Abstract
Adenosine acts as a key regulator of striatum activity, in part, through the antagonistic modulation of dopamine activity. Exercise can increase adenosine activity in the brain, which may impair dopaminergic functions in the striatum. Therefore, long-term repeated bouts of exercise may subsequently generate plasticity in striatal adenosine systems in a manner that promotes dopaminergic activity. This study investigated the effects of long-term voluntary wheel running on adenosine 1 (A1R), adenosine 2A (A2AR), dopamine 1 (D1R), and dopamine 2 (D2R) receptor protein expression in adult mouse dorsal and ventral striatum structures using immunohistochemistry. In addition, equilibrative nucleoside transporter 1 (ENT1) protein expression was examined after wheel running, as ENT1 regulates the bidirectional flux of adenosine between intra- and extracellular space. The results suggest that eight weeks of running wheel access spared age-related increases of A1R and A2AR protein concentrations across the dorsal and ventral striatal structures. Wheel running mildly reduced ENT1 protein levels in ventral striatum subregions. Moreover, wheel running mildly increased D2R protein density within striatal subregions in the dorsal medial striatum, nucleus accumbens core, and the nucleus accumbens shell. However, D1R protein expression in the striatum was unchanged by wheel running. These data suggest that exercise promotes adaptations to striatal adenosine systems. Exercise-reduced A1R and A2AR and exercise-increased D2R protein levels may contribute to improved dopaminergic signaling in the striatum. These findings may have implications for cognitive and behavioral processes, as well as motor and psychiatric diseases that involve the striatum.
1. Introduction
The striatum is a component of the basal ganglia that is involved in motor, learning, and motivational processes. Abnormalities in the striatum play a role in a diverse array of neurological and psychiatric disorders, including Huntington's disease, Parkinson's disease, schizophrenia, substance abuse, disordered eating, and depression [1–9]. Evidence indicates that disturbances to energy metabolism may contribute to the onset and progression of neurological and psychiatric disorders that involve striatal dysfunction [10–14]. This is interesting because the striatum may be involved in systemic energy homeostasis [15], as well as the expression of fatigue-related behaviors [16, 17]. Evidence indicates that the striatum is under metabolic demand during aerobic exercise [18, 19], which may disrupt metabolic homeostasis and impair striatal function. Therefore, long-term repeated bouts of physical activity may promote adaptations in factors involved with energy homeostasis to compensate for periods of metabolic demand. Exercise-induced adaptations to metabolic factors within the striatum may have important implications for cognitive and behavioral function, particularly during future energy challenges and neurological disease.
Adenosine signaling within the brain represents a key link between energy homeostasis and neural activity. Indeed, adenosine is a product of adenosine triphosphate (ATP) catabolism that can act like a neuromodulator under periods of metabolic demand [20]. Adenosine receptors are particularly concentrated in the striatum, making it an attractive candidate for exercise-induced adaptations [21–23]. High-affinity adenosine 1 (A1R) and adenosine 2A (A2AR) receptors are now recognized as potent regulators of striatal circuit activity, in part, due to their postsynaptic location on medium spiny neurons in the striatum [24]. A single episode of exercise can increase brain adenosine concentrations [25]. Therefore, recurrently elevated adenosine levels resulting from repeated bouts of exercise may produce compensatory changes to the expression of striatal A1Rs and A2ARs, as increased activity at these receptors can alter ligand binding affinities and cellular protein levels [26–32]. Consistent with this hypothesis, recent evidence indicates that mRNA for A1R and A2AR receptors become downregulated in the rodent striatum following six weeks of access to running wheels [33], suggesting that physical activity status may also alter receptor properties in the striatum. Potential exercise-induced adaptations to signaling through A1Rs and A2ARs could have significant implications for striatal function.
A1Rs and A2ARs are G protein-coupled receptors that can also mediate neural activity through their interaction with several neurotransmitter systems (for review, see [34, 35]). However, changes to A1R and A2AR expression following exercise could have a particularly potent influence on striatal function through its modulation of dopaminergic activity. Indeed, A1Rs and A2ARs form heteromeric complexes with dopamine 1 (D1R) with dopamine 2 (D2R) receptors, respectively [24, 36–40]. Agonist binding at A1Rs and A2ARs results in conformational changes to dopamine receptors and decreases dopamine receptor coupling to G-proteins, thereby reducing the effectiveness of dopaminergic signaling within the striatum [39–43]. Impaired dopaminergic signaling in the striatum may favor activity in the basal ganglia pathways that are involved with features of fatigue, including diminished locomotor activity, impaired motivation, and disturbances to executive function [44–50]. Thus, potential exercise-induced changes to the modulation of striatal dopamine activity by adenosine could impact striatum-involved cognitive and behavioral processes, as well as be relevant for managing symptoms of neurological and psychiatric disease related to motor control and motivation. However, whether or not long-term wheel running alters A1R and A2AR protein expression in the rodent striatum remains unknown.
This study investigates the impact of long-term voluntary wheel running on mouse A1R and A2AR protein expression in the striatum subregions: the dorsal medial striatum (DMS), dorsal lateral striatum (DLS), nucleus accumbens core (AcC), nucleus accumbens shell (AcS), and lateral nucleus accumbens shell (LAcS), as well as striatal output region: the globus palidus (GP). These striatal subregions were selected because evidence suggests they provide distinct contributions to processing features of affect, motivation, motor behavior, pain, and cognitive function [51–54]. Moreover, equilibrative nucleoside transporter 1 (ENT1) protein density was investigated throughout the striatum, as ENT1 closely balances adenosine concentrations in synaptic space and contributes to the modulation striatum-involved behaviors [55–57]. Finally, exercise-induced changes to striatal D1R and D2R protein density were also quantified due to their heteromeric interactions with adenosine receptors influencing striatal function [58]. Thus, the capacity of physical activity status to influence A1R, A2AR, ENT1, D1R, or D2R protein density in the striatum may be of relevance for understanding the mechanisms by which exercise improves striatum-involved processes and buffers against striatal dysfunction.
2. Materials and Methods
2.1. Subject and Husbandry
Upon arrival, 32 adult male C57BL/6J mice (Jackson Labs, Sacramento, CA, USA) weighing approximately 25 g were individually housed in standard laboratory cages (35 cm × 30 cm × 15 cm) (n = 16) or cages with locked running wheels (35 cm × 30 cm × 15 cm) (n = 16). Although stress induced by single housing could potentially impact our dependent measures, mice were singly housed in order to accurately monitor individual running distance. One week after arrival, mice were allowed to run freely (n = 16, runners) or kept in standard, nonwheel running cages (n = 16, sedentary). Daily wheel revolutions were recorded digitally using Vital View software (Starr Life Sciences, Oakmont, PA, USA). Mice were euthanized either three weeks (n = 8 sedentary, n = 8 runner) or 8 weeks (n = 8 sedentary, n = 8 runner). Rooms were controlled for temperature (21 ± 1°C) and photoperiod (12 : 12 L : D) for the entire study. Envigo Teklad 2014 chow and water were provided ad libitum. All procedures were approved by the Iowa State University Institutional Animal Care and Use Committee and adhered NIH guidelines. Special care was taken to minimize animal discomfort during all procedures.
