Abstract
Safranal, a plant secondary metabolite isolated from saffron, has been reported for several promising pharmacological properties toward the management of Alzheimer’s disease. In the present study, we observe and report for the first time about several druglike attributes of safranal, such as adherence to Lipinski’s rule of five; optimum lipophilicity; high permeability; low blood-to-plasma ratio; less to moderate propensity to interact with P-glycoprotein (P-gp) or breast cancer-resistant protein (BCRP) transporters; and high plasma protein binding as common to most of the marketed drugs using in vitro and ex vivo models. In spite of the above attributes, in vivo oral absorption was found to be very poor, which is linked to the structural integrity of safranal in simulated gastric fluid, simulated intestinal fluid, plasma, and liver microsomes. Moreover, the presence of unsaturated aldehyde moiety in safranal remains in equilibrium with its hydroxylated acetal form. Further research work is required to find out the stable oral absorbable form of safranal by derivatization of its aldehyde group without losing its potency.
1. Introduction
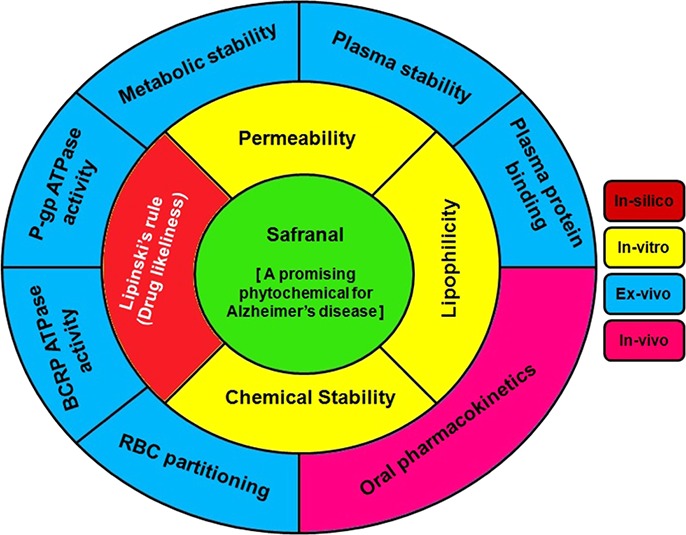
Natural products have immense potential for the discovery of new therapeutics to treat a wide range of diseases. More than 40% of the marketed drugs have been derived directly or indirectly from natural origin.1,2 Particularly, plant secondary metabolites have been used traditionally despite unprecedented advances in the modern system of medicine, which are predominantly associated with unwanted side effects. In this direction, safranal is one of the active constituents of Crocus sativus, commonly known as saffron. Safranal is present in the flower of this plant. It has been reported to possess numerous pharmacological activities for the treatment of several disease conditions.3,4 This promising phytochemical has been relentlessly explored for Alzheimer’s disease because of its beneficial action as neuroprotective and anti-inflammatory agent.5−14 Though hundreds of reports are available in the literature for the biological activity of safranal, there is hardly any report on its pharmacokinetics as well as allied properties. Early evaluation of pharmacokinetic profile is necessary in the drug development process because unfavorable pharmacokinetics is the major hurdle for a new candidate to be a drug.15 As the oral route is the most preferred and conventional route for drug administration, in vitro/ex vivo as well as in vivo investigations on the features of this molecule linked to oral exposure are needed. These examinations are crucial for any new therapeutics to establish a correlation between its in vitro and in vivo efficacy. With this background, it has become imperative to investigate the pharmacokinetics and allied properties of such a potent phytochemical like safranal (Figure 1).5−14
Figure 1.
Summary of the present research work plan.
2. Results and Discussion
Ongoing research on biological activities of safranal reveals that it has remarkable pharmacological actions toward the management of Alzheimer’s disease.5−14 Considering the success of plant-based natural products or their derivatives as marketed drugs, we aimed to investigate the drug-likeliness of safranal through pharmacokinetic investigations, which is the crucial step for any candidate to become a drug.
First, chemical structure-based prediction of drug-likeliness demonstrates the following: molecular weight is less than 500 Da, calculated log P is less than 5, the number of hydrogen-bond donors is less than 5, and the number of hydrogen-bond acceptors is less than 10. Therefore, safranal does not violate any criteria for drug-likeliness as per Lipinski’s rule of five, which helps to envisage drug-likeliness by acting as virtual filters.16
Lipophilicity is one such physicochemical property that influences the absorption characteristics of any molecule. We examined the partition coefficient (log P) as well as distribution coefficient (log D) of safranal, which is found to be in the range of 2.21 ± 0.14 to 2.47 ± 0.07 and 2.02 ± 0.28 to 2.38 ± 0.08, respectively, depending on the experimental ratios of octanol and water/phosphate-buffered saline, pH 7.4 (PBS). The obtained results for testosterone (3.09 ± 0.07) as standard have a good agreement with the reported value of around 3.28.17,18 Results illustrate that safranal has optimum lipophilicity (0 < log P < 3), which is favorable for good oral absorption behavior through the gastrointestinal tract.
