Abstract
Background
Pancreatic and peri-pancreatic neoplasms encompass a variety of histotypes characterized by a heterogeneous prognostic impact. miRNAs are considered efficient candidate biomarkers due to their high stability in tissues and body fluids. We applied Nanostring profiling of circulating exosomal miRNAs to distinct pancreatic lesions in order to establish a source for biomarker development.
Methods
A series of 140 plasma samples obtained from patients affected by pancreatic ductal adenocarcinoma (PDAC, n = 58), pancreatic neuroendocrine tumors (PanNET, n = 42), intraductal papillary mucinous neoplasms (IPMN, n = 20), and ampulla of Vater carcinomas (AVC, n = 20) were analyzed. Comprehensive miRNA profiling was performed on plasma-derived exosomes. Relevant miRNAs were validated by qRT-PCR and in situ hybridization (ISH).
Results
Lesion specific miRNAs were identified through multiple disease comparisons. Selected miRNAs were validated in the plasma by qRT-PCR and at tissue level by ISH. We leveraged the presence of clinical subtypes with each disease cohort to identify miRNAs that are differentially enriched in aggressive phenotypes.
Conclusions
This study shows that pancreatic lesions are characterized by specific exosomal-miRNA signatures. We also provide the basis for further explorations in order to better understand the relevance of these signatures in pancreatic neoplasms.
Keywords: Pancreatic lesions, Circulating miRNAs, Early biomarkers, Exosomes, NanoString profiling
Background
Pancreatic ductal adenocarcinoma (PDAC) represents approximately the 90% of all pancreatic cancers and is currently the third leading cause of cancer deaths in the United States with a reported 5-year survival rate of 8% [1]. Early symptoms of PDAC are non-specific, therefore more than half patients are diagnosed at a late stage when the tumor is unresectable [2, 3].
The second most common neoplasm of the pancreas is pancreatic neuroendocrine tumor (PanNET), a rare epithelial malignancy arising from pancreatic islet cells [4]. At present, surgical resection of PanNET with curative intent remains the most effective therapeutic option, with systemic therapies being largely ineffective for unresectable diseases [5]. Even if PDAC and PanNET represent the two most common pancreatic cancers, they show completely different genetic profiles [6, 7].
Several risk factors associated to PDAC development have been identified including genetic syndromes, smoking, alcohol and chronic pancreatitis (CP) [8, 9]. In particular, CP has been proposed as independent risk factor for pancreatic cancer development [10].
Three main PDAC precursor lesions have been identified: pancreatic intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN), whose early detection and management could prevent progression to malignancy [11]. Several studies have focused their attention on the genetic heterogeneity of pancreatic precursor lesions in order to elucidate the timing of specific alterations in pancreatic tumorigenesis especially in the contest of IPMN and PDAC [12–14].
Ampulla of Vater carcinomas (AVC) comprise a percentage ranging from 15 to 30% of all pancreatico-duodenectomies and 10 to 20% of all tumor-related obstructions of the common bile duct [15, 16].
Although AVC show a better prognosis than PDAC, it remains a deadly disease with a mortality rate of 60% [17, 18]. At present, diagnostic imaging is not able to distinguish between periampullary neoplasms such as periampullary PDAC or periampullary carcinoma of the duodenum and AVC [19].
The current therapeutic approaches for treating advanced pancreatic/peri-pancreatic cancers are poorly effective, and the known common biomarkers are inadequate for a reliable risk stratification as well as in the setting of early diagnosis. Therefore, new non-invasive strategies and more precise therapeutic targets are urgently needed to improve patients’ management and outcome. MicroRNAs (miRNAs) are a class of endogenous, small (19 to 25 nucleotides), non-coding RNAs that modulate the expression of at least one third of protein-coding genes [20–24]. Moreover, miRNAs have been detected within body fluids, such as plasma, serum, urine and breast milk. The so-called “circulating microRNAs” can be found either encapsulated in cell-secreted vesicles or vesicle-free and instead be associated to AGO protein-positive ribonucleoprotein (RNP) particles [25]. It has been demonstrated that secreted miRNAs can be taken up by recipient cells and act as biologically active molecules [26, 27].
It has been widely reported in literature the aberrant miRNAs expression in human cancers including PDAC, its precursor lesions, PanNETs and AVCs both on tissue and biofluids [28–32]. Many genomic aberrations have been associated to the differential expression of miRNAs between normal and malignant tissues as chromosomal alterations, DNA point mutations, epigenetic mechanisms and miRNA processing machinery alterations [21].
On these grounds, the aim of this study was to identify circulating miRNAs able in discriminating the different histotypes of pancreatobiliary neoplasms. Furthermore, we also sought to identify miRNAs differentially expressed in the same histotype, based on biological and clinical differences. In particular, we leveraged the presence of metastatic and localized PDAC in our cohort to identify markers of dismal disease. To our knowledge, this is one of the most extensive study cohort of circulating miRNAs profiling among patients with different pancreatobiliary lesions using digital technology.
Methods
Pancreatic lesion patients and clinical samples
A series of 155 plasma samples obtained from 58 PDAC, 42 PanNETs, 20 IPMN, 20 AVC (Additional file 1), and 15 CP patients were retrieved from the archives of the ARC-NET biobank of the Verona University Hospital. All experiments were performed in accordance with relevant guidelines and regulations. Clinicopathological features of PanNET, PDAC and AVC patients are reported in Table 1. Regarding IPMN samples, only 6 of 20 displayed associated invasive pancreatic ductal adenocarcinoma. All peripheral fasting blood samples were collected in 10-mL sodium heparin tubes (BD Vacutainer; Becton-Dickinson, Milan, Italy) and processed within 2 h by centrifugation at 2000 g at 4 °C for 10 min. The plasma obtained was then transferred to new tubes and centrifuged at 3000 g at 4 °C for 10 min to remove platelet contamination. Final plasma preparations were carefully collected from the upper portion of the supernatant and stored in aliquots at − 80 °C.
Table 1.
