Abstract
To determine ways to improve the utilization of corn stover, this study investigated methane production from different parts of corn stover using a simple co-culture of an anaerobic fungus (Pecoramyces species) and methanogen (Methanobrevibacter species). The simple co-culture was incubated with the stem pith, leaf blade, or stem bark of corn stover (as substrates) at 39°C for 72 h. The results showed that the stem bark had the lowest (P < 0.05) digestibility (38.0 ± 1.36%) and neutral detergent solubles, that is, cell solubles (31.6 ± 0.45%), and the highest (P < 0.05) lignin content (4.8 ± 0.56%). The leaf blade had a significantly higher methane conversion rate (56.6 ± 0.76 mL/g digested substrate) than the stem pith (49.2 ± 1.60 mL/g digested substrate), even though they showed similar levels of methane production (42.4 ± 1.0 mL and 40.9 ± 1.35 mL, respectively). Both the leaf blade and stem pith of corn stover have the potential to produce methane in a simple co-culture of an anaerobic fungus and methanogen.
Keywords: anaerobic fungus, methanogen, corn stover, leaf blade, stem pith
Introduction
The rational exploitation and utilization of energy are vital for sustainable social development. The increasing depletion of fossil fuels is one of the biggest challenges for the future development of the economy and society. The production of non-petroleum sources of energy has attracted increased attention in several countries. Today, dedicated energy crops, such as maize, sorghum, and wheat, are widely used to produce methane (Riva et al., 2014). However, an increasing energy demand has led to the need to consume large amounts of such energy crops to produce methane. This makes the cost of methane production very high, and leads to an inevitable conflict between the use of limited supplies of energy crops for both food/feed and energy production (Croce et al., 2016).
The use of corn stover, instead of energy crops, to produce methane is one way to address this challenge. Corn stover stores half of the organic matter of an entire crop and has a reasonably high nutritional value (Li et al., 2017). However, cellulose, which is complexed with lignin in crop residues, is highly resistant to anaerobic fermentation and thus to methane production (Berchem et al., 2017; Wu and Tian, 2018). Although there are many limitations in the production of methane from corn stover, it is still considered a good potential substrate for anaerobic fermentation. Furthermore, the production of methane from corn stover is greater than that produced from other crop straws (Croce et al., 2016). In recent years, corn stover has been used to produce methane in anaerobic digestors or in batch cultures (Haag et al., 2015).
Methane production from corn stover depends on its chemical composition (Fernando et al., 2006). At present, some physical and chemical pretreatment methods can improve the degradation of straw and thus improve the efficiency of methane production. Steam explosion, microwave, and other heat treatment methods can dissolve lignin, but they also produce toxic substances, which can inhibit anaerobic fermentation (Sapci et al., 2013; Theuretzbachera et al., 2015). Although cold treatment methods, such as extrusion and comminution, do not produce harmful substances, they do not remove lignin from the fiber structure, which leads to poor methane production (Hjorth et al., 2011).
Other processes, such as acid and alkali pretreatment, may also be applied separately before anaerobic fermentation. The application of acid for pretreatment is great extent restricted by high acid and energy consumption, equipment corrosion, and obligation for acid recovery (Ibrahim, 2012). Moreover, alkaline treatment can even produce secondary products, thereby reducing the production of methane during fermentation (Croce et al., 2016). When combined with some physical and chemical methods to simultaneously pretreat straw, the above listed drawbacks could be alleviated, and methane production increased. However, such pretreatment itself violates the original intention of producing new energy sustainably, owing to the considerable consumption of energy. In addition, the application of such treatments can be expensive.
Anaerobic fungi (AF), a constituent of the natural microbial communities found in the rumen of herbivores, are known for their fiber-degrading ability. Unlike fiber-degrading bacteria, AF have unique rhizoid systems that can colonize and degrade the plant cell wall for effective degradation (Gruninger et al., 2014; Solomon et al., 2016). Anaerobic fungi use many carbohydrate-degrading enzymes and their unique rhizoid system to physically destroy the ultrastructure of the plant cell wall to degrade lignocellulose. This action is considered to increase the surface area for bacterial colonization and further enzymatic digestion (Gruninger et al., 2018). The use of AF could preclude the requirement for pretreatment in methane production.
Compared with industrial preparations, AF are natural, harmless, efficient, and convenient and can be easily obtained. Methanogens can be found toward the end of the metabolic process in the rumen, which can utilize the metabolic products of AF and fibrolytic bacteria to produce methane (Jin et al., 2017). Many studies using co-cultured AF and methanogens have shown that co-cultured methanogens could not only use fungal metabolites to produce methane, but also shift the fungal metabolic pathway to confer significantly higher levels of fiber-degrading ability to the AF (Joblin et al., 1990). Thus, co-cultures of AF and methanogens have the potential for use in the degradation of lignocellulosic substrates for methane production (Theodorou et al., 1996; Bootten et al., 2011; Shi et al., 2019).
Zhou et al. (2015) reported that the structural components and nutrient utilization rates of different parts of the same straw were significantly different. Zhao et al. (2011) demonstrated that the content and structure of cellulose and hemicellulose in the stem bark (SB), leaf blade (LB), and stem pith (SP) of corn stover also differed. In order to improve the utilization of corn stover, this study investigated the methane production from different parts of corn stover using a simple co-culture of an anaerobic fungus and methanogen.
Materials and Methods
Co-culture of Anaerobic Fungus and Methanogen
The co-culture of an anaerobic fungus and methanogen used in the present study was isolated from a goat (Jin et al., 2011). The fungus was identified as Pecoramyces species (Li et al., 2017), and the methanogen was identified as Methanobrevibacter species (Jin et al., 2011). The anaerobic fungus was identified to the genus level through the use of traditional morphological identification and molecular phylogenetic analyses. Morphological identification included rhizoid, mycelium, and flagella identification and nuclear staining with DAPI (4′,6-diamidino-2-phenylindole). Molecular phylogenetic analyses were based on the amplification and sequencing of the genes encoding the 28S rRNA (LSU) and ITS (internal transcribed spacer) sequences with AF-LSU primers (Li et al., 2019). The 16S rRNA genes amplified from the total DNA extracted from the cultures were used for gene sequencing to evaluate the specific type of methanogen, using the Met86F and Met1340R primers, according to the methods of Jin et al. (2011).
