Abstract
Introduction
Several studies have explored the association between outdoor air pollution and semen quality. However, the results were inconsistent. We performed the current meta-analysis to evaluate the role of outdoor air pollution in semen quality. Material and Methods. Databases including PubMed, Web of Science, and Embase were searched to identify relevant studies. Relative data in participants under higher exposure and lower exposure to air pollution were extracted. Pooled weighted mean differences (WMDs) with corresponding 95% confidence intervals (CIs) were utilized to assess the effects of outdoor air pollution on semen quality. In addition, trial sequential analyses (TSAs) were performed to obtain a more comprehensive assessment of analyses.
Results
A total of 11 studies with 4562 males were enrolled in the current meta-analysis. Higher air pollution levels were associated with significant decreases in semen volume (WMD: -0.16, 95% CI: -0.27 to -0.05), sperm concentration (WMD: -5.52, 95% CI: -9.88 to -1.16), progressive motility (WMD: -6.23, 95% CI: -11.64 to -0.81), total motility (WMD: -7.65, 95% CI: -14.09 to -1.20), and normal sperm morphology rate (WMD: -3.71, 95% CI: -5.59 to -1.82). In addition, the DNA fragmentation index significantly increased (WMD: 4.11, 95% CI: 1.94 to 6.29).
Conclusions
Air pollution is associated with decreased semen volume, sperm concentration, motility, and normal morphology rate.
1. Introduction
According to the definition of the International Organization for Standardization (ISO), air pollution usually refers to the phenomenon that harmful or excessive quantities of substances enter the atmosphere due to human activities or natural processes. When the pollutants accumulate to enough concentration and sustained for enough time, air pollution will significantly impair the health of human beings. It can result in various diseases including cardiovascular and lung diseases, neurologic disorders, and infertility [1–5]. Recently, various studies have explored the effects of air pollution on male fertility [6, 7].
Human semen quality has been degraded for decades. Several studies have demonstrated that exposure to toxicants or air pollutants, electromagnetic waves from cell phones, obesity, drinking, smoking, psychological stress hypertension, and diabetes can be potential causes of this degradation [6, 8–14]. Considering the large number of affected populations, outdoor air pollution has become the hotspot recently. However, the specific role of air pollution in semen quality remains unclear. Epidemiologic studies have demonstrated nonsignificant or contrary results. Several studies demonstrated that air pollution can significantly reduce the sperm concentration [15–18] and total sperm count [16–18], but several studies did not show significant results. Concerning the sperm motility, air pollution was reported associated with decreased progressive sperm motility [15, 16, 19–21] and total sperm motility [16, 19–22]. However, some other studies did not demonstrate significant results.
Based on the data in the previous published studies, the current meta-analysis was performed to explore the overall impacts of air pollution on semen quality.
2. Materials and Methods
This study was strictly reported based on the PRISMA (Preferred Reporting Items for Systematic Review and Meta-analyses) statement [23]. The protocol of the present study was described previously [24] and registered in the international prospective register of systematic reviews (registration number CRD42019126060). We used the same research methods in the current study.
The quality of the enrolled studies was evaluated by Newcastle-Ottawa Scale (NOS) star system (range, 0 to 9 stars), which focuses on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest. The number of stars is positively associated with the quality of the study. Overall, the enrolled studies rated from 6 to 9 stars (Table 1).
Table 1.
Basic characteristics of the enrolled studies.
