Abstract
Background
Current cancer care increasingly incorporates psychosocial interventions. Cancer patients use dance/movement therapy to learn to accept and reconnect with their bodies, build new self‐confidence, enhance self‐expression, address feelings of isolation, depression, anger and fear and to strengthen personal resources.
Objectives
To update the previously published review that examined the effects of dance/movement therapy and standard care versus standard care alone or standard care and other interventions on psychological and physical outcomes in patients with cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 6), MEDLINE (OvidSP, 1950 to June week 4, 2014), EMBASE (OvidSP, 1980 to 2014 week 26), CINAHL (EBSCOhost, 1982 to July 15 2014), PsycINFO (EBSCOhost, 1806 to July 15 2014), LILACS (Virual Health Library, 1982 to July 15 2014), Science Citation Index (ISI, 1974 to July 15 2014), CancerLit (1983 to 2003), International Bibliography of Theatre and Dance (1989 to July 15 2014), the National Research Register (2000 to September 2007), Proquest Digital Dissertations, ClinicalTrials.gov, and Current Controlled Trials (all to July 15 2014). We handsearched dance/movement therapy and related topics journals, reviewed reference lists and contacted experts. There was no language restriction.
Selection criteria
We included all randomized and quasi‐randomized controlled trials of dance/movement therapy interventions for improving psychological and physical outcomes in patients with cancer. We considered studies only if dance/movement therapy was provided by a formally trained dance/movement therapist or by trainees in a formal dance/movement therapy program.
Data collection and analysis
Two review authors independently extracted the data and assessed the methodological quality, seeking additional information from the trial researchers when necessary. Results were presented using standardized mean differences.
Main results
We identified one new trial for inclusion in this update. In total, the evidence for this review rests on three studies with a total of 207 participants.
We found no evidence for an effect of dance/movement therapy on depression (standardized mean difference (SMD) = 0.02, 95% confidence interval (CI) ‐0.28 to 0.32, P = 0.89, I2 = 0%) (two studies, N = 170), stress (SMD = ‐0.18, 95% CI ‐0.48 to 0.12, P = 0.24, I2 = 0%) (two studies, N = 170), anxiety (SMD = 0.21, 95% CI ‐0.09 to 0.51 P = 0.18, I2 = 0%) (two studies, N = 170), fatigue (SMD = ‐0.36, 95% ‐1.26 to 0.55, P = 0.44, I² = 80%) (two studies, N = 170) and body image (SMD = ‐0.13, 95% CI ‐0.61 to 0.34, P = 0.58, I2 = 0%) (two studies, N = 68) in women with breast cancer. The data of one study with moderate risk of bias suggested that dance/movement therapy had a large beneficial effect on 37 participants' quality of life (QoL) (SMD = 0.89, 95% CI 0.21 to 1.57). One study with a high risk of bias reported greater improvements in vigor and greater reduction in somatization in the dance/movement therapy group compared to a standard care control group (N = 31). The individual studies did not find support for an effect of dance/movement therapy on mood, mental health, and pain. It is unclear whether this was due to ineffectiveness of the treatment, inappropriate outcome measures or limited power of the trials. Finally, the results of one study did not find evidence for an effect of dance/movement therapy on shoulder range of motion (ROM) or arm circumference in 37 women who underwent a lumpectomy or breast surgery. However, this was likely due to large within‐group variability for shoulder ROM and a limited number of participants with lymphedema.
Two studies presented moderate risk of bias and one study high risk of bias. Therefore, overall, the quality of the evidence is very low.
Authors' conclusions
We did not find support for an effect of dance/movement therapy on depression, stress, anxiety, fatigue and body image. The findings of individual studies suggest that dance/movement therapy may have a beneficial effect on QoL, somatization, and vigor. However, the limited number of studies prevents us from drawing conclusions concerning the effects of dance/movement therapy on psychological and physical outcomes in cancer patients.
Plain language summary
Dance/movement therapy for cancer patients
The issue Cancer may result in extensive emotional, physical and social suffering. Current cancer care increasingly incorporates psychosocial interventions to improve quality of life. Creative arts therapies such as dance/movement, music, art and drama therapy have been used to aid care and recovery. Following medical therapies, which can be invasive, people with cancer use dance/movement therapy to learn to accept and reconnect with their bodies, build new self‐confidence, enhance self‐expression, address feelings of isolation, depression, anger, fear and distrust and strengthen personal resources. It has also been used to improve range of arm motion and to reduce arm circumference after mastectomy or lumpectomy. For this review, studies were considered only if dance/movement therapy was provided by a formally trained dance/movement therapist or by trainees in a formal program.
The aim of the review This review is an update of a previous Cochrane review from 2011, which included two studies which did not find support for an effect of dance/movement therapy on body image, the only common outcome between the two studies. The aim was to examine the impact of dance/movement therapy on psychological and physical outcomes in people with cancer.
For this review update, we searched for additional trials on the effect of dance/movement therapy on psychological and physical outcomes in people with cancer. We searched for published and ongoing studies up toJuly 2014. We considered all studies in which dance/movement therapy was compared with any form of standard treatment.
What are the main findings? We identified one new study for this update. The three studies included a a total of 207 participants, which were women with breast cancer. The studies were small in size. We found no evidence of an effect for depression, stress, anxiety, fatigue, and body image. The findings of individual studies suggest that dance/movement therapy may have a beneficial effect on the quality of life, somatization (i.e. distress arising from perceptions of bodily dysfunction) and vigor of women with breast cancer. No adverse effects of dance/movement therapy interventions were reported.
Quality of the evidence The evidence is based on only three small studies and the quality of the evidence is not strong.
What are the conclusions? No conclusions could be drawn regarding the effect of dance/movement therapy on psychological and physical outcomes in cancer patients because of an insufficient number of studies. More research is needed. We did not identify any conflicts of interests in the included studies.
Summary of findings
Summary of findings 1. Dance/movement therapy versus standard care for improving psychological and physical outcomes in cancer patients.
| Dance/movement therapy versus standard care for improving psychological and physical outcomes in cancer patients | |||
| Patient or population: patients with improving psychological and physical outcomes in cancer patients Settings: Intervention: Dance/movement therapy versus standardcCare | |||
| Outcomes | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) |
| Depression Profile of Moods Scale (depression subscale) and Hospital Anxiety and Depression Scale | The mean depression in the intervention groups was 0.02 standard deviations higher (0.28 lower to 0.32 higher) | 170 (2 studies) | ⊕⊝⊝⊝ very low1,2 |
| Stress Symptom Checklist_90_Revised and Perceived Stress Scale | The mean stress in the intervention groups was 0.18 standard deviations lower (0.48 lower to 0.12 higher) | 170 (2 studies) | ⊕⊝⊝⊝ very low1,2 |
| Fatigue Profile of Moods Scale (Fatigue subscale) and Brief Fatigue Inventory | The mean fatigue in the intervention groups was 0.36 standard deviations lower (1.26 lower to 0.55 higher) | 170 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Body Image Borscheid, Walster, Bohrnstedt Body Image Scale and The Body Image Scale | The mean body image in the intervention groups was 0.13 standard deviations lower (0.61 lower to 0.34 higher) | 68 (2 studies) | ⊕⊝⊝⊝ very low2,4 |
| Anxiety Hospital Anxiety and Depression Scale and Symptom Checklist 90‐Revised | The mean anxiety in the intervention groups was 0.21 standard deviations higher (0.09 lower to 0.51 higher) | 170 (2 studies) | ⊕⊝⊝⊝ very low1,2 |
| CI: Confidence interval | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 One trial was at high risk of bias and one trial at moderate risk of bias 2 Wide confidence interval that includes zero 3 Results were inconsistent across studies as evidenced by I² = 80% 4 One study received low risk of bias rating but the other study received high risk of bias rating
Background
Description of the condition
Having cancer may result in extensive emotional, physical and social suffering. Study findings indicate that cancer patients experience elevated levels of psychological distress (Duivenvoorden 1997; Norton 2004; Sellick 1999) and depression (Massie 2004; Parle 1996; Raison 2003) in response to diagnosis and treatment. Therefore, current cancer care increasingly incorporates psychosocial interventions to help patients come to terms with changes in their body, address distorted body images, deal with unresolved grief, restore hope, increase emotional expression, reduce isolation and improve self‐esteem. Research results have indicated that such comprehensive care has improved cancer patients' quality of life (QoL) (Dibbell‐Hope 2000; Mannheim 2006).
Description of the intervention
Creative arts therapies such as dance/movement, music, art and drama therapy have been used to aid in the care of cancer patients and in their recovery. Following medical therapies, which can be invasive, cancer patients use dance/movement therapy to learn to accept and reconnect with their bodies, build new self‐confidence, enhance self‐expression, address feelings of isolation, depression, anger, fear and distrust and strengthen personal resources (Dibbell‐Hope 2000; Mannheim 2006). It is assumed that when physical changes in the body are a source of pain and distress, that a body‐focused approach to psychosocial support can have a meaningful impact (Goodill 2006). Dance/movement therapy has also been used to improve range of arm motion and shoulder function and to reduce arm circumference after mastectomy or lumpectomy, to decrease pain and fatigue and to improve vitality (Sandel 2005).
As defined by the American Dance Therapy Association, "Dance/movement therapy is the psychotherapeutic use of movement as a process which furthers the emotional, social, cognitive and physical integration of the individual" (ADTA). Dance/movement therapy may include a variety of dance/movement methods and is characterized by a goal‐oriented, systematic treatment process. For this review, we considered studies only if dance/movement therapy was provided by a formally trained dance/movement therapist or by trainees in a formal dance/movement therapy program.
How the intervention might work
Dance/movement therapy promotes individuals’ spontaneous movement expression, which allows them to enact thoughts and feelings that are often difficult to articulate in words. This experience not only creates an outlet through which emotions and psychological tension can be discharged, but also provides a vehicle for becoming aware of personal strengths and inner resources (Serlin 1997). The process of enactment, combined with the use of affirmative images and symbols, enables individuals to reinforce healthy parts of themselves as well as activate motivation for positive change. This, in turn, can result in enhanced self‐efficacy (Shim 2014). Self‐efficacy is a significant predictor for higher level of illness adaptation, symptom management and quality of life in cancer patients (Lev 2009; Porter 2008; Rottman 2010). Attentive self‐awareness may furthermore lead to valuing one’s own body, and this may contribute to better self‐care behaviors (Goodill 2005).The process of intentional embodiment, which is at the core of dance/movement therapy interventions, means that new experiences become analogous to active practicing. Thus, what is learnt in the session (e.g., new behaviors, interpersonal skills, expressive abilities, or affect regulation) is more likely to be mastered and generalized to contexts outside the therapy session.
In addition to facilitating expression of emotions and thoughts, dance/movement therapy encourages individuals to enhance awareness of their own body, as well as explore their perceptions of self and others in the interpersonal environment (Kleinman 2006). Dance/movement therapy can help cancer patients to restore a positive relationship with themselves and to develop a healthy body image by promoting a realistic understanding of the shape and function of their body; instilling a sense of acceptance about the changes their body might have gone through in the course of treatment; and helping them to restore the vital connection to their body (Dibbell‐Hope 2000; McKibben 1988).
