Abstract
Background
Despite recent improvements in the burden of cardiovascular disease (CVD) in the UK, deaths from CVD are relatively high compared with other high-income countries. An estimated 7 million people in the UK are living with CVD, and the healthcare cost is approximately £11 billion annually. In more than 90% of cases, the risk of a first heart attack is thought to be related to modifiable risk factors including smoking, poor diet, lipidemia, high blood pressure, inactivity, obesity and excess alcohol consumption. The aim of the study is to synthesise evidence for the comparative effectiveness and cost-effectiveness of different interventions for the primary prevention of CVD.
Methods
We will systematically search databases (for example, MEDLINE (Ovid), Embase (Ovid), Cochrane Library) and the reference lists of previous systematic reviews for randomised controlled trials that assess the effectiveness and cost-effectiveness of any form of intervention aimed at adult populations for the primary prevention of CVD, including but not limited to lipid lowering medications, blood pressure lowering medications, antiplatelet agents, nutritional supplements, dietary interventions, health promotion programmes, physical activity interventions or structural and policy interventions. Interventions may or may not be targeted at high-risk groups. Publications from any year will be considered for inclusion. The primary outcome will be all cause mortality. Secondary outcomes will be cardiovascular diseases related mortality, major cardiovascular events, coronary heart disease, incremental costs per quality-adjusted life years gained. If data permits, we will use network meta-analysis to compare and rank effectiveness of different interventions, and test effect modification of intervention effectiveness using subgroup analyses and meta-regression analyses.
Discussion
The results will be important for policymakers when making decisions between multiple possible alternative strategies to prevent CVD. Compared to results from existing multiple separate pairwise meta-analyses, this overarching synthesis of all relevant work will enhance decision-making. The findings will be crucial to inform evidence-based priorities and guidelines for policies and planning prevention strategies of CVD.
Systematic review registration
PROSPERO CRD42019123940.
Background
Cardiovascular disease (CVD) includes all the diseases of the heart and circulation including coronary heart disease (CHD) and stroke. CVD accounts for the highest proportion of non-communicable disease deaths, resulting in 160,000 deaths in the UK annually [1–3]. Cardiovascular risk is determined by a variety of ‘upstream’ factors (such as healthy food production and availability, access to a safe environment that encourages physical activity and access to health education) as well as ‘downstream’ behavioural issues (such as unhealthy diet, smoking and physical inactivity). In more than 90% of cases, the risk of a first heart attack is related to nine potentially modifiable risk factors [4, 5]: smoking/tobacco use, poor diet, high blood cholesterol, high blood pressure, high blood glucose, insufficient physical activity, overweight/obesity, diabetes, psychosocial stress and excess alcohol consumption. A significant proportion of CVD morbidity and mortality can be prevented through population strategies for primary prevention. There is a major potential population health impact of improving our understanding of CVD prevention.
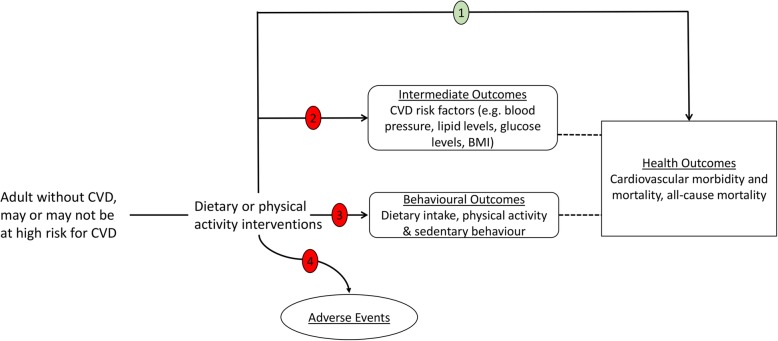
Though there are many pairwise systematic reviews and meta-analyses that have examined the effectiveness of drug, lifestyle and policy/structural interventions either separately and collectively (Additional file 1); there is no systematic review to date that has comprehensively synthesised all available evidence to understand the comparative effectiveness of these interventions for the primary prevention of CVD with the aim of supporting evidence-based recommendations to policymakers. The overarching aim of the proposed study is to fill this research gap by synthesising evidence for the comparative effectiveness of different interventions for the primary prevention of CVD using a network meta-analysis. The specific objectives are as follows: (1) to use comprehensive searches and to describe the scale and range of interventions that have been conducted and to categorise interventions and their components, (2) to determine which interventions, have the greatest probability of effectiveness for the primary prevention of CVD (see Fig. 1), (3) to identify which intervention components are associated with the greatest effectiveness for the primary prevention of CVD, (4) to examine reliability and conclusiveness of the available evidence on interventions for the primary prevention of CVD and to identify the areas with most potential benefit for future research, (5) to identify trial characteristics associated with prevention effect estimates, (6) to identify, appraise and synthesise any published economic evaluations and economic models of interventions for the primary prevention of CVD and (7) to determine the applicability and generalisability of interventions and the assessments of their cost-effectiveness to the UK NHS setting.