2.2. Tissue Preparation
Following [59], mice were deeply anesthetized with 100 mg/kg sodium pentobarbital (i.p.) in pairs (one runner and sedentary mouse). Mice were then transcardially perfused with 0.1 M phosphate buffer (PB) solution followed by 4% paraformaldehyde in PB solution. Brains were extracted and postfixed overnight in 4% paraformaldehyde and then transferred to a 30% sucrose solution in PB with saline (PBS) until sectioning. Brains were thinly sliced into 30 μm thick coronal sections using a microtome (Leica SM2010R) with an electronic freezing stage (Physitemp BFS-5MP) set to -22°C. Sections were separated into a 1-in-6 series (i.e., 240-micron increment sections through the rostral-caudal extent of the brain) and stored in tissue cryoprotectant at -20°C until immunohistochemistry.
2.3. Immunohistochemistry
Sections chosen for immunohistochemistry for each animal were selected across four locations spanning the rostral to caudal striatum using the Franklin and Paxinos Mouse Brain Stereotaxic Coordinates (4th edition), at approximately 1.54 mm, 0.98 mm, 0.38 mm, and -0.22 mm from the bregma. Immunohistochemistry for each primary antibody was completed on all sections for each mouse simultaneously, using the same solutions for each step. All four sections for each mouse were placed into 1.5 cm diameter net wells. Each wash and incubation period were performed on an orbital rotator for uniformity of solution exposure across all sections. A small number of the striatum-containing sections were incubated with and without (i.e., secondary antibodies only) primary antibodies to support the validity of staining. Furthermore, literature supporting the specificity of primary antibody binding to desired A1R, A2AR, D2, and ENT1 proteins can be found cited in [22, 60–63].
Following [59], the sections were washed three times in 0.1 M PBS for 5 minutes each and treated with 0.6% H2O2 for 15 min to block endogenous peroxidase activity. They were then washed thrice with 0.1 M PBS for 5 min each time. To block nonspecific binding, the sections were incubated with 5% heat-inactivated PBS-X (in 0.1 M PBS containing 5% goat serum albumin and 0.2% Triton X-100) for 1 h. After blocking was completed, the primary antibody was added for 48 hr incubation times. Primary antibodies were rabbit anti-A1R at 1 : 200 dilution (Millipore EMD, AB1587P), mouse anti-A2AR at 1 : 600 dilution (Millipore EMD, 05-717), mouse anti-ENT1 at 1 : 1,000 dilution (Santa Cruz Biotech, sc-377283), mouse anti-D1R at 1 : 10,000 dilution (Santa Cruz Biotech, sc-33660), or rabbit anti-D2R at 1 : 2,000 dilution (Millipore EMD, AB5084P). Both the primary and secondary antibody solutions were diluted in 0.1 M PBS containing 5% normal sera (NGS) and 0.2% Triton-X. Following the primary antibody incubation, the sections were washed with the antibody washing buffer (3% NGS) and 0.2% Triton X-100 in 0.1 M PBS) 4 times for 5 min each time prior to the secondary antibody application. The sections were then incubated using a biotinylated goat anti-rabbit or goat anti-mouse secondary antibody (Vector Laboratories, USA) at a dilution of 1 : 250. The secondary antibody was incubated for 90 minutes at room temperature then the previous washing method was repeated. The sections were then incubated in Vectastain AB solution. Sections were washed again in the same concentration PBS-X solution 4 times with 5 minutes per time. Following washing, the immunohistochemical complex was visualized by exposure to diaminobenzidine and nickel chloride (DABNi, Sigma, USA) for 10 min. The stained sections were rinsed with 0.1 M PBS and then dehydrated in ascending concentrations of alcohol (5 min in 70% ethanol, 5 min in 95% ethanol, 15 min in 100% ethanol, and then 5 min in xylenes), before being coverslipped with mounting medium.
2.4. Data Acquisition
A1R, A2AR, ENT1, D1R, or D2R protein density was estimated by semiquantitative computer-assisted optical densitometry. Monochrome images of the brain sections were captured digitally using a Leica MC 170 HD mounted camera to a Leica DM4 B digital microscope (Leica Microsystems, Buffalo Grove, IL) at 50x total magnification with constant intensity, exposure, and gain. Images were taken unilaterally alternating right and left hemispheres for each mouse. The corpus callosum (CC) was included as a potential control for regional specificity of physical activity-induced protein changes. However, it should be noted that A1R, A2AR, and ENT1 mRNA is expressed in oligodendrocytes, so the corpus callosum does not represent a good negative control for these receptors. Relative optical density of brain regions were determined in a blinded fashion using the LAS X software (Leica Microsystems, Buffalo Grove, IL) by placing a square frame of constant size over a region of interest for each brain region (see Table 1 for frame size and stereotaxic coordinates adapted from Franklin and Paxinos Mouse Brain Stereotaxic Coordinates 4th edition). Relative optical density of protein (i.e., light intensity) within the region of interest (see Table 1) was calculated in a computer-generated linear model using LAS X software. Results are reported as relative optical density, which represents the mean signal light intensity within each region of interest.
Table 1.
Stereotaxic coordinates and frame size used for densitometry.
| Section | Region | Frame (microns) | Bregma (mm) | M/L (mm) | D/V (mm) |
|---|---|---|---|---|---|
| 1 | DMS | 400 × 400 | 1.54 | ±0.8 | 3.0 |
| 2 | DMS | 400 × 400 | 0.98 | ±1.0 | 3.2 |
| 3 | DMS | 400 × 400 | 0.38 | ±1.1 | 3.3 |
| 4 | DMS | 400 × 400 | -0.22 | ±1.7 | 3.6 |
| 1 | DLS | 400 × 400 | 1.54 | ±2.0 | 2.8 |
| 2 | DLS | 400 × 400 | 0.98 | ±2.2 | 2.8 |
| 3 | DLS | 400 × 400 | 0.38 | ±2.6 | 2.8 |
| 4 | DLS | 400 × 400 | -0.22 | ±3.0 | 2.5 |
| 1 | AcC | 250 × 250 | 1.54 | ±1.0 | 1.1 |
| 2 | AcC | 250 × 250 | 0.98 | ±1.2 | 1.0 |
| 1 | AcS | 250 × 250 | 1.54 | ±0.8 | 1.0 |
| 2 | AcS | 250 × 250 | 0.98 | ±0.8 | 0.8 |
| 1 | LAcS | 250 × 250 | 1.54 | ±1.8 | 1.0 |
| 2 | LAcS | 250 × 250 | 0.98 | ±2.0 | 0.8 |
| 4 | GP | 400 × 400 | -0.22 | ±1.8 | 2.0 |
| 1 | CC | 75 × 75 | 1.54 | ±1.0 | 3.6 |
| 2 | CC | 75 × 75 | 0.98 | ±1.0 | 3.8 |
| 3 | CC | 75 × 75 | 0.38 | ±1.0 | 3.8 |
| 4 | CC | 75 × 75 | -0.22 | ±1.0 | 3.8 |
2.5. Statistical Analysis
Trends for A1R, A2AR, ENT1, D1R, and D2R relative optical density were consistent across the rostral to caudal sections containing striatal regions. Therefore, relative optical density for A1R, A2AR, ENT1, D1R, and D2R protein were reported as an average across the rostral to caudal sections for each striatal subregion.