Finally, a number of interacting factors contribute to the capacity for dance/movement therapy to improve mood state, and reduce self‐reported depression and anxiety (Jeong 2005; Koch 2014). In practice, dance/movement therapy combines the benefits of mobility and mild exercise with interpersonal/social support and the creative/expressive process. Independently, each of these components has mood‐elevating properties: expression of emotions, as opposed to the constraint of that expression, can lead to better health outcomes (Krantz 2007), and social support provides a context for that expression. Dance and dance/movement therapy both have been shown to bring about a sense of vitality (Erhardt 1989; Koch 2007). This is partially through the use of rhythmic action, which discharges and releases muscular tension. It may be this same combination of curative factors in dance/movement therapy that leads to stress reduction, as was demonstrated and discussed in a randomized controlled trial with 162 participants (Bräuninger 2012). Neurohormonal changes may also play a role in the mood‐elevating effect of dance/movement therapy (Jeong 2005).
Why it is important to do this review
Several research studies on the use of dance/movement therapy with cancer patients have reported positive results (Dibbell‐Hope 1989; Ho 2005a; Ho 2008; Sandel 2005). The majority of these studies, however, are small and lack statistical power. In addition, differences in factors such as study designs, methods of interventions and type and intensity of treatment have led to varying results. A systematic review is needed to more accurately gauge the efficacy of dance/movement interventions for cancer patients as well as to identify variables that may moderate its effects.
Objectives
1. To examine the effects of dance/movement therapy on psychological and physical outcomes in patients with cancer. 2. To compare the effects of different types of dance/movement therapy.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) and studies with quasi‐randomized methods of treatment allocation (e.g. alternate allocation of treatments) were eligible for inclusion.
Types of participants
This review included patients diagnosed with any type of cancer in active treatment or in recovery. There were no restrictions as to age, gender, ethnicity or stage of illness.
Types of interventions
The review included all studies in which standard treatment combined with dance/movement therapy is compared with: (a) standard care alone or (b) standard care combined with other therapies. We considered studies only if dance/movement therapy was provided by a formally trained dance/movement therapist or by trainees in a formal dance/movement therapy program.
Types of outcome measures
Primary outcomes
Psychological outcomes (e.g. depression, anxiety, anger, hopelessness, helplessness, mood, self‐esteem)
Symptom relief (e.g. fatigue, nausea, pain)
Physical outcomes (e.g. physical health, vitality, range of motion (ROM), arm circumference)
Secondary outcomes
Physiological outcomes (e.g. immunoglobulin A levels, cortisol levels)
Relationship and social support (e.g. family support, social activity, isolation)
Communication (e.g. verbalization, facial affect, gestures)
QoL
Body image
Survival
Search methods for identification of studies
There were no language restrictions for either searching or trial inclusion.
Electronic searches
We searched the following electronic databases and trials registers.
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, 2014, Issue 6) (Appendix 1).
MEDLINE (1950 to June, week 4 2014) (Appendix 2).
EMBASE (1980 to 2014 week 26) (Appendix 3).
CINAHL (1982 to July 15 2014) (Appendix 4).
PsycINFO (1967 to July 15 2014) (Appendix 5).
LILACS (1982 to July 15 2014) (Appendix 6).
The Science Citation Index (to July 15 2014) (Appendix 7).
CancerLit (1983 to 2003; database is no longer maintained) (Appendix 8).
International Bibliography of Theatre and Dance Full Text (1989 to July 15 2014) (Appendix 9).
Proquest Digital Dissertations (to July 15 2014) (Appendix 10).
ClinicalTrials.gov (http://www.clinicaltrials.gov/) (July 15 2014) (Appendix 11).
Current Controlled Trials (http://www.controlled-trials.com/) (July 15 2014) (Appendix 12).
National Research Register (http://www.update-software.com/National/) (2000 to September 2007; database is no longer maintained) (Appendix 13).
For this update, the search results for the databases were extended from 2011 to 2014 between July 1 and July 15 2014.
Searching other resources
We handsearched the following journals from first available date. For this update, the journals were handsearched from January 2011 until July 2014.
American Journal of Dance Therapy
Arts in Psychotherapy
Dance Research Journal
Human Movement Science
Journal of Physical Education, Recreation and Dance
Journal of Bodywork and Movement Therapies
Moving On, Journal of the Dance/movement Therapy Association of Australia
E‐motion, electronic journal of the Association for Dance/movement Therapy UK
Body, Movement and Dance in Psychotherapy
In an effort to identify further published, unpublished and ongoing trials, we searched the bibliographies of relevant studies and reviews, contacted experts in the field and searched available proceedings of dance/movement therapy conferences (e.g. Congress of Research in Dance). We consulted international dance/movement therapy association websites to help identify relevant research studies as well as dance/movement therapy practitioners and conference information. In addition, we posted a message on the listservs of the American Dance Therapy Association and the German Dance Therapy Association asking members to inform us about published and unpublished research that meets the inclusion criteria. Finally, we searched library catalogues of American Universities that offer dance/movement therapy training programs for relevant theses.
Data collection and analysis
Selection of studies
One review author (JB) conducted the searches as outlined in the search strategy. The same review author (JB) and two dance/movement therapy research assistants scanned titles and abstracts of each record retrieved from the search and deleted obviously irrelevant references. When a title/abstract could not be rejected with certainty, two review authors (JB and SG) independently inspected the full‐text article. We used an inclusion criteria form to assess the trial's eligibility for inclusion. Agreement was reached on all inclusion decisions. In case of disagreement, we would have sought the input of the third author (MS). If a trial appeared eligible but was excluded after inspection of the full text, we kept a record of both the article and the reason for exclusion.
Data extraction and management
One review author (JB) and a research assistant independently extracted data from the selected trials using a standardized coding form (Appendix 14). We encountered some issues with data extraction for one study (Dibbell‐Hope 1989). We sought the advice of a statistician, sought input of another review author (SG) and requested additional information from the chief investigator.
We extracted the following data.
General information
Author
Year of publication
Title
Journal (title, volume, pages)
If unpublished, source
Duplicate publications
Country
Language of publication
Intervention information
Type of intervention (e.g. authentic movement)
Length of intervention
Frequency of intervention
Comparison intervention
Participant information
Total sample size
Number of experimental group
Number of control group
Gender
Age
Ethnicity
Diagnosis
Illness stage
Setting
Inclusion criteria
Outcomes
We extracted pre‐test means, post‐test means, standard deviations and sample sizes for the treatment group and the control group for the following outcomes (if applicable).
Psychological outcomes (i.e., depression, anxiety, anger, hopelessness, helplessness, mood, self‐esteem).
Symptom relief (e.g., fatigue, nausea, pain).
Physical outcomes (e.g., physical health, vitality, range of motion, arm circumference).
Physiological outcomes (e.g., Immunoglobulin A levels, cortisol levels).
Relationship and social support (e.g., family support, social activity, isolation).
Communication (e.g., verbalization, facial affect, gestures).
QoL.
Body image.
Survival.
Assessment of risk of bias in included studies
Two review authors (JB and SG) assessed risk of bias, blinded to each other's assessment for trial quality of all included trials. Any disagreements were resolved by discussion. The review authors used the following criteria for assessment of risk of bias.
Random sequence generation
Low risk
Unclear risk
High risk
We rated random sequence generation as low risk if every participant had an equal chance to be selected for either treatment and if the investigator was unable to predict to which treatment the participant would be assigned. Use of date of birth, date of admission or alternation resulted in high risk of bias.
Allocation concealment
Low risk ‐ methods to conceal allocation include:
central randomization
serially numbered, opaque, sealed envelopes
other descriptions with convincing concealment
Unclear risk ‐ authors did not adequately report on method of concealment
High risk (e.g. alternation methods were used)
Blinding of participants and personnel
Low risk
Unclear risk
High risk
Blinding of participants is often not feasible in dance/movement therapy studies unless a comparative design is used.
Blinding of outcome assessors
Low risk: outcome assessors were blinded
Unclear risk: authors did not adequately report on method of blinding
High risk: (a) outcome assessors were not blinded or (b) self‐report measures were used and participants were not blinded
Incomplete outcome data
We recorded the proportion of participants whose outcomes were analyzed. We coded loss to follow‐up for each outcome.
Low risk: if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms
Unclear risk: if loss to follow‐up was not reported
High risk: if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms
Selective reporting
Low risk: reports of the study were free of suggestion of selective outcome reporting
Unclear risk
High risk: reports of the study suggest selective outcome reporting
Other sources of bias
Low risk: unlikely that other sources of bias influenced the results
Unclear risk: unclear if other sources of bias may have influenced the results
High risk: likely that other sources of bias influenced the results
We considered information on potential financial conflicts of interest as a possible source of additional bias.
We used the above criteria to give each article an overall quality rating (based on the Cochrane Handbook for Systematic Reviews of Interventions, section 8.7 (Higgins 2011)) A. Low risk of bias ‐ all criteria met. B. Moderate risk of bias ‐ one or more of the criteria only partly met. C. High risk of bias ‐ one or more criteria not met.
We did not exclude studies based on a low quality score.
Dealing with missing data
We did not impute missing outcome data. We analyzed data on an endpoint basis, including only participants for whom a final data point measurement was obtained (available‐case analysis). It was not assumed that participants who dropped out after randomization had a negative outcome.
Assessment of heterogeneity
We investigated heterogeneity by visual inspection of the forest plots as well as using the I2 statistic, with an I2 greater than 50% indicating significant heterogeneity.
Assessment of reporting biases
There were an insufficient number of trials to assess reporting biases. We had planned to compute funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small‐study effects such as publication bias.
Data synthesis
We entered data from the three trials included in this systematic review into Review Manager (Revman 5.3). These studies had five continuous variables in common. We calculated standardized mean differences (SMD) for these outcomes because the results were derived from different scales. We calculated a pooled estimate using the fixed‐effect model. In cases of significant heterogeneity, we used the random‐effects model. We determined the levels of heterogeneity by using the I2 statistic (Higgins 2002). We used a random‐effects model when the I2 value was more than 50%. We calculated 95% confidence intervals (CI) for the effect‐size estimate. For the other outcomes, we were limited to providing a narrative description of the results of individual trials.
We made the following treatment comparison.
Dance/movement therapy versus standard care alone.
We had planned to include the following additional treatment comparison but the three studies included in this review did not allow for such analysis.
Dance/movement therapy interventions versus other therapies.
Subgroup analysis and investigation of heterogeneity
We had determined a priori to perform subgroup analyses by (a) type of dance/movement therapy intervention and (b) stage of illness. However, these subgroup analyses could not be performed because of insufficient numbers of studies. Subgroup analyses would have been conducted as described by Deeks et al (Deeks 2001) as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, section 9.6 (Higgins 2011).
