Abstract
Background
Bacterial gas vesicles, composed of two major gas vesicle proteins and filled with gas, are a unique class of intracellular bubble-like nanostructures. They provide buoyancy for cells, and thus play an essential role in the growth and survival of aquatic and soil microbes. Moreover, the gas vesicle could be applied to multimodal and noninvasive biological imaging as a potential nanoscale contrast agent. To date, cylinder-shaped gas vesicles have been found in several strains of cyanobacteria. However, whether the functional gas vesicles could be produced in the model filamentous cyanobacteria Anabaena sp. PCC 7120 remains controversial.
Results
In this study, we found that an intact gvp gene cluster indeed exists in the model filamentous cyanobacteria Anabaena sp. PCC 7120. Real-time PCR assays showed that the gvpA gene is constitutively transcribed in vivo, and its expression level is upregulated at low light intensity and/or high growth temperature. Functional expression of this intact gvp gene cluster enables the recombinant Escherichia coli to gain the capability of floatation in the liquid medium, thanks to the assembly of irregular gas vesicles. Furthermore, crystal structure of GvpF in combination with enzymatic activity assays of GvpN suggested that these two auxiliary proteins of gas vesicle are structurally and enzymatically conserved, respectively.
Conclusions
Our findings show that the laboratory strain of model filamentous cyanobacteria Anabaena sp. PCC 7120 possesses an intact but partially degenerated gas vesicle gene cluster, indicating that the natural isolate might be able to produce gas vesicles under some given environmental stimuli for better floatation.
Keywords: Gas vesicle, Cyanobacteria, Natural isolate, Heterologous expression, Crystal structure, ATPase activity
Background
Gas vesicles (GVs), a unique class of intracellular bubble-like nanostructures, are found in many aquatic and soil microbes including halophilic archaea, photosynthetic bacteria, and heterotrophic bacteria [1]. Ambient gases could freely diffuse into and out of GVs, whereas water is impermeable, making the GV a gas-filled organelle [2, 3]. GVs could regulate the buoyancy of microbial cells, enabling the vertical floatation to an appropriate depth in aqueous environments for a better access of oxygen, light and even nutrients [4]. As an organelle composed of only proteins, GV adopts a spindle-shaped cylinder with conical end caps, usually of 45 ~ 250 nm in width and 100 ~ 2000 nm in length [5]. The unique physical properties allow GVs to serve as a potential nanoscale contrast agent for ultrasound and magnetic resonance imaging, which yields multimodal and noninvasive biological imaging with high spatial and temporal resolution [6].
As previously reported, formation of GVs is related to a conserved cluster of 8 ~ 14 genes (termed gas vesicle protein gene cluster, or gvp gene cluster for short), encoding two major structural proteins and several essential minor components that might putatively function as chaperones, nucleators and regulators [2, 5, 7]. The primary structural protein GvpA and the external scaffold protein GvpC constitute the 2-nm-thick outer amphiphilic shell of the GV [2, 5, 8]. GvpA, a 7.5-kDa highly conserved and hydrophobic protein, assembles into tandem arrays that form 4.6-nm-wide characteristic ribs running nearly perpendicular to the long axis of the GV [9, 10]. Notably, most cyanobacteria possess multiple copies of gvpA gene, for example, two in Calothrix sp. [11], three in Microcystis aeruginosa [12] and five in Anabaena flos-aquae [13]. In contrast, GvpC is a less-abundant, not conserved, and highly hydrophilic protein [14]. GvpC usually contains a number of conserved 33-residue repeating motif (33RR), and functions to connect GvpA molecules in the same and/or adjacent ribs to strengthen and stabilize the shell of GV [15]. In vitro experiments demonstrated that removal of GvpC leads to a three-fold decrease of the critical collapse pressure of GVs, whereas addition of GvpC helps GVs to restore normal strength [16, 17]. In addition, GvpF is reported to be a structural protein localized at the inner surface of GVs [18].
To date, a series of cyanobacteria have been found to produce GVs, such as A. flos-aquae, Calothrix sp. PCC 7601, M. aeruginosa PCC 7806, Oscillatoria sp. 6412, Pseudanabaena, Nostoc sp. 6705 [12, 19]. Notably, filamentous cyanobacteria Calothrix and Nostoc can differentiate hormogonia upon environmental stimuli, the process of which is characterized by the formation of GVs [2, 20]. Despite the laboratory strain of model filamentous cyanobacteria Anabaena sp. PCC 7120 fails in differentiating hormogonia [19, 21], it remains unknown whether the natural isolate could differentiate hormogonia and produce GVs. Here we found that Anabaena sp. PCC 7120 possesses an intact gvp gene cluster, which shares an organization similar to that of previously identified GV-forming cyanobacteria. The results of real-time PCR showed that gvpA is constitutively transcribed in vivo, and its expression level could be augmented at an altered light intensity and growth temperature. The complete gvp gene cluster could be heterologously expressed and assembled into irregular GVs in Escherichia coli. Moreover, structural combined with enzymatic investigations suggested that GvpF and GvpN are structurally and enzymatically conserved, respectively. These findings indicated that the natural isolate of Anabaena sp. PCC 7120 is most likely able to produce GVs under some given environmental stimuli.
Results
Organization and conservation of the gvp genes in Anabaena sp. PCC 7120
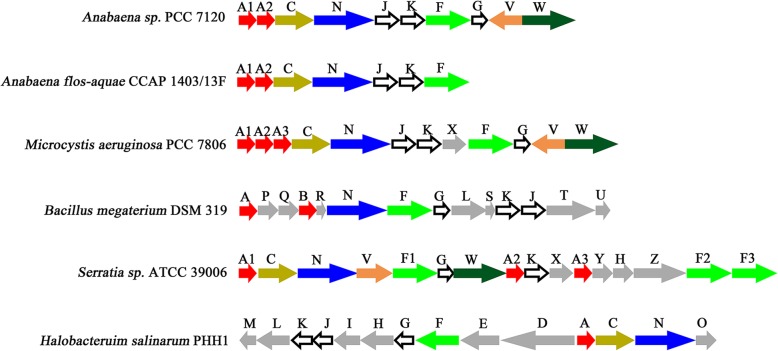
The entire genomic sequence of the model filamentous nitrogen-fixing cyanobacteria Anabaena sp. PCC 7120 was reported in 2001, which consists of a single circular genome of 6,413,771 bp and six plasmids [22]. Eight out of the 5368 putative open reading frames in the genome were annotated as gvp genes: gvpA, gvpB, gvpC, gvpN, gvpJ, gvpK, gvpF and gvpG, without annotations of gvpV and gvpW compared to some other gvp gene clusters. Using BlastP program, we found that the proteins encoded by alr2246 and alr2245, two genes at the downstream of gvpG, share a sequence similarity of 62% and 65% to GvpV and GvpW of M. aeruginosa PCC 7806, respectively. Thus we assigned alr2246 and alr2245 to gvpV and gvpW, respectively (Fig. 1). It suggested that Anabaena sp. PCC 7120 possesses an intact gvp gene cluster, which shares a gene organization similar to that in the previously reported GV-forming cyanobacteria, such as A. flos-aquae and M. aeruginosa PCC 7806. Notably, most of the gvp genes in GV-forming Haloarchaea and other bacteria are highly conserved [5], despite the gene organizations vary a lot (Fig. 1).