The co-culture was maintained in liquid media (Davies et al., 1993) with rice straw as a substrate and transferred every 3 days. Briefly, this method entailed the transfer of 1 mL of co-cultured anaerobic fungus and methanogen solution into a roll tube with 9 mL of fresh medium, according to the methods of Cheng et al. (2009). Each 1,000 mL of medium contained 150 mL of buffer solution A, 150 mL of buffer solution B, 550 mL of basal medium, (2.5 g yeast extract, 100 g tryptone, and 6 g NaHCO3), 150 mL of cell-free rumen fluid (centrifuged at 16,000g for 20 min at 4°C, with the supernatant decanted and stored at −20°C), 1 g of L-cysteine hydrochloride, and 1 mL of 0.1% (wt/vol) resazurin. Buffer solution A contained 0.3 g of K2HPO4 per 100 mL, and buffer solution B contained 0.3 g of KH2PO4, 0.6 g of NaCl, 0.6 g of (NH4)2SO4, 0.06 g of MgSO4 ⋅ 7H2O, and 0.06 g of CaCl2 ⋅ 2H2O per 100 mL. This medium was sterilized by autoclaving at 115°C for 20 min.
Penicillin–streptomycin solution was added to the medium to inhibit bacterial growth. The final concentrations of penicillin and streptomycin were 1,915 and 2,031 U mL–1, respectively. Antibiotic solution was sterilized by using a filtration membrane (size 0.22 μm, SCAA-102; ANPEL, Shanghai, China). At the end of the fermentation process, an aliquot of 10 mL of the supernatant from each bottle was transferred to new media, to which chloramphenicol was added to determine whether the medium became clear after 3 days of incubation. If the medium became clear, this indicated the presence of methanogens in the fermentation medium, and if the medium remained turbid, this indicated the presence of bacteria.
Different Parts of Corn Stover
The corn stover was collected, air dried, and separated into the LB, SP, and SB. The three parts were separately oven dried at 65°C and ground to be passed through a sieve (∼1 mm) for further use as substrates.
Experimental Design and Sample Collection
The experiment comprised three groups, each containing 1 g of SP, LB, or SB as substrates. Each group had four replicates. The media (90 mL) was pre-warmed at 39°C and inoculated with 10 mL of 3-day-old anaerobic fungus and methanogen co-culture. Based on the established growth characteristics of the anaerobic fungus (Li et al., 2017), the co-culture of the anaerobic fungus and methanogen was conducted under strict anaerobic conditions under a headspace of 100% CO2 at 39°C in a butyl rubber-stoppered 180 mL serum bottle containing 90 mL of media without shaking for 72 h. A blank group without inoculation was prepared for gas and analyte correction.
Before the fermentation process, 2 mL of the supernatants was collected from the SP, LB, and SB vessels and used to measure the concentrations of reducing sugars, glucose, and xylose. Gas production and methane production were measured every 6 h (at 0, 6, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, and 72 h). At the end of fermentation, the pH was immediately measured, and the supernatant was collected for the analysis of fermentation end products, fiber-degrading enzyme activities, and reducing sugars. The remaining substrates were collected for the analysis of the digestibility of dry matter (DM), neutral detergent fiber (NDF), acid detergent fiber (ADF), cellulose, and hemicellulose.
Analysis of Chemical Composition of Different Parts of Corn Stover
The contents of NDF, ADF, and lignin of the LB, SP, and SB were determined according to the methods of Van Soest et al. (1991). The cell solubles or the soluble solutes in the neutral detergent solution (NDS), cellulose, and hemicellulose were calculated according to the methods of Niu et al. (2018). In addition, NDF and ADF were determined using a fiber analyzer (Ankom A200i; Ankom Technology, Macedon, NY, United States). First, the samples were treated with NDS; the dissolved part was NDS, and the residue was NDF. The NDF was further treated with acid detergent solution to dissolve the hemicellulose and obtain the ADF. The ADF thus obtained was digested with 72% sulfuric acid, which dissolves cellulose, and the residue comprised a mixture of lignin and silicate. This residue was ashed, which removes the lignin, thereby facilitating the determination of silicate. The digestibility of DM, NDF, ADF, cellulose, and hemicellulose was calculated according to the methods of Li et al. (2016).
Each 1,000 mL of NDS contained 30 g of sodium dodecyl sulfate, 18.6 g of disodium ethylenediamine tetraacetic acid (Na2EDTA), 6.8 g of Na2B4O7, 4.6 g of Na2HPO4, and 10 mL of C6H14O4. Each 1,000 mL of acid detergent solution contained 20 g of cetrimonium bromide dissolved in 1,000 mL of 0.5 mol/L sulfuric acid solution.
Measurement of Gas and Methane Production
Gas production was determined using a pressure transducer, according to the methods of Theodorou et al. (1994). The pressure transducer determined the levels of gas production during fermentation at the top surface of the serum bottles with a capacity of 180 mL. Gas production was recorded every 6 h, and the gas was then released to bring the air pressure within the bottle to 0, to facilitate the determination of gas production at the next time point. The gas production volumes at each time point were added to determine the cumulative gas production. By recording the gas production at intermittent time points, a complete growth curve of the co-culture of the anaerobic fungus and methanogen was obtained. After each time point at which gas production was determined, 5 mL of gas was collected in an air bag to determine the methane concentration in the gas.
Methane content in the gas was determined by gas chromatography (Agilent 7890B; Agilent, Palo Alto, CA, United States), according to the methods of Hu et al. (2006). The conditions used were as follows: column temperature of 80°C; vaporization chamber temperature of 100°C; H2 ion flame detector, with a detection temperature of 120°C; and carrier gas (N2) pressure of 0.05 MPa; air pressure of 0.05 MPa; and H2 pressure of 0.05 MPa. The volume of methane was calculated according to the method of Li et al. (2018).
Analysis of Fermentation End Products
The reducing sugars were analyzed using the 3,5-dinitrosalicylic acid (DNS) method (Lowe et al., 1987b). The ratio of the sample to DNS reagent was 1:2, and absorbance was read at 640 nm. The concentrations of glucose, xylose, formate, acetate, and lactate were determined according to the protocol described by Shi et al. (2019). Glucose was determined using the glucose oxidase method, xylose was measured using the phloroglucinol color-developing method, and lactate was determined by the NAD+ transformation method. These analytes were all determined using the applicable assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Formate was measured with the Formate Assay Kit (Sigma, Santa Clara, CA, United States). Acetate was measured by gas chromatography (Daojin GC- 2014AFsc Instrument; Shimadzu, Kyoto, Japan) using a capillary column. An aliquot of 0.2 mL of crotonate metaphosphate solution (0.25 g/mL) was mixed with 1 mL of supernatant from the fermentation medium and centrifuged at 16,000g for 10 min. The supernatant was then analyzed by gas chromatography with a column temperature of 130°C; vaporization temperature of 180°C; H2 ion flame detector and detection temperature of 180°C; N2 carrier gas at a pressure of 60 kPa; H2 pressure of 50 kPa; and O2 pressure of 50 kPa. Ethanol was determined by gas chromatography (TRACE GC Ultra; Thermo Fisher, Waltham, Massachusetts, United States) using the method described by Edgardo et al. (2008).
