Abstract
Background:
It is hypothesised that cotargeting the androgen receptor (AR) and paracrine androgen biosynthesis with enzalutamide and abiraterone acetate in metastatic castration-resistant prostate cancer (mCRPC) will dissipate adaptive feedback loops observed with either agent alone.
Objective:
To assess the safety, efficacy, androgen signalling/metabolome, and drug-drug interactions (DDIs) of enzalutamide with abiraterone acetate in progressive bone mCRPC (bmCRPC).
Design, setting, and participants:
This open-label, single-centre interventional study was conducted in bmCRPC patients.
Intervention:
Enzalutamide 160 mg and abiraterone acetate 1000 mg once daily; prednisone 5 mg twice daily.
Outcome measurements and statistical analysis:
Adverse events (AEs), prostate-specific antigen (PSA) response, progression-free survival (PFS), tumour biomarker/metabolite expression, and Cmin plasma concentrations were evaluated.
Results and limitations:
Sixty patients were enrolled. Common AEs independent of grade/causality included fatigue (72%), hyperglycaemia (67%), alkaline phosphatase (ALP) elevation (53%), and hot flush (43%). Grade 3 AEs included hypertension (17%), alanine aminotransferase elevation (12%), ALP elevation (5%), and arthralgia (5%). No treatment-related grade 4 AEs or deaths were reported. Median treatment-discontinuation time was 312 d (95% confidence interval [CI] 196.0–483.0). Maximal PSA decline ≥50% and ≥90% occurred in 46 (77%) and 29 (48%) patients, respectively. Median PFS was 251 d (95% CI 147–337). At week 9, median tumour microenvironment androgens, precursors, and nuclear AR expression decreased (p < 0.001). The baseline tumour AR C/N terminal ratio of ≥80% was associated with treatment benefit. At enzalutamide steady state, abiraterone acetate Cmin was ~23% lower (range 14.05–200.5 ng/ml) than when given alone.
Conclusions:
Enzalutamide combined with abiraterone acetate has a manageable safety profile, without a meaningful DDI. Both agents are pharmacodynamically active with no feedback. Efficacy findings do not support significant benefit of combined treatment for unselected bmCRPC.
Patient summary:
This is the first study combining enzalutamide plus abiraterone in bone metastatic castration-resistant prostate cancer. Results show that this combination is safe.
Keywords: Abiraterone acetate, Bone metastases, Castration-resistant prostate, cancer, Enzalutamide, Safety, Tolerability
1. Introduction
Enzalutamide, a potent second-generation androgen receptor (AR) antagonist, and abiraterone acetate plus prednisone (henceforth referred to as “abiraterone”), a first-generation androgen biosynthesis inhibitor, individually prolong the lives of men with metastatic castration-resistant prostate cancer (mCRPC) [1–5]. These findings have informed the initial hypothesis that intracrine androgen biosynthesis [6] and alterations in the AR, such as amplification, mutation, and potentially splice variants [7], remain drivers of castration-resistant disease progression and suitable therapy targets.
As anticipated, not all patients benefit equally [1–5] and progression patterns differ. Our correlative clinical studies demonstrate that the clinical efficacy of enzalutamide and abiraterone may depend on, or vary according to, pretreatment AR signalling [8,9]. These observations are indicative of interpatient, intrapatient, and temporal tumour heterogeneity [10], allowing for the determination of two broad groups of prostate cancer: one group is primarily resistant to enhanced androgen deprivation with no benefit from further suppression of androgen signalling with existing agents and in the other group androgen signalling remains a meaningful, though not exclusive, “driver”. We hypothesised that within the latter group, the combination of enzalutamide and abiraterone may inhibit androgen signalling more completely than either agent alone. The rationale for the combination derives from previous single-agent studies with enzalutamide or abiraterone, pointing to a two-compartment, potentially adaptive, feedback loop [8,9]. The hypothesis tested is that the combination will dissipate the adaptive response observed with each agent alone. That may contribute to improved efficacy in a subset of patients if observed feedbacks are implicated in secondary resistance to androgen signalling inhibition.
This first reported combinatorial phase 2 study [11] evaluated the safety, efficacy, tumour microenvironment effects on androgen signalling/metabolome, and potential drug-drug interactions (DDIs) of cotargeting AR and androgen biosynthesis with enzalutamide and abiraterone in patients with progressive bone metastatic castration-resistant prostate cancer (bmCRPC).