The relative optical densities for A1R and A2AR across each striatum subregion were compared using two-way ANOVAs with exercise condition (sedentary vs. runner) and sample time point (three weeks vs. eight weeks) as between-subject factors. Post hoc analyses with Fisher's LSD corrections were completed following significant ANOVA interactions between exercise condition and time point.
Tissue availability was limited for three-week sedentary and runner mice, as these sections were used for other analyses (unpublished). Therefore, ENT1, D1R, and D2R relative optical density was compared only in eight-week sedentary and running mice using unpaired t tests. For all analyses, p < 0.05 was considered statistically significant. A power analysis was completed on statistically significant results to assure that all comparisons had at least a 0.8 likelihood of detecting an effect that is present.
3. Results
3.1. Wheel Running
Daily wheel running distance increased steadily for 3 weeks and thereafter maintained a plateau of averaging 7.73 km/d (±0.66 SE) over the final three weeks. The average distance ran for the entire experiment was 7.25 km/d (±0.61 SE). No differences in running distance were observed between mice that ran for three weeks and mice that ran for eight weeks over the first three weeks of wheel access.
3.2. A1R And A2AR Density following Three- and Eight-Weeks of Running
Representative images for immunohistochemistry staining of A1R can be found in Figures 1(a) and 1(b), and for A2AR in Figures 1(d) and 1(e) for running and sedentary mice.
Figure 1.
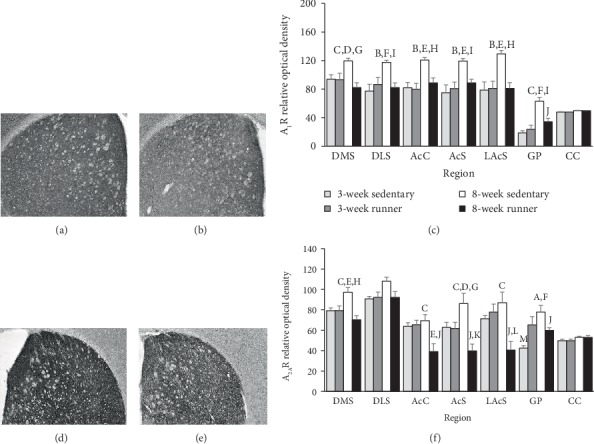
Striatal A1R and A2AR protein expression following three or eight weeks of wheel running. Representative immunohistochemistry images for A1R in the mouse striatum following eight weeks of (a) sedentary conditions and (b) running wheel access. (c) Relative levels of A1R protein density represented as averages across sections as detailed in the Methods. Representative immunohistochemistry images for A2AR in the mouse striatum following eight weeks of (d) sedentary conditions and (e) running wheel access. (f) Relative levels of A2AR protein density represented as averages across sections as detailed in Methods. Statistical significance denoted as follows: sedentary 8 weeks vs. runner 8 weeks at Ap < 0.05, Bp < 0.01, and Cp < 0.001. Sedentary 8 weeks vs. runner 3 weeks at Dp < 0.05, Ep < 0.01, and Fp < 0.001. Sedentary 8 weeks vs. sedentary 3 weeks at Gp < 0.05, Hp < 0.01, and Ip < 0.001. Runner 8 weeks vs. sedentary 3 weeks at Jp < 0.05. Runner 8 weeks vs. runner 3 weeks at Kp < 0.05 and Lp < 0.01. Sedentary 3 weeks vs. runner 3 weeks at Mp < 0.05.
Eight weeks of wheel running spared age-related increases of both A1R and A2AR across the DMS, AcC, AcS, LAcS, and GP. Age-related increases of A1R were spared in the DLS following eight-weeks of wheel running; however, this trend marginally failed to reach statistical significance for A2AR. There was no statistically significant interaction or effect of exercise condition for A1R and A2AR receptor density in the CC. However, age mildly increased the expression of both adenosine receptor subtypes in the CC. Test statistics for ANOVAs can be observed in Table 2. Approximate post hoc values can be found in Figures 1(c) and 1(f).
Table 2.
List of ANOVA results for adenosine 1 and adenosine 2A receptor protein density in striatal subregions. ∗p < 0.05 interaction between time point and condition.
| Region | Receptor | Time point | Exercise condition | Interaction |
|---|---|---|---|---|
| DMS | A1R∗ | F(1, 28) = 1.22, p = 0.280 | F(1, 28) = 7.90, p = 0.009 | F(1, 28) = 7.30, p = 0.012 |
| A2AR∗ | F(1, 28) = 1.26, p = 0.270 | F(1, 28) = 11.00, p = 0.002 | F(1, 28) = 11.26, p = 0.002 | |
| DLS | A1R∗ | F(1, 28) = 5.38, p = 0.028 | F(1, 28) = 2.79, p = 0.106 | F(1, 28) = 8.34, p = 0.007 |
| A2AR | F(1, 28) = 3.70, p = 0.065 | F(1, 28) = 2.48, p = 0.127 | F(1, 28) = 3.61, p = 0.068 | |
| AcC | A1R∗ | F(1, 28) = 12.13, p = 0.002 | F(1, 28) = 6.14, p = 0.019 | F(1, 28) = 4.62, p = 0.040 |
| A2AR∗ | F(1, 28) = 3.22, p = 0.084 | F(1, 28) = 5.98, p = 0.021 | F(1, 28) = 7.38, p = 0.011 | |
| AcS | A1R∗ | F(1, 28) = 11.12, p = 0.002 | F(1, 28) = 2.49, p = 0.126 | F(1, 28) = 5.35, p = 0.028 |
| A2AR∗ | F(1, 28) = 0.01, p = 0.924 | F(1, 28) = 10.41, p = 0.003 | F(1, 28) = 9.58, p = 0.005 | |
| LAcS | A1R∗ | F(1, 28) = 7.85, p = 0.009 | F(1, 28) = 6.51, p = 0.016 | F(1, 28) = 7.93, p = 0.009 |
| A2AR∗ | F(1, 28) = 1.73, p = 0.200 | F(1, 28) = 5.89, p = 0.022 | F(1, 28) = 10.34, p = 0.003 | |
| GP | A1R∗ | F(1, 28) = 33.28, p < 0.001 | F(1, 28) = 6.17, p = 0.019 | F(1, 28) = 12.31, p = 0.002 |
| A2AR∗ | F(1, 28) = 6.51, p = 0.016 | F(1, 28) = 0.17, p = 0.687 | F(1, 28) = 12.12, p = 0.002 | |
| CC | A1R | F(1, 28) = 18.70, p < 0.001 | F(1, 28) = 0.05, p = 0.819 | F(1, 28) = 0.03, p = 0.870 |
| A2AR | F(1, 28) = 4.55, p = 0.042 | F(1, 28) = 0.00, p = 0.993 | F(1, 28) = 0.00, p = 0.995 |
3.3. ENT1 and Dopamine Receptor Density following Eight Weeks of Running
Since differences of protein density for A1R and A2AR were observed following eight weeks of running, analyses of ENT1, D1R, and D2R protein density at this time point could provide useful information in the context of observed changes to adenosine receptor protein density. Therefore, analyses were completed following eight weeks of sedentary or running conditions for ENT1, D1R, and D2R protein density. Representative images for immunohistochemistry staining of ENT1, D2R, and D1R for sedentary and running mice are, respectively, located in Figures 2(a), 2(b), 2(d), 2(e), 2(g), and 2(h).