Analysis of Fiber-Degrading Enzyme Activity
The activities of carboxymethyl cellulase (CMCase), a cellulose-degrading enzyme, and xylanase, a hemicellulose-degrading enzyme, were measured according to the method of Lowe et al. (1987a). The fermentation supernatant was used to measure enzyme activities. To an appropriate volume of the supernatant solution (preheated at 50°C), either 1 mL of xylan solution or 10 mg of carboxymethylcellulose sodium in 0.1 mol/L citric acid disodium hydrogen phosphate buffer solution was added. After 30 min of reaction at 50°C, DNS reagent was added, and the mixture was then boiled for 10 min, and the absorbance was read at 640 nm. The ratio of the sample to DNS reagent was 1:2. One unit of CMCase activity was defined as 1 μmol of glucose released per mL of supernatant per minute (U mL–1 min–1). One unit of xylanase enzyme activity was defined as 1 μmol of xylose released per mL of supernatant per minute (U mL–1 min–1).
Statistical Analysis
Statistical analysis was performed using the SPSS 20.0 software (IBM SPSS Statistics, version 20.0; IBM Corp, Armonk, NY, United States) with one-way analysis of variance at a confidence interval of 95%. Duncan new multiple-range test was then used to compare the differences among the three groups. Data were presented as the mean ± standard error of the mean.
Results
Chemical Composition and Digestibility of the SB, LB, and SP of Corn Stover
The results are presented in Table 1. The SB had the lowest (P < 0.05) NDS content (31.6 ± 0.45%) and highest (P < 0.05) cellulose (40.9 ± 0.30%) and lignin (4.8 ± 0.56%) contents. The LB had the lowest (P < 0.05) cellulose (26.5 ± 0.09%) and lignin contents (2.4 ± 0.07%), and highest (P < 0.05) hemicellulose content (27.4 ± 0.55%). The SP had the highest (P < 0.05) NDS content (45.4 ± 0.55%).
TABLE 1.
Chemical composition of the stem bark, leaf blade, and stem pith of corn stover.
| Items |
Corn stover parts |
SEM | P | ||
| SB | LB | SP | |||
| NDS (%) | 31.6c | 42.8b | 45.4a | 0.73 | <0.001 |
| NDF (%) | 68.4a | 57.2b | 54.6c | 0.73 | <0.001 |
| ADF (%) | 46.6a | 29.8c | 31.4b | 0.48 | <0.001 |
| Cellulose (%) | 40.9a | 26.5c | 28.0b | 0.42 | <0.001 |
| Hemicellulose (%) | 21.8b | 27.4a | 23.3b | 0.90 | 0.002 |
| Lignin (%) | 4.8a | 2.4b | 2.6b | 0.62 | 0.013 |
Values in the same row with different superscript letters are significantly different (P < 0.05). SB, stem bark; LB, leaf blade; SP, stem pith; NDS, neutral detergent soluble solute; NDF, neutral detergent fiber; ADF, acid detergent fiber; SEM, standard error of the mean (n = 4).
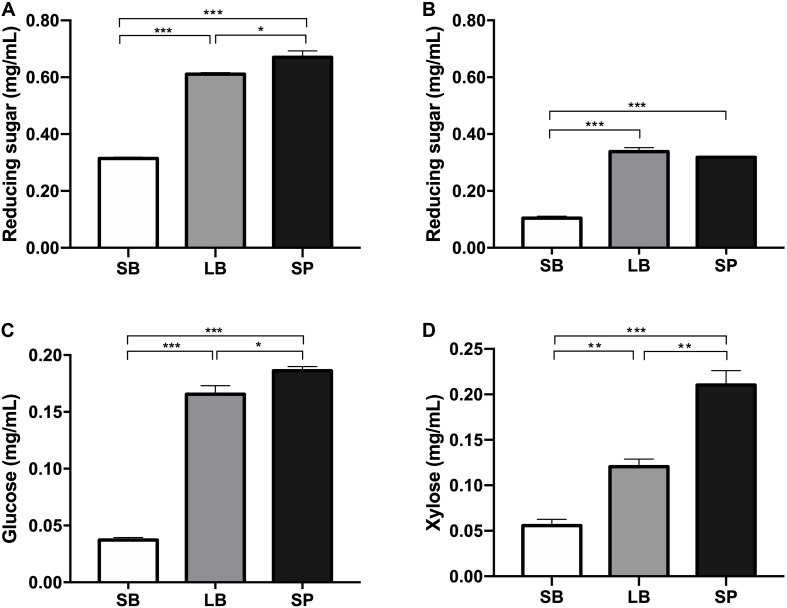
The degradation values of the SB, LB, and SP of corn stover in the co-culture are shown in Table 2. The DM digestibility (DMD) of the SP was the highest (82.0 ± 1.95%, P < 0.05), followed by those of the LB (74.8 ± 1.95%) and SB (38.0 ± 1.36%). The hemicellulose digestibility of the SP was highest (78.6 ± 1.64%), followed by those of the LB (77.4 ± 0.95%) and SB (25.7 ± 0.75%). As shown in Figure 1, the concentrations of reducing sugars, glucose, and xylose in the SP were the highest (P < 0.05), followed by those in the LB and SB.
TABLE 2.
Degradation of the stem bark, leaf blade, and stem pith of corn stover in a co-culture of an anaerobic fungus and methanogen.
| Items |
Corn stover parts |
SEM | P | ||
| SB | LB | SP | |||
| DMD (%) | 38.0c | 74.8b | 82.0a | 2.51 | <0.001 |
| NDFD (%) | 32.7c | 63.3b | 79.5a | 0.17 | <0.001 |
| ADFD (%) | 36.0c | 50.3b | 80.2a | 0.12 | <0.001 |
| NDSD (%) | 62.4c | 81.4b | 86.9a | 0.07 | <0.001 |
| HD (%) | 25.7b | 77.4a | 78.6a | 0.30 | 0.002 |
| CD (%) | 24.2c | 60.1b | 75.0a | 0.75 | < 0.001 |
Values in the same row with different superscript letters are significantly different (P < 0.05). SB, stem bark; LB, leaf blade; SP, stem pith; DMD, digestibility of dry matter; NDFD, digestibility of neutral detergent fiber; ADFD, digestibility of acid detergent fiber; NDSD, digestibility of neutral detergent solubles; HD, digestibility of hemicellulose; CD, digestibility of cellulose; SEM, standard error of the mean (n = 4).