Sensitivity analysis
We had planned to examine the impact of sequence generation by comparing the results of including and excluding studies that used inadequate or unclear randomization methods. This was not possible because only three studies were included in this review.
Results
Description of studies
See Figure 1.
1.
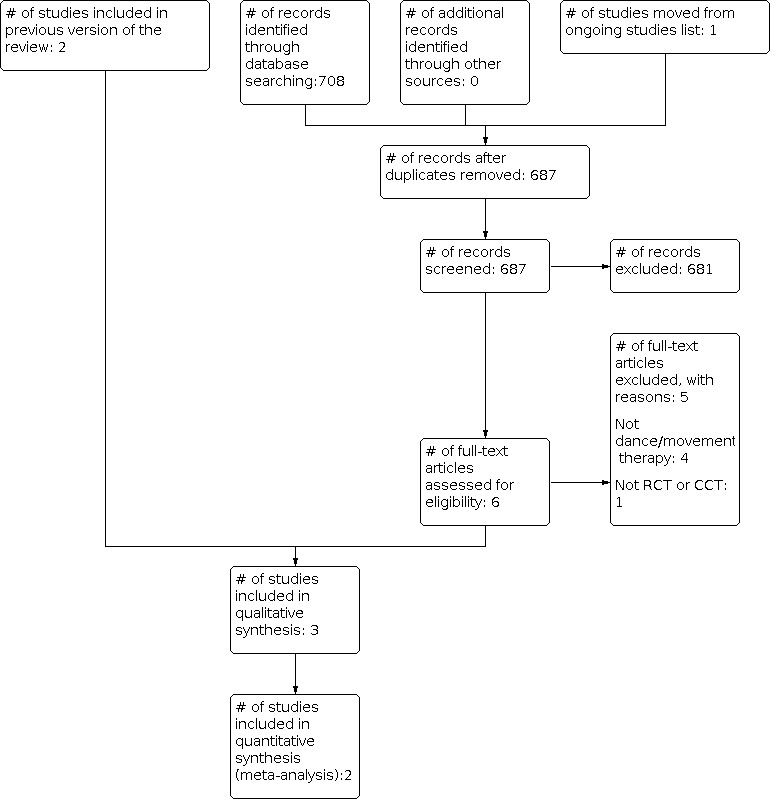
Study flow diagram.
Results of the search
For the original review, the database searches and handsearching of journals, conference proceedings and reference lists resulted in 770 unique citations. One review author (JB) and a research assistant examined the titles and abstracts and identified 15 studies as potentially relevant, which we retrieved for further assessment. These were then independently screened by the same review author (JB) and a research assistant. Another review author (SG) was consulted where needed.
We included three references reporting on two trials in this review (Dibbell‐Hope 1989; Sandel 2005). Where necessary, we contacted the chief investigators to obtain additional information on study details and data.
The 2014 update of the search resulted in 687 extra citations. One review author (JB) and two research assistants examined the titles and abstracts and retrieved full‐text articles where necessary. The two other reviewer authors (SG and MS) were consulted where needed. This resulted in the addition of one study (Ho 2014).
Included studies
We included three studies with a total of 207 participants. All studies included women with breast cancer who underwent treatment within five years of the onset of the study. We did not identify any studies with male patients or pediatric patients.
The average age of the participants was 53 years. In one study (Dibbell‐Hope 1989), 90% of the participants were Caucasian. All participants in the Ho study (Ho 2014) were Chinese. The other study (Sandel 2005) did not report the ethnicity of the participants. Two studies were conducted in the US (Dibbell‐Hope 1989; Sandel 2005). One study was conducted in Hong Kong (Ho 2014). Trial sample size ranged from 31 to 139 participants.
One study (Dibbell‐Hope 1989), used Authentic Movement, which is a simple form of self‐directed expressive movement and involves a mover/client (or group of movers) and a witness/therapist. The mover usually moves with eyes closed in order to attend to and bring a clearer focus to one's own inner experience. While the mover engages in her experience, listening to her own inner impulse and following where it may lead her, the witness observes the mover's experience as well as tracks her own somatic and imaginative processes (Stromsted 2001). Sandel and colleagues (Sandel 2005) used The Lebed MethodTM, Focus on Healing through Movement and Dance. This method, designed by Lebed‐Davis, is aimed at "restoring the range of motion of the shoulders and reduce lymphedema, coupled with dance movements designed to restore a sense of body symmetry, as well as femininity, grace and sexuality" (Sandel 2005, p. 302). Ho (Ho 2014) used a combination of dance/movement interventions specifically tailored to meet the needs of breast cancer patients, including guided movements to exercise the upper extremities, improvisational dance and movement to explore positive emotions, and group sharing to relate the movement process to participants’ personal experiences of breast cancer and cancer treatment.
Frequency and duration of treatment sessions varied greatly between the studies. One study (Dibbell‐Hope 1989), offered six, weekly sessions that lasted three hours each. The dance/movement therapy program in the Sandel study (Sandel 2005) was 12 weeks in duration, with two sessions per week for the initial six weeks and one session per week for the six subsequent weeks, for a total of 18 sessions. Each session lasted 50 to 60 minutes. Ho's study (Ho 2014) offered six sessions held twice a week for three consecutive weeks, with sessions lasting for 50 to 90 minutes. The rationale for program length and session frequency in the Sandel study was to allow for women in treatment or recovery to miss sessions due to fatigue or side effects.
All studies used a two‐arm parallel group design. Two studies used a wait‐list control group (Dibbell‐Hope 1989; Sandel 2005). One study used a standard care control group (Ho 2014). The studies did not measure all outcomes identified for this review.
Details of the studies included in the review are shown in the Characteristics of included studies table.
Excluded studies
The main reasons for exclusion of studies that appeared eligible for this review were (a) not a randomized or quasi‐randomized controlled trial (N = 15) and (b) not a dance/movement therapy intervention study (N = 4). One study was excluded because of unacceptable treatment allocation method (Goldov 2011).
Details of the excluded trials are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
Allocation
Two studies used an appropriate method of randomization, namely a computer‐generated number list (Ho 2014; Sandel 2005). Although Dibbell‐Hope stated that randomization was used, the author did not report the randomization method (Dibbell‐Hope 1989). Additional information received from the author revealed that an alternation method was used for group assignment. Sandel and colleagues and Ho used proper allocation concealment procedures. The use of an alternation method for group assignment prohibited the use of adequate allocation concealment in the Dibbell‐Hope study.
Blinding
Blinding for subjective outcomes was not possible in all three studies since study participants could not be blinded to the study intervention. However, outcome assessors for shoulder range of motion (ROM) were blinded in the Sandel study. Blinding of intervention allocation is often not possible in dance/movement therapy studies. This may introduce possible bias.
Incomplete outcome data
The drop‐out rate was small for all trials, namely between 6% and 12%. Detailed information on drop‐out reasons is included in the Characteristics of included studies table.
Selective reporting
Two studies did not show evidence of selective reporting (Dibbell‐Hope 1989; Sandel 2005). The Ho study is not yet published (Ho 2014). We received study results from the author. At the time of this review, study results for quality of life, were not yet available.
Other potential sources of bias
We did not identify any other potential sources of bias in the studies included in this review.
As a result of the 'Risk of bias' assessment, we concluded that two studies were at moderate risk of bias (Ho 2014; Sandel 2005). The third study presented a high risk of bias (Dibbell‐Hope 1989). Risk of bias is detailed for each study in the 'Risk of bias' tables included within the Characteristics of included studies table and an overall assessment of risk of bias can be viewed in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
Psychological outcomes
Mood
One study (Dibbell‐Hope 1989), examined the effects of dance/movement therapy on mood disturbance by means of the Profile of Mood States (POMS) (McNair 1971). The investigator reported that there was no statistically significant difference at post‐test between the treatment and the control group for this outcome.
Depression
The pooled effect of two studies (N = 170) (Dibbell‐Hope 1989; Ho 2014) suggested no effect for dance/movement therapy on depression (SMD = 0.02, 95% CI ‐0.28 to 0.32, P = 0.89, I2 = 0%) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Dance/movement Therapy versus Control, Outcome 1: Depression
Stress
Two studies examined the effects of dance/movement therapy on stress (Ho 2014) or distress (Dibbell‐Hope 1989).
Dibbell‐Hope's study was conducted at two sites (Northern and Southern sites in San Francisco) and significant differences were present for this outcome between the two sites (Dibbell‐Hope 1989). Therefore, the author presented the findings for the two sites separately. We computed an average mean and average standard deviation for the two sites in order to pool the data of this study with the data from the Ho study. It is important to point out that the large SDs reported for this outcome in the Dibbell‐Hope study indicate that the data were not normally distributed. Therefore, the results of this study need to be interpreted with caution.
The pooled estimate of these two studies (N = 170) did not find support for an effect of dance/movement therapy on stress in cancer patients (SMD = ‐0.18, 95% CI ‐0.48 to 0.12, P = 0.24). The results were consistent across the studies (I2 = 0%) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Dance/movement Therapy versus Control, Outcome 2: Stress
Anxiety
Two studies (N = 170) included anxiety as an outcome (Dibbell‐Hope 1989; Ho 2014). Their pooled estimate suggested that dance/movement therapy participants demonstrated slightly more anxiety than control group participants but this effect was not statistically significant (SMD = 0.21, 95% CI ‐0.09 to 0.51 P = 0.18, I2 = 0%) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Dance/movement Therapy versus Control, Outcome 3: Anxiety
Somatization
Dibbell‐Hope (Dibbell‐Hope 1989) examined the impact of dance/movement therapy on somatization using the somatization subscale of the Symptom Checklist 90‐Revised (SCL‐90‐R) (Derogatis 1979). The somatization subscale reflects distress arising from perceptions of bodily dysfunction. The results suggest a greater reduction in somatization in the dance/movement therapy than in the control group (SMD = ‐0.83, 95% CI ‐1.57 to ‐0.1, P = 0.03).
Mental Health
One study reported results on the effect of dance/movement therapy on participants' mental health (Sandel 2005), as measured by the subscale of the SF‐36 (Ware 1994). The mental health score improved for the treatment group (mean change score = 3.5, SD = 10.56) whereas, it slightly decreased for the control group (mean change score = ‐1.5, SD = 12.73), however, the difference between the groups was not statistically significant.
Symptom relief
Fatigue
A pooled estimate of two studies (N = 170) found no support for an effect of dance/movement therapy on fatigue (SMD = ‐0.36, 95% ‐1.26 to 0.55, P = 0.44) (Dibbell‐Hope 1989; Ho 2014). The results were not consistent across studies (I² = 80%) with Dibbell‐Hope reporting a greater decrease in fatigue in the treatment group than the control group.
Vigor
One study measured the impact of dance/movement therapy on vigor using the POMS vigor‐activity subscale (Dibbell‐Hope 1989). The results suggest that dance/movement therapy resulted in greater vigor than standard care (SMD = 1.44, 95% CI 0.64 to 2.24), P = 0.0004). Using general guidelines for the interpretation of intervention effects in the social sciences (Cohen 1988), this is considered a large treatment effect.