Fig. 1.
Organizations of the gvp gene cluster from different species of bacteria. Each alphabet above the arrow represents a gvp gene. Transcription direction of each gene is indicated by the arrow. The gvp genes absent in Anabaena sp. PCC 7120 are shown as grey arrows
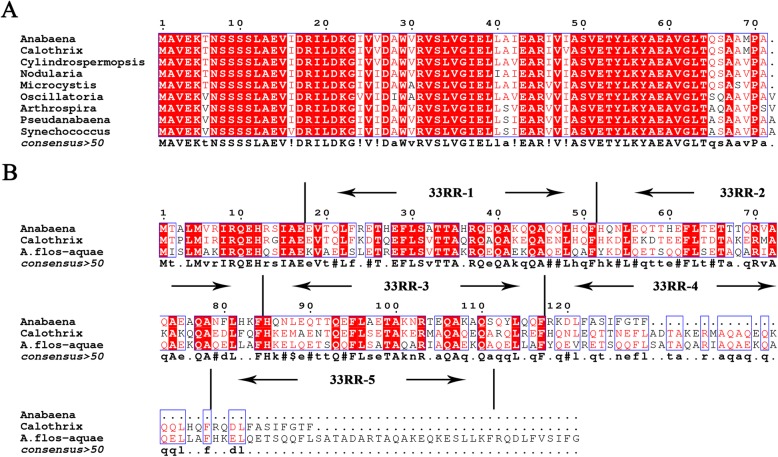
Multiple-sequence alignment showed that gvpB is nearly identical to gvpA in the gvp gene cluster of Anabaena sp. PCC 7120, suggesting that gvpB is an isoform of gvpA. Accordingly, gvpA and gvpB should be re-annotated to gvpA1 and gvpA2, respectively (Fig. 1). Moreover, GvpA of Anabaena sp. PCC 7120 shares a sequence similarity to those of other cyanobacterial strains up to 90% (Fig. 2a), indicating that the primary structural protein GvpA exhibits a rarely high conservativity in cyanobacteria. Further sequence analysis revealed that the external scaffold protein GvpC of Anabaena sp. PCC 7120 contains only three conserved 33RRs (Fig. 2b), which probably result in GVs of smaller diameter. In fact, a previous report revealed that A. flos-aquae GVs with a GvpC of five 33RRs have a larger diameter compared to those of Calothrix sp. PCC 7601 with a GvpC of four 33RRs [23].
Fig. 2.
Conservativity of GvpA and GvpC. a Multiple-sequence alignment of GvpA from different cyanobacterial strains. The alignment was performed with the program Multalin. All sequences were downloaded from the NCBI database (www.ncbi.nlm.nih.gov) with the following accession numbers: Anabaena sp. PCC 7120, WP_010996411; Calothrix sp. PCC 7103, WP_011316976; Nodularia spumigena CCY9414, AHJ27872; Microcystis aeruginosa PCC 7806, WP_084989880; Cylindrospermopsis raciborskii, WP_057178839; Oscillatoria sp. PCC 10802, WP_017721733; Arthrospira platensis, WP_006616598; Pseudanabaena sp. SR411, WP_009626980; Synechococcus sp. PCC 7502, WP_015167036. b Multiple-sequence alignment shows the 33RRs of GvpC. All sequences were downloaded from the NCBI database with the following accession numbers: Anabaena sp. PCC 7120, WP_010996410; A. flos-aquae, AAA58710; Calothrix sp. PCC 7103, EKF01074
The gvpA gene is upregulated at low light intensity and high temperature
Considering that the transcription of gvp genes is the prerequisite of GV formation, we investigated whether the gvpA gene, encoding the major structural component of GV, could be transcribed in vivo. The total RNA was extracted from Anabaena sp. PCC 7120 cells grown at a light intensity of 2000 lux at 28 °C, and applied to real-time PCR assays. The results showed that an expected fragment of gvpA could be detected (Fig. 3), suggesting that the gvpA gene is constitutively transcribed in Anabaena sp. PCC 7120 under normal laboratory growth condition.
Fig. 3.
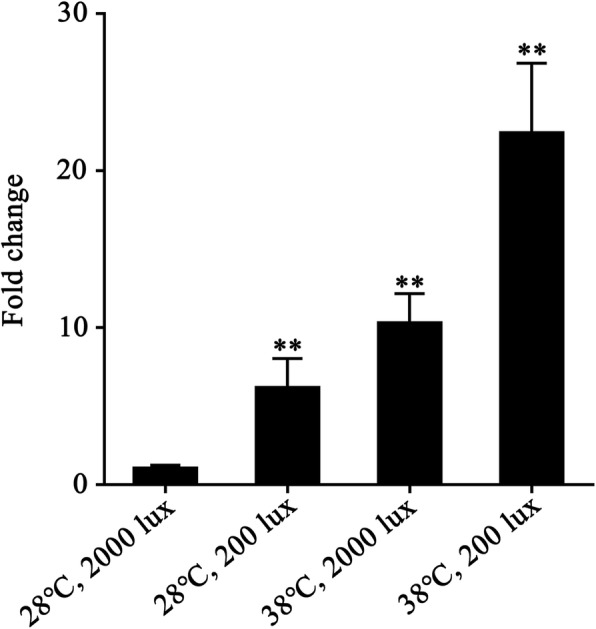
Expression levels of gvpA under different growth conditions detected by real-time PCR. Each histogram represents the average value of triplicate experiments, and a two-tailed Student’s t test was used for the comparison of statistical significance (**P < 0.01)
Afterwards, we shifted Anabaena sp. PCC 7120 cells to various external stimuli and detected the expression profiles of gvpA gene. The expression level of gvpA in Anabaena sp. PCC 7120 upon a single stimulus of low light intensity at 200 lux or high temperature at 38 °C were elevated to 6 and 11 folds, respectively (Fig. 3), compared to the constitutive expression level. Moreover, when Anabaena sp. PCC 7120 cells were grown under the condition of double stimuli of both low light intensity and high temperature, the expression level of gvpA was upregulated approximately 23 folds (Fig. 3). Considering that GvpA is the primary structural component of GV, we speculated that prototype GVs could be produced in Anabaena sp. PCC 7120 at some given conditions. However, we failed in observing the floatation of Anabaena sp. PCC 7120 cells in response to the above double stimuli. It implied that mature and functional GVs do not exist in the laboratory strain of Anabaena sp. PCC 7120, in consistence with its incapability of differentiating hormogonia.