Fig. 1.
Analytic framework Note: pathway 1 will be systematically reviewed (in green), while pathways 2, 3 and 4 will not be reviewed (in red)
Methods
The present protocol has been registered within the PROSPERO database (registration number CRD420119123940) and is reported in accordance with the Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) checklist [6], and the PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of healthcare interventions [7] (see checklist in Additional file 2). The record in PROSPERO and subsequent publications will be updated with any amendment made to the protocol.
Eligibility criteria
We will evaluate each identified study against the following selection criteria:
Study population: adult populations (≥18 years of age) included in population-based studies, which may or may not be targeted at moderate/high CVD risk groups (such as hypertension, obesity, hyperlipidaemia, type 2 diabetes or a combination of these). As the review focuses on the primary prevention of CVD, we will exclude trials that included those who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or per cutaneous transluminal coronary angioplasty (PTCA)), and those with angina or angiographically defined coronary heart disease (CHD). Studies with mixed populations, i.e. both population with and without CVD will be included if data for the relevant primary prevention can be extracted.
Intervention: any form of intervention aimed at the primary prevention of CVD, including but not limited to drugs (lipid lowering medications, blood pressure lowering medications, antiplatelet agents), diet (nutritional supplements, dietary interventions), physical activity or public health (health promotion programmes, structural and policy interventions) (Table 1).
Table 1.
Health technologies (interventions)
| Pharmacologic interventions | |||
|---|---|---|---|
| Lipid lowering medications | Blood pressure lowering medications | Nutritional supplements | Others |
|
Atorvastatin Fluvastatin Lovastatin Pitavastatin Pravastatin Rosuvastatin Fenofibrate Bezafibrate Ezetimibe |
ACE inhibitors Angiotensin receptor blockers (ARBs) Calcium channel blockers Thiazide diuretics Adrenergic receptor antagonists (alpha and beta blockers) Vasodilators Renin inhibitors |
Vitamin D, E, K and multivitamins Niacin Omega 3 and fatty acids Anti-oxidants Calcium Co-enzyme Q10 Selenium Folic acid Garlic |
Fixed dose combinations ‘polypill’ Antiplatelet agent (Aspirin) |
| Lifestyle-modification interventions | |||
| Dietary interventions | Health promotion | Exercise/physical activity in general | |
|
Mediterranean diet Fibres Nut consumption Chocolate Fruits and vegetables Green and black tea Reduced salt intake Reduced fat intake |
Smoking cessation Weight reduction Reduction in alcohol intake Multiple risk factors intervention Digital health promotion |
Endurance (or aerobic) exercise Strengthening exercise Balance Tai-chi Flexibility Yoga Aquatic Qiqong Transcendental meditation Combined exercise |
|
| Structural and policy-based interventions (population-wide interventions) | |||
|
Taxation and subsidies Mass media campaigns Food and menu labelling Local food environment Worksite wellness programmes Marketing restrictions Quality standards Healthy local environment Addressing air pollution |
|||
Comparators: other forms of intervention (such as a minimal intervention, active intervention, concomitant intervention), placebo, usual care or no intervention control group or wait list control.
