Abstract
Myocardial ischemia reperfusion syndrome is a complex entity where many inflammatory mediators play different roles, both to enhance myocardial infarction-derived damage and to heal injury. In such a setting, the establishment of an effective therapy to treat this condition has been elusive, perhaps because the experimental treatments have been conceived to block just one of the many pathogenic pathways of the disease, or because they thwart the tissue-repairing phase of the syndrome. Either way, we think that a discussion about the pathophysiology of the disease and the mechanisms of action of some drugs may shed some clarity on the topic.
1. Introduction
Myocardial infarction (MI) or acute myocardial infarction is a term used to refer to an event of heart attack. MI occurs when the cardiac muscle is injured by hypoxia, which happens when a coronary artery is blocked [1]. MI is classified as being either an ST-segment elevation myocardial infarction (STEMI) or a non-ST-segment elevation myocardial infarction (NSTEMI). Moreover, unstable angina (UA) is closely related to NSTEMI, and together, these entities are referred to as non-ST-segment elevation acute coronary syndromes (NSTEACS). Both STEMI and NSTEACS share an underlying pathophysiology: a superimposed thrombus caused by a disruption of an atherosclerotic plaque, which results in subtotal occlusion (NSTEACS) or total occlusion (STEMI) of a coronary artery [2], thus causing damage at the heart's muscle through hypoxia induction.
The principal symptoms of MI are chest pain, which travels to the left arm or left side of the neck, shortness of breath, sweating, nausea, vomiting, abnormal heart beating, anxiety, and fatigue [3]. Risk factors include an advanced age, tobacco smoking, high blood pressure, diabetes, lack of physical activity, obesity, and chronic kidney disease [4]. Risk factors can be categorized into nonmodifiable and modifiable. Nonmodifiable risk factors include age of more than 45 years in men and more than 55 years in women, family history of early heart disease, and African-American race [5]. Modifiable risk factors include hypercholesterolemia, specifically related to elevation of low-density lipoprotein cholesterols (LDL-C), hypertension, tobacco abuse, diabetes mellitus, obesity, lack of physical activity, metabolic syndrome, and/or mental distress and depression [5]. The difference between both types of risk factors evidently lies in what can be prevented and what cannot.
There is an estimated five-million emergency department visits each year in the US for acute chest pain. Annually, over 800,000 people experience an MI, of which 27% die, mostly before reaching the hospital [6]. On the other hand, heart disease is Mexico's leading cause of death [7], accounting for 18.8% of total deaths, of which 59% are attributable to myocardial infarction.
In several studies, reperfusion therapy (fibrinolysis and coronary angioplasty) has demonstrated to produce a decrease in the morbidity and mortality associated with myocardial infarction [8]. However, the process of myocardial reperfusion can, paradoxically, enhance myocardial injury through inflammation, finally contributing to 50% of the final MI size [9]. The precise role inflammation plays in the setting of MI has been debated since the 1980s with the infiltration of leukocytes now being recognized as inflammatory mediators, as opposed to the previous concept of them being bystanders of the damage [10].
Nonetheless, in the therapeutic setting, the requirement for best preserving myocardial structure and function upon MI is to restore coronary blood flow as early as possible, using thrombolytic therapy and/or angioplasty [11], but as soon as blood flow is restored, an inflammatory response arises in the damaged section of the heart. This immune response further expands the damage made by the occlusion, originating a phenomenon known as myocardial ischemia reperfusion injury, or myocardial ischemia reperfusion syndrome (MIRS). Actually, MIRS is a major challenge to the treatment of MI [12], because its characteristic local and systemic inflammatory response is able to greatly enhance MI-derived damage, worsening the patient's prognosis [13]. Moreover, current pharmacopeia lacks a specific treatment for such condition. The treatment has been elusive because the immune-muscular-vascular interplay that characterizes MIRS is very complex, and a midpoint between downregulating the inflammatory tissue-damaging response and allowing the leucocyte-orchestrated reparative phase must be achieved.
