples (r+ = 0.30 and 0.20, respectively; see Conner and Sparks, 2015, for equivalent findings). The corresponding average correlations were r+ = 0.18 and r+ = 0.15 in our study, which suggests that cancer survivors attach equal weight to the health benefits of physical activity as to its enjoyment, unlike their counterparts without a cancer diagnosis (O’Neill et al., 2013). It is apparent from reviews of both general population samples (Hagger et al., 2002; McEachan et al., 2016) and people with chronic illness (Rich et al., 2015) that norms are weakly associated with physical activity. This also proved to be the case among cancer survivors in the present review (r+ ≤ 0.14) and may reflect low societal expectations of physical activity among this population.
We observed few significant moderators of intention-behavior and self-efficacy-behavior relations among cancer survivors. Older samples demonstrated a stronger relationship between self-efficacy and physical activity, and samples with lower BMI exhibited reduced consistency between intentions and physical activity. Both of these findings would seem to align with TPB predictions. In particular, the TPB proposes that stronger relationships should be obtained for both of these predictors when participants possess greater actual control over the behavior (Ajzen, 2002; Sheeran et al., 2003). Thus, assuming that older samples have more accurate perceptions of control and that higher BMI reduces actual control over physical activity, it is understandable that age and BMI moderated these respective relationships. We should note, however, that these significant findings occur in the context of multiple tests of moderation, so replication of these results in primary studies would be desirable.
It is important to interpret the present findings in the context of limitations of our meta-analysis. First, even though we conducted computerized literatures searches guided by a medical librarian, and made efforts to access the gray literature, relatively few studies met the inclusion criteria for the review (k = 25, N = 5,897). Of the included studies, the majority (k = 14) used only self-report to measure physical activity while targeting psychological variables of interest. Second, we were unable to obtain full intercorrelation matrices for several studies, which limited the number of tests available for meta-analytic structural equation modeling. Third, while survivors of all types of cancer, in all stages of curative treatment were eligible for inclusion, most studies involved breast cancer survivors who had completed curative treatment; this consideration limits the generalizability of the present findings. The implication is that additional longitudinal studies using objective measures of physical activity among survivors of multiple types of cancer are needed, and full inter-correlation matrices should be reported to facilitate future quantitative syntheses. Finally, the scope of this meta-analysis excludes identification of confounding effects. We acknowledge that correlation is not causation and corroboration of the observational findings reported here with the results of experimental tests that manipulate intentions, self-efficacy, etc. would be valuable.
Notwithstanding these limitations, the present findings have implications both for health behavior theories and for interventions to promote physical activity among cancer survivors. Although we had no theoretical agenda at the outset of the review and included studies that used multiple, different theories, the present findings clearly fit the structure of the TPB. We also note that support for TPB constructs emerged in the context of primary studies that had a high average rigor score (M = 8.64) and used longitudinal designs that assess how psychosocial constructs predict subsequent behavior. The present findings offered little support for extensions to the TPB such as distinguishing between affective and instrumental attitudes or injunctive and descriptive norms (Conner & Sparks, 2015). At the same time, we acknowledge that our database did not include several key variables that predict physical activity after TPB variables have been taken into account, including anticipated regret (Sandberg & Conner, 2007), implementation intentions (Gollwitzer & Sheeran, 2006), and self-identity (Rhodes et al., 2016) . Empirical tests of these variables’ capacity to improve prediction of cancer survivors’ physical activity beyond that engendered by the TPB are warranted.