Pain
One study examined the impact of dance/movement therapy on pain severity in cancer patients ((Ho 2014). There was no significant difference between the dance/movement therapy group and the control group at post‐test (MD = ‐0.30, 95% CI ‐1.0 to 0.40, P = 0.4).
Physical outcomes
Range of motion (ROM)
One study (Sandel 2005), reported on the effects of dance/movement therapy on shoulder ROM of women who underwent a lumpectomy or more extensive breast surgery at least one month before the onset of the study. The authors reported that the ROM in the shoulder on the side of breast surgery increased 15° in the intervention group and 8° in the wait‐list control group. However, the difference between the two groups was not statistically significant (P = 0.58).
Arm circumference
Sandel and colleagues (Sandel 2005) also included arm circumference as an outcome in their study and reported that there were no changes in arm circumference from pre‐test to post‐test in either group, in either the involved or non‐involved arm.
Secondary outcomes
Body Image
Two studies (N = 68) (Dibbell‐Hope 1989; Sandel 2005) examined the impact of dance/movement therapy on participants' body image. Their pooled estimate indicated no evidence of effect of dance/movement therapy and the results were consistent across the two studies (SMD = ‐0.13, 95% CI ‐0.61 to 0.34, P = 0.58, I2 = 0%) (Analysis 1.5).
1.5. Analysis.

Comparison 1: Dance/movement Therapy versus Control, Outcome 5: Body Image
Quality of Life
Sandel and colleagues (Sandel 2005) reported on the effects of dance/movement therapy on health‐related QoL. Their results indicated that the QoL of the women who participated in dance/movement therapy (mean change score = 14.7, SD = 16.36) improved significantly compared with the wait‐list control group (mean change score = ‐1.9, SD = 19.95) after 12 weeks of treatment (P = 0.008). When expressed in SMD, the effect of dance/movement therapy was 0.89 (95% CI 0.21 to 1.57). This is considered a large treatment effect (Cohen 1988). This increase in QoL was maintained as indicated by follow‐up measures at 26 weeks.
We did not identify any studies that addressed the other secondary outcomes listed in the protocol, namely physiological outcomes, relationship and support, communication and survival.
Discussion
Summary of main results
Three studies met the inclusion criteria for this review. The following outcomes were shared by at least two studies: depression, stress, anxiety, fatigue and body image.Their pooled estimates did not find support for an effect of dance/movement therapy on any of these outcomes in women with breast cancer (Table 1). Results from individual studies suggest that dance/movement therapy may have a beneficial effect on quality of life (QoL), somatization, and vigor (Effects of interventions).
The data from individual studies indicated that there was no statically significant difference between treatment and control groups for mood, mental health, and pain. Finally, the results of one study did not find support for an effect of dance/movement therapy on shoulder range of motion (ROM) or arm circumference in women who underwent a lumpectomy or more extensive breast surgery.
Overall completeness and applicability of evidence
This review included two randomized controlled trials (RCTs) and one quasi‐RCT that examined the effects of dance/movement therapy on women with breast cancer who underwent treatment within five years of the onset of the study. The small number of studies included in this review prevents us from drawing conclusions concerning the applicability of the findings.
For dance/movement therapy no support of an effect was found for depression, stress, anxiety, fatigue, or body image. However, only two studies per outcome were included in these meta‐analyses and therefore, no conclusions can be drawn about the effectiveness of dance/movement therapy for these outcomes at this time.
One study with a moderate risk of bias rating reported a large effect of dance/movement therapy on QoL (Sandel 2005). Moreover, similar improvements were reported for the wait‐list control group during crossover to treatment. One study with a high risk of bias reported greater improvements in vigor and greater reduction in somatization in the dance/movement therapy group compared to a standard care control group (Dibbell‐Hope 1989).
No support was found in the individual studies for body image, mood, mental health, pain, ROM or arm circumference. It is unclear whether this was due to ineffectiveness of the treatment, inappropriate outcome measures (for subjective outcomes) or limited power of the studies. Sandel and colleagues pointed out that even though the ROM improvement in the treatment group was 7° greater than in the control group, a large within‐group variability and small sample size negatively impacted the statistical analysis of between‐group differences for this outcome (Sandel 2005). As for arm circumference, only a few participants reported a diagnosis of lymphedema. Therefore, it was difficult to determine a treatment effect for this outcome. As for body image, Dibbell‐Hope reported contradictory findings between objective and subjective data in the study (Dibbell‐Hope 1989). The objective data indicated no improvement in body image whereas the subjective data suggested that the participants experienced a marked improvement in body image. This discrepancy could be due to the fact that the measurement (Borscheid‐Walster‐Bohrnstedt Body Image Scale (BWB)) (Borscheid 1972) used in this study was not sensitive enough to measure the improvements reported by the participants in the post‐treatment interviews. Alternatively, it is possible that the women exaggerated their reports of improvement in the interviews to please the researcher. In contrast to the findings of these two individual studies, results of non‐controlled trials with cancer patients have suggested a beneficial effects of dance/movement therapy on mood (Ho 2007; Serlin 1997), distress (Ho 2005b; Ho 2008) and body image (Shin 2009). More research is needed to examine the impact of dance/movement therapy on these outcomes.
The results of this review pertain to dance/movement therapy with women with breast cancer. We did not identify trials that included patients with other types of cancer, male participants or children. Therefore, these findings cannot be generalized to these other population groups.
Quality of the evidence
The quality of the evidence (GRADE assessment) in this review is very low (Table 1) due to the small number of included studies (three), the small sample sizes (207 participants in total) and the high risk of bias of one of the included studies (Dibbell‐Hope 1989).
Two trials received a moderate risk of bias rating (Ho 2014; Sandel 2005). Although few trials were identified that met the inclusion criteria of this review, the Sandel study demonstrates that it is possible to conduct a high‐quality RCT on the effects of dance/movement therapy with cancer patients. As blinding of study participants and therapist is not possible in most dance/movement therapy studies, it is impossible for these types of clinical studies to receive a low risk of bias rating. Finally, the reporting of the Sandel study was excellent.
'Risk of bias' summaries are detailed in Figure 2 and Figure 3.
Potential biases in the review process
We searched all available databases and a large number of journals, checked reference lists of all relevant trials, contacted relevant experts for identification of unpublished trials, posted inquiries to national and international dance/movement therapy listservs and included publications without restricting language. In spite of such a comprehensive search, it is still possible that we missed some published and unpublished trials.
We requested additional data where necessary for all trials we considered for inclusion. This allowed us to get accurate information on the trial quality and data for most trials and helped us make well‐informed trial selection decisions.
Agreements and disagreements with other studies or reviews
This review is the first systematic review on the use of dance/movement therapy with cancer patients. Only three trials could be included in this review. By comparison, a recent systematic review by Koch and colleagues on the effects of dance/movement therapy and dance on health‐related psychological outcomes did not exclusively focus on cancer patients (Koch 2014). It differed from our present review in two additional ways. First, Koch and colleagues included a much larger range of studies. Specifically, their meta‐analysis included 23 controlled clinical trials (with or without randomization) of both dance/movement therapy and dance interventions, with a large variety of both clinical and non‐clinical populations. Thus, pooled estimates for outcome variables were obtained across heterogenous populations. Secondly, the inclusion of studies on dance, creative movement and dance/movement therapy interventions constitutes a difference from our review, which focuses entirely on dance/movement therapy interventions only. The broader scope of the Koch et al. review afforded a larger total sample for analysis (N = 1078), and more studies per outcome but increased heterogeneity.Their meta‐analysis reported effects for some of the same outcomes examined herein, as follows: body image (SMD = 0.27, 95% CI ‐0.04 to 0.57, P < 0.1, I2= 11%), for quality of life (QoL) (SMD = 0.37 (95% CI 0.18 to 0.55, P < 0.001, I2 =11%), and depression (SMD = 0.36, 95% CI 0.17 to 0.56, P < 0.001, I2 = 0%). The results of the Koch review contrast with our findings in that we found a smaller effect for body image (SMD = 0.13) (Analysis 1.5) and depression (SMD = 0.02) (Analysis 1.1) and a larger effect for quality of life (SMD = 0.89) (Sandel 2005), although the latter was based on just one study. The review by Koch and colleagues included two of the three studies in this review (Dibbell‐Hope 1989; Sandel 2005), and also included one study examined for inclusion in this review (Goldov 2011), but which we did not include because treatment allocation was based on self‐selection by participant into the treatment or control group.
A review by Kiepe and colleagues included 11 RCTs that evaluated the effect of dance/movement therapy (six studies) and ballroom dances (five studies) in adults with physical and mental illness (i.e., cancer, dementia, depression, and fibromyalgia) in comparison to other types of intervention or care as usual (Kiepe 2012). Of the six dance/movement therapy studies that were reviewed by Kiepe et al., two studies examined the effects of dance/movement therapy on cancer patients, both of which are included in this review (Dibbell‐Hope 1989; Sandel 2005). According to Kiepe and colleagues, dance/movement therapy had a positive effect on quality of life, distress, vitality (vigor), and depressive symptoms. However, these conclusions were based on results of single studies. Unfortunately, the discussion section provided by Kiepe and colleague is quite misleading. They state that dance/movement therapy has a positive impact on shoulder ROM and body image in cancer patients whereas no statistically significant between‐group differences were reported for these outcomes in the original study (Sandel 2005). Instead, Sandel's study reported significant changes over time for both the dance/movement therapy group and the control group but no difference between the groups over time differences.
Sandel's findings of a beneficial effect of dance/movement therapy on QoL (Sandel 2005) are supported by a non‐controlled pilot study in Germany (Mannheim 2005) that examined the effects of dance/movement therapy on quality of life, anxiety, depression and self‐worth in 77 women with cancer. This study used a one‐group pre‐test/post‐test design. Ninety‐minute dance/movement therapy group sessions were offered two to three times per week and most women (81%) participated in five to nine sessions. The results suggested that dance/movement therapy improved the women's quality of life (P < 0.001) as well as their anxiety (P < 0.001), depression (P < 0.001) and self worth (P < 0.001).
Authors' conclusions
Implications for practice.
Dance/movement therapy has been used with patients with cancer for provision of social support, reduction of stress, anxiety, depression and fatigue, improvement in role, social, emotional and physical functioning and enhancement of QoL variables such as spirituality and self‐esteem. The results of this review are based on two small‐scale trials and one moderately sized trial in women with breast cancer. The pooled effect of these studies did not find evidence for effect on depression, stress, anxiety, fatigue or body image. In contrast, the results of individual trials suggest that dance/movement therapy may be beneficial for QoL, somatization, and vigor in women with breast cancer. Data of individual studies included in this review did not find support for effect of dance/movement therapy on other outcomes included in this review such as mood, mental health, pain, ROM or arm circumference. The low drop‐out rate indicates that dance/movement therapy is well tolerated by these patients. However, in the absence of sufficient evidence, recommendations for clinical practice cannot be made at this time.