Expression of the gvp gene cluster of Anabaena sp. PCC 7120 in E. coli
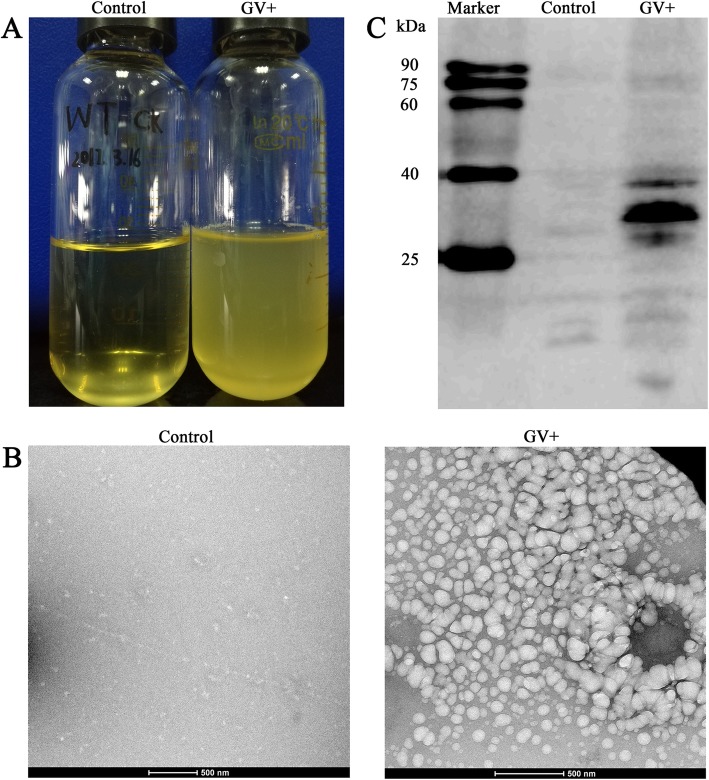
The gvp genes of Anabaena sp. PCC 7120 were constructed in the expression vectors and then transformed to E. coli cells. Interestingly, we observed that the E. coli cells transformed with the recombinant gvp plasmids exhibit a buoyancy phenotype, whereas the cells carrying the control vectors sink to the bottom of the tube (Fig. 4a). Then, the turbidity measurements showed that the upper fraction of the culture medium of the experimental group has an absorbance of 0.57 at 600 nm, compared to 0.02 of the control. The results suggested that GVs might be produced in the recombinant E. coli cells that harbor the gvp gene cluster of Anabaena sp. PCC 7120.
Fig. 4.
Expression of the gvp gene cluster of Anabaena sp. PCC 7120 in E. coli. a Photographs of E. coli cells transformed with recombinant gvp plasmids and control vectors, respectively. b Negative-staining electron microscopy images of the putative gas vesicles purified from E. coli cells expressing the gvp genes (right) and the control vectors (left). c Western blot of the purified gas vesicles. The probe is anti-His antibody. The prestained protein standards are displayed in the lane marked Marker and their molecular masses are indicated in kDa. The lanes marked control and GV+ indicate the control and the experimental group, respectively
Afterwards, the recombinant GVs were purified from E. coli cells and applied to transmission electron microscopy, following a previously reported protocol [18]. The image displayed a large number of round and oval bubble-like structures (Fig. 4b), similar to those irregular GVs via expressing Bacillus megaterium gvp gene cluster in E. coli [24]. In contrast, similar bubble-like structures were absent from the E. coli cells that expressing the control vectors (Fig. 4b). Western blot assays further showed that the purified GVs possess enriched His-tagged GvpA proteins (Fig. 4c). Altogether, transformation of the gvp gene cluster of Anabaena sp. PCC 7120 enabled E. coli to display a buoyancy phenotype, because of the assembly of GVs, indicating that Anabaena sp. PCC 7120 possesses an intact gvp gene cluster capable of heterogeneously producing irregular but functional GVs.
The crystal structure of GvpF
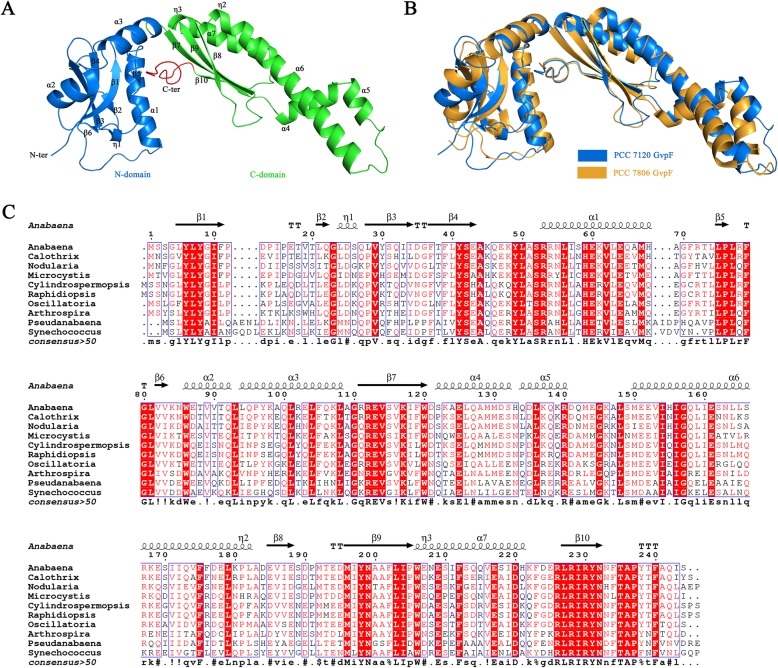
To further investigate the putative GVs of Anabaena sp. PCC 7120 from the structural point of view, all Gvp proteins, except for GvpA and GvpC, were successfully overexpressed, purified and applied to crystal screening; however, only the crystal structure of GvpF was eventually solved at 2.55 Å resolution in the space group C2221 (Table 1). Anabaena sp. PCC 7120 GvpF is composed of two structurally separated domains, both of which display a fold in α + β class, followed by a C-terminal tail inserted into the middle area of the two domains (Fig. 5a). Further structural analysis showed that the N-domain of GvpF displays an architecture in which a six-stranded β-sheet (β1-β6) is sandwiched by two α-helices (α1-α2) and the helix η1, whereas the C-domain adopts a modified ferredoxin fold owing to an extension region consisting of three consecutive helices (α4, α5 and the N-terminal segment of α6) (Fig. 5a). Moreover, the additional C-terminal tail provides an interface for the N-domain and C-domain to pack against each other, resulting in the structural stability and correct folding of GvpF (Fig. 5a).