On the other hand, ischemia reperfusion injury (IRI) is not exclusive to MI, as it also happens as a consequence to brain, kidney, liver, testis, or lung ischemia [14]. In such a tonic, we think that some lessons can be learned from these separate entities that may be applicable in the setting of MIRS. Also, information about MIRS-specific tissue-damaging and tissue-remodeling mediators is currently very vast, so that it may be useful to analyze the current baggage of knowledge on the topic, with aims to pinpoint some of the pathogenic pathways that may help to restrain MIRS upon blockage, as well as some strategies that may be of use for that purpose.
2. Pathophysiology of Myocardial Ischemia Reperfusion Syndrome
In general terms, MIRS must be understood as a complex phenomenon that arises upon blood flow restoration, where reperfused leukocytes find many damage-associated molecular patterns (DAMPs), such as extracellular Ca+ and ATP released by necrotic cells, which induce the activation of many TLR pathways to promote an inflammatory response. Thus, an acute Th1 response is rapidly induced to clean the necrotic debris, but such an immune response, unfortunately, expands MI-associated damage [9, 15]. Myocardial reperfusion is unavoidable, as it occurs as a consequence to common MI treatments such as thrombolysis, angioplasty [16], and coronary bypass [17, 18]. At a later stage, the Th1-immune response subsides to a Th2-driven immunity, where leukocytes shift their phenotype in order to orchestrate tissue remodeling to avoid cardiac rupture [19]. A highly potent Th2 response, nonetheless, may induce pathological scarring, rendering the whole phenomenon as highly dependent on a very precise immune regulation. Thus, the mediators of this immunopathology must be precisely understood to find areas of opportunity for the development of a specific treatment (Figures 1 and 2).
Figure 1.
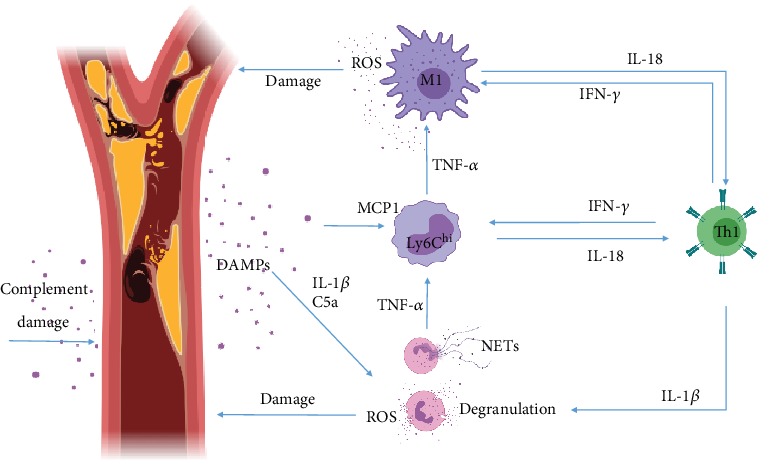
Inflammation during the Th1 tissue-damaging immune response of MIRS. Blood clots generate ischemia, which causes necrosis. Released DAMPs induce neutrophil and monocyte activation trough TLR and inflammasome activation, which in turn potentiate Th1 polarization. Inflammatory monocytes mature and become M1 macrophages. Tissue damage amplification comes in the form of NETs, granule components, and ROS produced by innate cells and direct complement attack.
Figure 2.
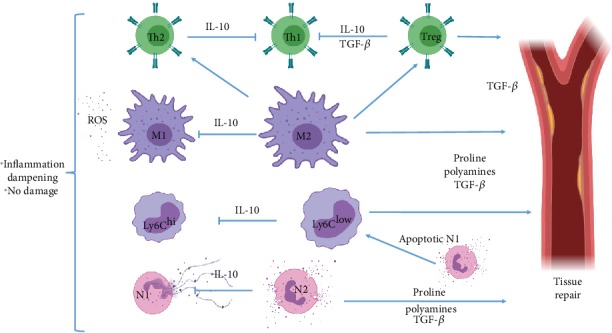
The Th2-mediated reparative phase of MIRS. N2 neutrophils and M2 macrophages both produce high levels of IL-10 to dampen N1, Ly6Chi, and M1-mediated degradation of tissue integrity. Also, M2 macrophages induce Th2 and Treg differentiation, while both suppress Th1 development, and Tregs thwart Th2 cells. M2 differentiation is possible by phagocytosis of the neutrophil apoptotic bodies. M2 and Treg cells mediate tissue repair.