Clinicopathological characteristics of considered PDAC, PanNET and AVC
| Variable | PDAC (n = 55) | PanNET (n = 42) | AVC (n = 19)b |
|---|---|---|---|
| Gender | |||
| F | 22 (40%) | 16 (38%) | 10 (53%) |
| M | 33 (60%) | 26 (62%) | 9 (47%) |
| Age (years) | 64.5 ± 10,5 | 51.1 ± 13.65 | 63.4 ± 11.18 |
| (median 67.0) | (median 49.5) | (median 63) | |
| Primary tumor | |||
| T1 | 5 (9%) | 20 (47%) | 3 (15%) |
| T2 | 25 (46%) | 15 (36%) | 6 (30%) |
| T3 | 9 (16%) | 7 (17%) | 9 (45%) |
| T4 | 6 (11%) | 0 (0%) | 0 (0%) |
| an.a. | 10 (18%) | 0 (0%) | 1 (5%) |
| Regional lymph nodes | |||
| N0 | 3 (6%) | 22 (52%) | 7 (35%) |
| N1 | 15 (27%) | 12 (29%) | 7 (35%) |
| N2 | 21 (38%) | 0 (0%) | 4 (20%) |
| n.a. | 16 (29%) | 8 (19%) | 1 (5%) |
| Distant metastasis | |||
| M0 | 38 (69%) | 40 (95%) | 18 (90%) |
| M1 | 11 (20%) | 2 (5%) | 1 (10%) |
| n.a. | 6 (11%) | 0 (0%) | 0 (0%) |
| Clinical stage | |||
| I | 3 (6%) | 11 (26%) | 6 (30%) |
| II | 15 (27%) | 11 (26%) | 0 (0%) |
| III | 20 (36%) | 10 (24%) | 12 (60%) |
| IV | 11 (20%) | 2 (5%) | 1 (5%) |
| n.a. | 6 (11%) | 8 (19%) | 0 (0%) |
| Grade | |||
| G1 | 0 (0%) | 29 (69%) | 2 (10%) |
| G2 | 25 (45.5%) | 13 (31%) | 12 (60%) |
| G3 | 14 (25.5%) | 0 (0%) | 4 (20%) |
| n.a. | 16 (29%) | 0 (0%) | 1 (5%) |
| Lymphovascular invasion | |||
| absent | 1 (2%) | 17 (40.5%) | 3 (15%) |
| present | 38 (69%) | 17 (40.5%) | 15 (75%) |
| n.a. | 16 (29%) | 8 (19%) | 1 (5%) |
| Perineural invasion | |||
| absent | 0 (0%) | 22 (52%) | 5 (25%) |
| present | 39 (71%) | 12 (29%) | 13 (65%) |
| n.a. | 16 (29%) | 8 (19%) | 1 (5%) |
an.a (not available data): patients who did not undergo radical surgery; bOne out of 20 AVCs is represented by an adenomatous lesion with high-grade dysplasia (this case has not been reported in the table)
Preparation of exosomal RNA from pancreatic lesion plasma samples
Plasma-derived exosomes were isolated as described in Casadei et al. [33] by using the ExoQuick system (System biosciences) according to the manufacturer’s protocol. The quality and size of particles were determined by Nanosight, qRT-PCR and western blot analyses (Additional file 2). The exosomal pellet was resuspended in 1 ml Trizol (Life Technologies), and total RNA was isolated with Norgen RNA clean-up and concentration kit (Norgen BioTek), following the provided instructions. Exosomal RNA was eluted in 20 μl of H2O; the average RNA yield per group per 1 ml of plasma was: for the CP group ~ 4 ng/ml, for IPMN ~ 6 nm/ml, for PDAC ~ 6.3 ng/ml, for AVC ~ 20 ng/ml, for PanNET ~ 4.7 ng/ml. Nanodrop 1000 spectrophotometer (Thermo Scientific) was used to exclude within our samples the presence of 230 nm peaks, due to organics compounds or chaotropic salts, which could represent a potential issue for the ligase enzymatic step during NanoString profiling.
Nanoparticle tracking analysis (NTA)
Concentration and particle size distribution in plasma-derived exosomal samples were determined by NTA using NanoSight NS300 system (Malvern Technologies, Malvern, UK). Nanosight was configured with a 532 nm green laser, a high sensitivity scientific CMOS camera, and samples were loaded by using a syringe. Samples were diluted in particle-free PBS (Sigma) to acceptable concentrations based off of the manufacturer’s recommendation. Sample data files were acquired under continuous flow (flow rate = 25–50) and at room temperature. Samples were recorded for 3 × 60 s successive videos and were captured with a camera level of 12. Data were analyzed using NTA 3.1.5 software with a detection threshold of 3–4.
NanoString nCounter assay and data analysis
A total of 155 samples were processed with Nanostring nCounter® Human v3 miRNA Expression Assay. NanoString analysis was performed as described in Drusco et al. [34]. Briefly, 3.5 μL of exosomal RNA were annealed with multiplexed DNA tags (miR-tag) and bridges target specifics. Mature miRNAs were then bond to specific miR-tags using a Ligase enzyme and all the tags in excess were removed by enzyme clean-up step. The tagged microRNAs product was diluted 1 to 5 and 5 μL were combined with 20 μL of Reported Probes in hybridization buffer and 5 μL of Capture probes. The overnight hybridization (16 to 20 h) at 65 °C allowed to complex probes sequence specific with targets. Probe excess was removed using two-step magnetic beads-based purification on an automated fluidic handling system (nCounter Prep Station) and target/probe complexes were immobilized on the cartridge for data collection. The nCounter Digital Analyzer collected the data by taking images of immobilized fluorescent reporters in the sample cartridge with a CCD camera through a microscope objective lens. For each cartridge, a high-density scan encompassing 325 fields of view was performed. Images were processed internally into a digital format (RCC files). NanoString raw data were analyzed with nSolver™, a software provided by NanoString Technologies. Negative controls were used to perform background subtraction. Positive controls were used to perform technical normalization to adjust any lane-by-lane variability due to differences in hybridization, purification or binding. After technical normalization, the data were biologically normalized by calculating the geometric mean of the top 100 miRNAs in all samples, as recommended by NanoString. p-values were calculated using the LIMMA package (Linear Models for Microarray Data) from the Bioconductor R project. The p-values were adjusted for multiple testing using the Benjamini and Hochberg method [35] to control the False Discovery Rate (FDR). For each pairwise analysis, we considered all those significant (p-value< 0.05) and expressed (≥20 counts in at least one condition, as 20 counts is approximately the average of the expression of the negative controls in the NanoString panel) deregulated miRNAs for the downstream analysis.