| Study | Exposure | Period | Study design | Location | Major ethnicity | Age | Groups | Positive findings | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Selevan, 2000 | PM10, TSPs, SO2, CO, Nox | 1993-1994 | Cross-sectional | Czech | Caucasian | 18 | Residents in urban or rural areas | Lower progressive motility, total motility, normal morphology rate, and chromatin structure | 8 |
| De Rosa, 2003 | Traffic pollutants | 2000-2002 | Cross-sectional | Italy | Caucasian | 23-62 | Workers at motorways or offices | Lower total motility, progressive motility, concentration, VSL, VCL, LIN, and ALH | 6 |
| Rubes, 2005 | PM10, PAH, SO2, NOx | 1995-1997 | Longitudinal | Czech | Caucasian | 19-25 | Residents in winter and summer | None | 9 |
| Guven, 2008 | Traffic pollutants | NM | Cross-sectional | Turkey | Caucasian | 35.2 ± 6.4; 33.7 ± 6.7 | Workers at motorways or offices | Lower normal morphology rate, concentration, progressive motility | 6 |
| Boggia, 2009 | Traffic pollutants | 2000-2004 | Cross-sectional | Italy | Caucasian | 23-57 | Workers at motorways or offices | Lower total motility and progressive motility | 6 |
| Rubes, 2010 | PM2.5, SO2, NO, CO, O3, PAH, Benzo | 2007 | Longitudinal | Czech | Caucasian | 33.6 ± 5.3 | Residents in winter and spring | Lower total motility and DFI | 9 |
| Calogero, 2011 | Traffic pollutants | NM | Cross-sectional | Italy | Caucasian | 20-47 | Workers at motorways or offices | Lower normal morphology rate, concentration, total sperm count, progressive motility, and DFI | 6 |
| Zhou, 2014 | PM10, SO2, NO2 | 2007 | Cross-sectional | China | Asian | 20-40 | Residents in urban or rural areas | Lower normal morphology rate, VSL, VCL, and VAP | 8 |
| Wu, 2016 | PM10 | 2013-2015 | Cross-sectional | China | Asian | 34.4 ± 5.4 | Residents with different PM exposure | Lower concentration and total count | 9 |
| Liu, 2017 | SO2 | 2013-2015 | Cross-sectional | China | Asian | 34.4 ± 5.4 | Residents with different PM exposure | Lower total count, concentration, progressive motility, and total motility | 9 |
| Lao, 2018 | PM2.5 | 2001, 2004 | Cross-sectional | China | Asian | 31.9 ± 4.3 | Residents with different PM exposure | Lower normal morphology and higher sperm concentration | 8 |
ALH: amplitude of lateral movement of sperm head; DFI: DNA fragmentation index; LIN: linearity of sperm motion; NM: not mentioned; NOS: Newcastle-Ottawa Scale; TSPs: PM-total suspended particulates; VCL: sperm curvilinear velocity; VSL: sperm linear velocity.
3. Results
3.1. Basic Characteristics of the Enrolled Studies
The study selection process was shown in Figure 1. In total, eleven studies with 4652 males met the inclusion criteria and were enrolled in the current meta-analysis [15–22, 25–27]. Notably, the outdoor air pollutants varied between the included studies. Four studies explored the role of traffic pollutants in male fertility and did not analyze the composition of the air pollutants. Among the 11 enrolled studies, nine were cross-sectional studies while the other two were longitudinal studies. Seven articles mainly focused on Caucasians and four focused on Asians. Participants were divided into different groups based on the extent of exposure to air pollution. Five, two, and four studies were grouped together according to the location, climate, and working conditions of the participants, respectively. Details of the aforementioned data are listed in Table 1.
Figure 1.
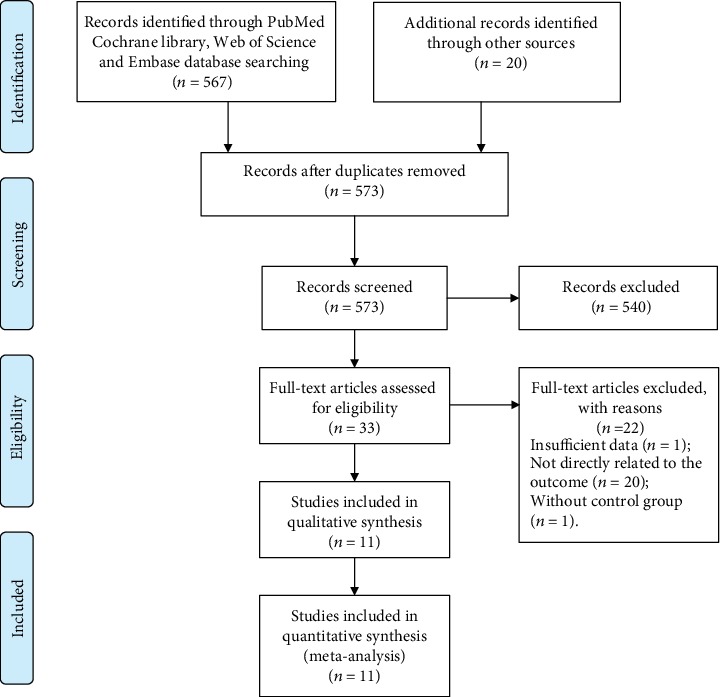
Flow diagram of the study selection process.
3.2. The Effects of Outdoor Air Pollution on Sperm Parameters
All eleven studies reported the role of outdoor air pollution in sperm concentration. Among them, six [15, 19, 20, 22, 25, 26] and five [16–19, 25] studies further explored the alterations in semen volume and total sperm count, respectively. The results indicated that higher air pollution levels were associated with significant decreases in semen volume (WMD: -0.16, 95% CI: -0.27 to -0.05) (Figure 2(a)) and sperm concentration (WMD: -5.52, 95% CI: -9.88 to -1.16) (Figure 2(b)). Notably, the decrease in total sperm count, which was obtained by multiplying semen volume by sperm concentration, was not significant (WMD: -38.19, 95% CI: -82.89 to 6.50) (Figure 2(c)). This may have partly resulting from the limited sample size.
Figure 2.