2.1. Immunopathological Mechanisms of MIRS
The main trigger for MIRS is the vascular and cardiomyocyte cell death [11], which by the release of fragments of mitochondrial DNA, ATP, high mobility group box 1 protein (HMBGB1), and Ca+ into the extracellular space acts as DAMPs [20], inducing the activation of the NLRP3-inflammasome [21] and TLR9 [22], which converge on the activation of the myeloid differentiation primary response gene 88 (MyD88) and nuclear factor-κB (NF-κB) pathways, thus inducing the release of a number of inflammatory mediators, including monocyte-chemoattractant protein 1 (MCP1), interleukin-1β (IL-1β), IL-6, tumor-necrosis factor-α (TNF-α), and IL-18 [23]. Inflammasome activation amplifies IL-1β and IL-18 secretion by cardiac fibroblasts and induces the caspase-1-dependent death of nearby cardiomyocytes, termed pyroptosis—a highly inflammatory form of cell death, characterized by features that are typical of both apoptosis and necrosis [23].
Macrophage inflammatory protein-2α (MIP-2α), leukotriene B4 (LTB4), cytokine-induced neutrophil chemoattractant 1 (CINC-1), IL-8, CXCL8, and complement 5a massively recruit neutrophils [24] to infiltrate the MI-damaged area in the first few hours following onset of ischemia [25], peaking at days 1–3, and starting to decline at day 5. Neutrophils then generate high levels of reactive oxygen species (ROS), produce neutrophil-extracellular traps (NETs), and secrete granule components including myeloperoxidase and proteases, which exacerbate local vascular and tissue injury [26] with the purpose of removing necrotic cell debris from the affected zone [27] (Figure 1).
Along with neutrophils, complement proteins infiltrate the reperfused area. The complement is composed of 30 proteins and protein fragments, many of which are circulating as proenzymes and are activated by proteases in response to DAMPs. In this setting, all these proteins converge on two of the three common (terminal) complement pathways, which result in (a) inflammation to attract additional phagocytes (complements C3a, C4a, and C5a) and (b) activation of the cell-killing membrane attack complex (complement C5b-9 or MAC). Thus, the complement cascade amplifies MIRS-derived inflammation and damage [28] (Figure 1).
Both complement elements like C3a, C4a, and C5a and chemokines like MCP1 rapidly recruit monocytes [29] into the reperfused area. Such cells are produced in the bone marrow and are released into the blood in 2 waves, the first one being dominated by inflammatory Ly6Chi monocytes (which peak at days 3–4 post MI) and the second one by anti-inflammatory Ly6Clow monocytes (which peak at day 7 post-MI). The infiltrating Ly6Chi cells contribute to debris clearing and vascular/muscular damage, mainly through phagocytosis (for the earlier function) and ROS production (for the latter function) [30]. Monocytes then differentiate into M1-type macrophages, which have enhanced abilities to the phagocyte, produce ROS, and amplify inflammation through local antigen presentation and costimulation [31] (Figure 1). Subsequently, Ly6Clow monocytes start to infiltrate the reperfused area, and M1 macrophages start to differentiate into M2-type cells (which suppress T-cell activation through negative costimulation and IL-10 production and orchestrate tissue remodeling and vascularization by the secretion of TGF-β), to orchestrate tissue remodeling (as it will be discussed in the following section). Nonetheless, high levels of Th1-inducing factors deter the shift from an M1-type of macrophages to an M2 phenotype, thereby reducing the healing potential of the chronic MIRS phase [32] (Figure 1).
The systemic release of diverse cytokines and chemokines induces the activation of CD4+ T-cells, which in the acute phase of MIRS differentiate into a Th1 phenotype, releasing chemokines like CCL7 and cytokines like interferon-γ (IFN-γ), IL-2, and TNF-α, which as a cluster reinforce Th1 differentiation; enhance N1, Ly6Chi, and M1 cells' tissue-damaging abilities [33]; recruit CD8+ T-cells [34]; and enhance B cell activity [35]. Both CD8+ and B cells have been described to amplify inflammation during this stage and to produce damage on their own, by degranulation, in the case of T-cytotoxic cells [34], and antibody-mediated complement activation, in the case of B-lymphocytes [35] (Figure 1).