FIGURE 1.
Concentrations of reducing sugar before (A) and after (B) fermentation and of glucose (C) and xylose (D) in the supernatant, following incubation using the stem bark (SB), leaf blade (LB), and stem pith (SP) of corn stover as substrates. The error bars represent the standard error of the mean (n = 4). *P < 0.05, **P < 0.01, and ***P < 0.001.
Gas and Methane Production From the SB, LB, and SP of Corn Stover
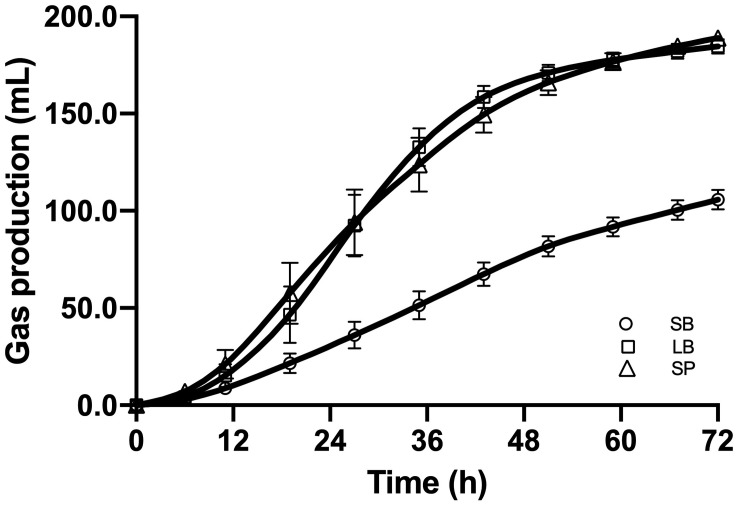
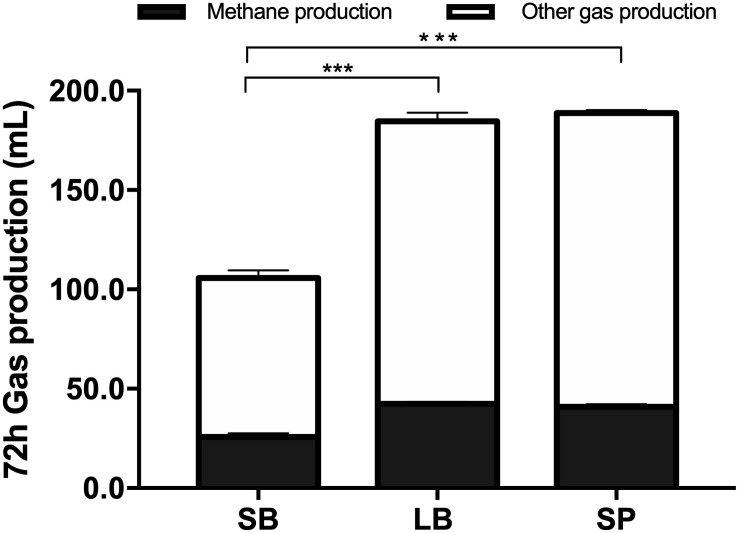
Figure 2 shows the cumulative gas production, over 72 h of fermentation, from the SB, LB, and SP of corn stover using the co-culture. The curves of methane production were similar to those of overall gas production. At the end of fermentation, the total gas production values of the LB (184.6 ± 3.74 mL) and SP (188.8 ± 2.60 mL) were significantly higher than those of the SB (105.8 ± 5.06 mL, P < 0.05), and no significant difference was observed between those of the LB and SP (P > 0.05) (Figure 3). Total methane production values from the LB (42.4 ± 0.99 mL) and SP (40.9 ± 1.35 mL) were significantly higher than those from the SB (25.8 ± 1.85 mL, P < 0.05), and no significant difference was observed between those from the LB and SP (P > 0.05) (Figure 3). The LB and SP showed similar levels of methane production, but the DMD of the LB was significantly lower than that of the SP (P < 0.05). This indicated that the methane conversion rate of the LB (56.6 ± 0.76 mL/g digested substrate) was significantly higher than that of the SP (49.2 ± 1.60 mL/g digested substrate) (P < 0.05).
FIGURE 2.
Cumulative gas production from the stem bark (SB), leaf blade (LB), and stem pith (SP) of corn stover using a co-culture of anaerobic fungus and methanogen. The error bars represent the standard error of the mean (n = 4).
FIGURE 3.
Total gas and methane production from the stem bark (SB), leaf blade (LB), and stem pith (SP) of corn stover using a co-culture of anaerobic fungus and methanogen. The error bars represent the standard error of the mean (n = 4). ***P < 0.001.
Fiber-Degrading Enzyme Activity and Fermentation Metabolites Following Incubation of the SB, LB, and SP of Corn Stover
As shown in Table 3, the activity of CMCase in the LB group was significantly higher (P < 0.05) than that in the SP and SB groups. Xylanase activity in the SB group was significantly lower (P < 0.05) than that in the LB and SP groups, and no significant difference (P > 0.05) was observed between the values in the LB and SP groups.
TABLE 3.
Activities of fiber-degrading enzymes of an anaerobic fungus following incubation using the stem bark, leaf blade, and stem pith of corn stover as substrates.
| Items |
Corn stover parts |
SEM | P | ||
| SB | LB | SP | |||
| CMCase (U mL–1 min–1) | 0.37c | 0.50a | 0.45b | 0.01 | <0.001 |
| Xylanase (U mL–1 min–1) | 9.51b | 14.53a | 13.80a | 0.71 | <0.001 |
Values in the same row with different superscript letters are significantly different (P < 0.05). SB, stem bark; LB, leaf blade; SP, stem pith; SEM, standard error of the mean (n = 4); CMCase, carboxymethyl cellulase.