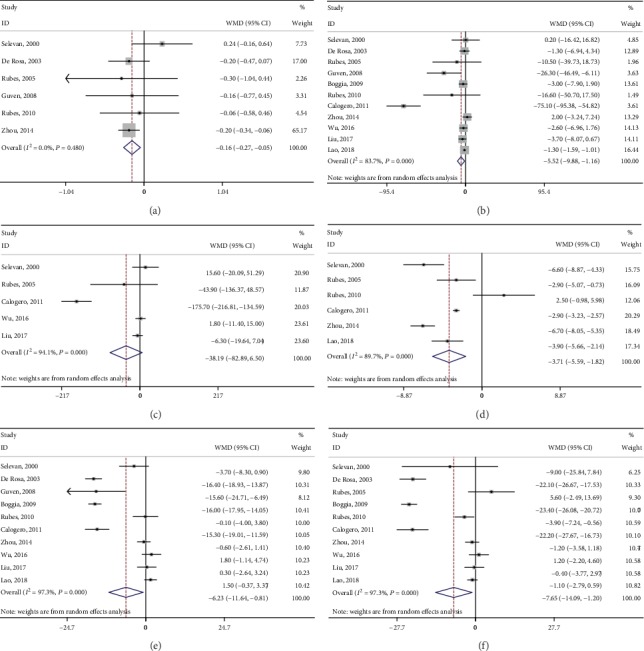
Forest plots of merged analyses of effects on sperm parameters by outdoor air pollution. (a–e) Forests plots of merged analyses of semen volume, sperm concentration, total sperm count, normal morphology rate, progressive motility, and total sperm motility, respectively.
Six studies explored the association between air pollution and normal sperm morphology rate [16, 19, 22, 25–27]. The pooled results demonstrated a significant decrease in normal morphology (WMD: -3.71, 95% CI: -5.59 to -1.82) (Figure 2(d)). Ten studies explored the association between outdoor air pollution and sperm motility [15–22, 26, 27]. The results indicated that air pollution was associated with significant decreases in progressive motility (WMD: -6.23, 95% CI: -11.64 to -0.81) (Figure 2(e)) and total motility (WMD: -7.65, 95% CI: -14.09 to -1.20) (Figure 2(f)). In addition, the DNA fragmentation index significantly increased based on the pooled result from four studies [16, 19, 22, 25] (WMD: 4.11, 95% CI: 1.94 to 6.29) (Figure 3(a)). Details of the aforementioned information of each enrolled study are listed in Table 2.
Figure 3.
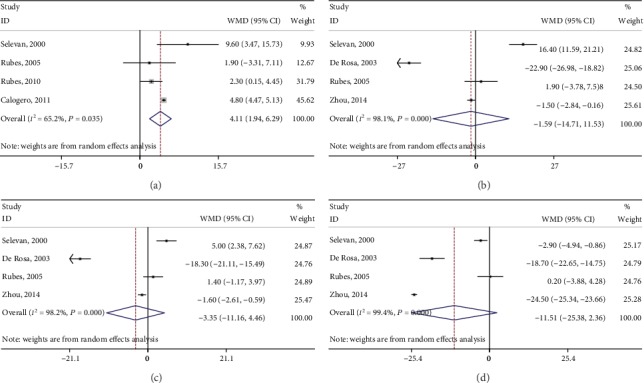
Forest plots of merged analyses of effects on DFI and CASA measures by outdoor air pollution. (a–d) Forests plots of merged analyses of DFI, VCL, VSL, and LIN, respectively.
Table 2.
Primary outcomes of the enrolled studies.