In this way, the immunopathology of MIRS can be succinctly described as the interplay between the innate and adaptive arms of the immune system, where a Th1-type immunity is critical for damage induction. In such a system, M1 macrophages and N1 neutrophils are key players on IRI induction, while the adaptive immunity component mainly amplifies the effector mechanisms of the aforementioned innate cells and complement.
2.2. The Th2-Mediated Reparative Phase of MIRS
On days 4-7 after MI, the Th1 tissue-destructive phase of MIRS enters a resolution stage, driven by a Th2 immune response induced by many changes in the cardiac microenvironment. This is regulated by the activation of endogenous inhibitory pathways that suppress the inflammatory phenotype in infiltrated leukocytes located in the MI zone [36].
After producing a high level of tissue damage, when most inflammatory debris have been cleared from the extracellular environment, neutrophils shift from their N1 phenotype to become N2-type cells. This change is accompanied by the production of high levels of IL-10, which aids in the suppression of the acute tissue-damaging Th1 response, by blocking the activation of CD4+, CD8+, B, N1, M1, and Ly6Chi cells [37]. Moreover, they produce phosphatidylserine (PS), which facilitates ingestion of apoptotic neutrophils by macrophages, resulting in a phenotypic change for macrophages from the M1 to the M2 type, which secrete anti-inflammatory and profibrotic cytokines such as IL-10 and TGF-β, thus promoting tissue repair and vascularization, while aiding in the suppression of inflammation [38] (Figure 2).
Also, the polarization of monocytes and macrophages (M/M) from the tissue-damaging and proinflammatory Ly6Chi and M1 phenotypes to the anti-inflammatory and tissue-repairing Ly6Clow and M2 phenotypes is critical to the reparative phase following MI [39], as these cells are able to produce an enzyme that is known as Arginase-1 (Arg-1). Such an enzyme catalyzes the conversion of L-arginine into L-ornithine, which is further metabolized into proline and polyamines. Both metabolites drive collagen synthesis and bioenergetic pathways that are critical for cell proliferation, respectively, thus contributing to tissue repair. Also, Arg-1 competes for the same substrate, but with more affinity, with the inducible-nitric oxide synthase (iNOS) enzyme, which is responsible for NO production [40]. In this way, M2 macrophages block ROS production by M1, N1, and Ly6Chi cells, thus limiting the extent of tissue damage by the remaining N1 and M1 cells (Figure 2).
The shift in M/M and neutrophil phenotype is mirrored by CD4+ T-cells, as Th1 cells subside to a vaster Th2 population that apparently amplifies the strength of the reparative actions of the M/M population [41]. This effect may be due to Th2-derived high levels of IL-4 and IL-13, which are able to induce M2 activation in macrophages [34]. Moreover, recent studies suggest that invariant natural killer (iNK) T-cells and γδT-cells have an important role in the settling of the Th1 acute inflammatory response through the secretion of anti-inflammatory cytokines such as TGF-β and IL-10, overall working with T-cells to dampen inflammation [42, 43]. Nonetheless, it has been observed that an enhanced Th2 response is able to induce pathological scarring with increased fibrosis in several settings [44, 45], in such a way that even the Th2 response must be controlled (Figure 2).
In the last decade, CD4+ CD25+ FoxP3+ T-regulatory (Tregs) cells have been recognized not only for their ability to dampen Th1 and Th2 lymphocyte activation and proliferation but also for their ability to downregulate innate immune cells' effector mechanisms [46, 47], while altering the cytokine milieu [48]. Tregs downregulate M1-macrophage activation and develop in parallel with M2 cells, presumably to control their level of activity [49]. In the setting of MIRS, Tregs have been shown to prevent cardiomyocyte apoptosis to limit further damage [48] and to downmodulate differentiation of fibroblasts into myofibroblasts, in order to avoid pathological scarring [50]. Their enhanced production of IL-10 has even been linked with a decrease in NKT cell activation [51]. In this way, they limit both the Th1- and Th2-mediated immunopathology [43] (Figure 2).