2. Patients and methods
2.1. Study design
This was a phase 2, open-label, single-centre interventional study. Key inclusion and exclusion criteria are included in the Supplementary material.
Oral enzalutamide 160 mg once daily, abiraterone 1000 mg once daily, and prednisone 5 mg twice daily were administered until disease progression (a composite endpoint comprising radiographic (per RECIST 1.1), prostate-specific antigen (PSA; per Prostate Cancer Working Group 2), and/or clinical disease progression (PSA progression alone was not sufficient), physician discretion, or patient withdrawal. This included discontinuation of any study drug or occurrence of an adverse event (AE), whereby continued treatment was not in the patient’s best interest.
Disease evaluations were performed every three cycles. Patients underwent bone marrow biopsy (BMB) and bone marrow aspirate (BMA) prior to treatment initiation, at week 9, and upon treatment discontinuation. Optional pharmacokinetic predosing blood draws were performed on cycle (C) 1on day (D) 4 and C2D1 for abiraterone, on C2D1 for enzalutamide, and on C3D1 for BMBs and BMAs.
2.2. Endpoints
2.2.1. Primary endpoints
Safety and tolerability were evaluated using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTC AE), version 4.03, per 28-d cycles.
2.2.2. Secondary endpoints
Efficacy was assessed by PSA decline from baseline (≥30%, ≥50%, and ≥90%), and progression-free survival (PFS) was defined as the time from treatment initiation until progression, study withdrawal, or death.
Changes in AR signalling, androgen levels, and other pathways of interest and proliferation within the tumour microenvironment were also evaluated.
2.2.3. Exploratory endpoints
DDIs were determined by assessment of plasma concentrations of enzalutamide and its metabolite M2, and abiraterone.
Nuclear expression of AR N and C terminals, ARV7, CYP17, NKX3.1, and other markers (not shown) was assessed in tumour infiltrate bone marrow samples by immunohistochemistry (Supplementary material) [8,9].
Androgen precursor and other associated metabolite concentrations in BMA and peripheral blood were assessed using liquid chromatography mass spectrometry.
2.3. Statistical analyses
Statistical analyses are outlined in the Supplementary material.
3. Results
3.1. Baseline patient and tumour characteristics
Sixty bmCRPC patients were enrolled between June 2012 and August 2015 (Supplementary Fig. 1). Baseline patient and tumour characteristics are presented in Table 1.
Table 1 –
Summary of demographic and baseline patient and tumour characteristics
| Parameter | Patient number (n = 60) |
|---|---|
| Race, n (%) | |
| White | 51 (85.0) |
| Black or African American | 6 (10.0) |
| Asian | 2 (3.3) |
| Other | 1 (1.7) |
| Median age, yr (range) | 66 (40–82) |
| Age category (yr), n (%) | |
| <55 | 7 (11.7) |
| ≥55 to <75 | 44 (73.3) |
| ≥75 | 9 (15.0) |
| Body mass index (kg/m2), mean (±SD) | 31.1 (±4.7) |
| Baseline PSA (μg/l), median (range) | 20.7 (1.0–670.2) |
| Duration of prostate cancer (yr), median (range) | 4.0 (0–19) |
| Prior treatments | |
| Median hormonal treatment lines (range) | 1 (1–4) |
| Antiandrogens, n (%) | 24 (40) |
| Oestrogens/prednisone, n (%) | 5 (8) |
| Chemotherapy, n (%) | 8 (13) |
| Other therapies, n (%) | 6 (10) |
| ECOG PS, n (%) | |
| 0 | 21 (35.0) |
| 1 | 35 (58.3) |
| 2 | 4 (6.7) |
| Gleason score at initial diagnosis,a n (%) | |
| ≤7 | 14 (23.3) |
| 8–10 | 39 (65.0) |
| Unknown/missinga | 7 (11.7) |
| Distribution of disease at screening,b n (%) | |
| Bone | 60 (100.0) |
| Lymph node | 10 (16.7) |
| Visceral metastasesc | 6 (10.0) |
| Number of bone lesions at screening,d n (%) | |
| 3–5 | 6 (10.0) |
| 6–9 | 12 (20.0) |
| 10–20 | 11 (18.3) |
| >20 | 31 (51.7) |
| Bone marrow infiltration, n (%) | |
| Baseline | 19 (31.2) |
| Any time point | 23 (38.4) |
ECOG PS = Eastern Cooperative Oncology Group performance status; PSA = prostate-specific antigen; SD = standard deviation.