Implications for research.
The results of individual studies suggest that dance/movement therapy may have a beneficial effect on QoL, somatization and vigor in women with breast cancer. However, more RCTs are needed to strengthen this evidence. The limited number of RCTs in dance/movement therapy with cancer patients may be due to lack of research training and few funding sources for dance/movement therapy research. There are few opportunities for doctoral research training specifically in dance/movement therapy and thus currently not enough researchers prepared to obtain funding for, and carry out, high‐quality large‐scale outcome studies.
As this review did not find support for an effect of dance/movement therapy for several outcomes included in this review, researchers should consider whether the poor results from the reviewed studies are possibly due to the lack of sensitivity or inappropriateness of the outcome measures that were employed. Several dance/movement therapy researchers have emphasized the importance of using outcome measures that can capture the effect of a unique intervention such as dance/movement therapy (Bojner Horwitz 2006; Koch 2014; Meekums 2010). Koch and colleagues discuss the challenge of measuring the impact of nonverbal interventions with verbal intervention tools (Koch 2014).
Brevity of the treatment period is recognized as one of the possible causes of lack of treatment effect in dance/movement therapy research. The treatment duration in the three studies included in this review ranged from three weeks to 12 weeks. Future research should investigate optimal treatment dose and duration for dance/movement therapy interventions with people with cancer. This must take into consideration that dance/movement therapy, like other treatment options in the complementary and integrative therapy realm, has been identified as requiring “systematic therapeutic learning” (Cassileth 1994, p. 293) meaning that a period of initial learning to become conversant in the therapy medium (in this case expressive movement) is typically necessary before benefits are manifest and measurable.
Dance/movement therapy is not a manualized therapy and the necessarily improvisational clinical methods render it challenging for researchers to systematize the intervention. Dance/movement therapy researchers should develop ways to ensure treatment fidelity in RCTs while retaining the spontaneous, client‐centered properties of the therapy. Berrol, Ooi and Katz (Berrol 1997) have demonstrated that this can be done in a large multi‐site dance/movement therapy project with older adults.
Although we strongly recommend that more RCTs are needed, it is important that qualitative research and results of non‐controlled research be considered, as these enhance our understanding of the qualitative aspects of a patients' experience and identify factors that may contribute to, or limit, the effectiveness of dance/movement therapy interventions. In addition, mixed methodology is appropriate for investigating emerging therapies such as dance/movement therapy. The use of rigorous mixed‐method designs will both generate useful outcome data and provide insight as to the possible mechanisms of dance/movement therapy with cancer patients. Qualitative findings can yield more targeted hypotheses for future RCTs as well.
Sandel and colleagues recommend that future trials include an active control group (e.g. exercise group without music and dance) to further differentiate the particular benefits of dance/movement therapy. One such study of a short‐term dance/movement therapy intervention successfully controlled for the effects of exercise alone and music alone, demonstrating the benefits of interactive dance for the reduction of depression in psychiatric patients (Koch 2007).
Researchers need to consider examining the effects of dance/movement therapy with population groups other than women with breast cancer. Future studies should explore the utility of this modality for men with cancer as well as for women with other types of cancer. Furthermore, the influence of factors such as gender, age and culture should be carefully examined.
Future trials will also need to examine the relationship between frequency and duration of dance/movement therapy interventions and treatment effects. Researchers should also evaluate the impact of treatment timing relative to diagnosis and treatment stage.
It is important that future studies include power analysis so that adequate sample sizes are used.
Finally, formal evaluation of the cost and benefit of dance/movement therapy is needed.
What's new
| Date | Event | Description |
|---|---|---|
| 20 May 2021 | Review declared as stable | One new report has been identified with a scoping search up to 20 April 2020, and added to Studies awaiting classification. This new information is unlikely to change the conclusions of the review. This review is considered stable. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 7 May 2020 | Amended | One new report has been identified with a scoping search up to 20 April 2020, and added to Studies awaiting classification. This new information is unlikely to change the conclusions of the review. |
| 7 January 2015 | New search has been performed | Literature search updated, text revised and author list amended. |
| 7 January 2015 | New citation required but conclusions have not changed | One new included study, conclusions unchanged, new author added, one author removed and new outcomes included. |
Notes
New information is unlikely to change the conclusions of the review. This review is considered stable.
Acknowledgements
We would like to thank and acknowledge Dr Clare Jess (Managing Editor), Dr Chris Williams (Co‐ordinating Editor (retired)), Lesley Smith, Helen Payne, Amy Godfrey and RT Ho for their help and editorial advice during the preparation of the review. We would also like to acknowledge Patricia Gonzalez, graduate assistant at Temple University, for her help in the handsearching of journals and retrieval of articles and Allison Linn and Emily Headrick, research assistants at Drexel University, for their help with handsearching, screening search outputs and retrieving full text articles. Finally we would like to thank Dr Cheryl Dileo for her contributions as co‐author in the original review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Neoplasms] explode all trees #2 malignan* or neoplasm* or cancer* or carcinoma* or tumour* or tumor* #3 #1 or #2 #4 MeSH descriptor: [Dance Therapy] this term only #5 MeSH descriptor: [Dancing] this term only #6 dance or dancing #7 movement next therap* #8 #4 or #5 or #6 or #7 #9 #3 and #8
Appendix 2. MEDLINE search strategy (OvidSp)
1 exp Neoplasms/ 2 (malignan* or neoplasm* or cancer* or carcinoma* or tumour* or tumor*).mp. 3 1 or 2 4 Dance Therapy/ 5 Dancing/ 6 (dance or dancing).mp. 7 (movement adj therap*).mp. 8 4 or 5 or 6 or 7 9 3 and 8 key: mp = title, original title, abstract, name of substance word, subject heading word
Appendix 3. EMBASE search strategy (OvidSp)
1 exp Neoplasm/ 2 (malignan* or neoplasm* or cancer* or carcinoma* or tumour* or tumor*).mp. 3 1 or 2 4 dance therapy/ 5 dancing/ 6 (dance or dancing).mp. 7 (movement adj therap*).mp. 8 4 or 5 or 6 or 7 9 3 and 8
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. CINAHL search strategy (EBSCO)
S8 S3 and S7 S7 S4 or S5 or S6 S6 MW dance S5 MH dance therapy S4 TX (dance OR (dance therapy) OR (dance/movement therapy) OR (movement therapy)) S3 S1 or S2 S2 TX (malignan$ or neoplasm$ or cancer or carcinoma$ or tumo$) S1 MH neoplasms
Appendix 5. PsycInfo search strategy (EBSCO)
| S9 | S8 |
| S8 | S3 and S7 |
| S7 | S4 or S5 or S6 |
| S6 | DE "Dance" |
| S5 | DE "Dance Therapy" |
| S4 | TX (dance OR (dance therapy) OR (dance/movement therapy) OR (movement therapy)) |
| S3 | S1 or S2 |
| S2 | TX (malignan$ or neoplasm$ or cancer or carcinoma$ or tumo$) |
| S1 | DE "Neoplasms" OR DE "Benign Neoplasms" OR DE "Breast Neoplasms" OR DE "Endocrine Neoplasms" OR DE "Leukemias" OR DE "Nervous System Neoplasms" OR DE "Terminal Cancer" |
Appendix 6. LILACS search strategy (Virtual Health Library)
((dance or "dance therapy" or "dance/movement therapy")) and ((((malignan$ or neoplasm$ or "CANCER" or carcinoma$ or tumo$)) or (("cancer"))))
Appendix 7. The Science Citation Index (ISI)
1. TS = neoplasm*2. TS=(malignan* or neoplasm* or cancer or carcinoma* or tumour or tumor) 3. #2 OR #1 4. TS=(dance OR (dance therapy) OR (dance/movement therapy) OR (movement therapy)) 5. #3 AND #4
Appendix 8. CancerLit search strategy
(((dance) OR dance/movement therapy[Title/Abstract]) OR dance movement therapy[Title/Abstract]) OR dance[Text Word] Limits: Cancer
Appendix 9. International Bibliography of Theatre and Dance search strategy
S1 MH neoplasms S2 TX (malignan$ or neoplasm$ or cancer or carcinoma$ or tumo$) S3 S1 or S2 S4 TX (dance OR (dance therapy) OR (dance/movement therapy) OR (movement therapy)) S5 MH dance therapy S6 S4 or S5 S7 S3 and S6 S8 Limiters ‐ Scholarly (Peer Reviewed) Journals Narrow by Journal6: ‐ Journal of Dance Medicine & Science Narrow by Journal5: ‐ Dance Chronicle Narrow by Journal4: ‐ Dance Research Journal Narrow by Journal3: ‐ Body, Movement & Dance in Psychotherapy Narrow by Journal2: ‐ American Journal of Dance Therapy Narrow by Journal3: ‐ Journal of Dance Education Narrow by Journal2: ‐ PAJ: A Journal of Performance & Art Narrow by Journal1: ‐ Body, Movement & Dance in Psychotherapy Narrow by Journal0: ‐ American Journal of Dance Therapy
Appendix 10. Proquest Digital Dissertations search strategy (Proquest)
(TI(dance or "dance therapy" or "dance/movement therapy") or AB(dance or "dance therapy" or "dance/movement therapy")) AND (TI(cancer or tumor or malignant or neoplasm) or AB(cancer or tumor or malignant or neoplasm))
Appendix 11. clinicaltrials.gov search strategy
dance or "dance therapy" or "dance/movement therapy"
Appendix 12. Current Controlled Trials search strategy
dance or "dance therapy" or "dance/movement therapy"
Appendix 13. National Research Register search strategy
dance or "dance therapy" or "dance/movement therapy"
Appendix 14. Study Selection, Quality Assessment & Data Extraction Form
Name coder:
Date:
Paper code:
| First author | Title | Journal/Conference Proceedings etc | Year | Language |
Other references to trial
If there are further references to this trial, link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan. (main paper should be [number]A; other publications related to the same trial should be [same number]B)
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year | Language |
Study Design
|
Study Design (circle or highlight): 2‐arm parallel group 3‐arm parallel group cross‐over trial Briefly describe experimental and control group/condition interventions: Experimental group: Control group: |
Participants and trial characteristics
| Participant characteristics | |
| Age (mean, median, range) | Experimental: Control: Total: Range: |
| Sex of participants (list n or %) | Experimental: F M Control: F M Total: F M |
| Ethnicity (list n or %, if available) | |
| Diagnosis/Disease status (list n or % per diagnosis, if available) | |
| Setting (please circle) | Inpatient Outpatient Other: |
Methodological quality
| Method of Randomization | |
| Was the trial reported as randomized? | Yes No |
| Random sequence generation | Low risk Unclear risk High risk |
| State here randomization method used and reasons for grading (circle ): | |
| 1. Computer‐generated number list 2. Table of random numbers 3. Draw of lots 4. Flip coin 5. Systematic, please specify: 6. other: |
|
| Concealment of allocation | |
| Concealment of allocation | Low risk Unclear risk High risk |
| State here the method used to conceal allocation and reasons for grading | |
| 1. Opaque sealed envelopes 2. Central randomization 3. Alteration method 4. Other___________________________________________ | |
Low risk: (1) central randomization, (2) serially numbered opaque envelopes, (3) other descriptions with convincing concealment
High risk: (1) alteration methods, (2) other manners in which allocation was not adequately concealed
Unclear risk: authors did not adequately report on method of concealment used