In a normal heart, there are a number of fibroblasts, which become activated during the reparative phase [52] mainly by the secretion of TGF-β [53], while the EDA-coated fibronectin produced by the newly transdifferentiated myofibroblasts induces extracellular matrix-protein (EMP) deposition [54]. Myofibroblast differentiation is also potentiated by the initial production of high levels of IL-1β and interferon-γ-inducible protein- (IP-) 10 [55], so that the extent of scarring is also determined by the significance of the Th1 response.
Moreover, activated myofibroblasts then modify the extracellular matrix environment, by the expression of EMPs like fibronectin and nonfibrillar collagens [55, 56], all of which support myofibroblast migration and adherence in order for them to close the wound.
On the other hand, from a wound-healing perspective, three phases of the process are recognized: (1) the inflammatory, (2) the proliferative, and (3) the remodeling stages, the first one being dominated by a Th1 response, the second one by Th2 immunity, and the third one being characterized by the reorganization, degradation, and resynthesis of the EM, in order to obtain maximum tensile strength. It is noteworthy that the latter process can last up to a year and only starts when Th2 cytokines have been downregulated, but also that in general, the strength and duration of each stage depends upon the strength and duration of the anterior phase [57]. In this way, Tregs have been linked to the transition from the Th1-mediated inflammatory stage to the Th2-mediated proliferative phase and finally to the remodeling phase, in such a way that these cells appear to promote the whole process of wound healing, while downregulating pathological scarring [58].
3. The Clinical Management of an MI Event
According to the European Society of Cardiology [59, 60], the best proceeding for the management of an MI is to obtain a 12-lead ECG as soon as possible, with the optimum proposed time lapse of 10 minutes in order to determine the precise location, extension, and kind of myocardial infarction for each patient, in order to personalize the surgical procedure. Pain relief should be practiced as soon as possible to avoid the increase of the heart's workload. It is usually done with the use of titrated opioids, although it is currently under debate if such drugs may interfere with the action of antiplatelet aggregation agents [61, 62]. Oxygen should also be administered in patients whose O2 saturation is less than 90%, along with a mild tranquilizer in order to reduce stress. When the diagnosis of STEMI is made in a prehospital setting, immediate activation of the catheterization laboratory is encouraged, in order to reduce treatment delays and patient mortality [63]. Either way, after diagnosis, pain management, and oxygenation, the next step is an attempt to lyse the blood clot by the use of thrombolytic drugs [59, 60].
Two scenarios may happen after thrombolysis: (1) the heart may recover blood flow or (2) the heart's blood flow alterations may persist. In the first case, MIRS starts upon thrombolysis, while in the second, primary percutaneous coronary intervention (PCI) is the preferred strategy that should be applied to patients with confirmed STEMI diagnosis within the first 12 h of symptom onset. In this second scenario, MIRS will happen after surgical reperfusion.
3.1. Periprocedural Pharmacotherapy
Patients undergoing primary PCI should receive aspirin and a P2Y12 inhibitor, in order to dampen platelet aggregation. The oral dose of aspirin should be administered without an enteric coat to ensure rapid action [59, 60].
Routine postprocedural anticoagulant therapy is not indicated after primary PCI, except when there is a separate indication for either full-dose anticoagulation or prophylactic doses for the prevention of venous thromboembolism in patients requiring prolonged bed rest, but ECG monitoring for arrhythmias and ST-segment deviations is recommended for at least 24 h after symptom onset in all STEMI patients. Afterwards, lifestyle changes are suggested to patients in order to prevent further risks [59, 60].
It should be noted that current medical guidelines do not mention any anti-inflammatory treatment to cope with MIRS, in such a way that the phenomenon still allows for an enhanced risk of post-MI injury progression [9].
4. Immunoregulation as a Modern Alternative to Immunosuppression
While the pathophysiological mechanisms of MIRS have been extensively studied, to the point where many inflammatory mediators, such as leukocytes and cytokines, and their role in the whole phenomenon are known, current pharmacopeia lacks a specific treatment to avoid MIRS. Despite this, much research has been done to attack the different pathways involved in postischemic injury progression, and it may be important to review these attempts in order to understand what has failed and what could be done.