Our findings have two key implications for interventions to promote physical activity among cancer survivors. First, the results indicate that techniques geared at strengthening intentions and enhancing self-efficacy are liable to be effective, and techniques that target attitudes, norms, and self-efficacy should strengthen intentions (Abraham & Michie, 2008). Second, we identified a substantial ‘gap’ between physical activity and both intentions and self-efficacy among cancer survivors that was similar to the cognition-behavior discrepancy observed for other patient groups (Rich et al., 2015), and is greater than the gap seen in general population samples (Hagger et al., 2002; McEachan et al., 2016). It was also the case that younger and overweight samples had particular difficulty in enacting their physical activity intentions and self-efficacy. These findings speak to the potential value of using volitional strategies such as prompting self-monitoring (e.g., via pedometers; review by Harkin et al., (2016) or forming implementation intentions (i.e., if-then plans that specify how to respond to opportunities for, or obstacles to, physical activity; review by Bélanger-Gravel, Godin, and Amireault, (2013), as these strategies are known to improve the translation of cognitions into action. In sum, the present meta-analysis signals several directions for predictive and interventive studies that can and should be tested in future research.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institute of Nursing Research (T32NR007091) and the Lineberger Cancer Outcomes Research Program.
We thank Xianming Tan for invaluable statistical consulting. We also thank the authors who provided additional, unpublished data to facilitate our analyses: Jennifer Brunet, Charles F. Physical Activity in Cancer Survivors Emery, Elizabeth A. Fallon, Scherezade Mama, Siobhan M. Phillips, Bernardine M. Pinto, and Jeff Vallance.
Contributor Information
Rachel Hirschey, University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center.
Ashley Leak Bryant, University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center.
Catherine Macek, University of North Carolina at Chapel Hill.
Claudio Battaglini, University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center.
Sheila Santacroce, University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center.
Kerry S. Courneya, University of Alberta
Jennifer S. Walker, University of North Carolina at Chapel Hill
Aya Avishai, University of North Carolina at Chapel Hill.
Paschal Sheeran, University of North Carolina at Chapel Hill and Lineberger Comprehensive Cancer Center.
References
* References for Studies Included in the Meta-Analysis
- *Abraham C & Michie S (2008). A taxonomy of behavior change techniques used in interventions. Health Psychol, 27(3), 379–387. doi: 10.1037/0278-6133.27.3.379 [DOI] [PubMed] [Google Scholar]
- *Ajzen I (1991). The Theory of Planned Behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- *Ajzen I (2002). Perceived Behavioral Control, Self-Efficacy, Locus of Control, and the Theory of Planned Behavior. Journal of Applied Social Psychology, 32(4), 665–683. doi:DOI 10.1111/j.1559-1816.2002.tb00236.x [DOI] [Google Scholar]
- *Albarracin D, & Wyer RS Jr. (2000). The cognitive impact of past behavior: influences on beliefs, attitudes, and future behavioral decisions. Journal of Personality and Social Psychology, 79(1), 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Balogh EP (2018). Incorporating Weight Management and Physical Activity Throughout the Cancer Care Continuum: Proceedings of a Workshop. doi: 10.17226/24925 [DOI] [PubMed] [Google Scholar]
- *Bandura A (2004). Health promotion by social cognitive means. Health Educ Behav, 31(2), 143–164. doi : 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- *Begg CB, & Mazumdar M (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics, 50(4), 1088–1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- *Bélanger-Gravel A, Godin G, & Amireault S (2013). A meta-analytic review of the effect of implementation intentions on physical activity. Health Psychology Review, 7(1), 23–54. doi: 10.1080/17437199.2011.560095 [DOI] [PubMed] [Google Scholar]