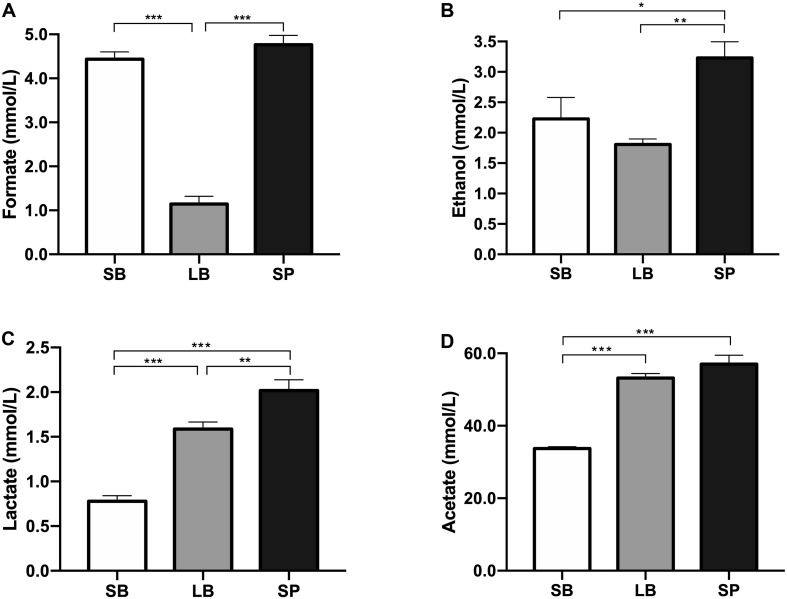
The pH values showed significant differences among the three groups (P < 0.05). The SB had the highest pH value (6.6 ± 0.01), which was significantly higher than those of the LB (6.4 ± 0.02) and SP (6.3 ± 0.01) (P < 0.05). The pH value of the LB was also significantly higher than that of the SP (P < 0.05). The concentrations of water-soluble metabolites in the supernatant of the co-culture of the anaerobic fungus and methanogen when incubated with SB, LB, and SP as substrates are shown in Figure 4. Acetate was the dominant metabolite in the supernatant, followed by formate, ethanol, and lactate. The concentrations of formate, ethanol, lactate, and acetate all showed significant differences among the three groups (P < 0.05).
FIGURE 4.
Concentrations of formate (A), ethanol (B), lactate (C), and acetate (D) in the supernatant of a co-culture of anaerobic fungus and methanogen, following incubation using the stem bark (SB), leaf blade (LB), and stem pith (SP) of corn stover as substrates. The error bars represent the standard error of the mean (n = 4). *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
The DMD of substrates reflects their utilization (Hao, 2011). In the present study, the DMD of the SP, LB, and SB of corn stover showed significant differences (P < 0.05). The DMD of the SB was significantly lower than that of the SP and LB, and the DMD of the LB was significantly lower than that of the SP (P < 0.05). These findings might be due to the differences in cell-soluble and lignin contents among the three parts. According to Ma et al. (2018), NDS mainly contains protein, fat, starch, and sugar, all of which can be easily degraded by microorganisms (Teunissen et al., 1992).
In the present study, NDS in the SP was the highest, and that in the SB was the lowest. In plant fibers, lignin and hemicellulose are assembled into a complex supramolecular network, which coats the cellulose fibrils. This complex network reduces the digestibility of plant fiber and is the major constraint in the sustainable production of biofuels (Gruber, 2009; Silveira et al., 2015). In the present study, the digestibility of hemicellulose and cellulose in the LB and SP was significantly higher than that in the SB, which might be due to the significantly higher lignin content in the SB compared with that in the LB and SP.
Sun et al. (2005) investigated the attachment on and fermentation profiles of substrates with different lignin contents exposed to ruminal AF. They found that the growth of AF was decreased with increasing lignin content in the substrates. Lignin is a complex compound composed of phenylpropane. It is the most difficult part of the plant cell wall to be degraded. Lignin and polysaccharide are linked by hydroxycinnamic acid, which forms an ester bond and ether linkage with carbonyl and phenolic groups of hydroxycinnamic acid. Although AF can degrade arabinoxylan and release hydroxycinnamic acid, they can neither break the ether bond between lignin and hydroxycinnamic acid, nor degrade lignin (Borneman et al., 1990). Therefore, AF can degrade plant tissues with a relatively low lignin content to a greater extent and at a higher rate.
Methane production by the SB was the lowest in this study, which might be due to the low DMD of the SB, resulting in less substrates (H2/CO2/formate) for the co-cultured methanogens. Zhu et al. (2001) determined the total gas and methane production in their study of the degradation of straw by an anaerobic fungus. They found that the DMD was positively correlated with total gas production and methane production. Jin (2009) also reported that methane production was positively correlated with the DMD of substrates following the co-culture of an anaerobic fungus and methanogen. The process of anaerobic fungal growth and the utilization of crude fiber can be divided into two stages: in the first phase, the polysaccharide hydrolytic enzyme secreted by the anaerobic fungus hydrolyzes the crude fiber into fermentable sugars (mainly glucose and xylose); in the second stage, the anaerobic fungus absorbs or transports these fermentable sugars into the cell and finally metabolizes them into H2, CO2, formate, acetate, lactate, and ethanol (Solomon et al., 2016). In this study, each part of corn stover was first degraded into a large number of fermentable carbon sources, and then these carbon sources generated H2, CO2, and soluble metabolites in the cytosol and hydrogenosome of anaerobic fungus. At the end of the fermentation, the measured amount of H2 accumulation was very low, accounting for only 2% of the total gas production, indicating that most of the H2 produced was used by methanogens, H2 reduces CO2 to generate methane.
Li (2017) reported that during co-culture methane production is accelerated during the period from 32 to 64 h, and methanogens can utilize both the H2 and formate produced by the anaerobic fungus. In our current study, the LB fraction with the highest methane conversion efficiency had the lowest formate concentration in the medium. In addition, gas production from the LB fraction was highest at 30–64 h of fermentation. These results suggest that a large amount of formate produced by the anaerobic fungus was utilized by methanogens during this fermentation stage, which is consistent with previous results.
The SP had the highest DMD in the present study, which might be explained by the higher content of NDS and lower contents of cellulose and lignin, as discussed above. It is interesting that the LB had a significantly lower DMD than the SP but showed similar levels of gas and methane production. Thus, the methane conversion efficiency of the LB was higher than that of the SP, which might be attributed to higher levels of hemicellulose degradation in the LB compared with that in the SP. Without considering economic performance, the methane conversion efficiency of the co-culture pretreatment strategy used in the present study was lower than that of the physical and chemical pretreatment mentioned in the introduction. Whether sulfuric acid, hydrochloric acid, sodium hydroxide, extrusion, or steam explosion pretreatment is used, the conversion efficiency of methane is consistently higher than 100 mL/g, which is more than twofold the conversion efficiency of the pretreatment strategy used in the present study. However, the pretreatment of sulfuric acid, hydrochloric acid, and sodium hydroxide took 9 days (7 days of acid treatment and 2 days of drying), and the following anaerobic digestion was up to 35 days, which indicated that the whole procedure was 44 days (Song et al., 2014; Croce et al., 2016). Meanwhile, the present strategy provided in this study took only 3 days for the whole procedure, which is much more efficient than the above discussed pretreatments.