As stated in the above sections, neutrophils have been identified as major targets in MIRS because of their ability to massively infiltrate the infarct area upon reperfusion [64], to locally produce high levels of tissue-damaging ROS, NETs [65], and granule components such as myeloperoxidase and proteases. As such, research using animal models has shown that the inhibition of their tissue-damaging mechanisms [66] and recruitment into the reperfusion site [67] may be a viable option to limit MIRS-associated damage. Nonetheless, clinical trials using αCD11/CD18 integrin blocking antibodies to avoid neutrophil recruitment during myocardial reperfusion have shown limited success in the reduction of MI size and the improvement of short-term (30 days after infarct) clinical outcome [68, 69].
Despite the inflammatory, tissue-damaging role that neutrophils have on the acute phase of MIRS, after ≈7 days, the inflammatory Ly6G+ CD206− neutrophil population is replaced by a Ly6G+ CD206+ population that has been described to play an important role in the orchestration of the reparative phase, as reviewed in [70]. Also, apoptotic neutrophils induce an M2 phenotype in infiltrated macrophages upon their phagocytosis, which inhibits the macrophage proinflammatory tissue-damaging response and leads them to produce IL-10 and TGF-β [71, 72]. Importantly, IL-10 may serve to dampen both Th1 and Th2 inflammation, thus inhibiting MIRS-derived damage, as well as excessive tissue scarring during the reparative phase, while TGF-β may also play an important role in infarct revascularization (Figure 3).
Figure 3.
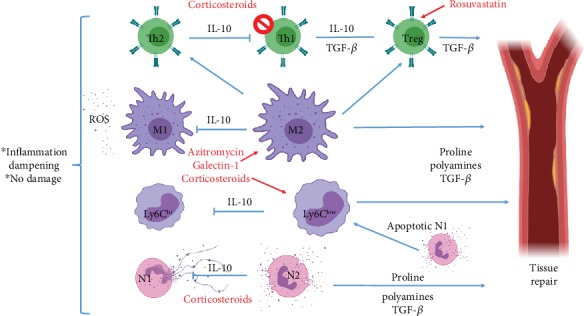
Immune-regulatory drugs could thwart destructive inflammation and promote tissue repair. Corticosteroids could enhance M2 differentiation while blocking NET, ROS, and granule-component deposition, thus blocking inflammatory damage. Also, azithromycin and rosuvastatin may induce cardioprotective leukocytes.
Thus, blocking neutrophil recruitment may not be a good alternative to reduce reperfusion-derived damage. Rather, the inhibition of the pathogenic effects of such cells may have a beneficial effect on MIRS. For instance, glucocorticoids have been shown to inhibit NET formation [73] and ROS production [74], while enhancing neutrophil mobilization [75], which renders them as good candidates for the reduction of neutrophil-derived damage (Figure 3).
Moreover, upon activation and apoptosis, neutrophils release proinflammatory alarmins that recruit inflammatory Ly6Chi monocytes [76], which are also important players in the acute production of ROS. In later stages (1-2 days after MI), these cells undergo differentiation (peaking at 3-4 days post MI) into the proinflammatory tissue-damaging M1-type of macrophages [77]. Also, M1 macrophages can be directly recruited and activated through MCP1 early production by damaged endothelial cells and cardiomyocytes [78, 79]. Either way, increased Ly6Chi cell counts after reperfusion have been associated with increased MIRS-derived damage [80, 81] as well as M1 macrophages, which further potentiate IRI [82]. At day 7 post MI, both the Ly6Chi and the M1-macrophage populations subside to the inflammation-resolving tissue-remodeling Ly6Clow monocytes and M2 macrophages, which by Arg-1 expression deplete NO production and produce IL-10, TGF-β, polyamines, and proline, thus undermining the inflammatory tissue-damaging acute phase of the MIRS and promoting tissue repair and vascularization. Nonetheless, an excess of both M2 macrophages and Ly6Clow monocytes has also been associated with pathologic myocardial scarring [19, 83]. In this way, both the proinflammatory and the anti-inflammatory M/M fractions can have a pathogenic role in MIRS, so that they represent an important target to limit MIRS-associated damage as a whole (Figure 3).