| Study | Sample size | Semen volume (mL) | Sperm concentration (106/mL) | Total count (106) | ||||
| High | Low | High | Low | High | Low | High | Low | |
| Selevan, 2000 | 47 | 162 | 2.2 ± 1.3 | 2.0 ± 1.1 | 60.1 ± 46.7 | 59.9 ± 64.3 | 129.1 ± 103.1 | 113.5 ± 130.7 |
| De Rosa, 2003 | 85 | 85 | 2.5 ± 0.9 | 2.7 ± 0.9 | 32.4 ± 22.1 | 33.7 ± 14.7 | NM | NM |
| Rubes, 2005 | 36 | 36 | 3.0 ± 1.7 | 3.3 ± 1.5 | 81.6 ± 42.09 | 92.1 ± 79.0 | 234.2 ± 141.1 | 278.1 ± 245.4 |
| Guven, 2008 | 38 | 35 | 3.2 ± 1.3 | 3.4 ± 1.4 | 44.6 ± 36.3 | 70.9 ± 50.0 | NM | NM |
| Boggia, 2009 | 100 | 64 | NM | NM | 34.3 ± 20.3 | 37.3 ± 11.7 | NM | NM |
| Rubes, 2010 | 47 | 47 | 3.2 ± 1.3 | 3.2 ± 1.3 | 134.2 ± 84.1 | 150.8 ± 84.6 | NM | NM |
| Calogero, 2011 | 36 | 32 | NM | NM | 24.1 ± 15.4 | 99.2 ± 56.7 | 64.9 ± 43.3 | 240.6 ± 111.4 |
| Zhou, 2014 | 429 | 917 | 2.3 ± 1.1 | 2.5 ± 1.4 | 79.4 ± 46.2 | 77.4 ± 44.6 | NM | NM |
| Wu, 2016 | 367 | 349 | NM | NM | 39.4 ± 29.1 | 42.0 ± 30.3 | 104.4 ± 82.9 | 102.6 ± 96.4 |
| Liu, 2017 | 370 | 327 | NM | NM | 39.4 ± 27.3 | 43.1 ± 31.1 | 108.4 ± 82.0 | 114.7 ± 96.0 |
| Lao, 2018 | 535 | 501 | NM | NM | 40.6 ± 2.5 | 41.9 ± 2.3 | NM | NM |
|
| ||||||||
| Study | Progressive motility (PR, %) | Total motility (PR + NP, %) | Normal morphology (%) | SCSA-DFI (%) | ||||
| High | Low | High | Low | High | Low | High | Low | |
| Selevan, 2000 | 32.5 ± 13.2 | 36.2 ± 17.1 | 41.6 ± 40.4 | 50.6 ± 79.6 | 13.2 ± 6.5 | 19.8 ± 8.5 | 28.8 ± 20.4 | 19.2 ± 12.2 |
| De Rosa, 2003 | 12.3 ± 11.0 | 28.7 ± 4.6 | 34.7 ± 20.2 | 56.8 ± 7.4 | NM | NM | NM | NM |
| Rubes, 2005 | NM | NM | 68.3 ± 12.1 | 62.7 ± 21.6 | 8.4 ± 2.6 | 11.3 ± 6.1 | 15.4 ± 12.6 | 13.5 ± 9.8 |
| Guven, 2008 | 54.7 ± 23.6 | 70.3 ± 15.6 | NM | NM | NM | NM | NM | NM |
| Boggia, 2009 | 15.0 ± 7.4 | 31.0 ± 5.3 | 37.0 ± 11.2 | 60.4 ± 6.3 | NM | NM | NM | NM |
| Rubes, 2010 | 58.0 ± 9.9 | 58.1 ± 9.4 | 70.5 ± 8.2 | 74.4 ± 8.3 | 21.3 ± 9.8 | 18.8 ± 7.2 | 12.4 ± 5.8 | 10.1 ± 4.8 |
| Calogero, 2011 | 12.4 ± 8.7 | 27.7 ± 6.9 | 29.6 ± 12.8 | 51.8 ± 10.2 | 17.2 ± 0.8 | 20.1 ± 0.6 | 9.3 ± 0.9 | 4.5 ± 0.4 |
| Zhou, 2014 | 51.7 ± 17.5 | 52.3 ± 17.5 | 69.8 ± 20.9 | 71.0 ± 20.4 | 23.5 ± 11.5 | 30.2 ± 12.3 | NM | NM |
| Wu, 2016 | 38.8 ± 17.7 | 37.0 ± 22.1 | 45.8 ± 20.6 | 44.6 ± 25.4 | NM | NM | NM | NM |
| Liu, 2017 | 38.9 ± 19.6 | 38.6 ± 19.9 | 45.9 ± 22.5 | 46.3 ± 22.8 | NM | NM | NM | NM |
| Lao, 2018 | 48.4 ± 15.4 | 46.9 ± 15.3 | 65.3 ± 14.0 | 66.4 ± 13.7 | 67.9 ± 15.2 | 71.8 ± 13.7 | NM | NM |
NM: not mentioned.
CASA measures were provided in four studies [19, 20, 25, 26], and our meta-analysis demonstrated nonsignificant decreases in VCL (WMD: -1.59, 95% CI: -14.71 to 11.53) (Figure 3(b)), VSL (WMD: -3.35, 95% CI: -11.16 to 4.46) (Figure 3(c)), and LIN (WMD: -11.51, 95% CI: -25.38 to 2.36) (Figure 3(d)). Detailed information concerning CASA measures of each enrolled study is listed in Table 3.
Table 3.