Investigation on the chemical stability of safranal in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) reveals that it was degraded up to 54 and 68%, respectively, in the experimental time frame of 2 and 4 h, respectively (Supporting Information). Therefore, this compound is found to be vulnerable to degradation and may be labile in the gastrointestinal tract that consequently can affect its oral exposure. This information will be beneficial to choose the proper route of administration as well as the need of formulation strategy during its further development.
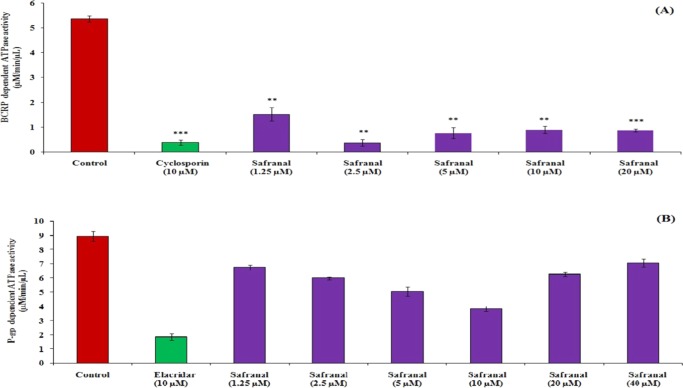
The presence of transporters in the gastrointestinal tract restricts the entry of drugs through cell membranes. It occurs when the drug is a substrate of that particular transporter. Interaction with transporters is considered as a liability for a new chemical entity. This is because a co-administered drug (inhibitor of a particular transporter system) hinders absorption of a drug (substrate of same transporter system) that leads to change in oral bioavailability as well as its pharmacological action.19 P-glycoprotein (P-gp) and breast cancer-resistant protein (BCRP) are the two important transporter systems that are involved in absorption for most of the marketed drugs. Therefore, the effect of safranal was evaluated on both the transporter systems through estimation of their ATPase activities using respective human protein. This assay is a simple, sensitive, rapid, and direct protocol to estimate the inorganic phosphate release linked to the activity of the transporters.20 Investigating the effect of safranal on BCRP transporters demonstrates that it can significantly interfere with BCRP-dependent ATPase activity in a concentration-independent manner. Results also displayed that safranal can hinder this above transporter activity at a concentration of 2.5 μM, which is equivalent to cyclosporine activity at 10 μM (Figure 2A). On the other hand, results of the P-gp-dependent ATPase activity of safranal suggest that it can interfere with P-gp in a concentration-dependent manner through a very less effective way than elacridar, which served as a positive control (Figure 2B). Safranal is more likely to interact with BCRP than P-gp. As safranal is often taken as a dietary/food supplement, its intake should be avoided with allopathic drugs having the substrate of BCRP transporters.21
Figure 2.
Effect of safranal on BCRP-dependent ATPase activity using cyclosporine as positive control (A) and P-gp-dependent ATPase activity using elacridar as positive control (B). Data are represented as mean ± standard error of the mean (SEM) (n = 2). Statistical significance level: *p < 0.05, **p < 0.01, and ***p < 0.001.
A panel of in vitro/ex vivo studies for pharmacokinetic profiling of safranal like permeability, plasma protein binding, red blood cells (RBC) partitioning, plasma stability, and metabolic stability in different microsomes like mice liver microsomes (MLM), rat liver microsomes (RLM), dog liver microsomes (DLM), and human liver microsomes (HLM) were also studied. In vitro permeability was accessed based on the parallel artificial membrane permeability assay (PAMPA) model, which is a straightforward and cost-effective method for the rapid determination of a molecule’s passive permeability for oral absorption. The permeability (log Pe) of safranal is found to be −3.88 ± 0.10, −3.93 ± 0.06, and −3.79 ± 0.11 in the concentration levels of 10, 25, and 50 μM, respectively. The experimental permeability of testosterone (−3.99 ± 0.18) is concordant with the reported results of −3.5 to −4.9 which may vary depending on parameters such as type of artificial membrane material, concentration of membrane material, incubation time, and lot-to-lot variation of plate.22,23 Results suggest that safranal has high permeability as experimental log Pe value > −5. Moreover, the permeability of safranal is found to be concentration-independent in experimental conditions. Though low-permeability (log Pe value < −5) drugs are also available in the market, a new chemical entity is expected to be of high permeability to show that permeability is not a rate-limiting factor for absorption.24
The extent of plasma protein binding for safranal varied from 87 to 92% in the experimental concentration range of 5–20 μM (Figure 3). Plasma protein binding of rifampicin as standard is found to be 91%, which matches with the reported results (82–92%).25,26 Plasma protein binding can be classified as very high protein binding (>98%), high protein binding (85–98%), and medium-to-low protein binding (<85%).27 Therefore, results indicate that plasma protein binding is high and concentration-independent. It is worth mentioning that the major proportion of marketed drugs is highly plasma protein-bound, and bioefficacy depends on free drug concentration but not on free drug fraction.28
Figure 3.