Table 1.
Crystal parameters, data collection, and structure refinement
| GvpF | |
|---|---|
| Data collection | |
| Space group | C2221 |
| Unit cell parameters | |
| a, b, c (Å) | 96.40, 147.09, 106.58 |
| α, β, γ (°) | 90.00, 90.00, 90.00 |
| Resolution range (Å) | 50.00–2.55 (2.62–2.55)a |
| Unique reflections | 24, 900 (2, 440) |
| Completeness (%) | 99.9 (100) |
| <I/σ(I)> | 16.3 (2.9) |
| Rmergeb (%) | 10.6 (69.1) |
| Average redundancy | 10.9 (10.9) |
| Structure refinement | |
| Resolution range (Å) | 44.46–2.55 |
| Rfactorc/Rfreed (%) | 19.57/25.61 |
| Number of protein atoms | 3, 962 |
| Number of water atoms | 28 |
| RMSDe bond lengths (Å) | 0.015 |
| RMSD bond angles (°) | 1.709 |
| Mean B factors (Å2) | 53.189 |
| Ramachandran plot (residues, %)f | |
| Most favored | 95.80 |
| Allowed | 4.00 |
| Outliers | 0.20 |
| Protein Data Bank entry | 6L5D |
a Highest resolution shell is shown in parenthesis. Rsym = ΣhΣi|Ih,i − Ih|/ΣhΣiIh,i, where Ih is the mean intensity of the i observations of symmetry related reflections of h. R = Σ|Fobs − Fcalc|/ΣFobs, where Fobs = Fp, and Fcalc is the calculated protein structure factor from the atomic model. RMSD in bond lengths and angles are the deviations from ideal values
Fig. 5.
Crystal structure of GvpF. a Cartoon representation of the overall structure. The N-domain, C-domain and C-terminal tail are colored in blue, green and red, respectively. The secondary structural elements are labeled sequentially. b Structural superposition of Anabaena sp. PCC 7120 GvpF (blue) on M. aeruginosa PCC 7806 GvpF (orange; PDB ID: 4QSG). c Multiple-sequence alignment of GvpF from different cyanobacterial strains. The alignment was performed with the program Multalin. The corresponding secondary structural elements of GvpF are displayed above the sequences. All sequences were downloaded from the NCBI database with the following accession numbers: Anabaena sp. PCC 7120, BAB73947; Calothrix sp. PCC 7103, WP_019492269; Nodularia spumigena CCY9414, EAW43904; Microcystis aeruginosa PCC 7806, CAE11906; Cylindrospermopsis raciborskii, WP_085729041; Raphidiopsis brookii D9, EFA73417; Oscillatoria sp. PCC 10802, WP_017721720; Arthrospira platensis, WP_006616264; Pseudanabaena sp. SR411, WP_094529688; Synechococcus sp. PCC 7502, AFY73702
DALI search [25] revealed that Anabaena sp. PCC 7120 GvpF shares a high structural homology to the previously reported GvpF of M. aeruginosa PCC 7806 (PDB code: 4QSG, Z score 27.5, sequence identity 67%), with a root-mean-square deviation of 1.8 Å over 238 Cα atoms. Superposition of the two structures showed a very similar structure, except that GvpF of Anabaena sp. PCC 7120 possesses a shorter helix α5 in the C-domain (Fig. 5b). Besides, structure-based multiple-sequence alignment revealed that GvpF proteins are highly conserved among diverse species of cyanobacteria (Fig. 5c). It indicated that Anabaena sp. PCC 7120 GvpF might also function as a structural protein involved in forming GVs as that of M. aeruginosa PCC 7806 [18].