| Blinding | |
| Blinding of study participants and dance/movement therapist | Low risk Unclear risk High risk |
| Blinding of outcome assessor(s) for objective outcomes | Low risk Unclear risk High risk |
| Blinding of outcome assessor(s) for subjective outcomes | Low risk Unclear risk High risk |
| Intention‐to‐treat | |
Number of withdrawals: Were withdrawals described? Yes No ? not clear ? Please add reasons for withdrawal + n or % here: |
Low risk Unclear risk High risk |
| Selective Reporting | |
|
Low risk Unclear risk High risk |
| Other Sources of Bias | |
| Are studies free of other problems that could have put them at high risk of bias (e.g. financial conflict of interest)? Please list other sources of bias: |
Low risk Unclear risk High risk |
| Data Reporting | |
| Is data reporting sufficient for inclusion in review (are means and SD for each outcome variable reported for experimental group/condition and for control group/condition)? If no, please detail what type of data is available: |
Yes / No |
Data extraction
| Outcomes relevant to your review | |||
| Reported in paper (circle) | Reported in paper (circle) | ||
| Psychological outcomes (depression, anxiety, etc) | Yes / No | Communication | Yes / No |
| Physical outcomes (pain, nausea) | Yes / No | Disease‐free survival | Yes / No |
| Physiological Outcomes (HR, RR, AP, SBP, DBP) | Yes / No | Social outcomes | Yes / No |
| Quality of Life | Yes / No | Body image | Yes / No |
| For Continuous data | ||||||||||
| Code of paper |
Outcomes |
Unit of measurement or scale used |
Intervention group | Control group | If mean(SD) are not reported, report either: ‐ t‐value and/or p‐value associated with t‐test ‐ SE of means calculated from within group ‐ confidence interval of means from within group ‐ description of results in text DETAIL whether pre‐test scores were significantly different. |
|||||
| n | Mean (SD) List pre‐test and post‐test values (and change scores, if available) |
n | Mean (SD) List pre‐test and post‐test values (and change scores, if available) |
|||||||
| Depression | ||||||||||
| Anxiety | ||||||||||
| Anger | ||||||||||
| Hopelessness | |
|||||||||
| Helplessness | ||||||||||
| Other psychological: | ||||||||||
| Other psychological: | ||||||||||
| Quality of Life | ||||||||||
| Fatigue | ||||||||||
| Nausea | ||||||||||
| Pain | ||||||||||
| Heart Rate | ||||||||||
| Respiratory Rate | ||||||||||
| Arterial Pressure | ||||||||||
| Systolic Blood Pressure | ||||||||||
| Diastolic Blood Pressure | ||||||||||
| Cortisol Levels | ||||||||||
| IgA Levels | ||||||||||
| Range of Motion | ||||||||||
| Other physical: | ||||||||||
| Social support. Specify: | ||||||||||
| Communication. Specify: | ||||||||||
| Disease‐free survival | ||||||||||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
| |
DMT Intervention
| Type of intervention used | |
| Group or individual therapy? | |
| Give detailed description of intervention used | |
| Intensity |
Number of sessions: Duration of each session: Time period over which sessions were spread for one patient/group (State weeks / months, etc, if cross‐over trial give length of time in each arm): |
| Trial characteristics: Further details | |
| Single centre / multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group (circle groups that are used for this review if 3‐arm parallel group) | Exp.group: Control: |
| Number of participants who received intended treatment |
Exp.group: Control: |
| Number of participants who were analyzed |
Exp.group: Control: |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Other |
|
Acknowledgements: We’d like to thank the Cystic Fibrosis Group for permission to modify their data extraction form.
References: Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001 Jul 7;323(7303):42‐6.
Appendix 15. Original search strategies
CENTRAL search strategy (2011 review)
#1 MeSH descriptor Neoplasms explode all trees #2 (malignan* or neoplasm* or cancer* or carcinoma* or tumour* or tumor*) in Clinical Trials #3 (#1 OR #2) #4 MeSH descriptor Dance Therapy explode all trees #5 MeSH descriptor Dancing explode all trees #6 (dance or dancing) #7 (movement next therap*) #8 (#4 OR #5 OR #6 OR #7) #9 (#3 AND #8)
MEDLINE search strategy (OvidSp)
1. Neoplasms/ 2. (malignan$ OR neoplasm$ OR cancer OR carcinoma$ OR tumour OR tumor).tw 3. 1 or 2 4. dance/ or dance therapy/ 5. danc$.tw. 6. (dance therapy).tw 7. (movement therapy).tw 8. ((dance OR movement) adj5 therapy).tw 9. or/4‐8 10. Randomized Controlled Trials/ 11. random allocation/ 12. Controlled Clinical Trials/ 13. control groups/ 14. clinical trials/ 15. double‐blind method/ 16. single‐blind method/ 17. Placebos/ 18. placebo effect/ 19. cross‐over studies/ 20. Multicenter Studies/ 21. Therapies, Investigational/ 22. Research Design/ 23. Program Evaluation/ 24. evaluation studies/ 25. randomized controlled trial.pt. 26. controlled clinical trial.pt. 27. clinical trial.pt. 28. multicenter study.pt. 29. evaluation studies.pt. 30. random$.tw. 31. (controlled adj5 (trial$ or stud$)).tw. 32. (clinical$ adj5 trial$).tw. 33. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 34. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 35. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 36. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 37. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 38. (coin adj5 (flip or flipped or toss$)).tw. 39. latin square.tw. 40. (cross‐over or cross over or crossover).tw. 41. placebo$.tw. 42. sham.tw. 43. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 44. controls.tw. 45. (treatment$ adj6 order).tw. 46. or/10‐45 47. 3 and 9 and 46 48. limit 47 to humans
EMBASE search strategy (Emtree)
#1 'neoplasm'/exp #2 malignan* OR neoplasm* OR cancer* OR carcinoma* OR tumour* OR tumor* #3 #1 OR #2 #4 ' dance therapy'/exp OR 'dance therapy' #5 'dancing'/exp OR 'dancing' #6 'dance'/exp OR dance OR 'dancing'/exp OR dancing OR 'dance/movement therapy' #7 #4 OR #5 OR #6 #8 #3 AND #7
PsycInfo Search strategy (OvidSp)
1. Neoplasms/ 2. (malignan$ or neoplasm$ or cancer or carcinoma$ or tumour or tumor).tw. 3. 1 or 2 4. dance/ or dance therapy/ 5. danc$.tw. 6. dance therapy.tw. 7. movement therapy.tw. 8. ((dance or movement) adj5 therapy).tw. 9. or/4‐8 10. empirical study.md. 11. followup study.md. 12. longitudinal study.md. 13. prospective study.md. 14. quantitative study.md. 15. "2000".md. 16. treatment effectiveness evaluation/ 17. exp hypothesis testing/ 18. repeated measures/ 19. exp experimental design/ 20. placebo$.ti,ab. 21. random$.ti,ab. 22. (clin$ adj25 trial$).ti,ab. 23. ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 24. or/10‐23 25. 3 and 24 and 9
The Science Citation Index (ISI)
1. TS = neoplasm* 2. TS=(malignan* or neoplasm* or cancer or carcinoma* or tumour or tumor) 3. #2 OR #1 4. #3 AND #4
Data and analyses
Comparison 1. Dance/movement Therapy versus Control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Depression | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.28, 0.32] |
| 1.2 Stress | 2 | 170 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.48, 0.12] |
| 1.3 Anxiety | 2 | 170 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.21 [‐0.09, 0.51] |
| 1.4 Fatigue | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.26, 0.55] |
| 1.5 Body Image | 2 | 68 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐0.61, 0.34] |
1.4. Analysis.

Comparison 1: Dance/movement Therapy versus Control, Outcome 4: Fatigue
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dibbell‐Hope 1989.
| Study characteristics | ||
| Methods | Quasi‐RCT 2‐arm wait‐list control group design |
|
| Participants | Women with breast cancer, stage I or stage II who completed treatment 6 to 60 months prior to study. 81% of the women had a modified radical mastectomy as the primary treatment. Other treatments included chemotherapy (21%), radiation (19%) and reconstruction (10%). 60% of the participants had completed treatment 24 to 60 months prior to the study. Mean age: 54.7 years N randomized: 33 (n of each group unclear) N analyzed for dance/movement therapy group: 15 N analyzed for control group: 16 Ethnicity: 90% Caucasian Setting: Churches Country: US |
|
| Interventions | Two study groups: 1. Dance/movement therapy group: Authentic Movement 2. Control groups: Wait‐list control The study was carried out at two sites resulting in four groups Number of sessions: 6 Length of sessions: 3 hours |
|
| Outcomes | Mood (Profile of Mood States), distress (Symptom Check List‐90‐Revised), body Image (Borscheid, Walster, Bohrnstedt Body‐Image Scale, 25‐item version)(Borscheid 1972), self‐esteem (Marlowe‐Crowne Social Desirability Scale)(Crowne 1960): post‐test scores per site. | |
| Notes | Mean post‐test scores and pooled SD for the two sites combined were computed by JB. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate assignment (personal communication with chief investigator) |
| Allocation concealment (selection bias) | High risk | Alternate assignment prohibited adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and therapist is not possible in dance/movement therapy interventions unless a comparative design is used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Study did not include objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | All subjective outcomes were measured via self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data of two women were eliminated because of extreme scores |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting for this study |
| Free from financial conflict of interest? | Low risk | No funding support |
Ho 2014.
| Study characteristics | ||
| Methods | RCT 2‐arm parallel group design |
|
| Participants | Adult patients with breast cancer, stage 0 (n = 9), stage 1 (n = 35), stage 2 (n = 58), stage 3 (n = 33), stage unknown (n = 4) Mean age: 49 years N randomized: 147 N analyzed for dance/movement therapy group: 69 N analyzed for control group: 70 Ethnicity: 100% Chinese Setting: Outpatient Country: Hong Kong |
|
| Interventions | Two study groups: 1. Dance/movement therapy group: The DMT program consisted of six 90‐minute long DMT sessions held twice a week for three consecutive weeks. The program contents were specially tailored to meet the needs of breast cancer patients, including guided movements to exercise upper extremities, improvisational dance and movement to explore positive emotions and for fun, as well as group sharing to relate the movement process to participants’ personal experiences of breast cancer and cancer treatment. 2. Control group: standard care Number of sessions: 6 Length of sessions: 90 min |
|
| Outcomes | Perceived stress (Perceived Stress Scale), anxiety (Hospital Anxiety and Depression Scale), depression (Hospital Anxiety and Depression Scale), fatigue (Brief Fatigue Inventory), and pain severity (Brief Pain Inventory): post‐test scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization using a computer‐generated list of random numbers (personal communication with chief investigator) |
| Allocation concealment (selection bias) | Low risk | Recruiter was unaware of randomization schedule (personal communication with chief investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and therapist is not possible in dance/movement therapy interventions unless a comparative design is used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No objective outcomes were included in this study |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | All subjective outcomes were measured via self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight women withdrew after randomization, nine patients dropped out during the study. Reasons for dropout include a lack of time or health issues (personal communication with chief investigator) |
| Selective reporting (reporting bias) | Unclear risk | Data on quality of life outcome are yet to be analyzed and are not included in this review (personal communication with chief investigator) |
| Free from financial conflict of interest? | Low risk | This study was funded by the Hong Kong Research Grants Council's General Research Fund (HKU745110H) |
Sandel 2005.