Figure 2.
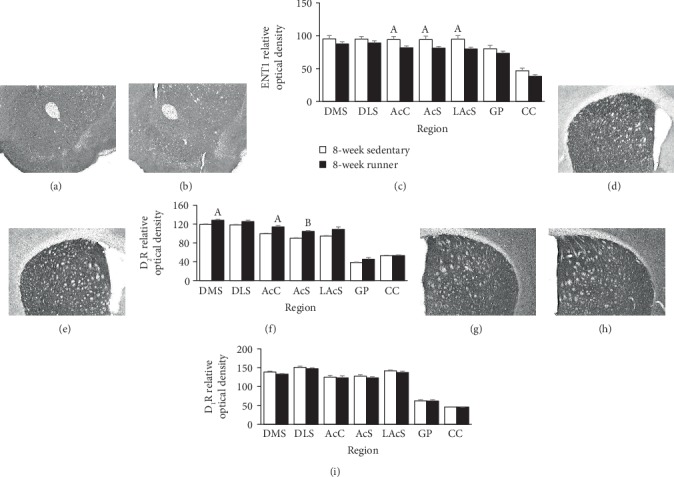
Striatal ENT1, D2R, and D1R protein expression following eight weeks of wheel running. Representative immunohistochemistry images for ENT1 in the mouse striatum following eight weeks of (a) sedentary conditions and (b) running wheel access. (c) Relative levels of ENT1 protein density represented as averages across sections as detailed in Methods. Representative immunohistochemistry images for D2R in the mouse striatum following eight weeks of (d) sedentary conditions and (e) running wheel access. (f) Relative levels of D2R protein density represented as averages across sections detailed in Methods. Representative immunohistochemistry images for D1R in the mouse striatum following eight weeks of (g) sedentary conditions and (h) running wheel access. (i) Relative levels of D1R protein density represented as averages across sections as detailed in Methods. Sedentary 8 weeks vs. runner 8 weeks at Ap < 0.05 and Bp < 0.01.
Eight weeks of running decreased ENT1 density across the ventral striatum subregions including the AcC [T(14) = 2.16, p < 0.05], AcS [T(14) = 2.17, p < 0.05], and LAcS [T(14) = 2.57, p = 0.02]. However, running produced no statistically significant differences or trends for ENT1 protein density in the DMS, DLS, GP, or CC (Figure 2(c)).
Eight weeks of running increased D2R protein density in the DMS [T(14) = 2.15, p < 0.05], AcC [T(14) = 2.67, p = 0.01], and the AcS [T(14) = 3.45, p = 0.004]. A statistically nonsignificant trend towards increased D2R protein density was observed in the DLS [T(14) = 1.94, p = 0.07] and the LAcS [T(14) = 1.94, p = 0.07]. Running did not impact D2R protein density in the GP or CC (Figure 2(f)).
A statistically nonsignificant trend towards decreased D1R protein density was observed following eight weeks of running in the DMS [T(14) = 1.97, p = 0.07]. However, running wheel access did not impact D1R protein density in the DLS, AcC, AcS, LAcS, GP, or CC (Figure 2(i)).
4. Discussion
The results of this study suggest that eight weeks of wheel running spared age-related increases of A1R and A2AR protein density across striatal subregions DMS, AcC, AcS, and the LAcS, as well as the striatal output region the GP (see Figure 1). Age-related increases of A1R protein density were also spared in the DLS; however, only a similar trend was observed for A2AR in this subregion. These findings are consistent with studies suggesting that treadmill running spared age-related increases of A2ARs in the young-adult rat hippocampus [64], and six weeks of wheel running reduced A1R and A2AR mRNA levels in the adult rat striatum [33]. Eight weeks of wheel running also decreased nucleoside transporter ENT1 protein density notably in the ventral striatum structures AcC, AcS, and LAcS (see Figure 2(c)). At the eight-week running time point, striatal D1R and D2R displayed distinct patterns of expression from their heteromeric adenosine receptor counterparts. Indeed, D2R protein density was subtly increased by wheel running in the DMS, AcC, and AcS compared to sedentary mice (see Figure 2(f)), despite overall trends towards greater density in other striatal subregions that failed to reach statistical significance. This finding is consistent with several reports of mildly increased D2R mRNA levels, protein density, and ligand binding affinity in the striatum following periods of exercise [65–71]. Finally, physical activity status had no impact on the density of D1R protein (see Figure 2(i)), which has also been reported in previous literature [70, 71]. The immunohistochemistry assessment of protein density provides some evidence that striatal A1R and A2AR concentrations are affected by physical activity status, which could reduce antagonistic heteromeric interactions with dopamine receptors in the striatum. Together, these data provide a potential mechanism in support of exercise-facilitated dopaminergic function in the striatum [67, 72–74].
An exercise-facilitated dopaminergic function that is consistent with observed changes to A1R, A2AR, and D2R receptor protein densities could alter the activity of striatal circuits in manners that promote locomotor activity, improve affect, and buffer against fatigue-related behavior, especially during challenges to energy homeostasis [72]. The principal neuron of the striatum, the medium spiny neuron, comprises two distinct neural circuits within the striatum, the direct and indirect pathways. Data from studies using gene modification and pharmacological approaches in conjunction with rodent behavior suggest that activation of the direct pathway contributes to reward and ambulation, whereas the indirect pathway activity contributes to aversion and stagnation [75–79]. Differences in the degree of direct and indirect pathway activation, thus, may underlie the varying expression of hedonic and ambulatory states during exposure to pleasurable or noxious stimuli [77]. Interestingly, through antagonistic A1R-D1R and A2AR-D2R heteromeric receptor complexes [36, 37, 40, 41, 80], adenosine can reduce postsynaptic D1R-mediated activation of direct pathway and D2-mediated inactivation of indirect pathway neurons in the striatum [40]. Therefore, the net effect of increased dopamine and adenosine levels acting on exercise-induced reductions of A1Rs and A2ARs, as well as increases of D2Rs in the striatum, could be to potentiate direct pathway and reduce indirect pathway neuron activity, thereby favoring positive hedonic states and locomotor activity [40], especially during episodes of heightened metabolic demand.