Compared with the NDS, hemicellulose and cellulose in plants need to be gradually degraded into usable sugars by plant cell wall-degrading enzymes secreted by the anaerobic fungus (Mountfort and Asher, 1985; Haitjema et al., 2014). These usable sugars are then metabolized by the anaerobic fungus into H2, CO2, formate, acetate, ethanol, and succinic acid (Li et al., 2016). Methanogens use H2, reduce the partial pressure of H2 in the system, remove the inhibition of hydrogenase, and thus catalyze more NAD(P)H to H2, which results in increased methane production (Bernalier et al., 1991). The formation of lactate and ethanol requires the participation of NAD(P)H; thus, the production of lactate and ethanol should be inhibited. In this study, the concentrations of ethanol and lactate in the LB group were significantly lower than those in the SP group (P < 0.05).
Solomon et al. (2016) showed that hemicellulase expression is more easily regulated than cellulolytic enzymes in AF. Recent studies have also found that under the same conditions the activity of xylanase produced by AF in the rumen is six times that of cellulase (Li et al., 2017) and four times more than that of the xylanase produced in some industrial fermentations (Lee et al., 1999). Yu (2017) studied the activity of plant cell wall–degrading enzymes in 12 strains of an anaerobic fungus and found that the xylanase activity in Piromyces species CN6 was significantly higher than that of cellulase. Jin (2009) studied the activities of several enzymes after 96 h of fermentation of rice straw by AF and found that xylanase activity of the AF was much higher than that of cellulase. The results of our current study also reflect this situation, in which xylanase activity was much higher than that of cellulase.
At the end of fermentation, the difference in pH values was due to the differences in the concentrations of metabolites. The accumulation of metabolites (acetate, lactate, and formate) can result in a reduction of the pH. Formate, lactate, acetate, and ethanol are reportedly the main water-soluble metabolites of AF (Boxma et al., 2004; Li et al., 2016). Ethanol and lactate are the end products of cytoplasmic metabolism by AF. Compared with the SP, the low concentrations of ethanol and lactate in the LB indicate that cytoplasmic metabolism by the anaerobic fungus was reduced, and more metabolites entered the hydrogenosome to generate H2 and acetate, which promoted the co-cultured methanogen to produce methane, as discussed above. When co-cultured with methanogen, the formate in the supernatant might be utilized to produce methane when H2 is limited (Jin, 2012; Wei et al., 2016).
In the present study, significantly lower levels of formate were observed in the LB group, which implied that the formate was used by the co-cultured methanogen to produce methane, resulting in higher levels of methane in this group. Previous studies have shown that the co-culture of an anaerobic fungus and methanogen could degrade lignocellulosic substrates and produce methane with very limited amounts of formate in the supernatant within 3 days (Kim et al., 2011; Tapio et al., 2017). In the present study, the concentrations of formate were all greater than 1 mmol/L, which implies that the co-culture needed more time to utilize formate to produce methane. Moreover, a large amount of acetate was accumulated in the culture. If acetate-utilizing methanogens could be added to the culture, much higher levels of methane could be produced.
The SB, LB, and SP showed significantly different chemical compositions, which resulted in different levels of digestibility and methane production. The LB and SP of corn stover had higher levels of digestibility and methane production, as they had higher levels of NDS and hemicellulose, which could be easily degraded by the anaerobic fungus. The co-culture of an anaerobic fungus and methanogen has the potential to degrade lignocellulosic substrates to produce methane. More studies are needed in this area to pave the way toward sustainable methane production from lignocellulosic substrates.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
YL and QS completed the experiment and data analysis. YC and ZH conceived and designed the manuscript. YL, YC, and WZ wrote and revised the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the Natural Science Foundation of China (31772627), the Fundamental Research Funds for the Central Universities (KYDK201701) and the “Belt and Road” Cooperation Program of Jiangsu Province (BZ2018055).
References
- Berchem T., Roiseux O., Vanderghem C., Boisdenghien A., Foucart G., Richel A. (2017). Corn stover as feedstock for the production of ethanol: chemical composition of different anatomical fractions and varieties. Biofuel. Bioprod. Biorefin. Biofpr. 11 430–440. 10.1002/bbb.1755 [DOI] [Google Scholar]
- Bernalier A., Fonty G., Gouet P. (1991). Cellulose degradation by two rumen anaerobic fungi in monoculture or in coculture with rumen bacteria. Anim. Feed Sci. Technol. 32 131–136. 10.1016/0377-8401(91)90016-l [DOI] [Google Scholar]
- Bootten T. J., Joblin K. N., McArdle B. H., Harris P. J. (2011). Degradation of lignified secondary cell walls of lucerne (Medicago sativa L.) by rumen fungi growing in methanogenic co-culture. J. Appl. Microbiol. 111 1086–1096. 10.1111/j.1365-2672.2011.05127.x [DOI] [PubMed] [Google Scholar]
- Borneman W. S., Hartley R. D., Morrison W. H., Akin D. E., Ljungdahl L. G. (1990). Feruloyl and p-coumaroyl esterase from anaerobic fungi in relation to plant cell wall degradation. Appl. Microbiol. Biotechnol. 33 345–351. 10.1007/BF00164534 [DOI] [Google Scholar]
- Boxma B., Voncken F., Jannink S., van Alen T., Akhmanova A., van Weelden S. W., et al. (2004). The anaerobic chytridiomycete fungus Piromyces sp. E2 produces ethanol via pyruvate:formate lyase and an alcohol dehydrogenase E. Mol. Microbiol. 51 1389–1399. 10.1046/j.1365-2958.2003.03912.x [DOI] [PubMed] [Google Scholar]