Outcome measures: The primary outcome will be all cause mortality. Secondary outcomes will be cardiovascular diseases related mortality, major cardiovascular events (defined as fatal and non-fatal myocardial infarction, sudden cardiac death, revascularisation, fatal and non-fatal stroke and fatal and non-fatal heart failure), coronary heart disease (fatal and non-fatal myocardial infarction and sudden cardiac death, excluding silent myocardial infarction), incremental costs per quality-adjusted life years gained reported alongside a randomised trial.
Study design: randomised controlled trials (RCTs) of at least 6 months’ duration of follow-up. Units of randomisation could be either individuals or clusters (such as family, workplace).
Information sources and search strategy
Clinical effectiveness
Due to the likelihood of a high volume of relevant trials to be included, we will follow standard guidelines for integrating existing systematic reviews into new reviews [8, 9]. Where existing systematic reviews with acceptable search and study selection methods (especially Cochrane reviews) are available for any of the intervention categories, these will be used as a starting point to identify relevant studies. Initial searches for relevant systematic reviews will not be restricted by date. Searches will not be restricted by language.
a) A comprehensive literature search for existing systematic reviews will be developed iteratively and undertaken in major medical and health-related electronic bibliographic databases including MEDLINE (Ovid), Embase (Ovid), Cochrane Database of Systematic Reviews (Wiley) and DARE (CRD). Systematic reviews that potentially include primary studies meeting our inclusion criteria will be selected. Records for the included (and, if available, excluded) studies in all selected systematic reviews will be identified and imported into EndNote using HubMed Citation Finder [10], systematically de-duplicated and screened.
The most recent systematic review for any intervention or intervention category will be assessed using AMSTAR-2 [11] items 4, 5 and 7 to help determine whether or not the search and study identification methods are acceptable for the purpose of identifying studies for this network meta-analysis. If not, the next most recent review where available will be assessed using the same criteria. An analysis of the search dates of the chosen reviews will inform the date limit used for the search for more recent trials (i.e. those that are not yet included in a published review). The aim of this is to ensure all time periods are covered.
b) The search for recent trials will be developed iteratively and will be informed by records of a broad cross-section of known studies. Searching based on the concepts of prevention and CVD outcomes, or on intervention terms and CVD outcomes, will be considered and tested. Scoping searches have retrieved very high numbers of trials in Cochrane Controlled Register of Trials (CENTRAL) (Wiley) using either approach. Our trial search will focus on CENTRAL (Wiley). For any interventions where no acceptable systematic review is available, a search for trials with no date limit and including relevant intervention terms will be performed. A draft search strategy in CENTRAL (Wiley) is available in Additional file 3.
c) In order to capture studies with more obscure records and those that may have been excluded by previous systematic reviews, we will run a highly sensitive search with no date limit and use machine learning to identify a proportion of these records for screening (see ‘Selection Process’ below).
d) Finally, the reference lists of included studies will be examined for additional relevant studies.
Cost-effectiveness
We will develop searches iteratively, referring to known articles, existing strategies and assessed search filters [12]. Search terms will include economic, cost and health-related quality of life-related terms combined with CVD terms. Other concepts may be added as necessary. Databases will include the following: MEDLINE (Ovid), MEDLINE In-Process Citations and Daily Update (Ovid) and Embase (Ovid). Searches will be limited to studies in the English language, and to humans. We will also check the reference lists of included studies and any relevant reviews.
Selection process
In order to reduce the workload of screening the searches result from the highly sensitive search with no date limit, we will develop a bespoke classifier/algorithm to identify potentially relevant studies. We will aim to achieve a high-performing algorithm comparable to human screening [13]. The computer will be fed with training data using the included and excluded studies found via our other searches. From this algorithm, the machine can make predictions (include or exclude) on other titles and abstracts that it has never seen. We will screen the titles/abstracts of a small proportion 10% of these results.