CASA measures of the enrolled studies.
| Study | VCL (μm/s) | VSL (μm/s) | LIN (%) | |||
|---|---|---|---|---|---|---|
| High | Low | High | Low | High | Low | |
| Selevan, 2000 | 107.8 ± 12.1 | 91.4 ± 21.7 | 48.3 ± 7.4 | 43.3 ± 10.0 | 44.7 ± 5.6 | 47.6 ± 8.2 |
| De Rosa, 2003 | 29.7 ± 18.4 | 52.6 ± 5.5 | 16.1 ± 12.0 | 34.4 ± 5.5 | 47.1 ± 15.6 | 65.8 ± 10.1 |
| Rubes, 2005 | 72.8 ± 11.3 | 70.9 ± 13.2 | 36.4 ± 4.7 | 35.0 ± 6.3 | 52.4 ± 8.1 | 52.2 ± 9.5 |
| Zhou, 2014 | 51.9 ± 12.1 | 53.4 ± 10.8 | 32.1 ± 9.0 | 33.7 ± 8.5 | 60.4 ± 8.4 | 84.9 ± 4.2 |
LIN: linearity of sperm motion; VCL: sperm curvilinear velocity; VSL: sperm linear velocity.
3.3. Trial Sequential Analysis Results
The TSA results indicated sufficient evidence that outdoor air pollution reduced semen volume (Figure 4(a)), sperm concentration (Figure 4(b)), normal morphology rate (Figure 4(c)), and total motility (Figure 4(d)). However, analysis of progressive motility showed a negative result, indicating that inaccuracy might exist (data not shown). Further studies are required to explore the role of outdoor air pollution in sperm progressive motility.
Figure 4.
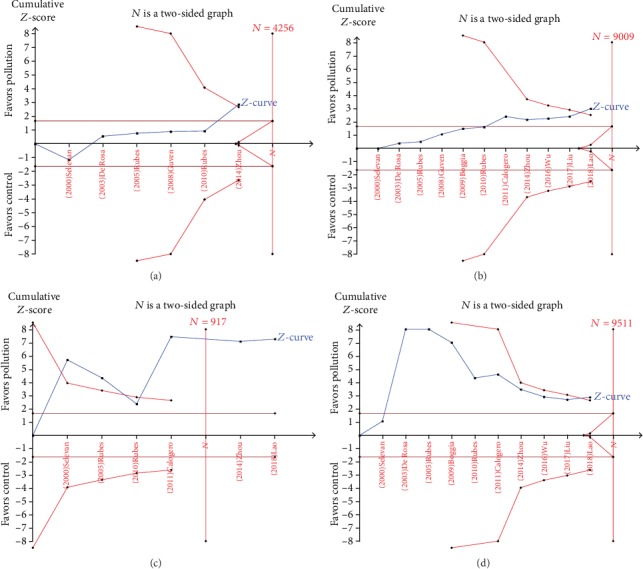
Trial sequential analysis of the effects of TST. (a–d) TSA of semen volume, sperm concentration, normal morphology rate, and total sperm motility.
3.4. Sensitivity Analysis
The influence of individual studies on the pooled WMDs was evaluated by sensitivity analyses (Figure S1). No significant alterations in pooled WMDs were observed after any single study was omitted, demonstrating that the results were robust.
3.5. Publication Bias
The results of Egger's linear regression tests demonstrated no potential publication bias of the enrolled studies (Semen volume: P = 0.433; sperm concentration: P = 0.124; total sperm count: P = 0.372; progressive motility: P = 0.854; total motility: P = 0.495; normal morphology rate: P = 0.528; DFI: P = 0.689; VCL: P = 0.984; VSL: P = 0.795; and LIN: P = 0.260). In addition, evidence of obvious asymmetry was not found in the funnel plots (Figure S2).
4. Discussion
Testicular function and sperm development can be affected by exposure to various environmental pollutants, including isoflavones, heavy metals, chlorination disinfection by-products in water, organic solvents, and particulate air pollution [14]. Recently, various studies focused on other harmful environment urban factors, especially the electromagnetic waves from cell phones and stations, can also decrease semen quality and their negative influence cannot be objectively separated from the other environmental pollutants [12, 13].
The effect of pollutants on sperm quality could be evaluated in humans or in laboratory animals. Several animal studies have been performed to investigate the negative effects of air pollution on semen parameters. Prenatal exposure to diesel exhaust has been associated with a significant reduction in daily sperm production, multinucleated giant cells in the seminiferous tubules, partial vacuolation of the seminiferous tubules, and elevated follicle-stimulating hormone receptor (FSHR) mRNA expression in mice [28]. The biological mechanisms of the effects of air pollution on semen quality remain uncertain, and relevant research is limited. One possible mechanism is disorder in the hypothalamic pituitary axis. Particulate matter, i.e., microscopic solid or liquid matter suspended in the atmosphere of Earth, can carry multiple trace elements and polycyclic aromatic hydrocarbons (PAHs). PAHs are a group of compounds that include several endocrine disruptors and can influence sexual hormones by interfering with the hypothalamic pituitary axis [29]. In addition, PAHs can directly impair spermatogenesis [30]. Several studies have demonstrated that the reactive metabolites of PM10 and PM2.5 can reach the testes and cause increased mitochondrial dysfunction, DNA fragmentation, and cell apoptosis [30, 31]. O3-induced oxidative stress is another possible mechanism. Sperm exist in a balanced physiological environment of reactive oxygen species (ROS) and antioxidants. O3 may result in inflammation in the male genital tract and formation of circulating toxic species and ROS. Excessive amounts of ROS can subsequently impair the integrity of the DNA in the sperm nucleus and accelerate the process of sperm apoptosis [32, 33]. Luo et al. demonstrated that gasoline exhaust can cause significant reduction in α6-integrin and β1-integrin in the rat testes, which may be a cause of decreased semen quality [34].