- Cheng Y. F., Edwards J. E., Allison G. G., Zhu W. Y., Theodorou M. K. (2009). Diversity and activity of enriched ruminal cultures of anaerobic fungi and methanogens grown together on lignocellulose in consecutive batch culture. Bioresour. Technol. 100 4821–4828. 10.1016/j.biortech.2009.04.031 [DOI] [PubMed] [Google Scholar]
- Croce S., Wei Q., D’Imporzano G., Dong R., Adani F. (2016). Anaerobic digestion of straw and corn stover: the effect of biological process optimization and pre-treatment on total bio-methane yield and energy performance. Biotechnol. Adv. 34 1289–1304. 10.1016/j.biotechadv.2016.09.004 [DOI] [PubMed] [Google Scholar]
- Davies D. R., Theodorou M. K., Lawrence M. I., Trinci A. P. (1993). Distribution of anaerobic fungi in the digestive tract of cattle and their survival in faeces. J. Gen. Microbiol. 139(Pt 6), 1395–1400. 10.1099/00221287-139-6-1395 [DOI] [PubMed] [Google Scholar]
- Edgardo A., Carolina P., Manuel R., Juanita F., Baeza J. J. E., Technology M. (2008). Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme Microb. Technol. 43 120–123. 10.1016/j.enzmictec.2008.02.007 [DOI] [Google Scholar]
- Fernando S., Adhikari S., Chandrapal C., Murali N. (2006). Biorefineries: current status, challenges, and future direction. Energy Fuel 20 1727–1737. 10.1021/ef060097w [DOI] [Google Scholar]
- Gruber L. (2009). Chemical composition, analyses and relevance of plant cell walls in the nutrition of ruminant livestock. Uebers. Tierernaehr. 37 45–86. [Google Scholar]
- Gruninger R. J., Nguyen T. T. M., Reid I. D., Yanke J. L., Pan W., Abbott D. W., et al. (2018). Application of transcriptomics to compare the carbohydrate active enzymes that are expressed by diverse genera of anaerobic fungi to degrade plant cell wall carbohydrates. Front. Microbiol. 9:1581 10.3389/fmicb.2018.01581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger R. J., Puniya A. K., Callaghan T. M., Edwards J. E., Youssef N., Dagar S. S., et al. (2014). Anaerobic fungi (phylum Neocallimastigomycota): advances in understanding their taxonomy, life cycle, ecology, role and biotechnological potential. FEMS Microbiol. Ecol. 90 1–17. 10.1111/1574-6941.12383 [DOI] [PubMed] [Google Scholar]
- Haag N. L., Nagele H. J., Fritz T., Oechsner H. (2015). Effects of ensiling treatments on lactic acid production and supplementary methane formation of maize and amaranth–an advanced green biorefining approach. Bioresour. Technol. 178 217–225. 10.1016/j.biortech.2014.08.048 [DOI] [PubMed] [Google Scholar]
- Haitjema C. H., Solomon K. V., Henske J. K., Theodorou M. K., O’Malley M. A. (2014). Anaerobic gut fungi: Advances in isolation, culture, and cellulolytic enzyme discovery for biofuel production. Biotechnol. Bioeng 111 1471–1482. 10.1002/bit.25264 [DOI] [PubMed] [Google Scholar]
- Hao J. X. (2011). Study on Nutritional Evaluation of Feed for Ruminants by in vitro. Nanjing: Nanjing agricultural university. [Google Scholar]
- Hjorth M., Gränitz K., Adamsen A. P. S., Møller H. B. (2011). Extrusion as a retreatment to increase biogas production. Bioresour. Technol. 102 4989–4994. 10.1016/j.biortech.2010.11.128 [DOI] [PubMed] [Google Scholar]
- Hu W. L., Wang J. K., Lv J. M., Guo Y. Q., Liu J. X. (2006). Rapid gas chromatogram determination of methane, organic acid in in vitro ruminal fermentation products. J. Z. Univ. 32 217–221. [Google Scholar]
- Ibrahim A. H. (2012). Pretreatment of straw for bioethanol production. Energy Procedia 14 542–551. 10.1016/j.egypro.2011.12.973 [DOI] [Google Scholar]
- Jin W. (2009). Isolation and Identification of Anaerobic Fungi and Their Associated Mehanogens from Herbivorous Animals and Their Characteristics of In Vitro Fermentation. Nanjing: Nanjing Agricultural University. [Google Scholar]
- Jin W. (2012). Rumen Cluster C: Distribution, Isolation and Hydrogenotrpphy. Nanjing: Nanjing Agricultural University. [Google Scholar]
- Jin W., Cheng Y. F., Mao S. Y., Zhu W. Y. (2011). Isolation of natural cultures of anaerobic fungi and indigenously associated methanogens from herbivores and their bioconversion of lignocellulosic materials to methane. Bioresour. Technol. 102 7925–7931. 10.1016/j.biortech.2011.06.026 [DOI] [PubMed] [Google Scholar]
- Jin W., Liu J. H., Li Y. F., Cheng Y. F., Zhu W. Y. (2017). Effect of methanogens on carbon metabolism of anaerobic fungi. Acta Microbiol. Sin. 57 162–167. [Google Scholar]
- Joblin K. N., Naylor G. E., Williams A. G. (1990). Effect of methanobrevibacter smithii on xylanolytic activity of anaerobic ruminal fungi. Appl. Environ. Microbiol. 56 2287–2295. 10.1128/aem.56.8.2287-2295.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Morrison M., Yu Z. (2011). Status of the phylogenetic diversity census of ruminal microbiomes. FEMS Microbiol. Ecol. 76 49–63. 10.1111/j.1574-6941.2010.01029.x [DOI] [PubMed] [Google Scholar]
- Lee S. S., Shin K. J., Kim W. Y., Ha J. K., Han I. K. (1999). The rumen ecosystem: as a fountain source of nobel enzymes - Review. AsianAustr. J. Anim. Sci. 12 988–1001. 10.5713/ajas.1999.988 [DOI] [Google Scholar]
- Li Y. (2017). Investigation of the Effect of Methanogens on the Metabolism of Anaerobic Fungi by Co-culture Technique. Nanjing: Nanjing Agricultural University. [Google Scholar]
- Li Y., Cheng Y., Zhu W. (2018). Enhancing the resistance of anaerobic fungus Piromyces sp. F1 to nitrovin by co-culture with Methanobrevibacter thauer F1. Microbiol. China 45 111–119. 10.13344/j.microbiol.china.170143 [DOI] [Google Scholar]
- Li Y., Jin W., Cheng Y., Zhu W. (2016). Effect of the associated methanogen methanobrevibacter thaueri on the dynamic profile of end and intermediate metabolites of anaerobic fungus Piromyces sp. F1. Curr. Microbiol. 73 434–441. 10.1007/s00284-016-1078-1079 [DOI] [PubMed] [Google Scholar]
- Li Y., Jin W., Mu C., Cheng Y., Zhu W. (2017). Indigenously associated methanogens intensified the metabolism in hydrogenosomes of anaerobic fungi with xylose as substrate. J. Basic Microbiol. 57 933–940. 10.1002/jobm.201700132 [DOI] [PubMed] [Google Scholar]
