Abstract
Although information on efficacy and adverse drug reactions is lacking, ribavirin has been used empirically for the treatment of severe acute respiratory syndrome (SARS). We report common adverse events in 110 patients with suspected or probable SARS who were treated with ribavirin. Sixty-one percent of the patients had evidence of hemolytic anemia, and hypocalcemia and hypomagnesmia were reported in 58% and 46% of patients, respectively.
Severe acute respiratory syndrome (SARS) has affected >8000 people worldwide and has an estimated case-fatality rate of 5%–19% [1]. In view of its broad-spectrum antiviral activity, treatment with ribavirin was initiated empirically very early after the recognition of the SARS outbreak.
Ribavirin is a synthetic nucleoside antiviral agent with inhibitory activity against both DNA and RNA viruses [2]. Ribavirin, usually in an aerosolized form, has been used for the treatment of respiratory syncytial virus pneumonitis in both adults [3] and children [4]. The combination of oral ribavirin and IFN has been shown to be efficacious in the treatment of chronic hepatitis C [5]. High-dose intravenous ribavirin has been used in the treatment of Lassa fever and hemorrhagic fever with renal syndrome [6, 7].
Despite the extensive recent use of ribavirin for the treatment of SARS, there has yet to be a detailed report outlining common adverse events (AEs) temporally associated with the use of ribavirin in this context. We describe the common AEs that were observed in a large Canadian cohort of patients with suspected or probable SARS treated with ribavirin.
Methods. All patients with suspected or probable SARS who were admitted to 5 hospitals in Toronto, Canada, were reviewed for the development of possible AEs temporally associated with the initiation of ribavirin therapy. The following common AEs that developed or worsened in association with ribavirin treatment were identified: hemolytic anemia (HA; decrease in the hemoglobin level of >20 g/L plus 1 of the following events: an increase in the bilirubin level to 1.5 times the baseline value, a haptoglobin level of <0.38 g/L, or a reticulocyte count of >110 reticuloyctes per 1000 erythrocytes), hypomagnesemia (decrease from the baseline magnesium level to <0.7 mmol/L), and hypocalcemia (decrease from the baseline calcium level to <2.2 mmol/L, corrected for albumin). Other drugs used by these patients included antibiotics (e.g., levofloxacin or azithromycin, with or without ceftriaxone) and corticosteroids.
Risk factors associated with the development of each of the AEs were compared using the χ2 test for categorical variables and Student's t test for continuous variables with a level of significance of P < .05. Data are shown as mean ± SD.
Results. A total of 110 patients with suspected or probable SARS who were treated with ribavirin were identified from a database compiled during phase 1 of a Canadian outbreak [8]. In view of the fact that, early in the Canadian outbreak, ribavirin therapy was administered to patients who met the case definition for suspected or probable SARS, an appropriate control group of patients who had not received ribavirin was unavailable. The mean age of the patients was 45.7 ± 17.2 years (range, 17–99 years). There were 71 female patients and 39 male patients. All patients received concurrent antibiotics after the initial assessment, and 50% received corticosteroids at some point during the course of their illness.
A total of 67 patients (61%) had evidence of HA. Nineteen (28%) of these patients received a transfusion of ⩾1 U of packed RBCs. The decrease in the hemoglobin level was 42.1 ± 14.0 g/L (range, 21–82 g/L). A significant decrease (>20 g/L) in the hemoglobin level was seen 6.8 ± 2.7 days after starting ribavirin therapy, and the hemoglobin level reached a nadir 13.0 ± 4.1 days after starting ribavirin therapy. HA was significantly associated with higher doses of ribavirin (P = .005) and prolonged hospital stay (P = .001) ( table 1). Neither age nor sex affected the development of HA.
Table 1.
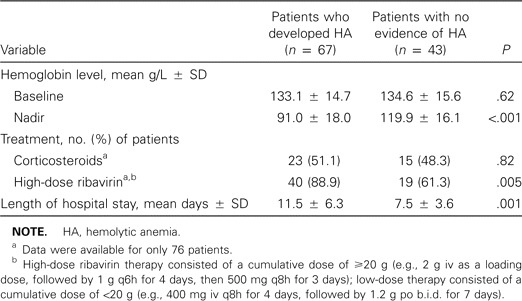
Clinical data for 110 patients with suspected or probable severe acute respiratory syndrome who were treated with ribavirin.