Quantitative real-time PCR
The expression of an exosomal individual mature miRNA was assessed in triplicate by using the TaqMan Stem-loop miRNA assay, according to the manufacturer’s protocol (ThermoFisher). For retro-trancription, 0.5 ng of RNA were used per reaction in a final volume of 7.5 μl. For qRT-PCR, 1 μl of cDNA was used per reaction. For RNA normalization, ath-miR-159a, osa-miR-414, and cel-miR-248 synthetic oligos (Integrated DNA Technologies) were added to each sample right after the exosomal pellet was resuspended in Trizol. Normalization was performed with the 2-Δct method. Results were analyzed by using a two-tailed Student t test.
Western blot
For the western blot analysis, exosomal proteins were isolated from 500 μl of plasma (the yield of exosomal protein was 9 mg/sample). The exosomal pellet was resuspended in RIPA buffer (Cell Signaling technology), supplemented with phosphatase and protease inhibitors (Roche), and incubated in ice for 20′. Samples were then harvested at 14,000 x g for 10′, and the supernatant collected in a new eppendorf. Protein concentration was determined by using Bradford Assay (Bio-Rad), following the manufacturer’s instructions. 80 μg of exosomal lysate were then loaded on a Criterion Tris-HCl 4–20% pre-cast gel (Bio-Rad), transferred onto a nitrocellulose membrane (Bio-Rad) and probed with anti-Alix (1:1000), anti-TSG101 (1:1000), anti-Calnexin (Sigma) (1:2000), and anti-CD9 (Cell Signaling Technology) (1:1000) primary antibodies, followed by isotype matched, horseradish-peroxidase-conjugated secondary antibodies. Finally, the proteins of interest were detected through chemi-luminescence reaction.
miRNA in situ hybridization analysis (ISH)
Locked nucleic acid (LNA) probes with complementarity to miR-4454, miR-106a-5p, and miR-17-5p were labelled with 5′-biotin and synthesized using Exiqon (Vedbaek, Denmark). Tissue sections were digested with ISH protease 1 (Ventana Medical Systems, Milan, Italy) and ISH was performed as we previously described [36]. Positive (U6; Exiqon) and negative scrambled LNA probes (Exiqon) were used as controls. Only cytoplasmic miRNA staining was retained for scoring purposes.
Results
Digital profiling identifies circulating miRNAs specific to the neoplastic state
Comprehensive miRNA profiling was performed in order to identify exosomal miRNAs differently expressed between pancreatic lesions (AVC, IPMN, PDAC and PanNET) and chronic pancreatitis (CP). Overall, we found 26, 23, 40 and 45 deregulated miRNAs between AVC vs CP, IPMN vs CP, PDAC vs CP and PanNET vs CP, respectively (Fig. 1 a-b-c-d). For each comparison, a linear fold change > 1.5 was used as threshold. Next, relevant miRNAs were filtered again considering only those with a number of counts greater than 20 (See Methods section, Table 2 and Additional file 3). In details, we found 5 deregulated miRNAs (3 upregulated and 2 downregulated miRNAs) between AVC and CP; 4 miRNAs between IPMN and CP (3 upregulated and 1 downregulated miRNAs); 9 miRNAs between PDAC and CP (3 upregulated and 6 downregulated miRNAs) and 11 miRNAs between PanNET and CP (6 upregulated and 5 downregulated miRNAs) (Table 2 and Additional file 3).
Fig. 1.
Differential expression of circulating miRNAs in pancreatic lesions compared to chronic pancreatitis. a-b-c-d Volcano plots of miRNAs expression showing significant (P < 0.05) and deregulated (with a |LinearFC| > 1.5) miRNAs in each comparison. e-f-g-h-i-j-k NanoString results were validated by qRT-PCR analysis. The expression levels of the indicated miRNAs in the CP group (here considered as a control group) were compared with the expression of the same miRNAs within the indicated pancreatic cancer groups. The square inside the box plot indicates the mean value, whereas the “x” outside the box indicates the 99-percentile. Student’s t-test was performed for statistical analysis. *, 0.01 < P ≤ 0.05; **, 0.001 < P ≤ 0.01
Table 2.
Summary of deregulated miRNAs from Nanostring profiling between pancreatic lesions
| Type of comparison | Upregulated miRNAs | Downregulated mRNAs |
|---|---|---|
| AVC Vs. CP |
miR-106a-5p; miR-17-5p; miR-520f-3p |
miR-4454; miR-7975 |
| IPMN Vs. CP |
miR-106a-5p; miR-17-5p; miR-16-5p |
miR-122-5p |
| PDAC Vs. CP |
miR-372-3p; miR-140-3p; miR-644a |
miR-1299; miR-146a-5p; miR-148b-3p; miR-130a-3p; miR-4454; miR-7975 |
| PanNET Vs. CP |
miR-451a; miR-372-3p; miR-106a-5p; miR-17-5p; miR-25-3p; miR-644a |
miR-22-3p; miR-1246; miR-4454; miR-7975; miR-320e |
| ACV Vs. PDAC |
miR-106a-5p; miR-17-5p; miR-2116-5p; miR-199a-3p; miR-199b-3p; miR-342-3p; miR-520d-5p; miR-527; miR-518a-5p; miR-93-5p; miR-30a-5p; miR-27b-3p; miR-146a-5p; miR-451a; miR-20a-5p; miR-20b-5p; miR-185-5p; miR-520 h; miR-25-3p; miR-1322; miR-19a-3p; miR-302e |
– |
| IPMN Vs. PDAC |
miR-4454; miR-7975; miR-2116-5p; miR-1910-5p; miR-16-5p; miR-451a; miR-19b-3p; miR-106a-5p; miR-17-5p; miR-629-5p; miR-376a-3p; miR-20a-5p; miR-20b-5p; miR-26b-5p; miR-93-5p; miR-25-3p; miR-590-5p; miR-30e-5p; miR-19a-3p; miR-608; let-7b-5p |