- Li Y., Li Y., Jin W., Sharpton T. J., Mackie R. I., Cann I., et al. (2019). Combined Genomic, transcriptomic, proteomic, and physiological characterization of the growth of Pecoramyces sp. F1 in Monoculture and Co-culture with a syntrophic methanogen. Front. Microbiol. 10:435 10.3389/fmicb.2019.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. (1987a). Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl. Environ. Microbiol. 53 1216–1223. 10.1128/aem.53.6.1216-1223.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. J. (1987b). Growth and fermentation of an anaerobic rumen fungus on various carbon-sources and effect of temperature on development. Appl. Environ. Microbiol. 53 1210–1215. 10.1128/aem.53.6.1210-1215.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S. N., Xu G. S., Cui K., Ma T., Diao Q. Y. (2018). Correlation analysis of CF, NDF and ADF in ruminant feedstuffs. Feed Ind. 39 52–59. 10.13302/j.cnki.fi.2018.21.012 [DOI] [Google Scholar]
- Mountfort D. O., Asher R. A. (1985). Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl. Environ. Microbiol. 49 1314–1322. 10.1128/aem.49.5.1314-1322.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu D., Zuo S., Jiang D., Tian P., Zheng M., Xu C. J. A. F., et al. (2018). Treatment using white rot fungi changed the chemical composition of wheat straw and enhanced digestion by rumen microbiota in vitro. Anim. Feed Sci. Technol. 237:S0377840117308350. [Google Scholar]
- Riva C., Schievano A., D’Imporzano G., Adani F. (2014). Production costs and operative margins in electric energy generation from biogas. Full-scale case studies. Waste Manag. 34 1429–1435. 10.1016/j.wasman.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Sapci Z., Morken J., Linjordet R. (2013). An investigation of the enhancement of biogas yields from lignocellulosic material using two pretreatment methods: microwave Ir- radiation and steam explosion. Bioresources 8 1976–1985. 10.15376/biores.8.2.1976-1985 [DOI] [Google Scholar]
- Shi Q., Li Y., Li Y., Cheng Y., Zhu W. (2019). Effects of steam explosion on lignocellulosic degradation of, and methane production from, corn stover by a co-cultured anaerobic fungus and methanogen. Bioresour. Technol. 290:121796 Epub ahead., 10.1016/j.biortech.2019.121796 [DOI] [PubMed] [Google Scholar]
- Silveira R. L., Stoyanov S. R., Gusarov S., Skaf M. S., Kovalenko A. (2015). Supramolecular interactions in secondary plant cell walls: effect of lignin chemical composition revealed with the molecular theory of solvation. J. Phys. Chem. Lett. 6 206–211. 10.1021/jz502298q [DOI] [PubMed] [Google Scholar]
- Solomon K. V., Haitjema C. H., Henske J. K., Gilmore S. P., Borges-Rivera D., Lipzen A., et al. (2016). Early-branching gut fungi possess a large, comprehensive array of biomass-degrading enzymes. Science 351 1192–1195. 10.1126/science.aad1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Yang G., Liu X., Yan Z., Yuan Y., Liao Y. (2014). Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS One 9:e93801 10.1371/journal.pone.0093801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Z., Mao S. Y., Jie C., Zhou L. F., Zhu W. Y. (2005). Effect of substrates with different lignin content on rumen fungal attachment and fermentation in the rumen of goats. Acta Pratac. Sci. 3 56–61. [Google Scholar]
- Tapio I., Snelling T. J., Strozzi F., Wallace R. J. (2017). The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 8:7 10.1186/s40104-017-0141-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen M. J., Kets E. P., Op den Camp H. J., Huis in’t Veld J. H., Vogels G. D. (1992). Effect of coculture of anaerobic fungi isolated from ruminants and non-ruminants with methanogenic bacteria on cellulolytic and xylanolytic enzyme activities. Arch. Microbiol. 157 176–182. 10.1007/bf00245287 [DOI] [PubMed] [Google Scholar]
- Theodorou M. K., Williams B. A., Dhanoa M. S., Mcallan A. B., France J. (1994). A simple gas-production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 48 185–197. 10.1016/0377-8401(94)90171-90176 [DOI] [Google Scholar]
- Theodorou M. K., Zhu W. Y., Rickers A., Nielsen B. B., Gull K., Trinci A. P. J. (1996). Biochemistry and Ecology of Anaerobic Fungi. Berlin: Springer. [Google Scholar]
- Theuretzbachera F., Lizasoaina J., Lefevera C., Saylora M. K., Enguidanosa R., Weranc N., et al. (2015). Steam explosion pretreatment of wheat straw to improve methane yields: investigation of the degradation kinetics of structural com- pounds during anaerobic digestion. Bioresour. Technol. 179 299–305. 10.1016/j.biortech.2014.12.008 [DOI] [PubMed] [Google Scholar]
- Van Soest P. J., Robertson J. B., Lewis B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74 3583–3597. 10.3168/jds.S0022-0302(91)78551-78552 [DOI] [PubMed] [Google Scholar]
- Wei Y. Q., Long R. J., Yang H., Yang H. J., Shen X. H., Shi R. F., et al. (2016). Fiber degradation potential of natural co-cultures of Neocallimastix frontalis and Methanobrevibacter ruminantium isolated from yaks (Bos grunniens) grazing on the Qinghai Tibetan Plateau. Anaerobe 39 158–164. 10.1016/j.anaerobe.2016.03.005 [DOI] [PubMed] [Google Scholar]
- Wu Y. N., Tian H. P. (2018). Advances in utilization of crop straw as forage resource for ruminants. Livestock Poul. Ind. 29:26. [Google Scholar]
- Yu D. D. (2017). Isolation, Identification and enzymatic characteristics of rumen fungi. J. Agric. Biotechnol. 120 571–587. 10.1111/jam.13035 [DOI] [Google Scholar]
- Zhao M. M., Jiang M., Zhou Z. W. (2011). the components analysis of several kinds of agricultural residues. Mater. Rev. 25 122–125. [Google Scholar]
- Zhou T., Yang Q. L., Zhang T. Y., Ren L. P. (2015). Chemical composition, energetic values and ruminal degradation characteristics of different portions of cornstalks derived from skin-pith separation. Chin. J. Anim. Nutr. 27 320–326. [Google Scholar]
- Zhu W., Mao S., Wang Q., Wen Y. (2001). Study on the screening of anaerobic fungi by in vitro fermentation. J. Nanjing Agric. Univ. 3 44–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.