Data collection process
Data will be independently extracted using a pre-specified piloted proforma by two reviewers, with discrepancies resolved by a third reviewer. We will use a data collection form for study characteristics and outcome data. One author will extract study characteristics from the included studies and a second author will spot-checked study characteristics for accuracy against the trial report. Any inconsistencies will be resolved by discussion.
We will extract the following characteristics:
Study citation: year(s) of study, registration number to trial registries, year of publication, location, setting, number of centres, sample size, diagnostic criteria, funding/sponsor
Methods: including study design (type of RCT), number of arms, risk of bias (see below)
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline measures of physiological functioning (e.g. cardiovascular function, blood pressure, body mass index, blood glucose, HbA1C, smoking history), inclusion and exclusion criteria
Interventions: intervention, comparison, concomitant medications and excluded medications
Outcomes: primary and secondary outcomes specified and collected, and time points reported including information on whether an intention to treat approach has been used and how it was defined
Data items and measurement of treatment effect
Clinical effectiveness
We will report dichotomous outcomes as risk ratios (RRs). For continuous outcomes, we will calculate mean differences (MDs) when the studies report homogenous outcomes. Time-to-event outcomes or generic inverse variance outcomes will be expressed as the logarithm of hazard ratio (HR).
If possible, we will use the intention-to-treat population for all analyses. When effect sizes are incompletely reported, we will contact the corresponding author. When the SDs of absolute changes from baseline are not available from individual trials, we will impute them as described in detail in the Cochrane Handbook [14]. In brief, we will assume a correlation of r = 0.5 between baseline and follow-up to estimate SD for change from baseline. Using the imputed correlation coefficient values, we will calculate SDs for the change from baseline for the studies with missing SDs using the following formula:
We will include cluster-randomised trials in the meta-analysis along with individually randomised trials (unit of analysis issues). Cluster-randomised trials will be labelled with a (C). For cluster-randomised trials to be included in the network meta-analyses, we will adjust for design effect using an ‘approximation method’ [15] if the trial did not use a cluster-adjusting analytical strategy. The ‘approximation method’ entails calculation of an ‘effective sample size’ for the comparison groups by dividing the original sample size by the ‘design effect’, which is 1 + (M − 1) ICC, where M is the average cluster size and ICC is the intra-cluster correlation coefficient. For dichotomous data, we will divide both the number of participants and the number who experience the event by the same design effect, while for continuous data, only the sample size will be reduced (means and standard deviations (SDs) will be left unchanged).
Cost-effectiveness
For each identified study that meets the selection criteria, we will extract the following data: country, study design, population, intervention(s), comparator(s), type of economic analysis, perspective, model type (structure and key assumptions), time horizon, effectiveness data, primary outcome, resource use and unit cost data, price year, discounting and the results of the base-case and sensitivity analyses. Data such as outcomes and characteristics will be synthesised quantitatively, where appropriate or narratively. For the primary outcome, the preferred measure will be cost per quality-adjusted life years (QALY) gained.
Geometry of the network
A network plot will be created to describe and present the geometry of the intervention network of comparisons across trials [16]. Intervention comparisons not connected to the rest of the network will be excluded from the network meta-analysis and describe that comparison separately. In the network diagram, each node represents an intervention, and the edges represent head-to-head comparisons between a pair of interventions. The size of a node reflects the sample size for the intervention, and the thickness of an edge reflects the number of trials that included the comparison [16].
Risk of bias in individual studies
We will use the Cochrane Collaboration’s tool for assessing risk of bias for quality assessment of the included trials at trial level [17]. The trials were graded (unclear, high or low risk of bias) based on sequence generation, allocation concealment, blinding of outcome assessor, incomplete outcome data and selective outcome reporting. Risk of bias for the included trials will be assessed using the Robot Reviewer [18]. We will use the quality assessment of economic modelling checklist developed by Philips et al. [19] to assess the quality of the economic evaluation studies. We will use the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [20] to assess the quality of the economic evaluation studies
For cluster-randomised trials, we will assess the following cluster-specific risks of bias as outlined in the Cochrane Handbook for Systematic Reviews of Interventions [14].