Primary and secondary Gleason grades were not available.
Patients can be included in more than one category.
Adrenal gland, one patient; prostate, one patient; lung, two patients; and right pelvic mass, two patients.
Includes metastatic lesions per bone lesion.
3.2. Safety and tolerability
All patients reported one or more AEs, independent of causality. The most frequent any-grade AEs (≥40% incidence) were fatigue (43 patients; 72%), hyperglycaemia (40 patients; 67%), blood alkaline phosphatase increase (32 patients; 53%), and hot flush (26 patients; 43%; Supplementary Table 1). Grade 3 AEs occurring in more than one patient are reported in Table 2. One patient (2%) reported grade 3 fatigue. One patient (2%) reported a grade 4 event (elevated creatinine level) that was considered unrelated to treatment. Ten serious AEs were reported in eight (13%) patients, two of which were considered treatment related (femur fracture and urosepsis). There were no grade 5 AEs and no deaths occurred.
Table 2 –
Summary of NCI CTCAE grade 3 adverse events occurring in more than one patient
| Adverse event | Patient number (n = 60), n (%) |
|---|---|
| Hypertension | 10 (16.7) |
| Alanine aminotransferase increase | 7 (11.7) |
| Arthralgia | 3 (5.0) |
| Alkaline phosphatase increase | 3 (5.0) |
| Aspartate aminotransferase increase | 2 (3.3) |
| Lymphocyte count decrease | 2 (3.3) |
| Bone pain | 2 (3.3) |
| Hypokalaemia | 2 (3.3) |
| Syncope | 2 (3.3) |
NCI CTCAE = National Cancer Institute Common Terminology Criteria for Adverse Events.
Two (4%) patients had liver function AEs classified as potential drug-induced liver injury (two times the upper limit of normal range for alanine aminotransferase [ALT] and/or two times the upper limit of normal range for aspartate aminotransferase in combination with two times the upper limit of normal range for total bilirubin). One patient discontinued treatment, while the other continued with a reduced dose of abiraterone (750 mg). Overall, combination treatment revealed no new safety concerns.
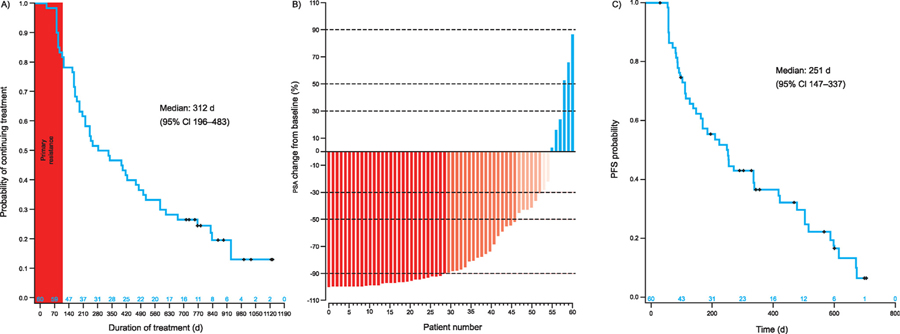
3.3. Exposure and discontinuation
Median time to treatment discontinuation was 312 d (95% confidence interval [CI] 196.0–483.0; range 0–1134 d; Fig. 1A). Ten patients were still on treatment at the data cut-off date, while 40 discontinued (Supplementary Fig. 1). Forty-one (68%) patients experienced radiographic or clinical disease progression: 16 (39%) experienced radiographic progression only, nine (22%) clinical progression only, and 16 (39%) more than one definition of progression. Three (5%) patients discontinued, as per physician decision, for PSA increase only. Five (8%) patients withdrew for one of the following reasons: grade 3 ALT and aspartate aminotransferase increases, withdrawal of consent, physician decision, intervention, and sponsor decision. Of note, 11 (18%) patients exhibited primary resistance with progression within 4 mo, as previously defined [8]. Associations between pretreatment patient and tumour characteristics and time to treatment discontinuation were observed. Prior chemotherapy, tumour-infiltrated lymph nodes, tumour-infiltrated biopsy, and time to castration-resistant prostate cancer <1 yr were associated with primary resistance (Supplementary Fig. 2).