In light of this hypothesis, evidence for greater direct pathway and attenuated indirect pathway activity has been reported in physically active, compared to sedentary, rats during exposure to a series of uncontrollable tail shocks (i.e., acute stress) [33]. Evidence indicates that exaggerated neural pathway activity resulting from exposure to this acute stressor produces widespread energy imbalances in the brain, thereby promoting a state of fatigue, which is a core symptom of depression [13, 81–85]. In fact, antagonism of adenosine receptors in the brain following exposure to acute stress can prevent impaired performance on the shuttle box escape task [81–83], which involves the striatum and is thought to model depression-like motivation deficits [82, 86–88]. Interestingly, six weeks of wheel running access also spares the development of depression-like shuttle box escape deficits in rats exposed to acute stress [89–91], suggesting that exercise may create plasticity in the striatal adenosine system that may contribute to the prevention of stress-induced motivation deficits. The observed exercise-induced changes to A1R, A2AR, and D2R protein densities are consistent with a reduced adenosine-mediated inhibition of striatal dopamine activity, which could favor the stimulation of direct pathway neurons. Greater direct pathway and less indirect pathway activity could contribute to positive emotional states, thereby buffering against motivation deficits resulting from periods of heightened energy expenditure, like exposure to acute stress. However, the modulation of neural activity in the striatum is complex and involves coordinated activity across several neurotransmitter systems and brain pathways. Moreover, immunohistochemical detection of changes to A1R, A2AR, and D2R protein density alone are not sufficient to provide convincing support for these observations. Therefore, future studies are required to determine if a causal relationship exists between these observations.
ENT1 is an integral protein responsible for the transportation of nucleosides, like adenosine, across cellular membranes [92]. ENT1 can mediate adenosine activity by bidirectionally regulating adenosine diffusion across several tissues, including within the central nervous system [56]. Given that ENT1 regulates adenosine flux both into and out of cells, it is not entirely clear how a potential small reduction of transporter protein (see Figure 2(c)) might influence overall adenosine activity in the striatum. On one hand, less available ENT1 could lower the amount of accumulating intracellular adenosine (e.g., during heightened neuron activity) that is transported into synaptic space, thereby limiting the adenosine-related modulation of neuron activity. On the other hand, a potential downregulation of ENT1 protein could also lead to a less efficient removal of adenosine from extracellular space, thereby potentiating adenosine activity at neural circuits. Any potential changes to intra- and extracellular adenosine concentrations by reduced ENT1 protein availability, thus, may be situational and contingent on the sources and amounts of accumulating adenosine. Moreover, extracellular adenosine concentrations are regulated through multiple mechanisms of transport and metabolism enzymes (e.g., adenosine kinase and adenosine deaminase), and therefore cannot be completely accounted for by the potential reduction of a single transporter protein [20, 56, 92, 93]. A more complete analysis of enzymes that metabolize adenosine and nucleoside uptake proteins must be considered to fully understand the influence of physical activity on the regulation of adenosine concentrations in synaptic space.
In conclusion, the current data provide novel evidence that exercise promotes adaptations in the striatal adenosine system. Reductions to A1R and A2AR protein expression could contribute to reduced efficacy of adenosine-mediated inhibition of dopamine activity in the striatum, which should be followed up in more detail by future studies. Indeed, changes in A1Rs and A2ARs that are dependent on physical activity status could affect therapeutic approaches for psychiatric and neurological diseases that involve abnormal dopamine signaling in the striatum, including addiction, depression, Parkinson's disease, and Huntington's disease, among many others [94–104]. Therefore, the current findings could be of importance for understanding the mechanisms contributing to exercise-improved cognitive function, as well as the prevention and treatment of mental health and neurobiological disorders that involve the striatum.
Acknowledgments
This work was supported by the College of Human Sciences Intramural Collaborative Seed Grant at Iowa State University. Special thanks are due to Iowa State University animal care facility.
Data Availability
The data used to support the findings of this study may be released upon request to Peter Clark at Iowa State University, who can be contacted at pjclark@iastate.edu.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article.
Authors' Contributions
Ella E. Bauer, Trevor J. Buhr, and Carter H. Reed contributed equally to data collection and preparation of the manuscript.
References
- 1.Gittis A. H., Kreitzer A. C. Striatal microcircuitry and movement disorders. Trends in Neurosciences. 2012;35(9):557–564. doi: 10.1016/j.tins.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plotkin J. L., Goldberg J. A. Thinking outside the box (and arrow): current themes in striatal dysfunction in movement disorders. The Neuroscientist. 2018;25(4):359–379. doi: 10.1177/1073858418807887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yager L. M., Garcia A. F., Wunsch A. M., Ferguson S. M. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson E. H., Kellendonk C., Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65(5):585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levitt J. J., Rosow L. K., Nestor P. G., et al. A volumetric MRI study of limbic, associative and sensorimotor striatal subregions in schizophrenia. Schizophrenia Research. 2013;145(1-3):11–19. doi: 10.1016/j.schres.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Avena N. M., Bocarsly M. E. Dysregulation of brain reward systems in eating disorders: neurochemical information from animal models of binge eating, bulimia nervosa, and anorexia nervosa. Neuropharmacology. 2012;63(1):87–96. doi: 10.1016/j.neuropharm.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank G. K. Altered brain reward circuits in eating disorders: chicken or egg? Current Psychiatry Reports. 2013;15(10):p. 396. doi: 10.1007/s11920-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoflich A., Michenthaler P., Kasper S., Lanzenberger R. Circuit mechanisms of reward, anhedonia, and depression. The International Journal of Neuropsychopharmacology. 2019;22(2):105–118. doi: 10.1093/ijnp/pyy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russo S. J., Nestler E. J. The brain reward circuitry in mood disorders. Nature Reviews. Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amano S., Kegelmeyer D., Hong S. L. Rethinking energy in parkinsonian motor symptoms: a potential role for neural metabolic deficits. Frontiers in Systems Neuroscience. 2015;8:p. 242. doi: 10.3389/fnsys.2014.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarnopolsky M. A., Beal M. F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Annals of Neurology. 2001;49(5):561–574. [PubMed] [Google Scholar]