- *Blanchard CM, Courneya KS, & Stein K (2008). Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. Journal of Clinical Oncology, 26(13), 2198–2204. doi: 10.1200/JCO.2007.14.6217 [DOI] [PubMed] [Google Scholar]
- *Brunet J, Burke SM, & Sabiston CM (2013). The benefits of being self-determined in promoting physical activity and affective well-being among women recently treated for breast cancer. Psychooncology, 22(10), 2245–2252. doi: 10.1002/pon.3287 [DOI] [PubMed] [Google Scholar]
- Cheung MW-L (2014). Fixed-and random-effects meta-analytic structural equation modeling: Examples and analyses in R. Behavior Research Methods, 46(1), 29–40. [DOI] [PubMed] [Google Scholar]
- Cheung MW-L (2018). metaSEM: Meta-Analysis using Structural Equation Modeling. R package version 1.2.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MW-L, & Chan W (2005). Meta-analytic structural equation modeling: A two-stage approach. Psychological Methods, 10(1), 40. [DOI] [PubMed] [Google Scholar]
- *Cialdini RB, Kallgren CA, & Reno RR (1991). A focus theory of normative conduct: A theoretical refinement and reevaluation of the role of norms in human behavior. Advances in Experimental Social Psychology, 24, 201–234. [Google Scholar]
- *Cohen J (1992). A power primer. Psychological Bulletin, 112(1), 155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- Conner M, & Norman P (2015). Predicting and Changing Health Behaviour: Research and Practice with Social Cognition Models: Open University Press. [Google Scholar]
- *Cormie P, Zopf EM, Zhang X, & Schmitz KH (2017). The Impact of Exercise on Cancer Mortality, Recurrence, and Treatment-Related Adverse Effects. Epidemiology Reviews, 39(1), 71–92. doi: 10.1093/epirev/mxx007 [DOI] [PubMed] [Google Scholar]
- *Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, & Jones LW (2004). Exercise motivation and adherence in cancer survivors after participation in a randomized controlled trial: an attribution theory perspective. International Journal of Behavioral Medicine, 11(1), 8–17. doi: 10.1207/s15327558ijbm1101_2 [DOI] [PubMed] [Google Scholar]
- *Denlinger CS, Carlson RW, Are M, Baker KS, Davis E, Edge SB, … Freedman-Cass D (2014). Survivorship: introduction and definition. Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network, 12(1), 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gardner B (2015). A review and analysis of the use of ’habit’ in understanding, predicting and influencing health-related behaviour. Health Psychology Review, 9(3), 277–295. doi: 10.1080/17437199.2013.876238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gollwitzer PM, & Sheeran P (2006). Implementation intentions and goal achievement: A meta-analysis of effects and processes. Advances in Experimental Social Psychology (38), 69–119. doi: 10.1016/50065-2601(06)38002-1 [DOI] [Google Scholar]
- *Hagger MS, Chatzisarantis NLD, & Biddle SJH (2002). A meta-analytic review of the theories of reasoned action and planned behavior in physical activity: Predictive validity and the contribution of additional variables. Journal of Sport & Exercise Psychology, 24(1), 3–32. doi:DOI 10.1123/jsep.24.1.3 [DOI] [Google Scholar]
- *Hagger MS, Polet J, & Lintunen T (2018). The reasoned action approach applied to health behavior: Role of past behavior and tests of some key moderators using meta-analytic structural equation modeling. Social Science & Medicine, 213, 85–94. doi: 10.1016/j.socscimed.2018.07.038 [DOI] [PubMed] [Google Scholar]
- *Harkin B, Webb TL, Chang BP, Prestwich A, Conner M, Kellar I, … Sheeran P (2016). Does monitoring goal progress promote goal attainment? A meta-analysis of the experimental evidence. Psychol Bull, 142(2), 198–229. doi: 10.1037/bul0000025 [DOI] [PubMed] [Google Scholar]
- *Higgins JP, Thompson SG, Deeks JJ, & Altman DG (2003). Measuring inconsistency in meta-analyses. BMJ, 327(7414), 557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Jak S (2015). Meta-analytic structural equation modelling. Dordrecht, Netherlands: Springer. [Google Scholar]