Fig. 1 –
(A) Kaplan-Meier plot of time to treatment discontinuation, (B) waterfall plot of prostate-specific antigen change from baseline at nadir, and (C) Kaplan-Meier plot of progression-free survival. CI = confidence interval; PFS = progression-free survival; PSA = prostate-specific antigen.
3.4. Efficacy
A maximal decline from baseline in PSA level of ≥50% occurred in 46 (77%) patients, with 52 (87%) having a ≥30% decline and 29 (48%) having a ≥90% decline. Six (10%) patients did not experience a decrease in PSA level (Fig. 1B). Median PFS was 251 d (95% CI 147–337; Fig. 1C).
Of the 16 patients with measurable soft tissue disease at screening and one or more postbaseline assessments, 11 (69%) had partial response, three (19%) had stable disease, and two (13%) had progressive disease as the best overall response per RECIST, version 1.1.
Mean and median urine N-telopeptide increased from baseline to week 9 (mean [standard deviation]: 72% [151%]; median: 38%) and at the end of treatment visit (mean [standard deviation]: 79% [78%]; median: 80%).
3.5. Effect of treatment on AR signalling and androgen levels
Twenty (33%) patients had bone marrow infiltration at pretreatment BMBs and 16 (27%) in subsequent C3D1 BMBs. Paired tumour-infiltrated specimens amenable to molecular characterisation (ie, tumour infiltration >10%) were available for 11 patients. Six (55%) tumours were primarily resistant to treatment (ie, progressed within 4 mo of treatment initiation per prior definition) [8], while five (45%) experienced confirmed treatment benefit and serum PSA decline. Reductions in tumour AR N/C terminal nuclear expression and downstream NKX3.1 expression were observed in these latter samples (Fig. 2).
Fig. 2 –
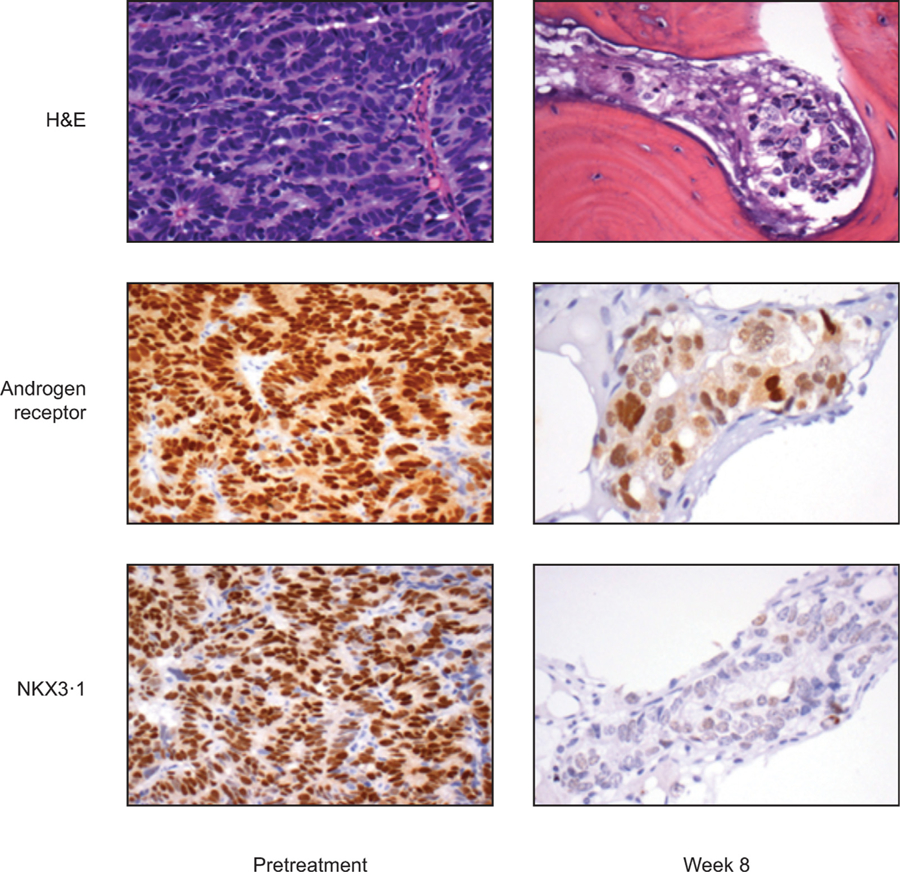
Tumour AR-N terminal nuclear expression and downstream NKX3.1 expression using immunohistochemistry. AR = androgen receptor; H&E = haematoxylin and eosin stain.