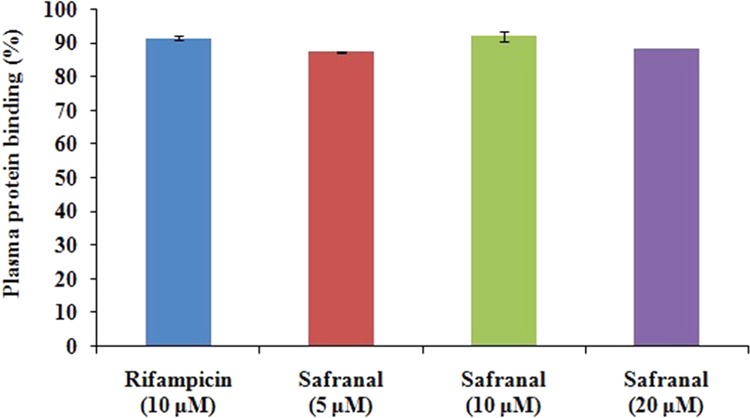
Plasma protein binding of safranal using rifampicin as standard. Data are represented as mean ± SEM (n = 4).
The penetration of a drug into the RBC to a larger extent can affect its activity. Therefore, RBC partitioning of a compound is an integral part of the preclinical investigation. Moreover, such information will help to choose the appropriate matrix during the assessment of the pharmacokinetic behavior of that molecule. We evaluated the blood-to-plasma ratio for safranal and found that its value (KRBC/Plasma) decreases from 0.88 ± 0.07 to 0.24 ± 0.03 at 0 and 60 min, respectively (Figure 4). The experimental result for propanolol (KRBC/Plasma from 1.07 ± 0.05 at 0 min to 0.90 ± 0.03 at 60 min) as standard is found to be well within the reported range in the literature (1.0 ± 0.2).29 From the obtained results, it can be illustrated that safranal has a lower propensity to accumulate in RBCs unlike marketed drugs like rapamycin (3.5 ± 0.5), chloroquine (8.5 ± 0.1), and chlorthalidone (20.0 ± 0.3).29
Figure 4.
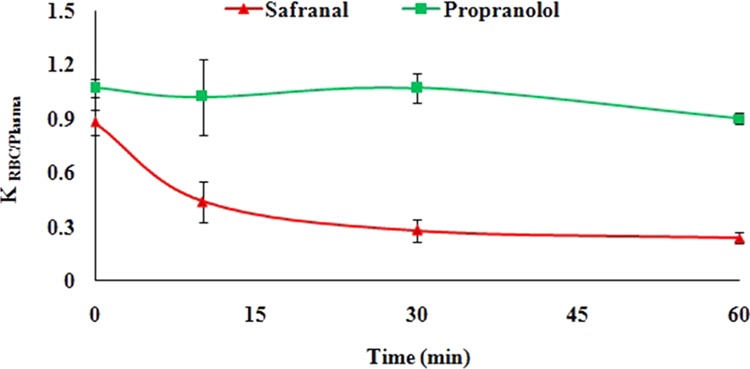
RBC partitioning of safranal using propanolol as standard. Data are represented as mean ± SEM (n = 3).
The stability study of safranal in both mice and rat plasma showed a substantial decrease in compound concentration to 31 and 22%, respectively, under the experimental time period, i.e., 0–4 h (Figure 5 and Supporting Information). Results indicate that the compound seems to be inadequately stable in plasma that may hamper with its oral exposure time. However, there are distinguished drugs in the market where the drug is very less stable in rat plasma but more stable in human plasma or vice verse like enalapril and procaine, respectively.30
Figure 5.