Electrolyte data were available for a smaller cohort of patients. Thirty-five (46%) of these 76 patients developed hypomagnesemia while receiving therapy with ribavirin. Hypocalcemia was observed in 36 (58%) of 62 patients with corrected calcium available. Twenty-two (29%) of 76 patients had evidence of both hypocalcemia and hypomagnesemia. Although most patients remained asymptomatic, 1 patient developed generalized tetany, and another patient developed severe muscle twitching and spasm of the face extending down the arms and the upper chest that was believed to be due to the combination of hypomagnesemia and hypocalcemia. The times to onset of hypomagnesemia and hypocalcemia were 4.15 ± 1.9 and 6.7 ± 3.5 days, respectively. Magnesium and calcium replacement were initiated for 49% of patients with hypomagnesemia and 47% of patients with hypocalcemia, respectively. Prophylactic calcium and magnesium supplementation were not effective in preventing either. Hypomagnesemia, but not hypocalcemia, was significantly associated with the dose of ribavirin, and hypocalcemia was associated with a longer length of hospital stay (table 2). Neither age nor sex contributed to the development of hypocalcemia or hypomagnesemia.
Table 2.
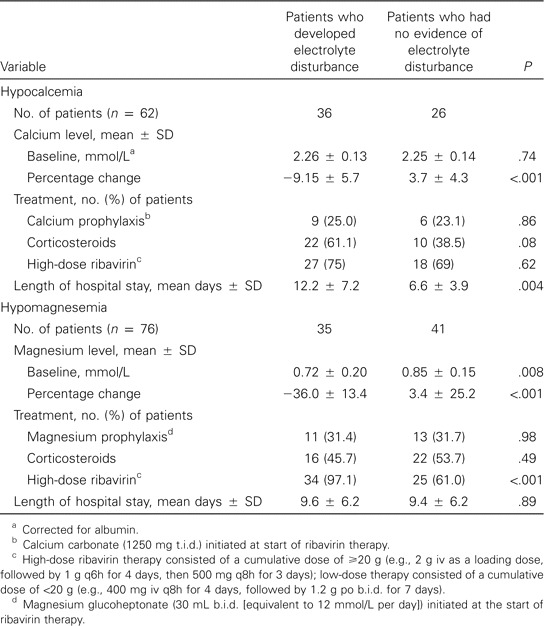
Clinical data associated with electrolyte disturbances in patients with suspected or probable severe acute respiratory syndrome who were treated with ribavirin.
Other AEs seen within the course of patients' illness, such as elevated transaminase levels, elevated lactate dehydrogenase levels, elevated creatinine kinase levels, thrombocytopenia, and lymphopenia, showed neither a clear dose-related effect nor a clear time-related effect with ribavirin and were probably more associated with the course of SARS illness (data not shown).
Discussion. Ribavirin is a broad-spectrum purine nucleoside analogue antiviral agent that has been empirically used in the treatment of SARS. In contrast to the experience in the Hong Kong outbreak, where ribavirin-associated AEs have not been detailed in published retrospective and prospective studies [9, 8], our experience with comparable doses of ribavirin suggests that associated AEs occur frequently, have a dose-response relationship, and correlate with a longer length of hospital stay.
The major established toxicity of ribavirin is reversible, dose-dependent HA [11]. HA developed in 61% of our patients with SARS who were treated with ribavirin and was significantly associated with the dose used (table 1). This is comparable to the 58% incidence of HA associated with high-dose intravenous ribavirin therapy for viral hemorrhagic fever syndromes [6], but it is significantly higher than the 7%–9% incidence associated with lower doses of oral ribavirin used for hepatitis C [11]. The nadir hemoglobin level occurred at day 13 after the start of treatment, even after the completion of ribavirin therapy. Also, not surprisingly, HA was associated with a prolonged hospital stay.