Fifteen pretreatment tumour samples were evaluable for further molecular characterisation, including expression of AR (AR-N/AR-C), CYP17, and ARV7, and AR C/N terminal ratio and association with primary resistance. The combination of AR N terminal overexpression and expression of an AR C/N terminal ratio of ≥80% was significantly associated with treatment benefit (p = 0.0002; Supplementary Table 2 and Fig. 3). Two of six (33%) baseline tumour specimens with primary resistance, and none of those exhibiting benefit, had nuclear ARV7 expression.
Fig. 3 –
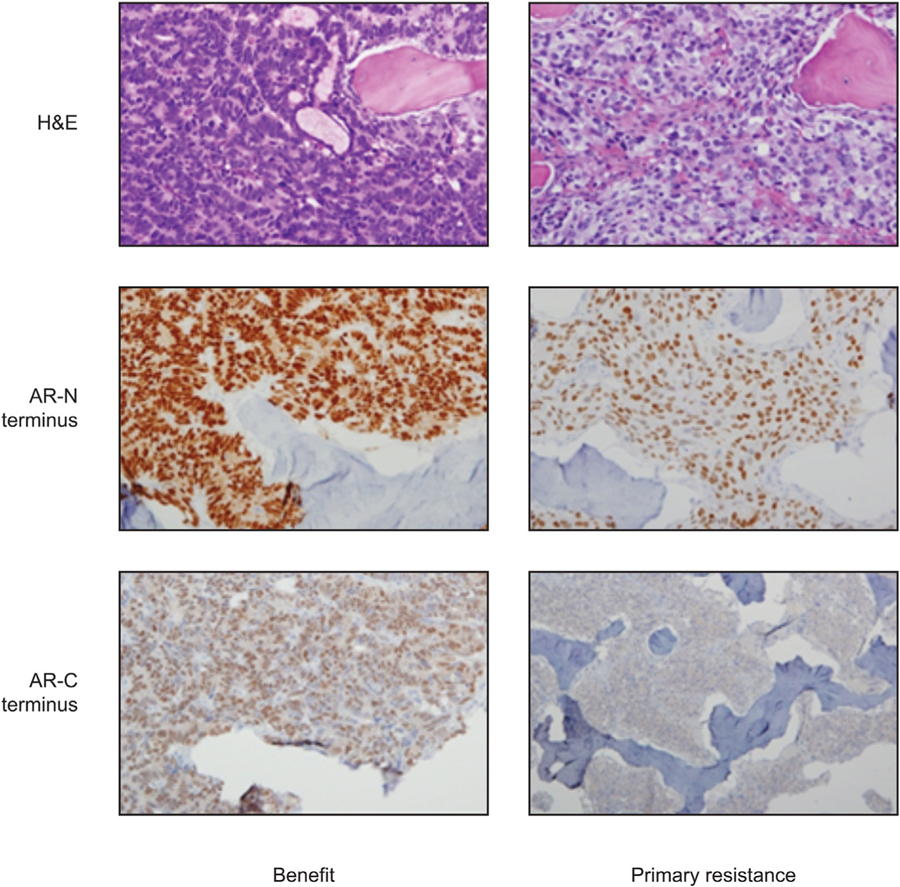
Ratio of AR-C/AR-N terminal expression and association with treatment benefit/primary resistance. AR = androgen receptor; H&E = haematoxylin and eosin stain.