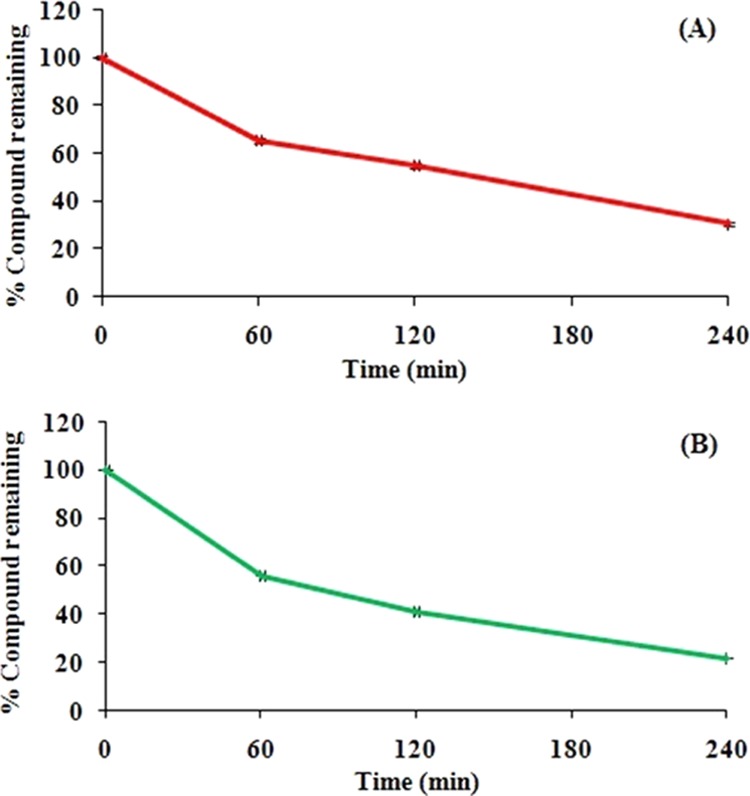
Stability of safranal in rat plasma (A) and mice plasma (B) at the initial concentration level of 75 μg/mL. Data are represented as mean ± SEM (n = 4).
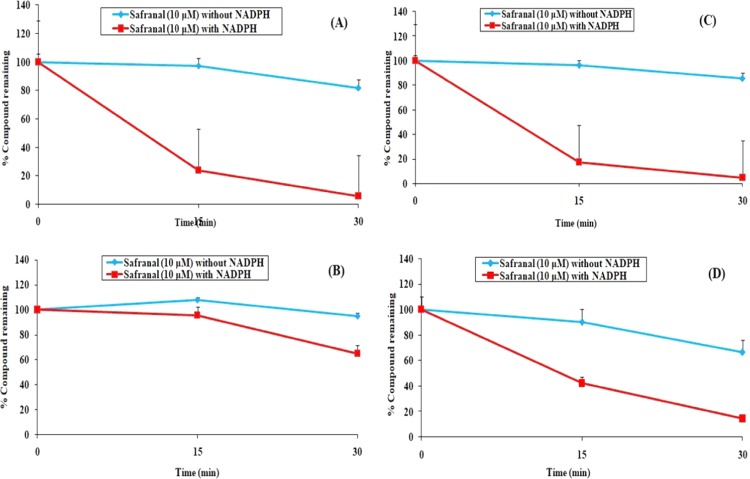
The liver plays a major role in the elimination of drugs from the body, and therefore, in vitro metabolic stability can demonstrate the extent of oral exposure of the compound. In the present study, the metabolic stability of safranal was assessed by the substrate depletion approach using a range of microsomes like MLM, RLM, DLM, and HLM. The results are depicted in Figure 6, which illustrates that safranal degraded rapidly in all of the microsomes under the experimental conditions. Two marketed drugs, namely, atenolol and verapamil, remained intact in RLM under the same experimental conditions by 10 and 90%, respectively.31
Figure 6.
Metabolic stability of safranal in mice liver microsomes (A), rat liver microsomes (B), dog liver microsomes (C), and human liver microsomes (D). Data are represented as mean ± SEM (n = 3).
The above-mentioned metabolic stability of safranal was assessed after quantitation of safranal remains in the reaction mixture by high-performance liquid chromatography (HPLC). It should be noteworthy to mention that the maximum wavelength for absorption (λmax) for safranal is 310 nm, which shifted to 259 nm for a few study samples where retention times of both peaks in HPLC were found to be the same. This is probably due to the presence of rapid equilibrium between safranal and its corresponding hydroxylated acetal form (Figure 7). In fact, the calculation of λmax for safranal and its acetal by empirical Woodward’s rule showed a hypsochromic shift of the parent molecule. For simplicity, metabolic stability in different liver microsomes was assessed based on the higher response of safranal at λmax of 310 nm in HPLC.
Figure 7.
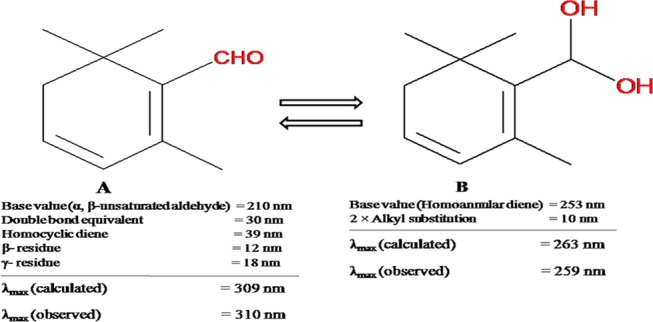
Calculated (309 and 263 nm) and observed (310 and 259 nm) λmax values of safranal (A) and its hydroxylated acetal form (B), respectively.