A large number of epidemiologic studies have explored the associations between outdoor air pollution and semen quality. However, the results were inconsistent. The current meta-analysis was performed to obtain conclusive results by pooling all qualified data. The results indicated that outdoor air pollution can significantly impair semen quality by increasing sperm DFI and decreasing semen volume, sperm concentration, motility, and normal morphology rate.
Notably, although semen volume and sperm concentration significantly decreased in participants with higher exposure to air pollution was revealed, the decrease in total sperm count, which is obtained by multiplying semen volume by sperm concentration, was not significant. There were several causes for the nonsignificant result. First, although most of the enrolled studies (11 studies) focused on the semen concentration, only 5 of them provided total sperm count data. Limited sample size can be one cause for the nonsignificant decrease in total sperm count. Second, the standard deviations of the total sperm count were larger than those of the semen volume and sperm concentration. Based on this, a larger sample size is required to reach statistical significance.
It should be noted that sperm development consists of three different key periods: spermatogenesis, development of sperm motility, and epididymal storage, which correspond to 70-90, 10-14, and 0-9 days before ejaculation, respectively. Several studies have reported relatively short-term effects (10-14 or 0-9 days before ejaculation) of air pollution on semen parameters but the results were inconclusive. Notably, the exposure assessment of most included studies in the current meta-analysis was based on the information from monitoring stations for at least 90 days before semen sampling, which provided information about relatively long-term effects of air pollution. Further animal researches and epidemiologic studies are required to explore the effects of air pollution on different periods of sperm development.
The current study has several strengths: (1) the sample size was relatively larger, which made our results more reliable; (2) sensitivity analyses, Egger's linear regression tests, and funnel plots indicated that there were no low-quality studies or publication bias; and (3) TSAs were first performed in the current meta-analysis and indicated sufficient evidence that outdoor air pollution can reduce semen volume, sperm concentration, normal morphology rate, and total motility. Notably, compared with those in a previous meta-analysis that included 6 studies [35], the cumulative Z-curves in the current meta-analysis crossed the trial sequential monitoring boundaries, meaning the total sample size was more than the estimated information size after adding another 5 studies.
Though this study had a relatively large sample size, several limitations should be stressed: (1) the sources and concentration of the air pollutants varied among the enrolled studies, which may increase the heterogeneity between studies and result in potential bias. One reason for this is that pollution levels were different in different regions or seasons, making it difficult to set the same standard. In this meta-analysis, all relevant researches were strictly scanned and most of the studies only provided information about the overall impacts of air pollution. Based on the existing data, the current meta-analysis is aimed at exploring the overall impacts of air pollution on semen quality. (2) The impacts of the single components of the air pollutants were not analyzed because studies provided information concerning single components were limited. Further studies are required to explore the impacts of each component such as SO2 and CO. (3) Other harmful environment urban factors, such as water pollution or electromagnetic waves from cell phones and stations, can decrease semen quality, and their negative influence cannot be objectively separated from the negative influence of toxic air components. (4) Most participants enrolled in this study were Caucasians, and relevant data in Africans and Asians were limited and required further study. (5) Only four studies focused on CASA measures with inconsistent conclusions, and more studies are needed to investigate the effects of air pollution on these indicators. (6) Though the results of TSA indicated a firm association between air pollution and decreased semen volume, concentration, progressive motility, and total motility, more high-quality studies are required to offer more individual data.
5. Conclusion
Air pollution is associated with decreased semen volume, sperm concentration, motility, and normal morphology rate.
Acknowledgments
This work is supported by the grant from the National Natural Science Foundation of China (Grant No. 81671448).
Data Availability
This is a systematic review and all the data were extracted from the enrolled studies. The original data can be accessed in these studies.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
HL conceived and designed the experiments. JZ and ZC searched for and selected the publications. JZ, CM, and JX analyzed the data. JZ, CM, and JX prepared the figures. JZ and ZC contributed materials/analysis tools. JZ and ZC wrote and revised the paper.
Supplementary Materials
Supplement Materials Figure S1: sensitivity of each included study in this meta-analysis. (A–K) Sensitivity analyses of semen volume, sperm concentration, total sperm count, normal morphology rate, progressive motility, total sperm motility, DFI, VCL, VSL, and LIN, respectively. Figure S2: Begg's funnel plots of the publication bias. (A–K) Funnel plots of semen volume, sperm concentration, total sperm count, normal morphology rate, progressive motility, total sperm motility, DFI, VCL, VSL, and LIN, respectively.