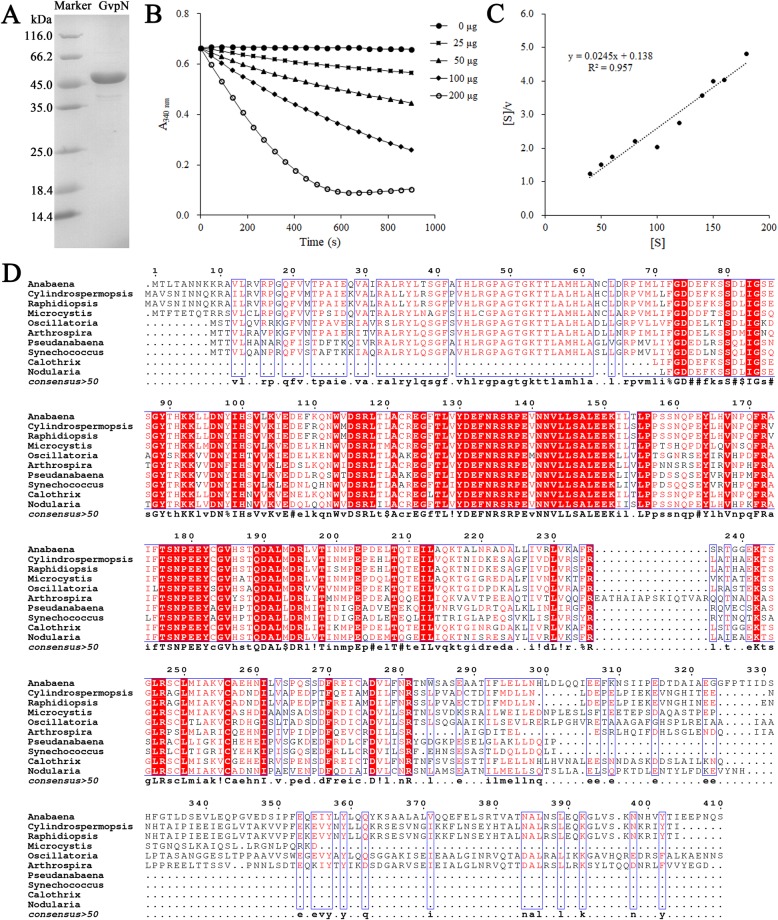
GvpN is an active ATPase
Sequence analysis against the Pfam database [26] showed that Anabaena sp. PCC 7120 GvpN contains an ATPases Associated with various cellular Activities (AAA) domain at the N-terminus, which was previously classified in the AAA+ protein superfamily of the ring-shaped P-loop NTPases [27]. Therefore, the recombinant GvpN of Anabaena sp. PCC 7120 was overexpressed in E. coli and purified (Fig. 6a), which was applied to the ATPase activity assays. Upon the addition of recombinant GvpN, the substrate ATP was gradually hydrolyzed over time (Fig. 6b). Upon the increase of GvpN added to the reaction, the ATP was hydrolyzed at a higher rate (Fig. 6b), suggesting that GvpN indeed possesses the ATPase activity. Using the Hanes-Woolf plot method (Fig. 6c), we determined the Michaelis-Menten parameters of GvpN towards ATP at a Km of 3.9 ± 1.5 μM, kcat of 35 ± 2 s− 1 and kcat/Km of 8.97 s− 1 μM− 1. Moreover, multiple-sequence alignment showed that the AAA domain of GvpN is highly conserved among different cyanobacterial species (Fig. 6d), indicating that the ATPase activity is a common feature of GvpN. In fact, a previous report showed that deletion of GvpN in Serratia sp. ATCC 39006 led to small bicone-shaped GVs and lack of cell buoyancy [28]. All together, it suggested that Anabaena sp. PCC 7120 possesses an enzymatically active GvpN, which might be necessary for the formation of mature GVs.
Fig. 6.
GvpN is an active ATPase. a SDS-PAGE of the purified GvpN protein. b The enzymatic profiles of GvpN. The final amounts of recombinant GvpN in the 200-μL system are 0, 25, 50, 100 and 200 μg, respectively. The decrease in absorbance at 340 nm was monitored using a DU800 spectrophotometer. c The Hanes-Woolf plot of GvpN. d Multiple-sequence alignment of GvpN from different species of cyanobacteria. The alignment was performed with the program Multalin. All sequences were downloaded from the NCBI database with the following accession numbers: Anabaena sp. PCC 7120, WP_010996409; Calothrix sp. PCC 7103, WP_019492266; Nodularia spumigena CCY9414, AHJ27875; Microcystis aeruginosa PCC 7806, WP_002747926; Cylindrospermopsis raciborskii, WP_061547066; Raphidiopsis brookii D9, EFA73420; Oscillatoria sp. PCC 10802, WP_017715028; Arthrospira platensis, WP_006616595; Pseudanabaena sp. SR411, WP_094529416; Synechococcus sp. PCC 7502, WP_015167038
Discussion
GVs play an essential role in the survival of prokaryotic species, and thus should be assembled or disassembled properly at the right time in response to diverse external stimuli. Actually, the formation of GVs or the expression of genes encoding Gvp proteins are affected by various environmental stimuli, such as temperature, light intensity, oxygen supply, pH and salinity, cell density and carbon source [5, 29–32]. For the cyanobacteria A. flos-aquae, Calothrix sp. PCC 7601 and Microcystis sp. BC 84/1, low light intensity could induce more GVs in order to enable the cells to move towards the surface of the aqueous habitat [5]. Mlouka and colleagues found that lack of nutrition, especially CO2 and light irradiance, leads to an augmented production of GVs in M. aeruginosa [12]. Moreover, for enterobacteria Serratia sp. ATCC39006, the formation of GVs depends on cell density under the control of quorum-sensing signals, and is responsive to oxygen shortage, resulting in facilitating the buoyancy of cells [28, 33]. In addition, for haloarchaea, two regulatory proteins GvpD and GvpE were shown to be involved in regulating the expression of gvp genes at both transcriptional and translational level [34]. Altogether, the formation of GVs is necessary for some bacteria in response to various environmental conditions.
The model filamentous and heterocyst-forming cyanobacteria Anabaena sp. PCC 7120 were isolated from the Lake Michigan in the late 1960s [22]. Theoretically, the environmental stimuli mentioned above should probably induce the formation of GV in Anabaena sp. PCC 7120, which possesses the gvp gene cluster. However, no one has observed GVs in the past 50 years of studying the physiology of this model organism. In this study, we found that Anabaena sp. PCC 7120 has an intact gvp gene cluster similar to those GV-forming cyanobacterial strains (Fig. 1). In fact, A. flos-aquae and M. aeruginosa PCC 7806 were proved to be able to produce cylinder-shaped GVs, the formation of which was regulated by light intensity [2, 12]. It is most likely that Anabaena sp. PCC 7120 is also capable of forming GVs under some given environmental stimuli. Indeed, the primary structural gene gvpA of Anabaena sp. PCC 7120 is constitutively transcribed, and could be upregulated at low light intensity and high temperature (Fig. 3). Heterologous expression of the intact Anabaena sp. PCC 7120 gvp gene cluster enabled E. coli cells to gain the capacity of floatation, thanks to the formation of irregular GVs (Fig. 4). However, we failed in either setting up a reproducible procedure to enable the floatation of Anabaena sp. PCC 7120, or seeing GVs inside the cells, or purifying GVs from this laboratory strain, after extensive trials of various stimuli and combinations. It indicated that the gvp gene cluster of Anabaena sp. PCC 7120 was partially degenerated in the 50-year laboratory culture.