Recruitment bias - whether the individuals participating in the trial were blinded to the type of cluster they were in before agreeing to participate
Baseline imbalance - whether there were differences in baseline characteristics between the randomised groups
Loss of clusters - whether any complete clusters were lost to follow-up and the reasons
Incorrect analysis - whether the proper statistical analysis was carried out for a cluster-randomised design
Comparability with individually randomised trials - whether the cluster-randomisation method could have resulted in different intervention effects than an individually randomised trial
When considering treatment effects, we will take into account the risk of bias of the studies that contribute to that outcome.
Planned methods of analysis
Characteristics of the included studies
We will produce descriptive statistics and study population characteristics across all eligible trials, describing the types of comparisons and other clinical or methodological variables, such as age, co-medication, country and study follow-up period.
Pairwise meta-analyses
In the first step we will perform series of conventional pairwise random-effects meta-analyses by combining studies that compared the same interventions, including the comparison between active treatments and the different control arms. If very few trials are available or the requirements of network meta-analysis are not met, it can be that network meta-analysis will not be appropriate and, in this case, conventional pairwise meta-analysis will be applied.
Assessment of heterogeneity
We will assess between-trials heterogeneity (variability in relative treatment effects within the same treatment comparison) using the tau-squared (the variance of the random effects distribution). The heterogeneity variance will be assumed common across the various treatment comparisons (grouped by comparison type) and we will compare the empirical distribution with predictive distributions [21–23]. We will explore the potential reasons for heterogeneity by subgroup analysis described below.
Assessment of the transitivity assumption
The transitivity assumption will be assessed by investigating the distribution of clinical and methodological variables that can act as effect modifiers across treatment comparisons [24].
We will assume that patients who fulfil the inclusion criteria are equally likely to be randomised to any of the interventions of interest (i.e. jointly randomisable). When additional evidence of intransitivity is lacking and potential effect modifiers have similar distributions across the included studies, network meta-analysis is likely to give valid results.
Network meta-analysis
We will conduct network meta-analyses [25, 26] to compare effectiveness of the different types of interventions for primary prevention of CVD. Given the substantial number of interventions and the limited evidence base available to construct the network of evidence, (in terms of both the number of trials and the number of direct comparisons between active interventions), we will use a two-level hierarchical network meta-analysis to borrow strength within the classes of intervention, strengthening inferences and potentially reducing the uncertainty around individual intervention effects. This will consequently increase our ability to rank these and to inform decision-making frameworks [27]. The two-level hierarchical network meta-analysis (level 1: intervention type and level 2: intervention class) will incorporate exchangeability between interventions of the same class to predict an effect estimate for each of the interventions individually [27].
We will calculate the probability of a given intervention having the largest beneficial effects as the proportion of simulations in which that intervention will be ranked as the ‘best’ according to the relative prevention effect estimate. In addition, we will calculate alternative rankings (second and third best, etc.) because in some policy and practice areas, the best intervention might be unavailable, too costly or contraindicated. Probability values will be summarised and reported as surface under the cumulative ranking (SUCRA) and graphically ranked using rankograms. SUCRA = 1 if an intervention always ranks first and SUCRA = 0 if it always ranks last.
All the network meta-analyses analyses [25, 26] will be conducted using a Markov chain Monte Carlo method. The models will account for the correlation between prevention effect estimates induced by three or more arm trials using the multi-arm trial code. At least two Markov chains will be run simultaneously using different arbitrary initial values. Convergence to a stable solution will be checked by viewing plots of the sampled simulations and using the Brooks-Gelman-Rubin diagnostic tool. All results will be reported as posterior medians of prevention effect estimate with corresponding 95% credible intervals (CrIs). Credible intervals are the Bayesian equivalent of classical confidence intervals. A 95% CrI can be interpreted as a 95% probability that the parameter takes a value in the specified range.