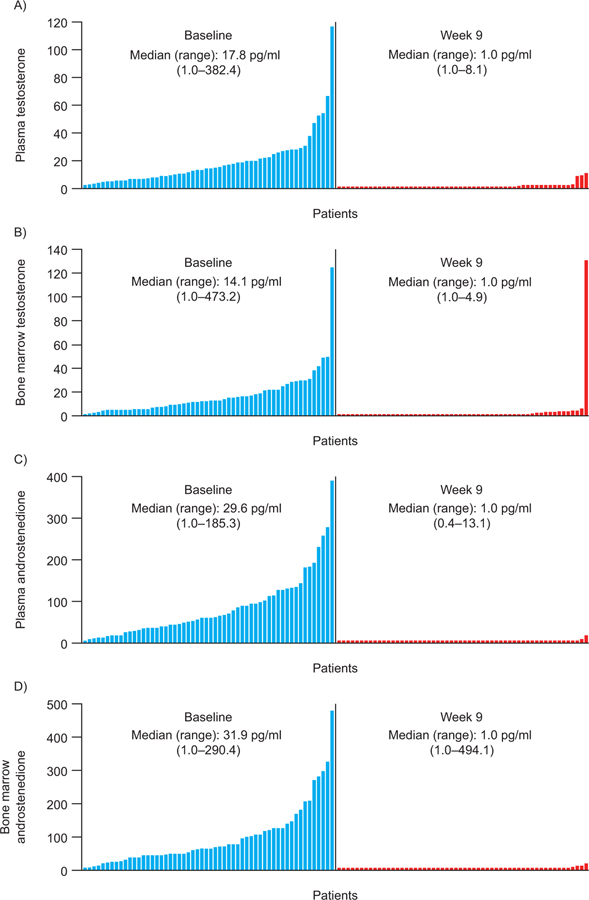
There was a statistically significant decrease (p < 0.001) in median BMA and plasma testosterone, cortisol, and androstenedione levels from baseline to week 9 (Supplementary Table 3 and Fig. 4). Conversely, median BMA, and plasma progesterone and pregnenolone levels increased significantly (p < 0.001) from baseline to week 9 (Supplementary Table 3).
Fig. 4 –
Waterfall plots of changes in (A and B) plasma and bone marrow testosterone and (C and D) plasma and bone marrow androstenedione at baseline and following 9 wk of treatment.
No monotonic correlation was established between any of the androgens or other associated metabolites and blood PSA levels at baseline or week 9: bone marrow and plasma testosterone (r = −0.1 and −0.2, respectively), cortisol (r = −0.3 and −0.1, respectively), androstenedione (-r = 0.1 and −0.2, respectively), progesterone (r = −0.02 and −0.01, respectively), and pregnenolone (r = −0.08 and 0.08, respectively).
3.6. Predose concentrations of enzalutamide and its metabolite M2, and abiraterone
Enzalutamide mean steady-state Cmin on C2D1 for patients in the current study (9785-CL-0011; n = 15) was similar to that observed for castration-resistant prostate cancer patients in the AFFIRM trial (n = 704; Astellas Pharma, Inc., Northbrook, IL, USA; data on file), suggesting that coadministration of enzalutamide and abiraterone has no influence on the exposure of enzalutamide (Supplementary Fig. 3A).
The least squares mean abiraterone concentrations indicate that Cmin of abiraterone was approximately 23% lower on C2D1 (37.9 ng/ml; range 14.09–200.5 ng/ml), when both enzalutamide and abiraterone were at steady state, compared with C1D4 (48.8 ng/ml; range 14.39–172.22 ng/ml), when abiraterone was given alone (n = 14; least squares mean ratio 77.7%; 90% CI 47.5–127.0; Supplementary Fig. 3B). The observed data do not suggest a significant DDI when the treatments are given in combination, considering the large intrasubject variability, with approximately 31% for area under the curve and 42% for Cmax.
4. Discussion
The goal of this study was to assess safety of the combination and build on previously reported pharmacodynamics and efficacy results of enzalutamide and abiraterone in bmCRPC [1–5].
Based on the results of this study, cotargeting the AR and androgen biosynthesis with enzalutamide and abiraterone is feasible. The safety profile of the combination did not differ significantly from that reported for either drug alone, as it pertains to AEs of grade 3 severity. However, 72% of patients experienced fatigue of grade 1–2 severity that may impact quality of life and 67% of patients experienced hyperglycaemia. Additionally, grade 3 ALT increase was reported in seven patients, with liver function AEs classified as potential drug-induced liver injury in two patients.
Though no objective, direct comparisons can be made with reported phase 3 outcomes for each agent alone, time to progression in COU-AA-302 [2] and PREVAIL [4] appears longer than that reported in this study, while PSA declines are similar. The median radiographic PFS in COU-AA-302 was 16.5 mo with abiraterone-prednisone and 8.3 mo with prednisone alone (hazard ratio for abiraterone-prednisone vs prednisone alone, 0.53; 95% CI 0.45–0.62; p < 0.001). In PREVAIL study, the rate of radiographic PFS at 12 mo was 65% among patients treated with enzalutamide, as compared with 14% among patients receiving placebo (81% risk reduction; hazard ratio in the enzalutamide group, 0.19; 95% CI 0.15–0.23; p < 0.001).