Notably, formation of GVs is a key feature accompanied with the differentiation of hormogonia, which has already been proved in filamentous cyanobacteria Calothrix and Nostoc [2, 20]. Recently, Gonzalez and colleagues reported that hormogonia differentiation is regulated by a hierarchal sigma factor cascade in the filamentous cyanobacteria Nostoc punctiforme, which retain the developmental complexity of natural isolates [21]. In detail, the sigma factor sigJ activates the expression of both sigC and sigF genes, as well as other hormogonium-specific genes; meanwhile, sigJ controls the transcription of gvpA gene via binding at the − 10 region, which is a consensus sigJ-dependent promoter (designated as J-Box, GGGaAtacT) [21]. However, we found that the highly conserved GGG stretch of J-Box was mutated to AGC at the upstream promoter region of gvpA in the laboratory strain of Anabaena sp. PCC 7120, which might result in the altered binding affinity towards sigJ, and eventually the failure of producing functional GVs. It indicated that the natural isolate of Anabaena sp. PCC 7120, without the mutations at the regulatory region of gvp gene cluster, might be capable of differentiating hormogonia and producing GVs for better floatation in response to some given environmental stimuli. More investigations including comparative genomics analyses might help us to clearly elucidate which mutations in the present laboratory strain of Anabaena PCC 7120 lead to the loss of function.
Conclusions
In this study we demonstrated that the laboratory model filamentous cyanobacterium Anabaena sp. PCC 7120 indeed possesses an intact but partially degenerated gene cluster encoding gas vesicles, which gives us the hint that its natural isolate was most likely able to produce GVs under some given environmental stimuli. Owing to the fast growth and non-toxicity of the model strain sp. PCC 7120, investigations that enable the large production of GVs in this strain will benefit the potential application of GVs in biological imaging.
Methods
RNA extraction and real-time PCR
The Anabaena sp. PCC 7120 cells were grown at 28 °C under a light intensity of 2000 lux (supplied from top) with a 12/12 photoperiod in BG-11 medium to an OD730nm of 0.8, and then induced with 200 lux light intensity, 38 °C and both for 24 h, respectively. The stressors were selected according to a previous report summarizing the environmental conditions that could induce the formation of gas vesicles [5]. The cells were harvested by centrifugation and washed twice with the PBS buffer. The total RNA was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The residual genomic DNA was removed by RNase-free DNase (Takara, Shiga, Japan) at 37 °C for 2 h. PCR assays were conducted to confirm the absence of genomic DNA contamination. The RNA quality was checked by agarose gel electrophoresis. The cDNA synthesis was carried out by reverse transcription using the PrimeScript™ RT reagent Kit (Takara, Shiga, Japan).
For real-time PCR, amplification was performed using the FastStart universal SYBR Green Master (Roche, Basel, Switzerland) with the StepOne™ Real-Time PCR System (Applied Biosystems, Carlsbad, USA). The primers for rnpB are 5′-GCGATTATCTATCTGGGACG and 5′-CAACTCTTGGTAAGGGTGC, whereas those for gvpA are 5′-TGGCAGAAGTTATTGACC and 5′-GAGAAACACGTACCCAAG. Notably, the rnpB gene encoding RNaseP subunit B was used as the internal reference gene according to previous real-time PCR experiments concerning cyanobacteria [35]. The PCR conditions were as follows: 1 cycle at 95 °C for 10 min, 40 cycles at 95 °C for 15 s, 60 °C for 60 s, and 72 °C for 20 s; then the melting curve stage was performed rising from 60 °C to 95 °C by every 0.3 °C. The transcription ratios of gvpA to rnpB were calculated using the relative quantification analysis module of 2-∆∆Ct method based on Ct values [36]. All real-time PCR experiments were performed in triplicate.
Buoyancy tests
The gvpABC, gvpNJKFG and gvpVW genes of Anabaena sp. PCC 7120 were amplified and cloned into pET-Duet, pET-28a and pCDFDuet-1 vectors (with different antibiotic markers), respectively. Notably, a His-tag was fused to the N-terminus of GvpA for detecting the expression of the gene cluster. Next, the three gvp recombinant plasmids were co-overexpressed in E. coli BL21 (DE3) strain. Cells were grown in liquid LB broth, induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) for 4 h at 37 °C, and resuspended in 35-mm-diameter test tubes. Then, the tubes were undisturbed at room temperature for about 24 h, at which time the cell buoyancy was determined by the turbidity of the upper fraction of the culture medium. The cells transformed with the empty vectors without gvp gene cluster were used as the control.
GV isolation, electron microscopy and western blot
According to a previously described protocol [18], GVs were purified from E. coli cells co-overexpressing the three recombinant plasmids that cover the complete gvp gene cluster. Carbon-coated copper grids (300-mesh) were immersed in the purified GVs for 1 min and excess liquid was removed with filter paper. GVs were negatively stained with 2% (w/v) uranyl acetate and then examined with a Tecnai G2 transmission electron microscopy (FEI, USA) running at 120 kV voltage. Images were taken using a CCD camera attached to the microscopy. The purified GVs were mixed with an equal volume of 2 × sample-loading buffer (100 mM Tris-HCl, pH 6.8, 4% SDS, 20% glycerol, 2% β-mercaptoethanol, 0.2% bromophenol blue), boiled for 10 min, and then applied to western blot using anti-His polyclonal antibodies.
Cloning, expression and purification of GvpF and GvpN
The coding region of gvpF was amplified from the genomic DNA of Anabaena sp. PCC 7120, and cloned into a modified pET-29a vector with a C-terminal 6 × His-tag. The E. coli BL21 (DE3) strain was used for the overexpression of recombinant protein. The transformed cells were grown at 37 °C until OD600 nm reached 0.8 and then induced with 0.2 mM IPTG for another 20 h at 16 °C. Cells were harvested by centrifugation (6000×g, 4 °C, 10 min) and resuspended in the lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl). After 10 min of sonication on ice and 30 min of centrifugation at 12,000×g, the supernatant was loaded onto a Ni-NTA column (GE Healthcare, Chicago, USA) equilibrated with the binding buffer, the same as the lysis buffer. The target protein was eluted with 300 mM imidazole and further applied to a Superdex 200 column (GE Healthcare, Chicago, USA) pre-equilibrated with the binding buffer. Fractions containing the target protein were collected and concentrated to 10 mg/mL for crystallization.
GvpN was expressed and purified in the same manner as GvpF. Samples for ATPase activity assays were collected at the highest peak fractions without concentration and stored at − 80 °C with 50% glycerol.