Assessment of inconsistency
The evaluation of transitivity will be supplemented with a statistical evaluation of consistency, the agreement between direct and indirect evidence. We will evaluate consistencies between direct and indirect comparisons in the network of evidence using the method of ‘node-splitting’ [28], by calculating the difference for each pair of interventions and the probability of whether direct estimates surpass the indirect estimate.
Investigation of heterogeneity and inconsistency
We anticipate several sources of heterogeneity relating to the content of the intervention and study design. We will test effect modification of intervention effectiveness using subgroup analyses and meta-regression analyses. For example, where there are sufficient data, we will stratify our analyses (subgroup) by the following: population risk groups (healthy versus high-risk), trial period (older versus recent), sex (male versus female) and age (young adult versus elderly population), by intervention components and by characteristics of outcome measures. Meta-regression analyses will be used to explore components of interventions, participant characteristics and outcome measure characteristics that can predict prevention effect estimates within and across different types of interventions. The network meta-regression will be performed by allowing for a common treatment-covariate interaction for each intervention in the network meta-analysis [29].
Publication bias
To assess small study effects and publication bias, we will use funnel plots of pairwise meta-analyses if 10 or more studies are included. We will also use a comparison-adjusted funnel plot for relative treatment effects between all active and control interventions [30].
Systematic review of cost-effectiveness studies
In order to make different incremental cost-effectiveness ratios (ICERs) comparable, we will convert them from their currencies to pounds sterling (£) using purchasing power parities [31]. Once converted to pounds sterling, the cost data will be inflated to 2019/20 prices using the Hospital and Community Health Services index [32]. For studies that do not report price year, the incremental cost-effectiveness ratios will be converted to pounds sterling using the published year of study as the assumed price year.
Statistical software
The analysis and presentation of results will be performed using R (meta, netmeta and BUGSnet packages) [33–36].
Assessment of the confidence in the evidence from NMA
The confidence in the relative treatment effect estimated in network meta-analysis for the primary outcome will be evaluated using the Confidence in Network Meta-Analysis framework [37], implemented in the web application. This tool evaluates the credibility of the findings across the domains of within-study bias, across-study bias, indirectness, imprecision, heterogeneity and incoherence.
Discussion
Despite recent improvements in the burden of cardiovascular disease in the UK, deaths from cardiovascular disease are relatively high compared with other high-income countries. There is a major potential population health impact of improving our understanding of CVD prevention. Identifying the most effective intervention however remains a challenge for researchers and policymakers.
This study will provide highly relevant findings using innovative methods to determine optimal strategies for primary prevention of CVD. The results will be important for policymakers when making decisions between the multiple possible alternative strategies to prevent CVD. Compared to results from the existing multiple separate pairwise meta-analyses, this overarching summary of all relevant work will enhance decision-making. The findings will be crucial to inform evidence-based priorities and guidelines for policies and planning prevention strategies. The public will have answers to which intervention or combination of interventions has the greatest probability of being most effective and most cost-effective in preventing CVD. Findings from our study will inform research funders and improve the targeting of subsequent research, and evaluation of additional population interventions.
Given the paucity of randomised evidence on population-wide interventions, this project will assess data from observational studies which are more prone to potential bias. We will mitigate this by using Bayesian approach to coherently synthesise evidence from both randomised and non-randomised evidence, which will take into account the potential bias in the observational studies using an extra variance component.
Findings from this study will be internationally relevant. Our findings will be widely shared with academics (as journal articles); policymakers (such as The National Institute for Health and Care Excellence), stakeholders and key organisations such as the British Heart Foundation (executive summaries) and patient groups (lay summaries).
Supplementary information
Additional file 1. List of existing of existing systematic reviews
Additional file 3. Cochrane CENTRAL search strategy
Acknowledgements
Not applicable.