This may be due partly to enrolling some chemotherapytreated individuals with more advanced disease and differences in study design, specifically as it relates to assessment of disease progression. The variable treatment-discontinuation time and PFS response may still suggest that enzalutamide and abiraterone in combination may offer benefit in a subset of patients who remain to be identified.
A nuclear to cytoplasmic AR shift and a corresponding rise in tumour-associated testosterone following enzalutamide [9], and depletion of tumour-associated androgens with increased nuclear AR expression and AR copy numbers following abiraterone [8], have previously been reported. This agent-specific androgen signalling modulation indicates a two-compartment adaptive loop. Our findings demonstrate that in men with “androgen signalling–responsive” mCRPC, simultaneous inhibition of AR signalling and biosynthesis avoids the adaptive response of either agent alone, and may more completely suppress androgen signalling. Based on this, we hypothesise that more complete suppression of androgen signalling may more effectively control a subset of prostate cancer as compared with delivering either agent alone. A recent report of a phase 4 study comparing sequential versus combinatorial strategy has not identified differences in efficacy, though the design may have hindered addressing the specific question [12].
Primary resistance occurred in 11/60 (18%) patients treated, which is in line with single-agent phase 2 and 3 experience, hence supporting our hypothesis that primary resistant tumours are not driven by androgen signalling, and should be identified a priori and treated with a different strategy.
This study allowed us to further investigate the predictors of outcome. Our first report of an association between the presence of nuclear ARV7 in tumour biopsy specimens and primary resistance to enzalutamide [9] was explored; however, nuclear ARV7 expression was found only in two baseline specimens of primary resistant tumours. In an attempt to detect AR C terminal loss as a potential contributor to primary resistance, we utilised an AR C/N terminal ratio [13] and found a strong association (p = 0.0002) between pretreatment AR N terminal overexpression with an AR-C/AR-N expression ratio of ≥80% and treatment benefit in this limited sample size (n = 9). Other markers of interest were investigated (data not shown) without findings of any correlation, possibly due to the availability of a limited number of samples (n = 15). This is in line with the understanding that robust androgen signalling axis is a requirement for efficient tumour targeting in mCRPC. Our methodology differs from those employed by others as it is biopsy based and identifies nuclear protein expression, which might account for more limited incidence than ribonucleic acid and circulating tumour cell assays [14–16]. Taken together, these “by-association” findings regarding the presence of AR variants suggest a deficit in “wild-type” androgen signalling that may be an epiphenomenon and not necessarily a driver of primary or secondary resistance. Importantly, methodologies employed are not validated with recent controversial results, adding to the complexity. Based on the reports suggestive of an association between glucocorticoid receptor overexpression and resistance to enzalutamide [17] and inclusion of prednisone in therapy schema, we assessed the expression of glucocorticoid receptor (data not shown) and identified no associations.
Given the potential for enzalutamide to induce CYP3A4 [18,19], pharmacokinetic assessments were an important component of our investigation. No significant DDIs that would limit this combination strategy were observed. Pharmacokinetic measurements were performed on C1D4 and C2D1. The latter measurement was consistent with enzalutamide steady state, though follow-up measurements may have provided more confidence in this finding.
Ongoing studies should test the hypothesis that cotargeting both AR and androgen biosynthesis inhibition in AR-responsive prostate cancer may further prolong survival in some mCRPC patients by delaying the emergence of resistance. This is an approach currently excluded from the treatment paradigm driven by a drug development strategy imposed by a lack of selection criteria. This is of particular relevance given the recent data supportive of a significant survival benefit by introducing androgen biosynthesis inhibition in the hormone-sensitive setting [20,21].