Crystallization, data collection and structure determination
Crystals of GvpF were grown at 16 °C using the hanging drop vapor diffusion method, with a drop of 1 μL protein solution mixed with an equal volume of the reservoir solution. Crystals were obtained against the reservoir solution of 20% (w/v) polyethylene glycol 4000, 0.2 M NaCl, and 0.1 M Tris-HCl, pH 8.0. Then, they were pooled and flash cooled with liquid nitrogen after transferring to cryoprotectant (reservoir solution supplemented with 30% sucrose). The X-ray diffraction data were collected at 100 K using beamline BL17U with an EIGER X 16 M detector at the Shanghai Synchrotron Radiation Facility.
The diffraction data were indexed, integrated and scaled with HKL-2000 to the highest resolution of 2.55 Å. The structure of M. aeruginosa PCC 7806 GvpF (PDB code: 4QSG) was used as the search model to determine the structure of Anabaena sp. PCC 7120 GvpF by molecular replacement using the Molrep program [37] in the CCP4i program suite [38]. Further refinement was performed by programs REFMAC5 [39] and COOT [40]. The final model was evaluated with MolProbity [41]. Crystallographic parameters and data-collection statistics are listed in Table 1. All structure figures were prepared with PyMOL (https://pymol.org).
ATPase activity assays of GvpN
The ATPase activity of GvpN was measured using an ATP/NADH coupled assay [42], in which the decrease of NADH is proportional to the rate of steady-state ATP hydrolysis. The reaction mixture contains 50 mM Tris-HCl, pH 8.0, 20 mM KCl, 5 mM MgCl2, 2.5 mM ATP, 1 mM phosphoenolpyruvate, 0.1 mM NADH, 12 U/mL pyruvate kinase (Sigma, Saint Louis, USA) and 12 U/mL lactate dehydrogenase (Sigma, Saint Louis, USA). The reaction was initiated by the addition of recombinant GvpN, final amounts of which in a 200-μL system are 0, 25, 50, 100 and 200 μg, respectively. Using a DU800 spectrophotometer (Beckman Coulter, Fullerton, USA), the decrease in absorbance at 340 nm was monitored at 25 °C at 45 s intervals for 15 min. Michaelis-Menten parameters of GvpN were calculated from the data at the concentration of NADH varying from 40 to 200 μM and in the presence of 50 μg GvpN using the Hanes-Woolf plot method.
Acknowledgements
We appreciate the assistance of the staff at the Shanghai Synchrotron Radiation Facility (SSRF) and the Core Facility Center for Life Sciences at University of Science and Technology of China.
Authors′ contributions
CZZ and QL conceived and designed the experiments; KC and YW performed the experiments; KC and YLJ solved and refined the structure; KC and BYX performed the ATPase activity assays; KC, YC, CZZ and QL analyzed the data; CZZ and QL wrote and revised the manuscript. All authors read and approved the final manuscript.
Abbreviations
- GV
Gas vesicle
- gvp gene cluster
Gas vesicle protein gene cluster
- 33RR
33-Residue repeating motif
- IPTG
isopropyl β-D-1-thiogalactopyranoside
- AAA protein
ATPases Associated with various cellular Activities protein.
Funding
This work is supported by the National Natural Science Foundation of China (Grant No. 31500602), the Ministry of Science and Technology of China (Grant No. 2016YFA0400900), National Synchrotron Radiation Laboratory (Grant No. UN2018LHJJ) and Chongqing Research Program of Basic Research and Frontier Technology (Grant No. cstc2015jcyjBX0142). The funding bodies had no role in study design, data collection, analysis and interpretation, and in writing of the manuscript.
Availability of data and materials
Structural coordinate of Anabaena sp. PCC 7120 GvpF has been deposited in the Protein Data Bank (https://www.rcsb.org/) under the accession number of 6L5D. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cong-Zhao Zhou and Qiong Li contributed equally to this work.
References
- 1.Walsby AE. Structure and function of gas vacuoles. Bacteriol Rev. 1972;36(1):1–32. doi: 10.1128/MMBR.36.1.1-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsby AE. Gas Vesicles. Microbiol Rev. 1994;58(1):94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, et al. Biogenic gas nanostructures as ultrasonic molecular reporters. Nat Nanotechnol. 2014;9(4):311–316. doi: 10.1038/nnano.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsby AE. Buoyancy-providing role of gas vacuoles in an aerobic bacterium. Arch Microbiol. 1976;109(1–2):135–142. doi: 10.1007/BF00425125. [DOI] [Google Scholar]
- 5.Pfeifer F. Distribution, formation and regulation of gas vesicles. Nat Rev Microbiol. 2012;10(10):705–715. doi: 10.1038/nrmicro2834. [DOI] [PubMed] [Google Scholar]
- 6.Lakshmanan A, Lu GJ, Farhadi A, Nety SP, Kunth M, Lee-Gosselin A, et al. Preparation of biogenic gas vesicle nanostructures for use as contrast agents for ultrasound and MRI. Nat Protoc. 2017;12(10):2050–2080. doi: 10.1038/nprot.2017.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinsman R, Hayes PK. Genes encoding proteins homologous to halobacterial Gvps N, J, K, F & L are located downstream of gvpC in the cyanobacterium Anabaena flos-aquae. DNA Seq. 1997;7(2):97–106. doi: 10.3109/10425179709020156. [DOI] [PubMed] [Google Scholar]
- 8.Englert C, Pfeifer F. Analysis of gas vesicle gene expression in Haloferax mediterranei reveals that GvpA and GvpC are both gas vesicle structural proteins. J Biol Chem. 1993;268(13):9329–9336. [PubMed] [Google Scholar]
- 9.Beard SJ, Hayes PK, Pfeifer F, Walsby AE. The sequence of the major gas vesicle protein, GvpA, influences the width and strength of halobacterial gas vesicles. FEMS Microbiol Lett. 2002;213(2):149–157. doi: 10.1111/j.1574-6968.2002.tb11299.x. [DOI] [PubMed] [Google Scholar]
- 10.Walsby AE, Hayes PK. Gas vesicle proteins. Biochem J. 1989;264(2):313–322. doi: 10.1042/bj2640313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damerval T, Houmard J, Guglielmi G, Csiszar K. Tandeau de Marsac N. A developmentally regulated gvpABC operon is involved in the formation of gas vesicles in the cyanobacterium Calothrix 7601. Gene. 1987;54(1):83–92. [DOI] [PubMed]