– |
| PanNET Vs. PDAC |
miR-451a; miR-26b-5p; miR-25-3p; miR-16-5p |
miR-1322; miR-1285-5p; miR-320e |
| Met. PDAC Vs. Loc. PDAC |
miR-106a-5p; miR-17-5p; miR-342-3p; miR-20a-5p; miR-20b-5p; miR-223-3p; miR-16-5p; miR-19b-3p; miR-130a-3p; miR-25-3p; miR-451a |
– |
| PB AVC Vs. INT. AVC |
miR-579-3p; miR-422a; miR-1253; miR-3144-3p; miR-1268a; miR-190a-3p |
miR-3613-3p; miR-582-5p; miR-1976; miR-885-5p; miR-122-5p |
| IPMN-C Vs. IPMN |
miR-1293; miR-450a-5p; miR-433-5p; miR-324-5p; miR-941; miR-499a-5p; miR-4787-5p; miR-139-3p; miR-516a-3p; miR-516b-3p; miR-665 |
miR-520f-3p; miR-126-3p |
Significant (Pvalue< 0.05) and expressed (≥20 counts in at least one condition) deregulated (with a |LinearFC| > 1.5) miRNAs in each comparison. miRNAs validated by qRT-PCR are indicated as bold text
Seven miRNAs were therefore selected for orthogonal validation by qRT-PCR on the same cohort of patients: miR-106-5p, miR-7975, miR-4454, miR-16-5p, miR-25-3p, miR-320e and miR-451a (Fig. 1 e-k and Table 2). In particular, since the NanoString assay is not able to discriminate between miR-106a-5p/miR-17-5p and miR-4454/miR-7975, we decided at first to perform a qRT-PCR analysis for these four miRNAs in order to understand their specific expression contribution within selected sample groups. At first, we found a significant upregulation of miR-106-5p in AVC and IPMN compared to CP (Fig. 1e) while miR-17-5p was not differentially deregulated between the same lesions (data not shown). The expression of both miRNAs was also investigated between PanNET and CP showing no different between groups (data not shown). Than we validated the downregulation of miR-7975 and miR-4454 in PDAC compared to CP while only miR-4454 was found downregulated in the comparison between PanNET and CP (Fig. 1 f-g). The upregulation of miR-16-5p was also validated by qRT-PCR in IPMN groups, while the upregulation of miR-25-3p and miR-451a and the downregulation of miR-320e were validated as deregulated in PanNET as compared to CP (Fig. 1 i-j-k).
Lesion specific circulating miRNAs
Differential miRNA profiling was also performed in order to identify relevant deregulated miRNAs between different pancreatic lesions (AVC vs PDAC, IPMN vs PDAC and PanNET vs PDAC). As reported in Fig. 2, we were able to identify 63 deregulated miRNAs between AVC and PDAC, 54 miRNAs between IPMN and PDAC and 16 miRNAs between PanNET and PDAC (Fig. 2 a-b-c). A linear fold change> 1.5 was applied as threshold for each comparison. After data filtering, we found 22 upregulated miRNAs in AVC compared to PDAC, 21 upregulated miRNAs in IPMN compared to PDAC and 7 deregulated miRNAs between PanNET and PDAC (4 upregulated and 3 downregulated miRNAs in PanNETs compared to PDAC) (Table 2 and Additional file 4). Selected miRNAs were than validated by qRT-PCR between different pancreatic lesions as reported in Additional file 3. Consistent with NanoString data, we found miR-17-5p and miR-106-5p significantly upregulated in AVC and IPMN as compared to PDAC (Fig. 2 d-e and Table 2). The upregulation of miR-16-5p and miR-19-3p in IPMN compared to PDAC was also validated as reported in Fig. 2 (f-g) and Table 2.
Fig. 2.
Differential expression of circulating miRNAs in pancreatic cancer patients. a-b-c Volcano plots of miRNA expression showing significant (P < 0.05), deregulated (with a |LinearFC| > 1.5) miRNAs in each comparison and an expression ≥20 counts in at least one condition. d-e-f-g NanoString results were validated by qRT-PCR. The differential expression of circulating miRNAs was assessed by using as a control the PDAC group with respect to the indicated pancreatic cancer groups. The square inside the box plot indicates the mean value, whereas the “x” outside the box indicates the 99-percentile. Student’s t-test was performed for statistical analysis. **, 0.001 < P ≤ 0.01
We also focused on the most deregulated circulating miRNAs associated with different biological and clinical subtypes of the same histotype: metastatic PDAC Vs localized PDAC (Met. PDAC vs Loc. PDAC), pancreatobiliary AVC vs intestinal AVC (PB AVC vs INT AVC) and IPMN with associated invasive adenocarcinoma and IPMN without associated invasive adenocarcinoma (IPMN-C VS IPMN) (Fig. 3). Using a linear fold change > 1.5 as threshold, we identified overall 9 deregulated miRNAs between metastatic and localized PDAC, 11 miRNAs between PB AVC and INT AVC and 12 miRNAs between malignant IPMN and benign IPMN (Fig. 3 a-b-c). As for previous analysis, data were filtered again leading to the identification of 9 downregulated miRNAs in metastatic PDAC compared to the localized one, 11 deregulated miRNAs between pancreatobiliary and intestinal IPMN (6 upregulated and 5 downregulated miRNAs) and 12 miRNAs between malignant and benign IPMN (10 upregulated and 2 downregulated miRNAs) (Table 2 and Additional file 4).
Fig. 3.