| Study characteristics | ||
| Methods | RCT 2‐arm wait‐list control group design |
|
| Participants | Women with breast cancer who had lumpectomy or more extensive breast surgery within 5 years of the onset of the study Mean age: 59.6years N randomized to dance/movement therapy group: 19 N randomized to control group: 19 N analyzed for dance/movement therapy group: 19 N analyzed for control group: 18 Ethnicity: Not reported Setting: Out‐patient Country: US |
|
| Interventions | Two study groups: 1. Dance/movement therapy group: movement intervention based on The Lebed methodTM, Focus on Healing through Movement and Dance. 2. Control group: wait‐list control group Number of sessions: 18 Length of sessions: 50 to 60 minutes |
|
| Outcomes | Quality of life (Functional Assessment of Cancer Therapy—Breast questionnaire) (Brady 1997), shoulder ROM, arm circumference, body image (The body Image Scale) (Hopwood 2001): post‐test scores | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was done by computer‐generated random numbers, with a separate list for each cancer center." (p.303) |
| Allocation concealment (selection bias) | Low risk | "Sequential sealed envelopes were opened at the conclusion of baseline testing."(p.303) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and therapist is not possible in dance/movement therapy interventions unless a comparative design is used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | "Shoulder ROM and arm circumferences were measured by an experienced physical therapist who was blind to group assignment." (p.304) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | Blinding was not possible for subjective outcomes (quality of life, body image) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients dropped out due to fatigue, other commitment or shoulder discomfort that was unrelated to the dance/movement therapy treatment. |
| Selective reporting (reporting bias) | Low risk | There are no indications of selective reporting of outcomes |
| Free from financial conflict of interest? | Low risk | Supported by the State of Connecticut Breast Cancer Research and Education Income Tax Check‐Off Fund. |
DMT: dance/movement therapy N: number RCT: randomized controlled trial ROM: range of motion SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chiquiar 1988 | Not RCT or CCT |
| Choi 2003 | Not RCT or CCT |
| Crane‐Okada 2012 | Intervention not implemented by a trained dance/movement therapist |
| Goldov 2011 | Unacceptable treatment allocation method: patients self‐selected into experimental or control group |
| Ho 2005a | Not RCT or CCT |
| Ho 2005b | Not RCT or CCT |
| Ho 2007 | Not RCT or CCT |
| Ho 2008 | Not RCT or CCT |
| Kaltsatouemail 2011 | Not dance/movement therapy intervention |
| Kanitz 2013 | Not dance/movement therapy intervention |
| Mannheim 2005 | Not RCT or CCT |
| Mannheim 2013 | Not RCT or CCT |
| Moskow 1996 | Not RCT or CCT |
| Perry‐Griffin 1989 | Not RCT or CCT |
| Pilarski 2008 | Not RCT or CCT |
| Schröder 2007 | Not RCT or CCT |
| Serlin 1997 | Not RCT or CCT |
| Shin 2009 | Not RCT or CCT |
| Sturm 2014 | Not dance/movement therapy intervention |
| Yuval 1995 | Not RCT or CCT |
CCT: controlled clinical trial RCT: randomized controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Ho 2018.
| Methods | RCT |
| Participants | Adult breast cancer patients |
| Interventions | Dance/movement therapy |
| Outcomes | Self‐report measures of perceived stress, fatigue, pain, and sleep disturbance and five salivary cortisol samples at baseline and post‐intervention. |
| Notes | Secondary data analysis of Ho 2014. |
Differences between protocol and review
We had planned to only include clinical trials that used appropriate methods of randomization. However, due to a limited number of available studies, we considered trials that used quasi‐randomized or systematic methods of treatment allocation for this review.
In the protocol, it was stated that the Trials Register of the Cochrane Cancer Network would be searched. However, that register is no longer functional.
Because of conversion from Revman 5.0 to Revman 5.3 the ‘Risk of bias' tool was changed during the preparation of the review as well as the 'Study selection, quality assessment and data extraction form' (Appendix 14).
Contributions of authors
Draft the protocol: Bradt (reviewed and approved by Dileo)
Search strategies, methods: Bradt (reviewed and approved by Dileo) Database searches and handsearches: Bradt, Goodill, Shim and graduate assistants Screening search results: Bradt, Shim and research assistants Organizing retrieval of papers: Bradt Screening retrieved papers against inclusion criteria: Bradt and research assistants Appraising quality of papers: Bradt and Goodill Abstracting data from papers: Bradt and research assistants Writing to authors of papers for additional information: Bradt and Goodill Providing additional data about papers: Bradt Obtaining and screening data on unpublished studies: Bradt and Goodill Data management for the review: Bradt Entering data into Review Manager: Bradt and Shim RevMan statistical data: Bradt Other statistical analysis not using RevMan: Bradt Interpretation of data: Bradt,Goodill, and Shim Statistical inferences: Bradt Writing the review: Bradt, Goodill and Shim Securing funding for the review: N/A Guarantor for the review (one author): Bradt Person responsible for reading and checking review before submission: Bradt
Sources of support
Internal sources
None, Other
External sources
None, Other
Declarations of interest
Two authors (Goodill and Shim) are dance/movement therapists.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Dibbell‐Hope 1989 {published and unpublished data}
- Dibbell-Hope S. Moving toward health: A study of the use of dance-movement therapy in the psychological adaptation to breast cancer. Doctoral Dissertation, California School of Professional Psychology, US 1989. [303682073]
- Dibbell-Hope S. The use of dance/movement therapy in psychological adaptation to breast cancer. Arts in Psychotherapy 2000;27(1):51-68. [DOI: 10.1016/S0197-4556(99)00032-5 ] [Google Scholar]
Ho 2014 {unpublished data only}
- Ho R. Randomized controlled trial of a dance/movement therapy program for breast cancer patients undergoing adjuvant radiotherapy: Effects on fatigue, pain and perceived stress. Personal communication with lead investigator.
- Ho RT, Lo PH, Luk MY. A good time to dance? a mixed-methods approach of the effects of dance movement therapy for breast cancer patients during and after radiotherapy. Cancer Nursing 2016;39(1):32-41. [DOI: 10.1097/NCC.0000000000000237] [DOI] [PubMed] [Google Scholar]
- Ho RTH, Cheung IKM, Leung ASL, Chan KP, Lo PHY, Chan CLW, et al. Dance/movement-based psychotherapy program: Its effect and impact on reducing radiotherapy-related symptoms and improving the quality of life in Chinese breast cancer patients. The 47th Annual Conference of the American Dance Therapy Association (ADTA), Albuquerque, NM., 11-14 October 2012. In: American Journal of Dance Therapy. Vol. 35. 2013:33-4.
- Ho RTH, Fong T. Effects of dance/movement therapy on the psychological distress in breast cancer patients undergoing radiotherapy. Abstracts from the 2013 48th Annual American Dance Therapy Association Research and Thesis Poster Session. In: American Journal of Dance Therapy. Vol. 36. 2014:54-5.
Sandel 2005 {published data only}
- Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality-of-life measures in breast cancer survivors. Cancer Nursing 2005;28(4):301-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chiquiar 1988 {published data only}
- Chiquiar M. Cancer: Working through anger and pain with authentic movement and entertainment and music. In: Presentation at the 23rd National Conference of American Dance Therapy Association. Baltimore, MD: American Dance Therapy Association, 1988.
Choi 2003 {unpublished data only}
- Choi H. Dance therapy for children who have bone marrow transplant. Master's Thesis, Pratt Institute, US 2003.
Crane‐Okada 2012 {published data only}
- Crane-Okada R, Kiger H, Sugerman F, Uman GC, Shapiro SL, Wyman-McGinty W, et al. Mindful movement program for older breast cancer survivors: A pilot study. Cancer Nursing 2012;35(4):E1-13. [DOI] [PubMed] [Google Scholar]
Goldov 2011 {unpublished data only}
- Goldov N. The effect of medical dance/movement therapy on body image wellness in women with breast cancer. Doctoral Dissertation, Argosy University, Seattle,US 2011.
Ho 2005a {published data only}
- Ho RTH. Dance movement therapy for Chinese cancer patients: A pilot group in Hong Kong. In: American Journal of Dance Therapy. Vol. 27. 2005:27. [DOI: 10.1007/s10465-005-6090-8] [DOI]
Ho 2005b {published data only}
- Ho RTH. Regaining balance within: Dance movement therapy with Chinese cancer patients in Hong Kong. In: American Journal of Dance Therapy. Vol. 27. 2005:87-99. [DOI: 10.1007/s10465-005-9002-z]
Ho 2007 {published data only}
- Ho RTH. Differential benefits of dance and dance/movement therapy for cancer patients. In: American Journal of Dance Therapy. Vol. 29. 2007:31-2. [DOI: 10.1007/s10465-007-9030-y] [DOI]
Ho 2008 {published data only}
- Ho RTH. Dance/movement therapy improves mental health in cancer patients. In: American Journal of Dance Therapy. Vol. 30. 2008:41-2. [DOI: 10.1007/s10465-008-9046-y] [DOI]
Kaltsatouemail 2011 {published data only}
- Kaltsatouemail A, Mameletzi D, Douka S. Physical and psychological benefits of a 24-week traditional dance program in breast cancer survivors. Journal of Bodywork and Movement Therapies 2011;15(2):162-7. [DOI] [PubMed] [Google Scholar]
Kanitz 2013 {published data only}
- Kanitz JL, Pretzer K, Calaminus G, Wiener A, Langler A, Henze G, et al. Eurythmy therapy in the aftercare of pediatric posterior fossa tumour survivors: A pilot study. Complementary Therapies in Medicine 2013;21(Suppl 1):S3-9. [DOI] [PubMed] [Google Scholar]
Mannheim 2005 {published data only}
- Mannheim EG, Weis J. Dance therapy with cancer patients: Results of a pilot study. Musik, Tanz, und Kunsttherapie 2005;16(3):121-8. [Google Scholar]
Mannheim 2013 {published data only}
- Mannheim EG, Helmes A, Weis J. Dance/movement therapy in oncological rehabilitation. Forsch Komplementmed 2013;20(1):33-41. [DOI] [PubMed] [Google Scholar]
Moskow 1996 {unpublished data only}
- Moskow J. Dance therapy as an intervention in breast cancer patients. Master's Thesis. Hunter College, US 1996.