- *Karvinen KH, Courneya KS, Plotnikoff RC, Spence JC, Venner PM, & North S (2009). A prospective study of the determinants of exercise in bladder cancer survivors using the Theory of Planned Behavior. Supportive Care in Cancer, 17(2), 171–179. doi: 10.1007/s00520-008-0471-8 [DOI] [PubMed] [Google Scholar]
- *Loprinzi PD, & Lee H (2014). Rationale for promoting physical activity among cancer survivors: literature review and epidemiologic examination. Oncology Nursing Forum, 41(2), 117–125. doi: 10.1188/14.ONF.117-125 [DOI] [PubMed] [Google Scholar]
- *Mack DE, Meldrum LS, Wilson PM, & 0Sabiston CM (2013). Physical activity and psychological health in breast cancer survivors: an application of basic psychological needs theory. Applied Psychology: Health and Well-Being, 5(3), 369–388. doi: 10.1111/aphw.12016 [DOI] [PubMed] [Google Scholar]
- McEachan R, Conner M, Taylor N, & Lawton R (2011). Prospective prediction of health-related behaviours with the Theory of Planned Behaviour: a meta-analysis. Health Psychology Review, 5(2), 97–144. doi: 10.1080/17437199.2010.521684 [DOI] [Google Scholar]
- McEachan R, Taylor N, Harrison R, Lawton R, Gardner P, & Conner M (2016). Meta-Analysis of the Reasoned Action Approach (RAA) to Understanding Health Behaviors. Ann Behav Med, 50(4), 592–612. doi: 10.1007/s12160-016-9798-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan D, Beauchamp MR, Kouvousis C, Ray CM, Wyrough A, & Rhodes RE (2019). Examining the active ingredients of physical activity interventions underpinned by theory versus no stated theory: a meta-analysis. Health Psychology Review, 13(1), 1–17. doi: 10.1080/17437199.2018.1547120 [DOI] [PubMed] [Google Scholar]
- Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, & French DP (2011). A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychology & Health, 26(11), 1479–1498. doi: 10.1080/08870446.2010.540664 [DOI] [PubMed] [Google Scholar]
- *Moher D, Liberati A, Tetzlaff J, Altman DG, & Group P (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoSMed, 6(7), e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *National Cancer Institute. (2018). Cancer Statistics. Retrieved from https://www.cancer.gov/about-cancer/understanding/statistics
- *O’Neill SC, DeFrank JT, Vegella P, Richman AR, Henry LR, Carey LA, & Brewer NT (2013). Engaging in health behaviors to lower risk for breast cancer recurrence. PLoS One, 8(1), e53607. doi: 10.1371/journal.pone.0053607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Prochaska JO, & Velicer WF (1997). The transtheoretical model of health behavior change. American Journal of Health Promotion, 12(1), 38–48. doi: 10.4278/0890-1171-12.1.38 [DOI] [PubMed] [Google Scholar]
- *Rhodes RE, Mark RS, & Temmel CP (2012). Adult sedentary behavior: A systematic review. American Journal of Preventive Medicine, 42(3), e3–28. 10.1016/j.amepre.2011.10.020 [DOI] [PubMed] [Google Scholar]
- *Rhodes R, McEwan D, & L. Rebar A (2018). Theories of physical activity behaviour change: A history and synthesis of approaches. [Google Scholar]
- Rhodes RE, McEwan D, & Rebar AL (2019). Theories of physical activity behaviour change: A history and synthesis of approaches. Psychology of Sport and Exercise, 42, 100–109. 10.1016/j.psychsport.2018.11.010 [DOI] [Google Scholar]
- Rhodes RE, & De Bruijn G-J (2013a). How big is the physical activity intention-behaviour gap? A meta-analysis using the action control framework. British Journal of Health Psychology, 18(2), 296–309. 10.1111/bjhp.12032 [DOI] [PubMed] [Google Scholar]
- Rhodes RE, & De Bruijn G-J (2013b). What predicts intention-behavior discordance? A review of the Action Control Framework. Exercise and Sport Sciences Reviews, 41(4), 201–207. 10.1097/JES.0b013e3182a4e6ed [DOI] [PubMed] [Google Scholar]
- Rhodes RE, Kausal N, & De Quinlan A (2016). Is physical activity a part of who I am? A review and meta-analysis of idnetity, schema, and physical activity. Health Psychology Review, 10(3), 204–225. [DOI] [PubMed] [Google Scholar]