A single-dose oral pharmacokinetic study of safranal was performed in Balb/C mice. The dose was administered at 100 mg/kg, considering the results mentioned above for chemical, plasma, and metabolic stability. Results showed that the plasma concentration of safranal at different time points is detectable up to 2 h, but most of the concentration data are below the lowest point of the calibration curve. If these plasma concentration data are used, it can be stated that safranal absorbs rapidly but have a very less half-life that leads to lower exposure of safranal after oral administration even at a high dose level (Supporting information). Inadequate oral exposure is also correlated to the in vitro results obtained in the chemical stability, plasma stability, and metabolic stability experimentations. Numbers of biological activities are reported in the literature for safranal after oral administration despite this unfavorable pharmacokinetic behavior, which is observed in the present investigation. This may be due to its excellent potency in native form and/or its degraded products and/or any of its metabolites. A similar phenomenon is also observed for phenolic compounds like curcumin.32
3. Conclusions
In spite of quite a few promising pharmacokinetic properties, poor oral exposure of safranal can be explained by its moderate chemical stability in SGF/SIF related to the unavailability of intact amount for absorption, low plasma stability linked to lesser extent of bioavailability, and petite metabolic stability connected to rapid clearance by liver. Therefore, this present investigation suggests the following key information for this useful phytoconstituent: (a) formulation approaches have to be applied for better oral exposure, (b) consideration for the presence of free aldehyde or hydroxylated acetal form during estimation, (c) contribution of degradation products/metabolites to the reported biological activities through orally, and (d) further research is required for stable form of safranal through derivatization of aldehyde group without losing its potency.
4. Materials and Methods
4.1. Chemicals and Reagents
Safranal (purity ≥ 90%), testosterone (purity ≥ 98%), elacridar (purity ≥ 98%), cyclosporine (purity ≥ 98.5%), rifampicin (purity ≥ 98%), propanolol (purity ≥ 99%), reduced nicotinamide adenine dinucleotide phosphate (NADPH), ATP/GTP ATPase activity assay kit, formic acid (LC-MS grade), and olive oil were purchased from Sigma-Aldrich. BCRP human membrane (Lot #7304003) and P-gp human membrane (Lot #6314001) were purchased from Corning Gentest. Rapid equilibrium dialysis (RED) device was procured from Pierce, Thermo-Fisher Scientific. MLM (Lot #MS035-B), RLM (Lot #RT053-F), DLM (Lot #DG031-B), and HLM (Lot #PL050C-E) were purchased from Gibco. Magnesium chloride, acetonitrile, methanol, dimethyl sulfoxide (DMSO), hexane, and hydrochloric acid were purchased from Fischer Scientific. n-Octanol and ethylenediaminetetraacetic acid (EDTA) were procured from Loba Chemie and SD Fine Chemicals, respectively. All other chemicals/reagents used were of research grade. Ultrapure water from Direct-Q3 water purification system (Merck-Millipore) was used for all experimentations.
4.2. In Silico Evaluation of Drug-likeliness
Drug-likeliness based on Lipinski’s rule was determined using online software (Schrodinger 2015-4, QikProp 4.6, New York, 2015), which is based on computational models. Drug-likeliness was evaluated in terms of violations in Lipinski’s rule of five.16
4.3. In Vitro/Ex Vivo Biopharmaceutical Profiling
log P of safranal was evaluated by conventional shake-flask method in octanol and water system using 1:2, 1:1, and 2:1 (v/v) ratios. Safranal concentration in both octanol and water layer was analyzed by HPLC (Table 1). Testosterone (1:1 ratio) was used as standard where analysis of samples was carried out by liquid chromatography tandem mass spectrometry (LC-MS/MS) (Table S1; Supporting Information). log D of safranal was evaluated in the same manner using phosphate-buffered saline, pH 7.4 instead of water.33
Table 1. HPLC (Model: Prominence; Make: Shimadzu) Conditions for Quantitation of Safranal.