Different expression of circulating miRNAs in different biological and clinical subtypes of the same pancreatic lesion histotype. a-b-c Volcano plots of miRNAs expression showing significant (P value< 0.05), deregulated (with a |LinearFC| > 1.5) miRNAs in each comparison and an expression ≥20 counts in at least one condition
In situ analysis confirmed exosomal miRNA profiling
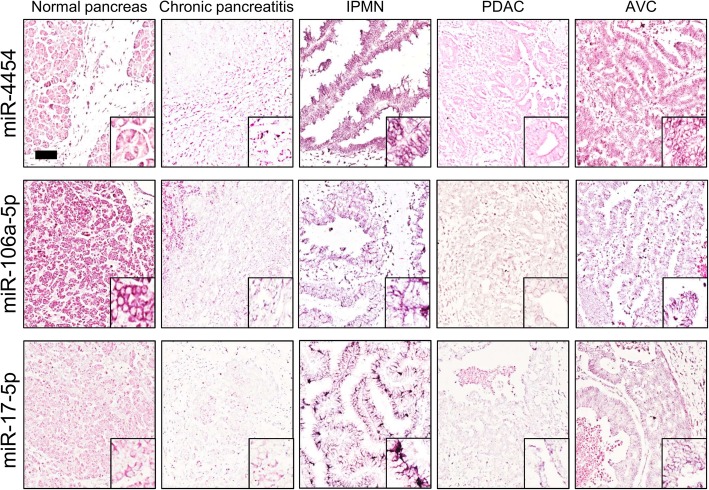
To further support our findings, we performed in situ hybridization (ISH) assay on matched formalin-fixed paraffin-embedded (FFPE) tissue sections of CP, IPMN, AVC, PDAC patients and normal pancreas for miR-4454, miR-106a-5p and miR-17-5p (Fig. 4). Despite ISH analyses lack the required sensitivity to identify subtle changes in expression levels of miRNA, they largely reflected qRT-PCR results. MiR-4454, which was significantly upregulated in CP compared to PDAC through qRT-PCR, showed the same expression trend by ISH (Fig. 4). MiR-106-5p and miR-17-5p qRT-PCR data also well matched with ISH experiments. As reported in Fig. 4, miR-106-5p was more expressed in IPMN and AVC tissue section as compared to PDAC and CP, while the expression of miR-17-5p was more evident in AVC and IPMN compared to PDAC.
Fig. 4.
Representative in situ hybridization (ISH) of miR-4454, miR-106-5p, miR-17-5p in tissue sections of pancreatic cancers. ISH assays demonstrate a significant miRNA expression dysregulation among different tumor hystotypes. Normal grey matter specimens showed a negative/faint expression of miR-106-5p and miR-17-5p in CP. On the other hand, IPMN and AVC showed a moderate/strong expression of miR-106-5p and miR-4454. Columns denote the different tumor subtype while rows the different miRNAs analyzed. The presence of miRNA is shown by a grainy blue cytoplasmatic stain; slides counterstained in fast red. (Scale bars 200 μm; Original magnifications 10x and 5x)
Discussion
In the last few years, many studies have highlighted the involvement of miRNAs in tumorigenesis and cancer progression supporting their possible role as new biomarkers for cancers [37]. Moreover, the identification of circulating miRNAs in the body fluids such as plasma/serum underline their potential application for detection, monitoring and predicting prognosis of cancer patients [24, 38]. At present, several miRNA expression profiling studies identified miRNA signatures associated to clinico-pathological features in PDAC as staging, progression, prognosis and response to treatment both on tissues [39–43] and biofluids [44–49] while only few data have been published on miRNAs and gastroenteropancreatic neuroendocrine tumors [31, 50–52]. Genome-wide miRNA expression analyses were also performed on IPMN tissues [53–56] but only two studies evaluated blood-based miRNA expression in early stage PDAC patients versus controls [45, 55]. As for IPMN, few information has been provided on circulating miRNAs and AVC, with plasma miR-192 up-regulation in periampullary carcinoma and its correlation with tumor stage and aggressiveness as the only identified biomarker [32, 57].
Despite this huge amount of data, miRNA expression profiling has not been introduced into the clinical practice, so far, maintaining radiological imaging and biopsy as the gold standards for tumor detection and diagnosis [58]. The causes of this failure may be found in the inconsistent results observed among studies or the unfeasibility to compare each other results due to different analytic methods, detection technologies or circulating components (e. g. plasma, serum or whole blood) analyzed [53]. On this ground, we applied digital profiling of circulating exosomal miRNAs to distinct pancreatic lesions in order to establish a bioresource for biomarker development. This is one of the most extensive exosomal miRNA study able to identify simultaneously peculiar miRNA signatures associate to different pancreatic lesions and within biological and clinical different subgroup of the same tumor histotype.
Recent evidences pointed out that exosomes represent a big bioresource of miRNAs, showing several advantages comparing to cell free miRNAs. First, exosomal miRNAs are protected from degradation even if in the presence of RNase and are stable under different storage conditions [59, 60]. Secondly, it has been reported that they are representative of the matched parental tumor in terms of miRNA signatures [61, 62] and finally, it is known that malignant tissues secrete a higher amount of exosomes compared to the normal counterpart in biofluids such as plasma and urine [63, 64]. These peculiar features render exosomal miRNAs a promising candidate for miRNAs’ profile studies.
In our study, plasma-derived exosomal RNA of 140 pancreatic lesions and 15 cases of chronic pancreatitis were processed by NanoString technology. Data analysis allowed us to select differentially expressed miRNAs in the majority of comparison between histotypes and CP patients. Selected miRNAs (miR-106-5p; miR-4454; miR-7975; miR-320e; miR-19b-3p; miR-16-5p and miR-17-5p) were validated between groups by qRT-PCR underling the powerful of NanoString technique for miRNAs profiling, especially for small RNA quantities as in our case (Figs. 1 and 2). Three selected miRNAs (miR-4454; miR-106a-5p and miR-17-5p) were then validated also by in situ hybridization, which largely reflected the results of plasma profiling and showed that most of circulating miRNAs were derived from the epithelial components of the lesions.
For the first time miR-4454 was found significantly upregulated in the plasma of patients with chronic pancreatitis compared to PDAC and PanNET (Fig. 1g); notably, this result has been also validated by ISH on tissue samples, further corroborating its reliability (Fig. 4). In line with our findings, in the literature there is evidence supporting the role of miR-4454 in inflammation. For example, it has been reported in lung that miR-4454 targets include cytokines and matrix metalloproteinases that could have a relevant impact on pulmonary inflammation and fibrosis [65]. Moreover miR-4454 was also identified as mediators of facet cartilage degeneration promoting inflammatory, catabolic and cell death activity [66].
Next, we aimed to identify miRNAs associated to biological and clinical different subtypes within the same histotype. Since recent studies highlighted the role of exosomes in the formation of a premetastatic niche in the liver [67] and in tumor proliferation [68], we profiled, for the first time, exosomal miRNAs between localized and metastatic pancreatic ductal adenocarcinoma. Our analysis identified a signature of 11 miRNAs significantly downregulated in metastatic PDAC compared to localized disease (Table 2). Further functional studies should investigate the biological role of this signature as useful non-invasive tool able to monitor disease progression.