References
- 1.Kihal-Talantikite W., Legendre P., le Nouveau P., Deguen S. Premature adult death and equity impact of a reduction of NO2, PM10, and PM2.5 levels in Paris-a health impact assessment study conducted at the census block level. International Journal of Environmental Research and Public Health. 2018;16(1):p. 38. doi: 10.3390/ijerph16010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tajudin M. A. B. A., Khan M. F., Mahiyuddin W. R. W., et al. Risk of concentrations of major air pollutants on the prevalence of cardiovascular and respiratory diseases in urbanized area of Kuala Lumpur, Malaysia. Ecotoxicology and Environmental Safety. 2019;171:290–300. doi: 10.1016/j.ecoenv.2018.12.057. [DOI] [PubMed] [Google Scholar]
- 3.Soleimani Z., Darvishi Boloorani A., Khalifeh R., Griffin D. W., Mesdaghinia A. Short-term effects of ambient air pollution and cardiovascular events in Shiraz, Iran, 2009 to 2015. Environmental Science and Pollution Research International. 2019;26(7):6359–6367. doi: 10.1007/s11356-018-3952-4. [DOI] [PubMed] [Google Scholar]
- 4.Fu P., Guo X., Cheung F. M. H., Yung K. K. L. The association between PM2.5 exposure and neurological disorders: a systematic review and meta-analysis. Science of The Total Environment. 2019;655:1240–1248. doi: 10.1016/j.scitotenv.2018.11.218. [DOI] [PubMed] [Google Scholar]
- 5.Conforti A., Mascia M., Cioffi G., et al. Air pollution and female fertility: a systematic review of literature. Reproductive Biology and Endocrinology. 2018;16(1):p. 117. doi: 10.1186/s12958-018-0433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafuente R., Garcia-Blaquez N., Jacquemin B., Checa M. A. Outdoor air pollution and sperm quality. Fertility and Sterility. 2016;106(4):880–896. doi: 10.1016/j.fertnstert.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Jurewicz J., Dziewirska E., Radwan M., Hanke W. Air pollution from natural and anthropic sources and male fertility. Reproductive Biology and Endocrinology. 2018;16(1):p. 109. doi: 10.1186/s12958-018-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Yang B., Cai Z., Li H., Han T., Wang Y. The negative impact of higher body mass index on sperm quality and erectile function: a cross-sectional study among Chinese males of infertile couples. American Journal of Men's Health. 2019;13(1) doi: 10.1177/1557988318822572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundhun P. K., Janoo G., Bhurtu A., et al. Tobacco smoking and semen quality in infertile males: a systematic review and meta-analysis. BMC Public Health. 2019;19(1):p. 36. doi: 10.1186/s12889-018-6319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardneck F., Israel G., Pool E., Maree L. Quantitative assessment of heavy metal effects on sperm function using computer-aided sperm analysis and cytotoxicity assays. Andrologia. 2018;50(10):p. e13141. doi: 10.1111/and.13141. [DOI] [PubMed] [Google Scholar]
- 11.Ricci E., Noli S., Ferrari S., et al. Alcohol intake and semen variables: cross-sectional analysis of a prospective cohort study of men referring to an Italian Fertility Clinic. Andrology. 2018;6(5):690–696. doi: 10.1111/andr.12521. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A., Singh A., Hamada A., Kesari K. Cell phones and male infertility: a review of recent innovations in technology and consequences. International Braz J Urol. 2011;37(4):432–454. doi: 10.1590/s1677-55382011000400002. [DOI] [PubMed] [Google Scholar]
- 13.Gorpinchenko I., Nikitin O., Banyra O., Shulyak A. The influence of direct mobile phone radiation on sperm quality. Central European Journal of Urology. 2014;67(1):65–71. doi: 10.5173/ceju.2014.01.art14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Nisio A., Foresta C. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reproductive Biology and Endocrinology. 2019;17(1):p. 4. doi: 10.1186/s12958-018-0449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guven A., Kayikci A., Cam K., Arbak P., Balbay O., Cam M. Alterations in semen parameters of toll collectors working at motorways: does diesel exposure induce detrimental effects on semen? Andrologia. 2008;40(6):346–351. doi: 10.1111/j.1439-0272.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 16.Calogero A. E., la Vignera S., Condorelli R. A., et al. Environmental car exhaust pollution damages human sperm chromatin and DNA. Journal of Endocrinological Investigation. 2011;34(6):e139–e143. doi: 10.1007/BF03346722. [DOI] [PubMed] [Google Scholar]