| parameter | condition |
|---|---|
| column | LiChrospher C18 (250 mm × 4.6 mm, 5 μm) |
| mobile phase | water:acetonitrile = 24:76 (% v/v) |
| elution | isocratic |
| flow rate | 0.75 mL/min |
| column temperature | ambient |
| wavelength for UV detection | 310 nm |
| run time | 12 min |
| retention time | ∼8.2 min |
| stock solution | acetonitrile |
| dilution | acetonitrile |
| software | LabSolutions |
The P-gp-dependent ATPase activity for safranal (1.25–40 μM) was evaluated using human P-gp membrane where elacridar (10 μM) was used as standard.34 This is a colorimetric assay to measure the release of inorganic phosphate in the reaction mixture composed of membrane protein, test compound, and ATP. BCRP-dependent ATPase activity for safranal (1.25–20 μM) was also performed using human BCRP membrane in the same manner as mentioned in the case of P-gp-dependent ATPase activity except cyclosporine (10 μM) was used as standard.35 The chemical stability of safranal in SGF and SIF was determined by incubating safranal (500 μM) in the respective fluid for 2 and 4 h, respectively.36 The sample spiked with safranal at the same concentration level but without incubation was considered as 0 h. Safranal concentration was determined by HPLC as described in Table 1. Permeability of safranal was determined by PAMPA,33 where membrane material was 5% hexadecane in hexane (v/v) and safranal concentration was 10, 25, and 50 μM. Testosterone (25 μM) was used as a standard. The concentration of safranal and testosterone in both donor and acceptor plates was quantified by HPLC as mentioned in Tables 1 and S2 (Supporting Information), respectively. Plasma protein binding of safranal was measured by the rapid equilibrium dialysis (RED) method.33 The study was performed using blank rat plasma and safranal concentrations of 5, 10, and 20 μM. The presence of safranal in both sample chamber and buffer chamber was determined by HPLC (Table 1). Rifampicin (10 μM) was used as a standard where rifampicin concentration was analyzed by HPLC (Table S3; Supporting Information). The blood-to-plasma ratio of safranal was assessed by incubating blank rat plasma as reference plasma and blood where safranal was spiked at the concentration level of 1000 nM, and incubation was done separately for 0, 10, 30, and 60 min. Propanolol was used as standard at the same concentration level.34 Safranal was estimated by HPLC as mentioned above, and propanolol was determined by LC-MS/MS (Table S4; Supporting Information). The stability of safranal in mice plasma as well as rat plasma was investigated at 500 μM for 0, 1, 2, and 4 h.37 Quantification of safranal was done by HPLC (Table 1). The metabolic stability of safranal was explored in MLM, RLM, DLM, and HLM using substrate depletion approach where substrate concentration was 10 μM, protein concentration was 250 μg/mL, NADPH concentration was 1.2 mM, and incubation time was 0, 15, and 30 min. The reaction mixture without NADPH served as a negative control.34,36 The concentration of safranal was analyzed by HPLC (Table 1). All of the above-mentioned parameters were evaluated, considering that the experimental solvents/buffers have no effect on safranal. Detail experimental procedures are provided in the Supporting Information.
4.4. In Vivo Pharmacokinetic Study
The pharmacokinetic study of safranal was performed in healthy Balb/C mice with prior approval from Institutional Animal Ethics Committee, CSIR-IIIM, Jammu. Prior to experimentation, the animals were housed under standard laboratory conditions for acclimatization. Overnight fasted animals were divided into groups (five animals/group) for sparse sampling. Dose formulation of safranal was prepared in olive oil (100%, v/v) with a dose volume of 10 mL/kg. Safranal was administered through the oral route at 100 mg/kg single dose followed by the collection of the blood sample at time points of 0, 0.25, 0.5, 1, and 2 h from retro-orbital plexus into a microcentrifuge tube containing aqueous EDTA solution. Plasma was obtained through centrifugation, and then, the sample was processed by the protein precipitation technique using acetonitrile. Safranal concentration in the sample was determined by HPLC (Table 1) using a matrix match calibration curve where standards were prepared by spiking a known amount of safranal into blank plasma. After that, the obtained plasma concentration data at respective time points were analyzed to determine the pharmacokinetic parameters by PK Solutions software (Summit Research Services) using the noncompartmental method.35,36
4.5. Statistical Analysis
Statistical significance was assessed using online Student’s t-test (QuickCalcs, GraphPad software) between alone group and combined treatment group for in vivo experimentations or control vs treatment data for in vitro experimentations. Data are represented as mean ± SEM. p-value of less than 0.05, 0.01, and 0.001 was considered statistical significant, highly statistical significant, extremely statistical significant, respectively.
Acknowledgments
The authors are grateful to Director (CSIR-IIIM) for carrying out this research work. They are thankful to CSIR, UGC, and DST (New Delhi, India) for providing research fellowship support. IIIM publication number: CSIR-IIIM/IPR/00109
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00160.
Detailed methodology for the assessment of log P and log D, ATPase activity, chemical stability, permeability, plasma protein binding, plasma stability, RBC partitioning, metabolic stability, and pharmacokinetic study; HPLC chromatograms for chemical stability and plasma stability; and plasma concentration vs time profile for pharmacokinetic study of safranal (PDF)
Author Contributions
A.D., P.K., S.B., and A.G. performed all of the in vitro and in vivo profiling of safranal including the HPLC or LC-MS/MS method development. Acquisition of consumables and proof reading were carried out by G.S. Chemical data interpretation was performed by D.M. Overall study design, statistical data evaluation, and MS preparation were performed by U.N.