Despite these evidences, blocking the inflammatory M/M recruitment into the MI zone might not be beneficial, as the adoptive transfer of M2 macrophages and Ly6Clow monocytes has shown to reduce MIRS-associated damage [84–86], so that the avoidance of M/M recruitment in the first place may limit the reparative phase of MIRS. On the other hand, M/M phenotype modulation to dampen such a cell's ability to produce oxidative and inflammatory stress may be a better strategy. Following this line of thought, IL-1β-blocking antibodies have been proposed as therapeutic alternatives to limit IRI, but results obtained from clinical trials have been contradictory, ranging from promising to discouraging [87–89]. Disregarding the results from clinical trials, animal models of this disease have shown a good limitation of MIRS-associated damage in relation to the use of IL-1β-blocking antibodies administered to diabetic rats, which has more translational value because most MI patients are diabetic. Importantly, the MIRS blockage with this kind of antibody was effective to improve systolic function even when it was administered 80 days after reperfusion [90, 91] (Table 1).
Table 1.
Main perspectives for the treatment of MIRS.
| Clinical trial or animal model | Treatment | Proposed mechanisms of action | Findings | Reference |
|---|---|---|---|---|
| CT | αCD11/CD18 | Reduction of neutrophil recruitment | No difference in baseline, angiographic of angioplasty characteristics | [68] |
| CT | αCD18 | Reduction of neutrophil recruitment | No differences in coronary blood flow, infarct size, or ECG ST-segment elevation resolution | [69] |
| AM | Chemerin-15 | Enhanced AAMs and IL-10; reduced ROS, neutrophils, IL-6, and TNF-α | Amelioration of MI | [85] |
| AM | Netrin-1 | Reduction of neutrophil and macrophage recruitment, induction of AAMs | Decreased cardiomyocyte apoptosis | [86] |
| CT | αIL-1β | Reduction of M/M inflammatory activation | Enhanced hemodynamics and left ventricular remodeling | [87] |
| CT | αIL-1β | Reduction of CRP | No differences with the placebo-treated group | [88] |
| AM | αIL-1β | n.a. | Reduces infarct size and improves left ventricle remodeling | [90] |
| AM | Azithromycin | Induction of AAMs, inhibition of the PI3K/Akt pathway | Neuroprotection on an animal model of stroke | [97] |
| AM | Rosuvastatin | Treg expansion, reduction of inflammatory infiltrates | Reduced cardiac troponin I, infarct size | [106] |
| CT | αC5 | n.a. | No reduction of infarct size but improved survival | [108] |
| CT | αC5 | n.a. | Enhanced survival | [109] |
| CT | αC5 | n.a. | Reduction of troponin T and creatine kinase-MB | [110] |
| CT | C1-esterase inhibitor | C1, C3c, and C4 reduction | No difference in postoperative complications, hospital stay, or in-hospital mortality | [111] |
| CT | C1-esterase inhibitor | C3a and C4a reduction | Enhanced mean arterial pressure, cardiac index, and stroke volume. Lower levels of cardiac troponin | [112] |
| AM | Low dose of methylprednisolone | n.a. | Reduced infarction size and scar | [118] |
| CT | Alpha-1 antitrypsin (AAT) | Reduction of CRP | Lower creatine kinase myocardial levels | [116] |
| AM | Galectin-1 | Reduction of macrophages, NK cells, and T lymphocytes. Increase in Tregs | Enhanced heart's contractility | [100] |
| AM | Intranasal troponin | Increased IL-10 and reduced IFN-γ | Reduction of infarct size | [104] |
| AM | Super-antagonistic αCD28 antibody | Treg and AAM induction | Increased collagen de novo expression, decreased rates of left ventricular ruptures | [49] |
Abbreviations: CT: clinical trial; AM: animal model; αCD11/18: anticluster of differentiation 11/18; αC5: anti-C5 complement protein; AAM: alternatively activated macrophages; ROS: reactive oxygen species; M/M: monocyte/macrophage; CRP: C-reactive protein; Treg: T-regulatory cell, IL-1β: interleukin-1β; IL-10: interleukin-10, ECG: electrocardiogram; n.a.: not available; MI: myocardial infarction.