- 12.Agren H., Niklasson F. Creatinine and creatine in CSF: indices of brain energy metabolism in depression. Journal of Neural Transmission. 1988;74(1):55–59. doi: 10.1007/bf01243575. [DOI] [PubMed] [Google Scholar]
- 13.Minor T. R., Hanff T. C. Adenosine signaling in reserpine-induced depression in rats. Behavioural Brain Research. 2015;286:184–191. doi: 10.1016/j.bbr.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 14.Zuccoli G. S., Saia-Cereda V. M., Nascimento J. M., Martins-de-Souza D. The energy metabolism dysfunction in psychiatric disorders postmortem brains: focus on proteomic evidence. Frontiers in Neuroscience. 2017;11:p. 493. doi: 10.3389/fnins.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ter Horst K. W., Lammers N. M., Trinko R., et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Science Translational Medicine. 2018;10(442, article eaar3752) doi: 10.1126/scitranslmed.aar3752. [DOI] [PubMed] [Google Scholar]
- 16.Lorist M. M., Tops M. Caffeine, fatigue, and cognition. Brain and Cognition. 2003;53(1):82–94. doi: 10.1016/s0278-2626(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhuri A., Behan P. O. Fatigue and basal ganglia. Journal of the Neurological Sciences. 2000;179(1-2):34–42. doi: 10.1016/S0022-510X(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey D. P., Adamo D. S., Anderson B. J. Exercise increases metabolic capacity in the motor cortex and striatum, but not in the hippocampus. Brain Research. 2001;891(1-2):168–175. doi: 10.1016/s0006-8993(00)03200-5. [DOI] [PubMed] [Google Scholar]
- 19.De Bruin L. A., Schasfoort E. M., Steffens A. B., Korf J. Effects of stress and exercise on rat hippocampus and striatum extracellular lactate. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1990;259(4):R773–R779. doi: 10.1152/ajpregu.1990.259.4.R773. [DOI] [PubMed] [Google Scholar]
- 20.Dunwiddie T. V., Masino S. A. The role and regulation of adenosine in the central nervous system. Annual Review of Neuroscience. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 21.Wojcik W. J., Neff N. H. Differential location of adenosine A1 and A2 receptors in striatum. Neuroscience Letters. 1983;41(1-2):55–60. doi: 10.1016/0304-3940(83)90222-7. [DOI] [PubMed] [Google Scholar]
- 22.Rosin D. L., Robeva A., Woodard R. L., Guyenet P. G., Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. The Journal of Comparative Neurology. 1998;401(2):163–186. [PubMed] [Google Scholar]
- 23.Rivkees S. A., Price S. L., Zhou F. C. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Research. 1995;677(2):193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- 24.Ferre S., Fuxe K., Fredholm B. B., Morelli M., Popoli P. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends in Neurosciences. 1997;20(10):482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 25.Dworak M., Diel P., Voss S., Hollmann W., Strüder H. K. Intense exercise increases adenosine concentrations in rat brain: implications for a homeostatic sleep drive. Neuroscience. 2007;150(4):789–795. doi: 10.1016/j.neuroscience.2007.09.062. [DOI] [PubMed] [Google Scholar]
- 26.Mundell S., Kelly E. Adenosine receptor desensitization and trafficking. Biochimica et Biophysica Acta. 2011;1808(5):1319–1328. doi: 10.1016/j.bbamem.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Hettinger B. D., Leid M., Murray T. F. Cyclopentyladenosine-induced homologous down-regulation of A1 adenosine receptors (A1AR) in intact neurons is accompanied by receptor sequestration but not a reduction in A1AR mRNA expression or G protein alpha-subunit content. Journal of Neurochemistry. 1998;71(1):221–230. doi: 10.1046/j.1471-4159.1998.71010221.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S., Millhorn D. E. Stimulation of expression for the adenosine A2A receptor gene by hypoxia in PC12 cells. A potential role in cell protection. The Journal of Biological Chemistry. 1999;274(29):20358–20365. doi: 10.1074/jbc.274.29.20358. [DOI] [PubMed] [Google Scholar]
- 29.Vendite D., Sanz J. M., López-Alañon D. M., Vacas J., Andrés A., Ros M. Desensitization of adenosine A1 receptor-mediated inhibition of adenylyl cyclase in cerebellar granule cells. Neurochemical Research. 1998;23(2):211–218. doi: 10.1023/a:1022437110269. [DOI] [PubMed] [Google Scholar]
- 30.Leon D. A., Castillo C. A., Albasanz J. L., Martín M. Reduced expression and desensitization of adenosine A1 receptor/adenylyl cyclase pathway after chronic (−)N6-phenylisopropyladenosine intake during pregnancy. Neuroscience. 2009;163(2):524–532. doi: 10.1016/j.neuroscience.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 31.Saitoh O., Saitoh Y., Nakata H. Regulation of A2a adenosine receptor mRNA expression by agonists and forskolin in PC12 cells. Neuroreport. 1994;5(11):1317–1320. [PubMed] [Google Scholar]
- 32.Ruiz M. A., León D. A., Albasanz J. L., Martín M. Desensitization of adenosine a(1) receptors in rat immature cortical neurons. European Journal of Pharmacology. 2011;670(2-3):365–371. doi: 10.1016/j.ejphar.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Clark P. J., Ghasem P. R., Mika A., et al. Wheel running alters patterns of uncontrollable stress-induced cfos mRNA expression in rat dorsal striatum direct and indirect pathways: a possible role for plasticity in adenosine receptors. Behavioural Brain Research. 2014;272:252–263. doi: 10.1016/j.bbr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheth S., Brito R., Mukherjea D., Rybak L. P., Ramkumar V. Adenosine receptors: expression, function and regulation. International Journal of Molecular Sciences. 2014;15(2):2024–2052. doi: 10.3390/ijms15022024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferre S., Ciruela F., Quiroz C., et al. Adenosine receptor heteromers and their integrative role in striatal function. ScientificWorldJournal. 2007;7:74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gines S., Hillion J., Torvinen M., et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(15):8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuxe K., Ferré S., Canals M., et al. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. Journal of Molecular Neuroscience. 2005;26(2-3):209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- 38.Rivera-Oliver M., Moreno E., Álvarez-Bagnarol Y., et al. Adenosine A1-Dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Molecular Neurobiology. 2019;56(2):797–811. doi: 10.1007/s12035-018-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonaventura J., Navarro G., Casadó-Anguera V., et al. Allosteric interactions between agonists and antagonists within the adenosine A2A receptor-dopamine D2 receptor heterotetramer. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(27):E3609–E3618. doi: 10.1073/pnas.1507704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferre S., Bonaventura J., Zhu W., et al. Essential control of the function of the striatopallidal neuron by pre-coupled complexes of adenosine A2A-dopamine D2 receptor heterotetramers and adenylyl cyclase. Frontiers in Pharmacology. 2018;9:p. 243. doi: 10.3389/fphar.2018.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciruela F., Fernández-Dueñas V., Llorente J., et al. G protein-coupled receptor oligomerization and brain integration: focus on adenosinergic transmission. Brain Research. 2012;1476:86–95. doi: 10.1016/j.brainres.2012.04.056. [DOI] [PubMed] [Google Scholar]