Abbreviations
- AMSTAR
A MeaSurement Tool to Assess systematic Reviews
- CABG
Coronary artery bypass grafting
- CEA
Cost-effectiveness analysis
- CHD
Coronary heart disease
- CHEERS
Consolidated Health Economic Evaluation Reporting Standards
- CRD
Centre for Reviews and Dissemination
- CVD
Cardiovascular disease
- HR
Hazard ratio
- HTA
Health technology assessment
- ICC
Intra-cluster correlation
- ICER
Incremental cost-effectiveness ratio
- MD
Mean difference
- MI
Myocardial infarction
- NHS
National Health Scheme
- NIHR
National Institute for Health Research
- NMA
Network meta-analysis
- PTCA
Percutaneous transluminal coronary angioplasty
- QALY
Quality-adjusted life year
- RCT
Randomised controlled trial
- RR
Risk ratio
- SD
Standard deviation
- SUCRA
Surface under the cumulative ranking
- TSA
Trial Sequential Analysis
Authors’ contributions
OAU and AC developed the initial review concept. OAU, LA, CUN, RC, HM, GJMT, STP and AC developed the review design further. All authors drafted the manuscript, and all authors read, contributed to, and approved the final draft.
Funding
This project was funded by the NIHR HTA (17/148/05). This research was supported by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care West Midlands (NIHR CLAHRC WM), now recommissioned as NIHR Applied Research Collaboration West Midlands. Sian Taylor-Phillips is supported by an NIHR Career Development Fellowship (CDF – 2016-09-018. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Availability of data and materials
Not applicable since all data that are referred to in this article will have been obtained through reading original studies or contacting the authors of cited studies.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Olalekan A. Uthman, Email: olalekan.uthman@warwick.ac.uk
Lena Al-Khudairy, Email: Lena.al-Khudairy@warwick.ac.uk.
Chidozie U. Nduka, Email: C.Nduka@warwick.ac.uk
Rachel Court, Email: R.A.Court@warwick.ac.uk.
Hema Mistry, Email: Hema.Mistry@warwick.ac.uk.
G . J. Melendez-Torres, Email: G.J.Melendez-Torres@exeter.ac.uk
Sian Taylor-Phillips, Email: S.Taylor-Phillips@warwick.ac.uk.
Aileen Clarke, Email: Aileen.Clarke@warwick.ac.uk.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13643-020-01366-x.
References
- 1.Steel N, Ford JA, Newton JN, Davis ACJ, Vos T, Naghavi M, Glenn S, Hughes A, Dalton AM, Stockton D, et al. Changes in health in the countries of the UK and 150 English Local Authority areas 1990-2016: a systematic analysis for the Global Burden of Disease Study. Lancet (London, England) 2018. 2016;392(10158):1647–1661. doi: 10.1016/S0140-6736(18)32207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatnagar P, Wickramasinghe K, Wilkins E, Townsend N. Trends in the epidemiology of cardiovascular disease in the UK. Heart (British Cardiac Society) 2016;102(24):1945–1952. doi: 10.1136/heartjnl-2016-309573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatnagar P, Wickramasinghe K, Williams J, Rayner M, Townsend N. The epidemiology of cardiovascular disease in the UK. Heart (British Cardiac Society) 2015. 2014;101(15):1182–1189. doi: 10.1136/heartjnl-2015-307516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S et al: Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet (London, England) 2016, 388(10046):761-775. [DOI] [PubMed]
- 5.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet (London, England) 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, Group P-P Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ (Clinical research ed) 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 7.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 8.Robinson KA, Whitlock EP, Oneil ME, Anderson JK, Hartling L, Dryden DM, Butler M, Newberry SJ, McPheeters M, Berkman ND, et al. Integration of existing systematic reviews into new reviews: identification of guidance needs. Systematic reviews. 2014;3:60. doi: 10.1186/2046-4053-3-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson KA, Chou R, Berkman ND, Newberry SJ, Fu R, Hartling L, Dryden D, Butler M, Foisy M, Anderson J, et al. Twelve recommendations for integrating existing systematic reviews into new reviews: EPC guidance. J Clin Epidemiol. 2016;70:38–44. doi: 10.1016/j.jclinepi.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Eaton AD: HubMed: a web-based biomedical literature search interface. Nucleic Acids Res 2006, 34(Web Server issue):W745-747. [DOI] [PMC free article] [PubMed]
- 11.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical research ed) 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glanville J, Kaunelis D, Mensinkai S. How well do search filters perform in identifying economic evaluations in MEDLINE and EMBASE. International journal of technology assessment in health care. 2009;25(4):522–529. doi: 10.1017/S0266462309990523. [DOI] [PubMed] [Google Scholar]
- 13.Marshall IJ, Wallace BC. Toward systematic review automation: a practical guide to using machine learning tools in research synthesis. Systematic reviews. 2019;8(1):163. doi: 10.1186/s13643-019-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. 2. Chichester (UK): John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Deeks JJ, Altman DG: Chapter 16: Special topics in statistics. . In: Cochrane Handbook for Systematic Reviews of Interventions Version 510 [updated March 2011]. edn. Edited by Higgins JPT, Green S. Oxford, UK: The Cochrane Collaboration; 2011.