The current standard histopathologic evaluation is often not able to identify the precise origin of a periampullary lesions, including neoplasms from other subtypes as could appended for ampullary carcinomas [69]. Moreover, since AVC may be classified as pancreatobiliary or intestinal according to the histologic type of differentiation [70], we explored the possibility to identify peculiar exosomal miRNAs signatures able to discriminate between PDAC and AVC and within AVC subtypes. In particular, we found 22 exosomal miRNAs differently expressed between AVC and PDAC and 11 exosomal miRNAs between pancreatobiliary and intestinal AVC (Table 2). These signatures could represent an important bioresources for further validations aimed to discriminate between different lesions.
IPMN showed a wide histological spectrum ranging from benign adenomas to invasive carcinoma and represent an important precursor of aggressive pancreatic ductal adenocarcinoma. Since IPMN with an associated PDAC have a significantly worse prognosis compared to IPMN without PDAC and since the identification of this association is important especially prior the surgery [71], we investigated potential different exosomal miRNAs signature between these two entities identifying 13 differently expressed miRNAs between lesions (Table 2).
Conclusion
In this study we showed that pancreatic lesions are characterized by specific exosomal miRNAs signatures. We also provided a bioresource for future explorations aimed to understand the biological and clinical relevance of such signatures.
Supplementary information
Additional file 1: Table S1. Summary of clinicopathological characteristics of pancreatic neoplasm patients.
Additional file 2: Figure S1. Quality and size analysis of pancreatic plasma-derived exosomes.
Additional file 3: Table S2. Deregulated miRNAs from Nanostring profiling associated to different pancreatic lesions.
Additional file 4: Table S3. Deregulated miRNAs from Nanostring profiling associated to biological and clinical different subtypes within the same histotype.
Acknowledgments
We thank Alexander Ridenour and Alex Cornwell from The Ohio State University Analytical Cytometry Shared Resources for the support with Nanosight analysis. We thank S. Grimaldi and G. Bonizzato from ARC-NET Research Centre for the support with the collection of materials.
Abbreviations
- PDAC
pancreatic ductal adenocarcinoma
- PanNET
pancreatic neuroendocrine tumors
- IPMN
intraductal papillary mucinous neoplasms
- AVC
ampulla of Vater carcinomas
- PanIN
pancreatic intraepithelial neoplasia
- MCN
mucinous cystic neoplasm
- CP
chronic pancreatitis
- Met. PDAC
metastatic PDAC
- Loc. PDAC
Localized PDAC
- PB AVC
pancreatobiliary AVC
- INT AVC
intestinal AVC
- IPMN-C
IPMN with associated invasive adenocarcinoma
Authors’ contributions
C.V., M.F., V.C., A.S., C.M.C., conceived the study. F.C., P.F., M.S., V.C., M. F, C. V designed the validation experiment. N.S. collected materials and clinical data. R.T.L. provided facilities. A.S., M.F., C.L. analyzed histopathological data. F.C., G.N., P.F. carried out NanoString profiling and raw data analysis. G. N, P.F. performed bioinformatic analyses. C.V., M.F., V.C., A.S., F. C, C.M.C. drafted the manuscript. All authors approved the final version of the manuscript.
Funding
This research was supported by Associazione Italiana Ricerca sul Cancro (AIRC, # 12182 to AS, and # 18718 to VC) and by NIH R35CA197706 and R01CA190740 to CMC in study design, data analysis, validation experiments design. The funding agencies had no role in the collection, analysis and interpretation of data and in the writing of the manuscript. NanoString nCounter and qRT-PCR analyses were performed at The Ohio State Genomics Shared Resources (GSR). All the Shared Resources at The Ohio State University that contributed to this paper were supported by the Cancer Center Support Grant P30CA016058.
Availability of data and materials
Supporting data and protocols are made available without restrictions from Prof. Aldo Scarpa.
Ethics approval and consent to participate
Materials used in the experiments have been collected under the Program 1885 with protocol 52438 approved on November 2010. The protocol includes written informed consent of the patient and were approved by the local ethics committee of the Integrated University Hospital Trust of Verona.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare. The corresponding author of the present manuscript and the co-author Dr. Claudio Luchini are Editorial Board Member of BMC Gastroenterology.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Caterina Vicentini and Federica Calore shared first authorship
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12876-020-01287-y.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY, Wang L, et al. Early detection of pancreatic cancer: where are we now and where are we going? Int J Cancer. 2017;141(2):231. doi: 10.1002/ijc.30670. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 4.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, et al. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63(5):318. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capelli P, Fassan M, Scarpa A. Pathology - grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26(6):705. doi: 10.1016/j.bpg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network Electronic address aadhe, Cancer Genome Atlas Research N. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32(2):185. doi: 10.1016/j.ccell.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scarpa A, Chang DK, Nones K, Corbo V, Patch AM, Bailey P, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017;543(7643):65. doi: 10.1038/nature21063. [DOI] [PubMed] [Google Scholar]
- 8.Becker AE, Hernandez YG, Frucht H, Lucas AL. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World J Gastroenterol. 2014;20(32):11182. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham A, Forsmark C. Chronic pancreatitis: review and update of etiology, risk factors, and management. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed]
- 10.Sadr-Azodi O, Oskarsson V, Discacciati A, Videhult P, Askling J, Ekbom A. Pancreatic Cancer following acute pancreatitis: a population-based matched cohort study. Am J Gastroenterol. 2018;113(11):1711. doi: 10.1038/s41395-018-0255-9. [DOI] [PubMed] [Google Scholar]