Ribavirin-induced hemolytic anemia is typically managed with reduction in the dosage or discontinuation of the ribavirin regimen, but transfusion of packed RBCs is occasionally required in cases of severe or symptomatic anemia. HA led to blood transfusions in almost one-third of our patients. Erythropoietin has been used in the treatment of anemia associated with ribavirin and IFN-α [12]. One of our patients received erythropoietin in addition to a transfusion for treatment of HA.
Ribavirin-induced anemia may exacerbate cardiovascular events in some patients [13]. It is recommended that caution be exercised when starting ribavirin therapy for patients with preexisting cardiovascular disease. No cardiovascular events were observed in any of our ribavirin-treated patients.
Although the association between ribavirin and hypocalcemia has not been well established, it was noted in 9 of 20 patients receiving ribavirin plus consensus IFN and in only 2 of 20 patients receiving consensus IFN alone for chronic hepatitis C, suggesting that the decrease in the calcium level may be, at least in part, related to ribavirin therapy [14]. Hypocalcemia has also been reported in 6 adult patients with severe measles pneumonitis who were treated with ribavirin [15]; although the hypocalcemia was attributed to disease, ribavirin may have contributed to the development of this AE. Hypomagnesemia secondary to ribavirin use has not been previously described. A large proportion of our patients developed hypocalcemia (58%) and/or hypomagnesemia (46%) within the first week after initiation of ribavirin. Baseline hypocalcemia was seen in 33% of patients; in contrast, only 10% of patients had baseline hypomagnesemia. This implies that ribavirin may be unmasking or accentuating what is, at least in part, a disease-related effect. Neither calcium nor magnesium prophylactic supplementation reduced the development of hypocalcemia and/or hypomagnesemia.
Ribavirin has a significant teratogenic and mutagenic effect. Animal studies have demonstrated various congenital malformations, as well as growth retardation and fetal deaths. In addition, because of possible accumulation of ribavirin in spermatozoa, the concerns of mutagenic effects in the offspring of men treated with ribavirin, and the unknown risk for teratogenic effects of ribavirin in humans, strict contraceptive measures must be taken by both men and women treated with ribavirin, both during treatment and for several months after cessation [16]. The extensive accumulation of ribavirin nucleotides and their slow dephosphorylation contribute to the long half-life (∼300 h) after receipt of multiple doses. To prevent potential teratogenic effects, it is recommended that 15 half-lives (or 6 months) is required to ensure complete washout after discontinuation of ribavirin [17].
Serious AEs, including HA and electrolyte disturbances, occurred early and frequently in Canadian patients with SARS who were treated with ribavirin. Ascribing causality in this situation is difficult in view of the lack of a comparably ill control group not treated with ribavirin, the likely contribution of the underlying disease, the long intracellular half-life of ribavirin, and the lack of rechallenge information. Health authorities in Canada have restricted the use of ribavirin on the basis of a lack of in vitro antiviral effect against SARS coronavirus at therapeutic concentrations [18] and the high incidence of ribavirin-associated AEs in treated patients [19]. A recent retrospective Canadian study highlights that ribavirin may not have clinical efficacy, may actually adversely affect outcome, and may need to be prematurely discontinued because of AEs in almost 20% of patients [20]. However, widespread use of ribavirin continues in Hong Kong, where AEs comparable to the Canadian experience have not been reported and where continued use has been justified by the potential nonspecific immunomodulatory activity of the drug and in vivo activity in the mouse hepatitis coronavirus model [10]. In view of the long half-life of ribavirin, the potential for teratogenicity, and the potential for significant drug-associated morbidity and mortality, vigilant monitoring for both short-term and long-term AEs is needed for patients exposed to this drug. The Canadian experience with ribavirin in the treatment of SARS suggests that the benefits of use may not outweigh the risk of AEs and the potential for ribavirin to have negative economic consequences on hospitals endorsing its use.
acknowledgments
We thank Gulzar Karmali (West Park Healthcare Centre, Toronto, Ontario, Canada) and Archie Kwan (Scarborough Hospital, General and Grace Division, Toronto) for their work and dedication in the data collection at their respective institutes. A special thank-you is extended to Dr. Nicole Mittmann (HOPE Research at Sunnybrook & Women's College Health Sciences Centre, Toronto) and Dr. Janet Raboud (Mount Sinai Hospital, Toronto) for their assistance and review of the statistical analysis.