- 17.Wu L., Jin L., Shi T., et al. Association between ambient particulate matter exposure and semen quality in Wuhan, China. Environment International. 2017;98:219–228. doi: 10.1016/j.envint.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y., Zhou Y., Ma J., et al. Inverse association between ambient sulfur dioxide exposure and semen quality in Wuhan, China. Environmental Science & Technology. 2017;51(21):12806–12814. doi: 10.1021/acs.est.7b03289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selevan S. G., Borkovec L., Slott V. L., et al. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environmental Health Perspectives. 2000;108(9):887–894. doi: 10.1289/ehp.00108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Rosa M., Zarrilli S., Paesano L., et al. Traffic pollutants affect fertility in men. Hum Reprod. 2003;18(5):1055–1061. doi: 10.1093/humrep/deg226. [DOI] [PubMed] [Google Scholar]
- 21.Boggia B., Carbone U., Farinaro E., et al. Effects of working posture and exposure to traffic pollutants on sperm quality. Journal of Endocrinological Investigation. 2009;32(5):430–434. doi: 10.1007/bf03346481. [DOI] [PubMed] [Google Scholar]
- 22.Rubes J., Rybar R., Prinosilova P., et al. Genetic polymorphisms influence the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutation Research. 2010;683(1-2):9–15. doi: 10.1016/j.mrfmmm.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D. G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine. 2009;151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Cai Z., Yang B., Li H. Association between outdoor air pollution and semen quality: protocol for an updated systematic review and meta-analysis. Medicine. 2019;98(20):p. e15730. doi: 10.1097/md.0000000000015730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubes J., Selevan S. G., Evenson D. P., et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Human Reproduction. 2005;20(10):2776–2783. doi: 10.1093/humrep/dei122. [DOI] [PubMed] [Google Scholar]
- 26.Zhou N., Cui Z., Yang S., et al. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environmental Pollution. 2014;187:145–152. doi: 10.1016/j.envpol.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Lao X. Q., Zhang Z., Lau A. K. H., et al. Exposure to ambient fine particulate matter and semen quality in Taiwan. Occupational and Environmental Medicine. 2018;75(2):148–154. doi: 10.1136/oemed-2017-104529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono N., Oshio S., Niwata Y., et al. Prenatal exposure to diesel exhaust impairs mouse spermatogenesis. Inhalation Toxicology. 2007;19(3):275–281. doi: 10.1080/08958370601069257. [DOI] [PubMed] [Google Scholar]
- 29.Jeng H. A., Yu L. Alteration of sperm quality and hormone levels by polycyclic aromatic hydrocarbons on airborne particulate particles. Journal of Environmental Science and Health. Part A, Toxic/Hazardous Substances & Environmental Engineering. 2008;43(7):675–681. doi: 10.1080/10934520801959815. [DOI] [PubMed] [Google Scholar]
- 30.Hammoud A., Carrell D. T., Gibson M., Sanderson M., Parker-Jones K., Peterson C. M. Decreased sperm motility is associated with air pollution in Salt Lake City. Fertility and Sterility. 2010;93(6):1875–1879. doi: 10.1016/j.fertnstert.2008.12.089. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Liu J., Ren L., et al. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Science of The Total Environment. 2018;634:1435–1444. doi: 10.1016/j.scitotenv.2018.03.383. [DOI] [PubMed] [Google Scholar]
- 32.Sokol R. Z., Kraft P., Fowler I. M., Mamet R., Kim E., Berhane K. T. Exposure to environmental ozone alters semen quality. Environmental Health Perspectives. 2006;114(3):360–365. doi: 10.1289/ehp.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuchakulla M., Masterson T., Arora H., Kulandavelu S., Ramasamy R. Effect of nitroso-redox imbalance on male reproduction. Translational Andrology and Urology. 2018;7(6):968–977. doi: 10.21037/tau.2018.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo L., Li E., Zhao S., et al. Gasoline exhaust damages spermatogenesis through downregulating α6-integrin andβ1-integrinin the rat model. Andrologia. 2018;50(7):p. e13045. doi: 10.1111/and.13045. [DOI] [PubMed] [Google Scholar]
- 35.Deng Z., Chen F., Zhang M., et al. Association between air pollution and sperm quality: a systematic review and meta-analysis. Environmental Pollution. 2016;208:663–669. doi: 10.1016/j.envpol.2015.10.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Materials Figure S1: sensitivity of each included study in this meta-analysis. (A–K) Sensitivity analyses of semen volume, sperm concentration, total sperm count, normal morphology rate, progressive motility, total sperm motility, DFI, VCL, VSL, and LIN, respectively. Figure S2: Begg's funnel plots of the publication bias. (A–K) Funnel plots of semen volume, sperm concentration, total sperm count, normal morphology rate, progressive motility, total sperm motility, DFI, VCL, VSL, and LIN, respectively.
Data Availability Statement
This is a systematic review and all the data were extracted from the enrolled studies. The original data can be accessed in these studies.