- 42.Ciruela F., Gómez-Soler M., Guidolin D., et al. Adenosine receptor containing oligomers: their role in the control of dopamine and glutamate neurotransmission in the brain. Biochimica et Biophysica Acta. 2011;1808(5):1245–1255. doi: 10.1016/j.bbamem.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Ferre S., von Euler G., Johansson B., Fredholm B. B., Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dreher J. K., Jackson D. M. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Research. 1989;487(2):267–277. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- 45.Gepshtein S., Li X., Snider J., Plank M., Lee D., Poizner H. Dopamine function and the efficiency of human movement. Journal of Cognitive Neuroscience. 2014;26(3):645–657. doi: 10.1162/jocn_a_00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salamone J. D., Pardo M., Yohn S. E., López-Cruz L., SanMiguel N., Correa M. Mesolimbic dopamine and the regulation of motivated behavior. Current Topics in Behavioral Neurosciences. 2016;27:231–257. doi: 10.1007/7854_2015_383. [DOI] [PubMed] [Google Scholar]
- 47.Olivetti P. R., Balsam P. D., Simpson E. H., Kellendonk C. Emerging roles of striatal dopamine D2 receptors in motivated behaviour: implications for psychiatric disorders. Basic & Clinical Pharmacology & Toxicology. 2019 doi: 10.1111/bcpt.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka K. F., Hamaguchi T. translational approach to apathy-like behavior in mice: from the practical point of view. Psychiatry and Clinical Neurosciences. 2019;73(11):685–689. doi: 10.1111/pcn.12915. [DOI] [PubMed] [Google Scholar]
- 49.Aoyama S., Kase H., Borrelli E. Rescue of locomotor impairment in dopamine D2 receptor-deficient mice by an adenosine A2A receptor antagonist. The Journal of Neuroscience. 2000;20(15):5848–5852. doi: 10.1523/JNEUROSCI.20-15-05848.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barraco R. A., Martens K. A., Parizon M., Normile H. J. Adenosine A2a receptors in the nucleus accumbens mediate locomotor depression. Brain Research Bulletin. 1993;31(3-4):397–404. doi: 10.1016/0361-9230(93)90233-2. [DOI] [PubMed] [Google Scholar]
- 51.Saddoris M. P., Sugam J. A., Cacciapaglia F., Carelli R. M. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Frontiers in Bioscience (Elite Edition) 2013;E5(1):273–288. doi: 10.2741/e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang S. E., Holland P. C. Effects of nucleus accumbens core and shell lesions on autoshaped lever-pressing. Behavioural Brain Research. 2013;256:36–42. doi: 10.1016/j.bbr.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baliki M. N., Mansour A., Baria A. T., et al. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. The Journal of Neuroscience. 2013;33(41):16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voorn P., Vanderschuren L. J., Groenewegen H. J., Robbins T. W., Pennartz C. M. Putting a spin on the dorsal-ventral divide of the striatum. Trends in Neurosciences. 2004;27(8):468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Anderson C. M., Xiong W., Geiger J. D., et al. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. Journal of Neurochemistry. 1999;73(2):867–873. doi: 10.1046/j.1471-4159.1999.0730867.x. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen M. D., Ross A. E., Ryals M., Lee S. T., Venton B. J. Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism. Pharmacology Research & Perspectives. 2015;3(6, article e00189) doi: 10.1002/prp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nam H. W., Hinton D. J., Kang N. Y., et al. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. The Journal of Neuroscience. 2013;33(10):4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferre S., Díaz-Ríos M., Salamone J. D., Prediger R. D. New developments on the adenosine mechanisms of the central effects of caffeine and their implications for neuropsychiatric disorders. Journal of Caffeine and Adenosine Research. 2018;8(4):121–130. doi: 10.1089/caff.2018.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clark P. J., Brzezinska W. J., Puchalski E. K., Krone D. A., Rhodes J. S. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–950. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song A., Zhang Y., Han L., et al. Erythrocytes retain hypoxic adenosine response for faster acclimatization upon re-ascent. Nature Communications. 2017;8(1):p. 14108. doi: 10.1038/ncomms14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hettinger B. D., Lee A., Linden J., Rosin D. L. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. The Journal of Comparative Neurology. 2001;431(3):331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 62.Cooke H. J., Wang Y., Liu C. Y., Zhang H., Christofi F. L. Activation of neuronal adenosine A1 receptors suppresses secretory reflexes in the Guinea pig colon. The American Journal of Physiology. 1999;276(2):G451–G462. doi: 10.1152/ajpgi.1999.276.2.G451. [DOI] [PubMed] [Google Scholar]
- 63.Wang H., Pickel V. M. Dopamine D2 receptors are present in prefrontal cortical afferents and their targets in patches of the rat caudate-putamen nucleus. The Journal of Comparative Neurology. 2002;442(4):392–404. doi: 10.1002/cne.10086. [DOI] [PubMed] [Google Scholar]
- 64.Costa M. S., Ardais A. P., Fioreze G. T., et al. Treadmill running frequency on anxiety and hippocampal adenosine receptors density in adult and middle-aged rats. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2012;36(1):198–204. doi: 10.1016/j.pnpbp.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 65.Thompson A. B., Stolyarova A., Ying Z., Zhuang Y., Gómez-Pinilla F., Izquierdo A. Methamphetamine blocks exercise effects on Bdnf and Drd2 gene expression in frontal cortex and striatum. Neuropharmacology. 2015;99:658–664. doi: 10.1016/j.neuropharm.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Foley T. E., Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Medicine. 2008;10(2):67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- 67.Rabelo P. C. R., Horta N. A. C., Cordeiro L. M. S., et al. Intrinsic exercise capacity in rats influences dopamine neuroplasticity induced by physical training. Journal of Applied Physiology. 2017;123(6):1721–1729. doi: 10.1152/japplphysiol.00506.2017. [DOI] [PubMed] [Google Scholar]
- 68.Gilliam P. E., Spirduso W. W., Martin T. P., Walters T. J., Wilcox R. E., Farrar R. P. The effects of exercise training on [3H]-spiperone binding in rat striatum. Pharmacology, Biochemistry, and Behavior. 1984;20(6):863–867. doi: 10.1016/0091-3057(84)90008-X. [DOI] [PubMed] [Google Scholar]
- 69.MacRae P. G., Spirduso W. W., Cartee G. D., Farrar R. P., Wilcox R. E. Endurance training effects on striatal D2 dopamine receptor binding and striatal dopamine metabolite levels. Neuroscience Letters. 1987;79(1-2):138–144. doi: 10.1016/0304-3940(87)90686-0. [DOI] [PubMed] [Google Scholar]
- 70.Vuckovic M. G., Li Q., Fisher B., et al. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson's disease: In vivo imaging with [18F]fallypride†. Movement Disorders. 2010;25(16):2777–2784. doi: 10.1002/mds.23407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robison L. S., Swenson S., Hamilton J., Thanos P. K. Exercise reduces dopamine D1R and increases D2R in rats: implications for addiction. Medicine and Science in Sports and Exercise. 2018;50(8):1596–1602. doi: 10.1249/MSS.0000000000001627. [DOI] [PubMed] [Google Scholar]