- 11.Riva G, Pea A, Pilati C, Fiadone G, Lawlor RT, Scarpa A, et al. Histo-molecular oncogenesis of pancreatic cancer: from precancerous lesions to invasive ductal adenocarcinoma. World J Gastrointest Oncol. 2018;10(10):317. doi: 10.4251/wjgo.v10.i10.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, et al. Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol. 2014;233(3):217. doi: 10.1002/path.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosoda W, Chianchiano P, Griffin JF, Pittman ME, Brosens LA, Noe M, et al. Genetic analyses of isolated high-grade pancreatic intraepithelial neoplasia (HG-PanIN) reveal paucity of alterations in TP53 and SMAD4. J Pathol. 2017;242(1):16. doi: 10.1002/path.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felsenstein M, Noe M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, et al. IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 2018;67(9):1652. doi: 10.1136/gutjnl-2017-315062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pea A, Riva G, Bernasconi R, Sereni E, Lawlor RT, Scarpa A, et al. Ampulla of Vater carcinoma: molecular landscape and clinical implications. World J Gastrointest Oncol. 2018;10(11):370. doi: 10.4251/wjgo.v10.i11.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Ahuja N, Makary MA, Cameron JL, Eckhauser FE, Choti MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16(1):83. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100(7):598. doi: 10.1002/jso.21374. [DOI] [PubMed] [Google Scholar]
- 18.Mafficini A, Amato E, Cataldo I, Rusev BC, Bertoncello L, Corbo V, et al. Ampulla of Vater carcinoma: sequencing analysis identifies TP53 status as a novel independent prognostic factor and potentially actionable ERBB, PI3K, and WNT pathways gene mutations. Ann Surg. 2018;267(1):149. doi: 10.1097/SLA.0000000000001999. [DOI] [PubMed] [Google Scholar]
- 19.Nikolaidis P, Hammond NA, Day K, Yaghmai V, Wood CG, 3rd, Mosbach DS, et al. Imaging features of benign and malignant ampullary and periampullary lesions. Radiographics. 2014;34(3):624. doi: 10.1148/rg.343125191. [DOI] [PubMed] [Google Scholar]
- 20.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Leva G, Croce CM. miRNA profiling of cancer. Curr Opin Genet Dev. 2013;23(1):3. doi: 10.1016/j.gde.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina PP, Slack FJ. microRNAs and cancer: an overview. Cell Cycle. 2008;7(16):2485. doi: 10.4161/cc.7.16.6453. [DOI] [PubMed] [Google Scholar]
- 23.Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, et al. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13(10):1668. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turchinovich A, Tonevitsky AG, Burwinkel B. Extracellular miRNA: a collision of two paradigms. Trends Biochem Sci. 2016;41(10):883. doi: 10.1016/j.tibs.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70(21):8259. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aucher A, Rudnicka D, Davis DM. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J Immunol. 2013;191(12):6250. doi: 10.4049/jimmunol.1301728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Li M, Wang H, Fisher WE, Lin PH, Yao Q, et al. Profiling of 95 microRNAs in pancreatic cancer cell lines and surgical specimens by real-time PCR analysis. World J Surg. 2009;33(4):698. doi: 10.1007/s00268-008-9833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18(4):981. doi: 10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Permuth-Wey J, Chen DT, Fulp WJ, Yoder SJ, Zhang Y, Georgeades C, et al. Plasma MicroRNAs as novel biomarkers for patients with Intraductal papillary mucinous neoplasms of the pancreas. Cancer Prev Res (Phila) 2015;8(9):826. doi: 10.1158/1940-6207.CAPR-15-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thorns C, Schurmann C, Gebauer N, Wallaschofski H, Kumpers C, Bernard V, et al. Global microRNA profiling of pancreatic neuroendocrine neoplasias. Anticancer Res. 2014;34(5):2249. [PubMed] [Google Scholar]
- 32.Sandhu V, Bowitz Lothe IM, Labori KJ, Lingjaerde OC, Buanes T, Dalsgaard AM, et al. Molecular signatures of mRNAs and miRNAs as prognostic biomarkers in pancreatobiliary and intestinal types of periampullary adenocarcinomas. Mol Oncol. 2015;9(4):758. doi: 10.1016/j.molonc.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casadei L, Calore F, Creighton CJ, Guescini M, Batte K, Iwenofu OH, et al. Exosome-derived miR-25-3p and miR-92a-3p stimulate Liposarcoma progression. Cancer Res. 2017;77(14):3846. doi: 10.1158/0008-5472.CAN-16-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drusco A, Fadda P, Nigita G, Fassan M, Bottoni A, Gardiman MP, et al. Circulating Micrornas Predict Survival of Patients with Tumors of Glial Origin. EBioMedicine. 2018;30:105. doi: 10.1016/j.ebiom.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B. 1995;57:289-300.
- 36.Carotenuto P, Fassan M, Pandolfo R, Lampis A, Vicentini C, Cascione L, et al. Wnt signalling modulates transcribed-ultraconserved regions in hepatobiliary cancers. Gut. 2017;66(7):1268. doi: 10.1136/gutjnl-2016-312278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berindan-Neagoe I, Monroig Pdel C, Pasculli B, Calin GA. MicroRNAome genome: a treasure for cancer diagnosis and therapy. CA Cancer J Clin. 2014;64(5):311. doi: 10.3322/caac.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollis M, Nair K, Vyas A, Chaturvedi LS, Gambhir S, Vyas D. MicroRNAs potential utility in colon cancer: early detection, prognosis, and chemosensitivity. World J Gastroenterol. 2015;21(27):8284. doi: 10.3748/wjg.v21.i27.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson NB, Morran DC, Morton JP, Ali A, Dickson EJ, Carter CR, et al. MicroRNA molecular profiles associated with diagnosis, clinicopathologic criteria, and overall survival in patients with resectable pancreatic ductal adenocarcinoma. Clin Cancer Res. 2012;18(2):534. doi: 10.1158/1078-0432.CCR-11-0679. [DOI] [PubMed] [Google Scholar]
- 40.Giovannetti E, Funel N, Peters GJ, Del Chiaro M, Erozenci LA, Vasile E, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528. doi: 10.1158/0008-5472.CAN-09-4467. [DOI] [PubMed] [Google Scholar]
- 41.Papaconstantinou IG, Manta A, Gazouli M, Lyberopoulou A, Lykoudis PM, Polymeneas G, et al. Expression of microRNAs in patients with pancreatic cancer and its prognostic significance. Pancreas. 2013;42(1):67. doi: 10.1097/MPA.0b013e3182592ba7. [DOI] [PubMed] [Google Scholar]