references
- 1.World Health Organization Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS). 26 May. 2003 Available at: http://www.who.int/csr/sars/country/2003_05_26/en/ [Google Scholar]
- 2.Sidwell R, Huffman H, Khare J. Broad spectrum antiviral activity of virazole: b-d-ribofuranosyl 1-2-4 triaxole-3-carboxamide. Science. 1972;177:705–6. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 3.Dominguez K, Mercier R. Treatment of RSV pneumonia in adults—evidence of ribavirin effectiveness? Ann Pharmacother. 1999;33:739–41. doi: 10.1345/aph.18243. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Infectious Diseases Use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics. 1993;92:501–4. [PubMed] [Google Scholar]
- 5.McHutchison J, Gordon S, Schiff E, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–92. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 6.Huggins J, Hsiang C, Cosgriff T, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991;164:1119–27. doi: 10.1093/infdis/164.6.1119. [DOI] [PubMed] [Google Scholar]
- 7.McCormick J, King I, Webb P, et al. Lassa fever: effective therapy with ribavirin. N Engl J Med. 1986;314:20–6. doi: 10.1056/NEJM198601023140104. [DOI] [PubMed] [Google Scholar]
- 8.Health Canada Severe acute respiratory syndrome (SARS): definition of persons under investigation. 30 May. 2003 Available at: http://www.hc-sc.gc.ca/pphb-dgspsp/sars-sras/sars-pui_e.html. [Google Scholar]
- 9.Tsang K, Ho P, Ooi G, et al. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–85. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 10.Peiris J, Chu C, Cheng V, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–72. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang C, Chen K, Lai M, Chan K. Meta-analysis: ribavirin-induced haemolytic anaemia in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2002;16:1623–32. doi: 10.1046/j.1365-2036.2002.01326.x. [DOI] [PubMed] [Google Scholar]
- 12.Talal A, Weisz K, Hau T, Kreisworth S, Dieterich D. A preliminary study of erythropoietin for anemia associated with ribavirin and interferon-α. Am J Gastroenterol. 2001;96:2802–3. doi: 10.1111/j.1572-0241.2001.04149.x. [DOI] [PubMed] [Google Scholar]
- 13.Chutaputti A. Adverse effects and other safety aspects of the hepatitis C antivirals. J Gastroenterol Hepatol. 2000;15((Suppl)):156–63. doi: 10.1046/j.1440-1746.2000.02114.x. [DOI] [PubMed] [Google Scholar]
- 14.Pockros P, Reindollar RJ, Reddy R, Wright T, Boyd D, Wilkes L. The safety and tolerability of daily infergen plus ribavirin in the treatment of naive chronic hepatitis C patients. J Viral Hepat. 2003;10:55–60. doi: 10.1046/j.1365-2893.2003.00402.x. [DOI] [PubMed] [Google Scholar]
- 15.Forni A, Schluger N, Roberts R. Severe measles pneumonitis in adults: evaluation of clinical characteristics and therapy with intravenous ribavirin. Clin Infect Dis. 1994;19:454–62. doi: 10.1093/clinids/19.3.454. [DOI] [PubMed] [Google Scholar]
- 16.Ribavirin: new preparation An advance, but still no cure-all. Prescrire Int. 2000;9:72–5. [PubMed] [Google Scholar]
- 17.Glue P. The clinical pharmacology of ribavirin. Semin Liver DIs. 1999;19:17–14. [PubMed] [Google Scholar]
- 18.Severe acute respiratory syndrome (SARS) and coronavirus testing—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:297–302. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5214a1.htm. [PubMed] [Google Scholar]
- 19.Health Canada Onine. Update #43: severe acute respiratory syndrome. 29 April. 2003 Available at: http://www.hc-sc.gc.ca/english/protection/warnings/sars/update43.html. [Google Scholar]
- 20.Booth C, Matukas L, Tomlinson G, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289:2801–9. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]