- *Rich A, Brandes K, Mullan B, & Hagger MS (2015). Theory of planned behavior and adherence in chronic illness: a meta-analysis. J Behav Med, 38(4), 673–688. doi: 10.1007/s10865-015-9644-3 [DOI] [PubMed] [Google Scholar]
- *Rivis A, & Sheeran P (2003). Descriptive Norms as an Additional Predictor in the Theory of Planned Behaviour: A Meta-Analysis. Current Psychology, 22(3), 218–233. [Google Scholar]
- *Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Gansler T (2012). Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin, 62(4), 243–274. doi: 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- *Ryan RM, & Deci EL (2017). Self-determination theory: Basic psychological needs in motivation, development, and wellness. New York, NY, US: Guilford Press. [Google Scholar]
- *Sallis JF, Grossman RM, Pinski RB, Patterson TL, & Nader PR (1987). The development of scales to measure social support for diet and exercise behaviors. Preventative Medicine, 16(6), 825–836. 10.1016/0091-7435(87)90022-3 [DOI] [PubMed] [Google Scholar]
- *Sandberg T, & Conner M (2007). Anticipated regret as an additional predictor in the theory of planned behavior: A meta-analysis (Vol. 47). [DOI] [PubMed] [Google Scholar]
- Sandberg T, & Conner M (2010). Anticipated regret as an additional predictor in the theory of planned behaviour: A meta-analysis. British Journal of Social Psychology, 47(4), 589–606. 10.1348/014466607X258704 [DOI] [PubMed] [Google Scholar]
- *Sheeran P, Abraham C, & Orbell S (1999). Psychosocial correlates of heterosexual condom use: A meta-analysis. Psychological Bulletin, 125(1), 90–132. doi: 10.1037/0033-2909.125.1.90 [DOI] [PubMed] [Google Scholar]
- *Sheeran P, Klein WM, & Rothman AJ (2017). Health Behavior Change: Moving from Observation to Intervention. Annual Reviews Psychology, 68, 573–600. doi: 10.1146/annurev-psych-010416-044007 [DOI] [PubMed] [Google Scholar]
- *Sheeran P, Trafimow D, & Armitage CJ (2003). Predicting behaviour from perceived behavioural control: tests of the accuracy assumption of the theory of planned behaviour. British Journal of Social Psychology, 42(Pt 3), 393–410. doi: 10.1348/014466603322438224 [DOI] [PubMed] [Google Scholar]
- *Sheeran P, & Webb TL (2016). The intention-behavior gap. Social and Personality Psychology Compass, 10(9), 503–518. 10.1111/spc3.12265 [DOI] [Google Scholar]
- Sniehotta FF, Nagy G, Scholz U, & Schwarzer R (2011). The role of action control in implementing intentions during the first weeks of behaviour change. British Journal of Social Psychology, 45(1), 87–106. 10.1348/014466605X62460 [DOI] [PubMed] [Google Scholar]
- *Tooth L, Ware R, Bain C, Purdie DM, & Dobson A (2005). Quality of reporting of observational longitudinal research. American Journal of Epidemiology, 161(3), 280–288. doi: 10.1093/aje/kwi042 [DOI] [PubMed] [Google Scholar]
- *Trafimow D, & Sheeran P (1998). Some Tests of the Distinction between Cognitive and Affective Beliefs. Journal of Experimental Social Psychology, 34, 378–397. doi: 10.1006/jesp.1998.1356 [DOI] [Google Scholar]
- *Vallerand JR, Rhodes RE, Walker GJ, & Courneya KS (2016a). Explaining the Aerobic Exercise Intention-behavior Gap in Cancer Survivors. Am J Health Behav, 40(5), 675–684. doi: 10.5993/AJHB.40.5.15 [DOI] [PubMed] [Google Scholar]
- *Vallerand JR, Rhodes RE, Walker GJ & Courneya KS Understanding strength exercise intentions and behavior in hematologic cancer survivors: an analysis of the intention-behavior gap. J Cancer Surviv 10, 945–955, doi: 10.1007/s11764-016-0540-9 (2016). [DOI] [PubMed] [Google Scholar]
- *van Leeuwen M, Husson O, Alberti P, Arraras JI, Chinot OL, Costantini A, … Eortc QLG (2018). Understanding the quality of life (QOL) issues in survivors of cancer: towards the development of an EORTC QOL cancer survivorship questionnaire. Health and Quality of Life Outcomes, 16(1), 114. doi: 10.1186/s12955-018-0920-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Webb TL, & Sheeran P (2006). Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychological Bulletin, 132, 249–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