- 42.Giovannetti E, van der Velde A, Funel N, Vasile E, Perrone V, Leon LG, et al. High-throughput microRNA (miRNAs) arrays unravel the prognostic role of MiR-211 in pancreatic cancer. PLoS One. 2012;7(11):e49145. doi: 10.1371/journal.pone.0049145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vila-Navarro E, Vila-Casadesus M, Moreira L, Duran-Sanchon S, Sinha R, Gines A, et al. MicroRNAs for detection of pancreatic Neoplasia: biomarker discovery by next-generation sequencing and validation in 2 independent cohorts. Ann Surg. 2017;265(6):1226. doi: 10.1097/SLA.0000000000001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA. 2014;311(4):392. doi: 10.1001/jama.2013.284664. [DOI] [PubMed] [Google Scholar]
- 45.Ganepola GA, Rutledge JR, Suman P, Yiengpruksawan A, Chang DH. Novel blood-based microRNA biomarker panel for early diagnosis of pancreatic cancer. World J Gastrointest Oncol. 2014;6(1):22. doi: 10.4251/wjgo.v6.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Raimondo M, Guha S, Chen J, Diao L, Dong X, et al. Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic Cancer. J Cancer. 2014;5(8):696. doi: 10.7150/jca.10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58(3):610. doi: 10.1373/clinchem.2011.172767. [DOI] [PubMed] [Google Scholar]
- 48.Kong X, Du Y, Wang G, Gao J, Gong Y, Li L, et al. Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: miR-196a could be a potential marker for poor prognosis. Dig Dis Sci. 2011;56(2):602. doi: 10.1007/s10620-010-1285-3. [DOI] [PubMed] [Google Scholar]
- 49.Kishikawa T, Otsuka M, Ohno M, Yoshikawa T, Takata A, Koike K. Circulating RNAs as new biomarkers for detecting pancreatic cancer. World J Gastroenterol. 2015;21(28):8527. doi: 10.3748/wjg.v21.i28.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruebel K, Leontovich AA, Stilling GA, Zhang S, Righi A, Jin L, et al. MicroRNA expression in ileal carcinoid tumors: downregulation of microRNA-133a with tumor progression. Mod Pathol. 2010;23(3):367. doi: 10.1038/modpathol.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24(29):4677. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 52.Li SC, Essaghir A, Martijn C, Lloyd RV, Demoulin JB, Oberg K, et al. Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol. 2013;26(5):685. doi: 10.1038/modpathol.2012.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Permuth-Wey J, Chen YA, Fisher K, McCarthy S, Qu X, Lloyd MC, et al. A genome-wide investigation of microRNA expression identifies biologically-meaningful microRNAs that distinguish between high-risk and low-risk intraductal papillary mucinous neoplasms of the pancreas. PLoS One. 2015;10(1):e0116869. doi: 10.1371/journal.pone.0116869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lubezky N, Loewenstein S, Ben-Haim M, Brazowski E, Marmor S, Pasmanik-Chor M, et al. MicroRNA expression signatures in intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2013;153(5):663. doi: 10.1016/j.surg.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 55.Caponi S, Funel N, Frampton AE, Mosca F, Santarpia L, Van der Velde AG, et al. The good, the bad and the ugly: a tale of miR-101, miR-21 and miR-155 in pancreatic intraductal papillary mucinous neoplasms. Ann Oncol. 2013;24(3):734. doi: 10.1093/annonc/mds513. [DOI] [PubMed] [Google Scholar]
- 56.Matthaei H, Schulick RD, Hruban RH, Maitra A. Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2011;8(3):141. doi: 10.1038/nrgastro.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murali Manohar K, Sasikala M, Kvsrr Y, Sunil V, Talukdar R, Murthy H, et al. Plasma microRNA192 in combination with serum CA19-9 as non-invasive prognostic biomarker in periampullary carcinoma. Tumour Biol. 2017;39(3):1010428317695018. doi: 10.1177/1010428317695018. [DOI] [PubMed] [Google Scholar]
- 58.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20(24):7864. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed]
- 60.Ge Q, Zhou Y, Lu J, Bai Y, Xie X, Lu Z. miRNA in plasma exosome is stable under different storage conditions. Molecules. 2014;19(2):1568. doi: 10.3390/molecules19021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng J, Yu L, Chen W, Lu X, Fan X. Circulating exosomal microRNAs reveal the mechanism of Fructus Meliae Toosendan-induced liver injury in mice. Sci Rep. 2018;8(1):2832. doi: 10.1038/s41598-018-21113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9(4):e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 64.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 65.Armstrong DA, Nymon AB, Ringelberg CS, Lesseur C, Hazlett HF, Howard L, et al. Pulmonary microRNA profiling: implications in upper lobe predominant lung disease. Clin Epigenetics. 2017;9:56. doi: 10.1186/s13148-017-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamura A, Rampersaud YR, Sharma A, Lewis SJ, Wu B, Datta P, et al. Identification of microRNA-181a-5p and microRNA-4454 as mediators of facet cartilage degeneration. JCI Insight. 2016;1(12):e86820. doi: 10.1172/jci.insight.86820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, et al. Connective tissue growth factor (CCN2) and microRNA-21 are components of a positive feedback loop in pancreatic stellate cells (PSC) during chronic pancreatitis and are exported in PSC-derived exosomes. J Cell Commun Signal. 2014;8(2):147. doi: 10.1007/s12079-014-0220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, et al. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. doi: 10.1186/1471-2407-8-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou H, Schaefer N, Wolff M, Fischer HP. Carcinoma of the ampulla of Vater: comparative histologic/immunohistochemical classification and follow-up. Am J Surg Pathol. 2004;28(7):875. doi: 10.1097/00000478-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Sohn TA, Yeo CJ, Cameron JL, Hruban RH, Fukushima N, Campbell KA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239(6):788. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of clinicopathological characteristics of pancreatic neoplasm patients.
Additional file 2: Figure S1. Quality and size analysis of pancreatic plasma-derived exosomes.
Additional file 3: Table S2. Deregulated miRNAs from Nanostring profiling associated to different pancreatic lesions.
Additional file 4: Table S3. Deregulated miRNAs from Nanostring profiling associated to biological and clinical different subtypes within the same histotype.
Data Availability Statement
Supporting data and protocols are made available without restrictions from Prof. Aldo Scarpa.