- 12.Mlouka A, Comte K, Castets AM, Bouchier C. Tandeau de Marsac N. The gas vesicle gene cluster from Microcystis aeruginosa and DNA rearrangements that lead to loss of cell buoyancy. J Bacteriol. 2004;186(8):2355–65. [DOI] [PMC free article] [PubMed]
- 13.Hayes PK, Powell RS. The gvpA/C cluster of Anabaena flos-aquae has multiple copies of a gene encoding GvpA. Arch Microbiol. 1995;164(1):50–57. doi: 10.1007/BF02568734. [DOI] [PubMed] [Google Scholar]
- 14.Walsby AE, Hayes PK. The minor cyanobacterial gas vesicle protein, Gvpc, is attached to the outer surface of the gas vesicle. J Gen Microbiol. 1988;134:2647–2657. [Google Scholar]
- 15.Hayes PK, Buchholz B, Walsby AE. Gas vesicles are strengthened by the outer-surface protein. GvpC Arch Microbiol. 1992;157(3):229–234. doi: 10.1007/BF00245155. [DOI] [PubMed] [Google Scholar]
- 16.Kinsman R, Walsby AE, Hayes PK. GvpCs with reduced numbers of repeating sequence elements bind to and strengthen cyanobacterial gas vesicles. Mol Microbiol. 1995;17(1):147–154. doi: 10.1111/j.1365-2958.1995.mmi_17010147.x. [DOI] [PubMed] [Google Scholar]
- 17.Dunton PG, Mawby WJ, Shaw VA, Walsby AE. Analysis of tryptic digests indicates regions of GvpC that bind to gas vesicles of Anabaena flos-aquae. Microbiology. 2006;152(Pt 6):1661–1669. doi: 10.1099/mic.0.28755-0. [DOI] [PubMed] [Google Scholar]
- 18.Xu BY, Dai YN, Zhou K, Liu YT, Sun Q, Ren YM, et al. Structure of the gas vesicle protein GvpF from the cyanobacterium Microcystis aeruginosa. Acta Crystallogr D Biol Crystallogr. 2014;70(Pt 11):3013–3022. doi: 10.1107/S1399004714021312. [DOI] [PubMed] [Google Scholar]
- 19.Damerval T, Castets AM, Guglielmi G, Houmard J, Demarsac NT. Occurrence and distribution of gas vesicle genes among cyanobacteria. J Bacteriol. 1989;171(3):1445–1452. doi: 10.1128/JB.171.3.1445-1452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damerval T, Guglielmi G, Houmard J, De Marsac NT. Hormogonium differentiation in the cyanobacterium Calothrix: a photoregulated developmental process. Plant Cell. 1991;3(2):191–201. doi: 10.2307/3869288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez A, Riley KW, Harwood TV, Zuniga EG, Risser DD. A tripartite, hierarchical sigma factor cascade promotes hormogonium development in the filamentous cyanobacterium Nostoc punctiforme. Msphere. 2019;4(3):e00231-19. [DOI] [PMC free article] [PubMed]
- 22.Kaneko T, Nakamura Y, Wolk CP, Kuritz T, Sasamoto S, Watanabe A, et al. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001;8(5):205–213. doi: 10.1093/dnares/8.5.205. [DOI] [PubMed] [Google Scholar]
- 23.Dunton PG, Walsby AE. The diameter and critical collapse pressure of gas vesicles in Microcystis are correlated with GvpCs of different length. FEMS Microbiol Lett. 2005;247(1):37–43. doi: 10.1016/j.femsle.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Cannon MC. Gas vesicle genes identified in Bacillus megaterium and functional expression in Escherichia coli. J Bacteriol. 1998;180(9):2450–2458. doi: 10.1128/JB.180.9.2450-2458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holm L, Rosenstrom P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Gebali S, Mistry J, Bateman A, Eddy SR, Luciani A, Potter SC, et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019;47(D1):D427–DD32. doi: 10.1093/nar/gky995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 28.Tashiro Y, Monson RE, Ramsay JP, Salmond GPC. Molecular genetic and physical analysis of gas vesicles in buoyant enterobacteria. Environ Microbiol. 2016;18(4):1264–1276. doi: 10.1111/1462-2920.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao H, Zhu T, Xu M, Wang S, Xu XD, Kong RQ. pH-dependent gas vesicle formation in Microcystis. FEBS Lett. 2016;590(18):3195–3201. doi: 10.1002/1873-3468.12370. [DOI] [PubMed] [Google Scholar]
- 30.Englert C, Horne M, Pfeifer F. Expression of the major gas vesicle protein gene in the halophilic archaebacterium Haloferax Mediterranei is modulated by salt. Mol Gen Genet. 1990;222(2–3):225–232. doi: 10.1007/BF00633822. [DOI] [PubMed] [Google Scholar]
- 31.Hechler T, Frech M, Pfeifer F. Glucose inhibits the formation of gas vesicles in Haloferax volcanii transformants. Environ Microbiol. 2008;10(1):20–30. doi: 10.1111/j.1462-2920.2007.01426.x. [DOI] [PubMed] [Google Scholar]
- 32.DasSarma P, Zamora RC, Muller JA, DasSarma S. Genome-wide responses of the model archaeon halobacterium sp strain NRC-1 to oxygen limitation. J Bacteriol. 2012;194(20):5530–5537. doi: 10.1128/JB.01153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsay JP, Williamson NR, Spring DR, Salmond GPC. A quorum-sensing molecule acts as a morphogen controlling gas vesicle organelle biogenesis and adaptive flotation in an enterobacterium. P Natl Acad Sci USA. 2011;108(36):14932–14937. doi: 10.1073/pnas.1109169108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeifer F. Haloarchaea and the formation of gas vesicles. Life (Basel) 2015;5(1):385–402. doi: 10.3390/life5010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto F, Pacheco CC, Ferreira D, Moradas-Ferreira P, Tamagnini P. Selection of suitable reference genes for RT-qPCR analyses in cyanobacteria. Plos One. 2012;7(4):e34983. [DOI] [PMC free article] [PubMed]
- 36.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (−Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 37.Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J Appl Crystallogr. 1997;30:1022–1025. doi: 10.1107/S0021889897006766. [DOI] [Google Scholar]
- 38.Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 41.Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharschmidt BF, Keeffe EB, Blankenship NM, Ockner RK. Validation of a recording spectrophotometric method for measurement of membrane-associated mg- and NaK-ATPase activity. J Lab Clin Med. 1979;93(5):790–9. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Structural coordinate of Anabaena sp. PCC 7120 GvpF has been deposited in the Protein Data Bank (https://www.rcsb.org/) under the accession number of 6L5D. The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.