5. Conclusions
In this study conducted in bmCRPC patients, enzalutamide and abiraterone were used safely without adverse pharmacologic or pharmacodynamic interactions, differentiating patients into those with primary resistance and those who benefit from combination treatment. Our findings indicate that enzalutamide and abiraterone in combination prevent the “adaptive response” observed with either agent alone. However, we did not see an alteration in the incidence of primary resistance. Although it is hypothesised that treatment combination may contribute to increased efficacy in a subset of patients with bmCRPC, employing this adaptive feedback loop in treatment resistance warrants further investigation and validation of predictive markers that will help in the identification of these patients. We are looking forward to ongoing phase 3 study results interrogating the efficacy of enzalutamide and abiraterone in combination in bmCRPC to provide further insight.
Supplementary Material
Acknowledgements:
Medical writing and editorial assistance were provided by Stephanie Rippon, Mei Lye, and Lauren Smith from Complete HealthVizion, all funded by the sponsor companies.
Funding/Support and role of the sponsor: This study was funded by Astellas Pharma Inc. and Medivation LLC (which was acquired by Pfizer Inc. in September 2016), the codevelopers of enzalutamide. This work was supported by National Institutes of Health/National Cancer Institute award number P30 CA016672 and the Genitourinary Cancers Program of the Cancer Center Support Grant shared resources at the MD Anderson Cancer Center. Dr. Eleni Efstathiou received a career development award from the Prostate Cancer Foundation.
Footnotes
Financial disclosures: Eleni Efstathiou certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Eleni Efstathiou received a grant from Sanofi and personal fees from Sanofi, Johnson & Johnson, Astellas Pharma, Inc., and Medivation, Inc. Carl Dmuchowski, Amal Melhem-Bertrandt, and Shiva Patil are employees of Astellas Pharma, Inc. Christopher J. Logothetis received grants from Astellas Pharma, Inc., BMS, Janssen, Novartis, Sanofi, and Bayer, and consulting fees from Astellas Pharma, Inc., Janssen, Sanofi, and Bayer. Mark Titus, Sijin Wen, Patricia Troncoso, Anh Hoang, Paul Corn, Ina Prokhorova, and John Araujo have nothing to disclose.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.euo.2019.01.008.
References
- [1].de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 2010;375:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- [6].Geller J, Liu J, Albert J, Fay W, Berry CC, Weis P. Relationship between human prostatic epithelial cell protein synthesis and tissue dihydrotestosterone level. Clin Endocrinol (Oxf) 1987;26:155–61. [DOI] [PubMed] [Google Scholar]
- [7].Claessens F, Helsen C, Prekovic S, et al. Emerging mechanisms of enzalutamide resistance in prostate cancer. Nat Rev Urol 2014;11:712–6. [DOI] [PubMed] [Google Scholar]
- [8].Efstathiou E, Titus M, Tsavachidou D, et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J Clin Oncol 2012;30:637–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Efstathiou E, Titus M, Wen S, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol 2015;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Logothetis CJ, Gallick GE, Maity SN, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov 2013;3:849–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Efstasthiou E, Titus M, Wen S, et al. Enzalutamide (ENZA) in combination with abiraterone acetate (AA) in bone metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol 2014;32 (15_Suppl):5000. [Google Scholar]
- [12].Attard G, Borre M, Gurney H, et al. A phase IV, randomized, double-blind, placebo (PBO)-controlled study of continued enzalutamide (ENZA) post prostate-specific antigen (PSA) progression in men with chemotherapynaive metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017;35(15_Suppl):5004. [Google Scholar]
- [13].Zhang X, Morrissey C, Sun S, et al. Androgen receptor variants occur frequently in castration resistant prostate cancer metastases. PLoS One 2011;6:e27970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol 2017;71:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Grande E, Fernández Pérez MP, Font Pous A, et al. Early responses to enzalutamide in AR-V7 positive first line metastatic castration-resistant prostate cancer (mCRPC). A prospective SOGUG clinical trial: the PREMIERE study. Ann Oncol 2016;27(suppl_6):726PD. [Google Scholar]
- [17].Arora VK, Schenkein E, Murali R, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gibbons JA, de Vries M, Krauwinkel W, et al. Pharmacokinetic drug interaction studies with enzalutamide. Clin Pharmacokinet 2015;54:1057–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Narayanan R, Hoffmann M, Kumar G, Surapaneni S. Application of a “fit for purpose” PBPK model to investigate the CYP3A4 induction potential of enzalutamide. Drug Metab Lett 2016;10:172–9. [DOI] [PubMed] [Google Scholar]
- [20].Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. [DOI] [PubMed] [Google Scholar]
- [21].James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med 2017;377:338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.