- 72.Greenwood B. N. The role of dopamine in overcoming aversion with exercise. Brain Research. 2019;1713:102–108. doi: 10.1016/j.brainres.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Cordeiro L. M. S., Rabelo P. C. R., Moraes M. M., et al. Physical exercise-induced fatigue: the role of serotonergic and dopaminergic systems. Brazilian Journal of Medical and Biological Research. 2017;50(12, article e6432) doi: 10.1590/1414-431X20176432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petzinger G. M., Holschneider D. P., Fisher B. E., et al. The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plasticity. 2015;1(1):29–39. doi: 10.3233/bpl-150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwak S., Jung M. W. Distinct roles of striatal direct and indirect pathways in value-based decision making. eLife. 2019;8 doi: 10.7554/eLife.46050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morita M., Hikida T. Distinct roles of the direct and indirect pathways in the basal ganglia circuit mechanism. Nihon Shinkei Seishin Yakurigaku Zasshi. 2015;35(5-6):107–111. [PubMed] [Google Scholar]
- 77.Lenz J. D., Lobo M. K. Optogenetic insights into striatal function and behavior. Behavioural Brain Research. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 78.Kravitz A. V., Tye L. D., Kreitzer A. C. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience. 2012;15(6):816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hikida T., Kimura K., Wada N., Funabiki K., Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66(6):896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 80.Nishi A., Kuroiwa M., Shuto T. Mechanisms for the modulation of dopamine D1 receptor signaling in striatal neurons. Frontiers in Neuroanatomy. 2011;5:p. 43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minor T. R., Winslow J. L., Chang W. C. Stress and adenosine: II. adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behavioral Neuroscience. 1994;108(2):265–276. doi: 10.1037//0735-7044.108.2.265. [DOI] [PubMed] [Google Scholar]
- 82.Minor T. R., Hunter A. M. Stressor controllability and learned helplessness research in the United States: sensitization and fatigue processes. Integrative Physiological and Behavioral Science. 2002;37(1):44–58. doi: 10.1007/bf02688805. [DOI] [PubMed] [Google Scholar]
- 83.Minor T. R., Chang W. C., Winslow J. L. Stress and adenosine: I. effect of methylxanthine and amphetamine stimulants on learned helplessness in rats. Behavioral Neuroscience. 1994;108(2):254–264. doi: 10.1037//0735-7044.108.2.254. [DOI] [PubMed] [Google Scholar]
- 84.Woodson J. C., Minor T. R., Job R. F. Inhibition of adenosine deaminase by erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) mimics the effect of inescapable shock on escape learning in rats. Behavioral Neuroscience. 1998;112(2):399–409. doi: 10.1037//0735-7044.112.2.399. [DOI] [PubMed] [Google Scholar]
- 85.Hunter A. M., Balleine B. W., Minor T. R. Helplessness and escape performance: glutamate-adenosine interactions in the frontal cortex. Behavioral Neuroscience. 2003;117(1):123–135. doi: 10.1037//0735-7044.117.1.123. [DOI] [PubMed] [Google Scholar]
- 86.Strong P. V., Christianson J. P., Loughridge A. B., et al. 5-Hydroxytryptamine 2C receptors in the dorsal striatum mediate stress-induced interference with negatively reinforced instrumental escape behavior. Neuroscience. 2011;197:132–144. doi: 10.1016/j.neuroscience.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maier S. F., Watkins L. R. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Maier S. F., Seligman M. E. Learned helplessness at fifty: insights from neuroscience. Psychological Review. 2016;123(4):349–367. doi: 10.1037/rev0000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Greenwood B. N., Foley T. E., Burhans D., Maier S. F., Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research. 2005;1033(2):164–178. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 90.Greenwood B. N., Fleshner M. Exercise, learned helplessness, and the stress-resistant brain. Neuromolecular Medicine. 2008;10(2):81–98. doi: 10.1007/s12017-008-8029-y. [DOI] [PubMed] [Google Scholar]
- 91.Greenwood B. N., Foley T. E., Day H. E., et al. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. The Journal of Neuroscience. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pastor-Anglada M., Perez-Torras S. Who is who in adenosine transport. Frontiers in Pharmacology. 2018;9:p. 627. doi: 10.3389/fphar.2018.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mackiewicz M., Nikonova E. V., Zimmerman J. E., et al. Enzymes of adenosine metabolism in the brain: diurnal rhythm and the effect of sleep deprivation. Journal of Neurochemistry. 2003;85(2):348–357. doi: 10.1046/j.1471-4159.2003.01687.x. [DOI] [PubMed] [Google Scholar]
- 94.Zheng J., Zhang X., Zhen X. Development of adenosine A2A receptor antagonists for the treatment of Parkinson’s disease: a recent update and challenge. ACS Chemical Neuroscience. 2019;10(2):783–791. doi: 10.1021/acschemneuro.8b00313. [DOI] [PubMed] [Google Scholar]
- 95.Morelli M., Carta A. R., Jenner P. Adenosine A2A receptors and Parkinson's disease. Handbook of Experimental Pharmacology. 2009;193:589–615. doi: 10.1007/978-3-540-89615-9_18. [DOI] [PubMed] [Google Scholar]
- 96.Lee C. F., Chern Y. Adenosine receptors and Huntington's disease. International Review of Neurobiology. 2014;119:195–232. doi: 10.1016/B978-0-12-801022-8.00010-6. [DOI] [PubMed] [Google Scholar]
- 97.Gomes C. V., Kaster M. P., Tomé A. R., Agostinho P. M., Cunha R. A. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochimica et Biophysica Acta. 2011;1808(5):1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 98.Fuxe K., Marcellino D., Borroto-Escuela D. O., et al. Adenosine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neuroscience & Therapeutics. 2010;16(3):e18–e42. doi: 10.1111/j.1755-5949.2009.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brown R. M., Short J. L. Adenosine a(2A) receptors and their role in drug addiction. The Journal of Pharmacy and Pharmacology. 2008;60(11):1409–1430. doi: 10.1211/jpp/60.11.0001. [DOI] [PubMed] [Google Scholar]
- 100.Ferre S., Ciruela F., Canals M., et al. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism & Related Disorders. 2004;10(5):265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 101.van Calker D., Biber K., Domschke K., Serchov T. The role of adenosine receptors in mood and anxiety disorders. Journal of Neurochemistry. 2019;151(1):11–27. doi: 10.1111/jnc.14841. [DOI] [PubMed] [Google Scholar]
- 102.Al-Attraqchi O. H. A., Attimarad M., Venugopala K. N., Nair A., Al-Attraqchi N. H. A. Adenosine A2A receptor as a potential drug target-current status and future perspectives. Current Pharmaceutical Design. 2019;25(25):2716–2740. doi: 10.2174/1381612825666190716113444. [DOI] [PubMed] [Google Scholar]
- 103.Domenici M. R., Ferrante A., Martire A., et al. Adenosine A2A receptor as potential therapeutic target in neuropsychiatric disorders. Pharmacological Research. 2019;147:p. 104338. doi: 10.1016/j.phrs.2019.104338. [DOI] [PubMed] [Google Scholar]
- 104.Uchida S., Soshiroda K., Okita E., et al. The adenosine A2A receptor antagonist, istradefylline enhances anti-parkinsonian activity induced by combined treatment with low doses of L-DOPA and dopamine agonists in MPTP-treated common marmosets. European Journal of Pharmacology. 2015;766:25–30. doi: 10.1016/j.ejphar.2015.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study may be released upon request to Peter Clark at Iowa State University, who can be contacted at pjclark@iastate.edu.