This research was supported by Council of Scientific and Industrial Research, New Delhi, India (MLP6006).
The authors declare no competing financial interest.
Supplementary Material
References
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Dias D. A.; Urban S.; Roessner U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee R.; Hosseinzadeh H. Safranal: from an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic Med Sci. 2013, 16, 12–26. 10.22038/ijbms.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk J. P.; Wang S.; Marcone M. F. Chemical and biological properties of the world’s most expensive spice: saffron. Food Res. Int. 2010, 43, 1981–1989. 10.1016/j.foodres.2010.07.033. [DOI] [Google Scholar]
- Sadeghnia H. R.; Shaterzadeh H.; Forouzanfar F.; Hosseinzadeh H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 3, 206–213. 10.5114/fn.2017.70485. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Ma J.; Fan L.; Zou Y.; Dang X.; Wang K.; Song J. Neuroprotective effects of safranal in a rat model of traumatic injury to the spinal cord by anti-apoptotic, anti-inflammatory and edema-attenuating. Tissue and Cell 2015, 47, 291–300. 10.1016/j.tice.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Ali M. S.; Al-Lohedan H. A. Experimental and computational investigation on the molecular interactions of safranal with bovine serum albumin: binding and anti-amyloidogenic efficacy of ligand. J. Mol. Liq. 2019, 278, 385–393. 10.1016/j.molliq.2019.01.034. [DOI] [Google Scholar]
- Bharti S.; Golechha M.; Kumari S.; Siddiqui K. M.; Arya D. S. Akt/GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia–reperfusion injury in rats. Eur. J. Nutr. 2012, 51, 719–727. 10.1007/s00394-011-0251-y. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H.; Sadeghnia H. R. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005, 8, 394–399. [PubMed] [Google Scholar]
- Sadeghnia H. R.; Kamkar M.; Assadpour E.; Boroushaki M. T.; Ghorbani A. Protective effect of safranal, a constituent of Crocus sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran. J. Basic Med. Sci. 2013, 16, 73–82. [PMC free article] [PubMed] [Google Scholar]
- Samarghandian S.; Azimi-Nezhad M.; Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp. Anim. 2015, 64, 65–71. 10.1538/expanim.14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K. J.; Yang J. S. Anti-allodynia effect of safranal on neuropathic pain induced by spinal nerve transection in rat. Int. J. Clin. Exp. Med. 2014, 7, 4990–4996. [PMC free article] [PubMed] [Google Scholar]
- Fukui H.; Toyoshima K.; Komaki R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus). Phytomedicine 2011, 18, 726–730. 10.1016/j.phymed.2010.11.013. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H.; Younesi H. M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7–15. 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns E. H. High throughput physicochemical profiling for drug discovery. J. Pharm. Sci. 2001, 90, 1838–1858. 10.1002/jps.1134. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A.; Lombardo F.; Dominy B. W.; Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Huesgen A. G.Determination of Log P for Compounds of Different Polarity Using the Agilent 1200 Infinity Series HDR-DAD Impurity Analyzer System. Application Note Small Molecule Pharmaceuticals & Generics; Agilent Technologies, Inc.: Waldbronn, Germany, 2019. [Google Scholar]
- Pyka A.; Babuska M. Lipophilicity of Selected Steroid Compounds. I. Investigations on RP18W Stationary Phase by RP-HPTLC. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1891–1903. 10.1080/10826070600757748. [DOI] [Google Scholar]
- Breedveld P.; Beijnen J. H.; Schellens J. H. M. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol. Sci. 2006, 27, 17–24. 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Rule C. S.; Patrick M.; Sandkvist M. Measuring in vitro ATPase activity for enzymatic characterization. J. Visualized Exp. 2016, 114, 54035 10.3791/54305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha W. Transporter-mediated natural product-drug interactions for the treatment of cardiovascular diseases. J. Food Drug Anal. 2018, 26, S32–S44. 10.1016/j.jfda.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohnsland F.; Faller B. High-Throughput permeability pH profile and high throughput alkane/water Log P with artificial membranes. J. Med. Chem. 2001, 44, 923–930. 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- Schmidt D.; Lynch J.. Application Note on Evaluation of the Reproducibility of Parallel Artificial Membrane Permeation Assays (PAMPA); Millipore Corporation, Life Science Division: Danvers, MA, 2003; pp 1–12. [Google Scholar]