- 16.Tonin FS, Borba HH, Mendes AM, Wiens A, Fernandez-Llimos F, Pontarolo R. Description of network meta-analysis geometry: a metrics design study. PLoS One. 2019;14(2):e0212650. doi: 10.1371/journal.pone.0212650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed) 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall IJ, Kuiper J, Wallace BC. RobotReviewer: evaluation of a system for automatically assessing bias in clinical trials. J Am Med Inform Assoc. 2016;23(1):193–201. doi: 10.1093/jamia/ocv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, Woolacoot N, Glanville J: Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health technology assessment (Winchester, England) 2004, 8(36):iii-iv, ix-xi, 1-158. [DOI] [PubMed]
- 20.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, Loder E. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ (Clinical research ed) 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes KM, Turner RM, Higgins JP. Empirical evidence about inconsistency among studies in a pair-wise meta-analysis. Res Synth Methods. 2016;7(4):346–370. doi: 10.1002/jrsm.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes KM, Turner RM, White IR, Jackson D, Spiegelhalter DJ, Higgins JP. Implementing informative priors for heterogeneity in meta-analysis using meta-regression and pseudo data. Stat Med. 2016;35(29):5495–5511. doi: 10.1002/sim.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41(3):818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. doi: 10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ (Clinical research ed) 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in medicine. 2004;23(20):3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 27.Owen RK, Tincello DG, Keith RA. Network meta-analysis: development of a three-level hierarchical modeling approach incorporating dose-related constraints. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18(1):116–126. doi: 10.1016/j.jval.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Statistics in medicine. 2010;29(7-8):932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 29.Dias S, Sutton AJ, Welton NJ, Ades AE. Evidence synthesis for decision making 3: heterogeneity--subgroups, meta-regression, bias, and bias-adjustment. Med Decis Making. 2013;33(5):618–640. doi: 10.1177/0272989X13485157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Synth Methods. 2012;3(2):161–176. doi: 10.1002/jrsm.57. [DOI] [PubMed] [Google Scholar]
- 31.Prices and purchasing power parities (PPP) [http://www.oecd.org/sdd/prices-ppp/].
- 32.Curtis L, Burns A: Unit costs of health and social care. Kent, UK: Personal Social Services Research Unit (PSSRU), University of Kent; 2018.
- 33.Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of Bayesian network meta-analyses. BMC medical research methodology. 2019;19(1):196. doi: 10.1186/s12874-019-0829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rücker G, Petropoulou M, Schwarzer G. Network meta-analysis of multicomponent interventions. Biom J. 2019. [DOI] [PMC free article] [PubMed]
- 35.Rücker G, Schwarzer G. Automated drawing of network plots in network meta-analysis. Res Synth Methods. 2016;7(1):94–107. doi: 10.1002/jrsm.1143. [DOI] [PubMed] [Google Scholar]
- 36.Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019;41:e2019013. doi: 10.4178/epih.e2019013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of existing of existing systematic reviews
Additional file 3. Cochrane CENTRAL search strategy
Data Availability Statement
Not applicable since all data that are referred to in this article will have been obtained through reading original studies or contacting the authors of cited studies.