Perry‐Griffin 1989 {unpublished data only}
- Perry-Griffin K. Dance/movement therapy and the effects on body image with women who have had a mastectomy. Master's Thesis. Goucher College, US 1989.
Pilarski 2008 {unpublished data only}
- Pilarski D. The experience of young women diagnosed with breast cancer involved in dance/movement therapy with regards to body image and sexuality. Master's Thesis. Drexel University. US 2008.
Schröder 2007 {published data only}
- Schröder A. Dance therapy for women with breast cancer [Tanztherapie für Mammakarzinom-Patientinnen]. Diagnostik + Therapie 2007;48:2-6. [Google Scholar]
Serlin 1997 {published data only}
- Serlin IA, Frances B, Vestevich K, Bailey T, Lavaysse L. The effect of dance/movement therapy on women with breast cancer. Alternative Therapies in Health and Medicine 1997;3:103. [Google Scholar]
Shin 2009 {unpublished data only}
- Shin S. The effect of dance/movement therapy on the body image and depression of the women with breast cancer. Master’s Thesis. Seoul Women’s University, Korea 2009.
Sturm 2014 {published data only}
- Sturm I, Baak J, Storek B, Traore A, Thuss-Patience P. Effect of dance on cancer-related fatigue and quality of life. Supportive Care in Cancer 2014;22(8):2197-206. [DOI] [PubMed] [Google Scholar]
Yuval 1995 {unpublished data only}
- Yuval M. Dance movement therapy with pediatric oncology patients. Master's Thesis. Hunter College. US 1995.
References to studies awaiting assessment
Ho 2018 {published data only}
- Ho RTH, Fong TCT, Yip PSF. Perceived stress moderates the effects of a randomized trial of dance movement therapy on diurnal cortisol slopes in breast cancer patients. Psychoneuroendocrinology 2018;87:119-26. [DOI: 10.1016/j.psyneuen.2017.10.012] [DOI] [PubMed] [Google Scholar]
Additional references
ADTA
- ADTA. American Dance Therapy Association website. http://www.adta.org/.
Berrol 1997
- Berrol CF, Ooi WL, Katz SS. Dance/movement therapy with older adults who have sustained a neurological insult: a demonstration project. American Journal of Dance Therapy 1997;19(2):135-60. [Google Scholar]
Bojner Horwitz 2006
- Bojner Horwitz E, Kowalski J, Theorell T, Anderberg UM. Dance/movement therapy in fibromyalgia patients: Changes in self-figure drawings and their relation to verbal self-rating scales. The Arts in Psychotherapy 2006;33(1):11-25. [Google Scholar]
Borscheid 1972
- Borscheid E, Walster E, Bohrnstedt G. Body image scale. Psychology Today 1972;6:58-64. [Google Scholar]
Brady 1997
- Brady MJ, Cella DF, Mo F. Reliability and validity of the functional assessment of cancer therapy–breast quality-of- life instrument. Journal of Clinical Oncology 1997;15:974-86. [DOI] [PubMed] [Google Scholar]
Bräuninger 2012
- Bräuninger I. The efficacy of dance movement therapy group on improvement of quality of life: A randomized controlled trial. The Arts in Psychotherapy 2012;39:296-303. [Google Scholar]
Cassileth 1994
- Cassileth B, Jonas W, Cassidy CM. Research methodologies. In: Berman BM, Larson DB, editors(s). Alternative Medicine: Expanding Medical Horizons: A Report to the NIH on Alternative Medical Systems and Practices in the US, Prepared under the auspices of the Workshop on Alternative Medicine. Washington, D.C.: Government Printing Office, National Institutes of Health, 1994:289-98. [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edition. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
Crowne 1960
- Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. Journal of Consulting Psychology 1960;24:349-54. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
Derogatis 1979
- Derogatis LR. Breast and gynecologic cancers. Their unique impact on body image and sexual identity in women. Frontiers of Radiation Therapy in Oncology 1979;14:1-11. [DOI] [PubMed] [Google Scholar]
Dibbell‐Hope 2000
- Dibbell-Hope S. The use of dance/movement therapy in psychological adaptation to breast cancer. The Arts in Psychotherapy 2000;27(1):51-68. [Google Scholar]
Duivenvoorden 1997
- Duivenvoorden HJ. Psychological sequelae of cancer diagnosis: A meta-analytical review of 58 studies. Psychosomatic Medicine 1997;59:280-93. [DOI] [PubMed] [Google Scholar]
Erhardt 1989
- Erhardt BT, Hearn MB, Novack C. Clients' attitudes towards healing processes in dance therapy. American Journal of Dance Therapy 1989;11:39-60. [Google Scholar]
Goodill 2005
- Goodill SW. Research Letter: Dance/movement therapy for adults with cystic fibrosis: Pilot data on mood and adherence. Alternative Therapies in Health & Medicine 2005;11(1):76-7. [PubMed] [Google Scholar]
Goodill 2006
- Goodill SW. Dance/movement therapy for people living with medical illness. In: Koch SC, Brauninger I, editors(s). Advances in Dance/Movement Therapy. Theoretical Perspectives and Empirical Findings. Berlin: Logos Verlag, 2006:52-60. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. Available from www.cochrane-handbook.org.
Hopwood 2001
- Hopwood P, Fletcher I, Lee A. A body image scale for use with cancer patients. European Journal of Cancer 2001;37:189-97. [DOI] [PubMed] [Google Scholar]
Jeong 2005
- Jeong YL, Hong SC, Lee MS, Park MC, Kim YK, Suh CM. Dance movement therapy improves emotional responses and modulates neurohormones in adolescents with mild depression. International Journal of Neuroscience 2005;115(12):1711-20. [DOI] [PubMed] [Google Scholar]
Kiepe 2012
- Kiepe MS, Stockigt B, Keil T. Effects of dance therapy and ballroom dances on physical and mental illnesses: A systematic review. The Arts in Psychotherapy 2012;39(5):404-11. [Google Scholar]
Kleinman 2006
- Kleinman S, Hall T. Dance/movement therapy: A method for embodying emotions. In: Davis W, Kleinman S, editors(s). The Renfrew Center Foundation Healing Through Relationship Series: Contributions to Eating Disorder Theory and Treatment. Fostering Body-Mind Integration. Philadelphia, PA: The Renfrew Center Foundation, 2006. [Google Scholar]
Koch 2007
- Koch SC, Morlinghaus K, Fuchs T. The joy dance. Specific effect of a single dance intervention on psychiatric patiens with depression. The Arts in Psychotherapy 2007;34:340-9. [Google Scholar]
Koch 2014
- Koch SC, Kunz T, Kolter A, Lykou S, Cruz R. Effects of dance movement therapy and dance on psychological outcomes: A meta-analysis. The Arts in Psychotherapy 2014;41:46-64. [Google Scholar]
Krantz 2007
- Krantz A, Pennebaker JW. Expressive dance, writing, trauma, and health: When words have a body. In: Serlin IA Sonke-Henderson J, Brandman R, Graham-Pole J, editors(s). Whole Person Healthcare. Vol. 3. Westport, CT: Praeger Publishers, 2007:201-29. [Google Scholar]
Lev 2009
- Lev EL, Eller LS, Gejerman G, Kolassa J, Colella J, Pezzino J, et al. Quality of life of men treated for localized prostate cancer: outcomes at 6 and 12 months. Support Care Cancer 2009;17(5):509-17. [DOI] [PubMed] [Google Scholar]
Mannheim 2006
- Mannheim E, Weis J. Dance/movement therapy with cancer patients. Evaluation of process and outcome parameters. In: Koch SC, Brauninger I, editors(s). Advances in Dance/Movement Therapy. Theoretical Perspectives and Empirical Findings. Berlin: Logos Verlag, 2006:61-72. [Google Scholar]
Massie 2004
- Massie MJ. Prevalence of depression in patients with cancer. Journal of the National Cancer Institute. Monographs 2004;32:57-71. [DOI] [PubMed] [Google Scholar]
McKibben 1988
- McKibben H. Dance-movement therapy with the medical population. In: Presentation at the 23rd National Conference of ADTA. Baltimore, MD, 1988.
McNair 1971
- McNair PM, Lorr M, Droppleman L. EITS manual for the profile of mood states. San Diego: Educational and Industrial Testing Services, 1971. [Google Scholar]
Meekums 2010
- Meekums B. Moving towards evidence for dance movement therapy: Robin Hood in dialogue with the King. The Arts in Psychotherapy 2010;37(1):35-41. [Google Scholar]
Norton 2004
- Norton TR, Manne SL, Rubin S, Carlson J, Hernandez E, Edelson MI, et al. Prevalence and predictors of psychological distress among women with ovarian cancer. Journal of Clinical Oncology 2004;22(5):919-26. [DOI] [PubMed] [Google Scholar]
Parle 1996
- Parle M, Jones B, Maguire P. Maladaptive coping and affective disorders among cancer patients. Psychological Medicine 1996;26:735-44. [DOI] [PubMed] [Google Scholar]
Porter 2008
- Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: Associations with symptoms and distress. Pain 2008;137(2):306-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raison 2003
- Raison CL, Miller AH. Depression in cancer: New developments regarding diagnosis and treatment. Biological Psychiatry 2003;54:283-94. [DOI] [PubMed] [Google Scholar]
Revman 5.3
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen. The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rottman 2010
- Rottmann N, Dalton SO, Christensen J, Frederiksen K, Johansen C. Self-efficacy, adjustment style and well-being in breast cancer patients: a longitudinal study. Quality of Life Research 2010;19(6):827-36. [DOI] [PubMed] [Google Scholar]
Sellick 1999
- Sellick SM, Crooks DL. Depression and cancer: An appraisal of the literature for prevalence, detection, and practice guideline development for psychological interventions. Psycho-Oncology 1999;8:315-33. [DOI] [PubMed] [Google Scholar]
Shim 2014
- Shim M. The use of creative arts therapies for chronic pain management: embodied interventions. In: Presentation at the 33rd Scientific Meeting of the American Pain Society. Tampa, FL, 2014.
Stromsted 2001
- Stromsted T. Re-inhabiting the female body: Authentic Movement as a gateway to transformation. The Arts in Psychotherapy 2001;28:39-55. [Google Scholar]
Ware 1994
- Ware J. SF-36 Physical and Mental Health Summary Scales: A Users’ Manual. Boston: The Health Institute, 1994. [Google Scholar]
References to other published versions of this review
Bradt 2011
- Bradt J, Goodill SW, Dileo C. Dance/movement therapy for improving psychological and physical outcomes in cancer patients. Cochrane Database of Systematic Reviews 2011, Issue 10. Art. No: CD007103. [DOI: 10.1002/14651858.CD007103.pub2] [DOI] [PubMed] [Google Scholar]