- Bennion B. J.; Be nA.; McNerney M. W.; Lao V.; Carlson E. M.; Valdez C. A.; Malfatti M. A.; Enright H. A.; Nguyen T. H.; Lightstone F. C.; Carpenter T. S. Predicting a drug’s membrane permeability: a computational model validated with in vitro permeability assay data. J. Phys. Chem. B 2017, 121, 5228–5237. 10.1021/acs.jpcb.7b02914. [DOI] [PubMed] [Google Scholar]
- Boman G.; nd Ringberger V. A. Binding of rifampicin by human plasma proteins. Eur. J. Clin. Pharmacol. 1974, 7, 369–373. 10.1007/BF00558209. [DOI] [PubMed] [Google Scholar]
- Litjens C. H. C.; Aarnoutse R. E.; Kolmer E. W. J. E.; Svensson E. M.; Colbers A.; Burger D. M.; Boeree M. J.; Brake L. H. M. Protein binding of rifampicin is not saturated when using high-dose rifampicin. J. Antimicrob. Chemother. 2019, 74, 986–990. 10.1093/jac/dky527. [DOI] [PubMed] [Google Scholar]
- Buscher B.; Laakso S.; Mascher H.; Pusecker K.; Doig M.; Dillen L.; Wagner-Redeker W.; Pfeifer T.; Delrat P.; Timmerman P. Bioanalysis for plasma protein binding studies in drug discovery and drug development: views and recommendations of the European Bioanalysis Forum. Bioanalysis 2014, 6, 673–682. 10.4155/bio.13.338. [DOI] [PubMed] [Google Scholar]
- Smith D. A.; Di L.; Kerns E. H. The effect of plasma protein binding on in vivo efficacy: misconceptions in drug discovery. Nat. Rev. Drug Discovery 2010, 9, 929–939. 10.1038/nrd3287. [DOI] [PubMed] [Google Scholar]
- Yu S.; Li S.; Yang H.; Lee F.; Wu J. T.; Qian M. G. A novel liquid chromatography/tandem mass spectrometry based depletion method for measuring red blood cell partitioning of pharmaceutical compounds in drug discovery. Rapid Commun. Mass Spectrom. 2005, 19, 250–254. 10.1002/rcm.1777. [DOI] [PubMed] [Google Scholar]
- https://www.cyprotex.com/product_sheets/Cyprotex_Plasma_Stability_Product_Sheet.pdf.
- Mondal S. K.; Mondal N. B.; Banerjee S.; Mazumder U. K. Determination of drug like properties of a novel antileishmanial compound: in vitro absorption, distribution, metabolism, and excretion studies. Indian J. Pharmacol. 2009, 41, 176–181. 10.4103/0253-7613.56075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler M.; Pfeiffer E.; Schulz S. I.; Dempe J. S. Curcumin uptake and metabolism. BioFactors 2013, 39, 14–20. 10.1002/biof.1042. [DOI] [PubMed] [Google Scholar]
- Magotra A.; Sharma A.; Singh S.; Ojha P. K.; Kumar S.; Bokoli N.; Wazir P.; Sharma S.; Khan I. A.; Singh P. P.; Vishwakarma R. A.; Singh G.; Nandi U. Physicochemical, pharmacokinetic, eficacy and toxicity profiling of a potential nitrofuranyl methyl piperazine derivative IIIM-MCD-211 for oral tuberculosis therapy via in-silico–in-vitro–in-vivo approach. Pulm. Pharmacol. Ther. 2018, 48, 151–160. 10.1016/j.pupt.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Magotra A.; Gour A.; Sharma D. K.; Dash A. K.; Singh G.; Mukherjee D.; Nandi U. Pharmacokinetic evaluation of medicinally important synthetic N, N′ diindolylmethaneglucoside: improved synthesis and metabolic stability. Bioorg. Med. Chem. Lett. 2019, 29, 1007–1011. 10.1016/j.bmcl.2019.02.010. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Gour A.; Bhatt S.; Rath S. K.; Malik T. A.; Dogra A.; Sangwan P. L.; Koul S.; Abdullah S. T.; Singh G.; Nandi U. Effect of IS01957, a para-coumaric acid derivative on pharmacokinetic modulation of diclofenac through oral route for augmented efficacy. Drug Dev. Res. 2019, 80, 948–957. 10.1002/ddr.21574. [DOI] [PubMed] [Google Scholar]
- Sharma A.; Magotra A.; Dogra A.; Rath S. K.; Rayees S.; Wazir P.; Nandi U.; et al. Pharmacokinetics, pharmacodynamics and safety profiling of IS01957, a preclinical candidate possessing dual activity against inflammation and nociception. Regul. Toxicol. Pharmacol. 2017, 91, 216–225. 10.1016/j.yrtph.2017.10.033. [DOI] [PubMed] [Google Scholar]
- Singh R.; Kumar V.; Bharate S. S.; Vishwakarma R. A. Synthesis, pH dependent, plasma and enzymatic stability of bergenin prodrugs for potential use against rheumatoid arthritis. Bioorg. Med. Chem. 2017, 25, 5513–5521. 10.1016/j.bmc.2017.08.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.