Current data on the phenomenon does not allow an exact explanation of this phenomenon, but it can be speculated that the lack of effect in some cases may be due to a vast array of M1-inducing cytokines and Ly6Chi-recruiting chemokines, other than IL-1β, being secreted at the MI zone upon reperfusion. Several cytokines and chemokines produced during MIRS, like TNF-α, IFN-γ, and MCP1, are known to have concomitant effects on the activation of inflammatory pathways like NF-κB [92, 93], PI3K/Akt [94], and JAK/STAT [95] in such a way that the inhibition of just one of the cytokines that signal through any of those pathways would not be able to have a consistent effect on the reduction of MIRS-associated damage (Figure 3).
In such line of thought, chemerin-15 [85] and netrin-1 [86] have been used in animal models to induce an M2 phenotype in macrophages during ischemia reperfusion, with the effect of reducing lesion size. Concordantly, glucocorticoid administration has shown to induce an alternative activation in macrophages, in such a way that they protect against inflammatory injury and are able to induce Treg expansion [96]. Such an effect may be attributable to the inhibition of the NF-κB pathway. Also, widely available drugs like azithromycin have shown to induce M2-type activation in macrophages to protect from ischemic stroke injury [97], in an effect associated with the inhibition of the PI3K/Akt pathway [98]. Moreover, the modulation of innate immunity using the C-type lectin, galectin-1, has also been proven to effectively dampen inflammation, mainly through AAM induction [99]. Interestingly, galectin-1 knockout mice showed enhanced cardiac inflammation (characterized by increased numbers of macrophages, natural killer cells, and T-cells) and a reduced frequency of regulatory T-cells that are associated with impaired cardiac function and ventricular remodeling. In the same study, the authors treated infarcted mice with recombinant galectin-1, which led to attenuated cardiac damage [100] (Table 1).
Whether this strategy induces pathological scarring was not evaluated, but the possibility should not be ruled out. To our notice, no clinical trials have been made exploring any immunoregulatory drug that has a direct effect on the M/M phenotype, and it may be important to gather such data due to a wide variation between the characteristics of MI in animal models and the clinical reality in human patients [19] (Figure 3).
On the other hand, CD4+ T lymphocytes and B cells are recruited within the first 90 minutes after reperfusion and appear to play a pathogenic role during the acute stage of MIRS, presumably because of their ability to promote an inflammatory tissue-damaging phenotype in M/M cells [33, 101]. Furthermore, in the tissue-remodeling stage of MIRS CD4+, T-cells may also play a pathological role, as they have been described to induce excessive scarring [102]. Nonetheless, there is an increasingly clear role for Treg cells in the dampening of both the pathogenic Th1 and Th2 inflammation phenomena [103] that is supported by several data (Figure 3).
Firstly, the induction of IL-10 secreting Treg cells by intranasal troponin administration shortly after reperfusion has shown to reduce MIRS-associated damage by 50%, evaluated 1.5 months after reperfusion [104]. Moreover, pharmacologic activation/recruitment of CD4+ CD25+ FoxP3+ cells using a super-antagonistic αCD28 antibody has been linked to a change in the phenotype of macrophages from M1 to M2, which promotes an enhanced, but not pathogenic, healing through the local production of TGF-β [49]. The observed suppression of pathogenic scarring may be due to a direct effect for Treg cells in the modulation of a fibroblast phenotype, in such a way that the latter cells migrate less, thus limiting their ability to form bigger scars [50] (Figure 3) (Table 1).
Another potentially important strategy to limit MIRS may be the use of statins, as they have been rendered as potent cardioprotectors that have an interesting effect on T-cell activation [105]. For instance, rosuvastatin has been shown to limit MIRS through Treg expansion in a murine model [106], but the effect may not be exclusive to animal models, as a meta-analysis performed by Sorathia et al. shows a vast increase in Tregs in patients that use rosuvastatin [107] (Figure 3) (Table 1).
Another important early player in the field of MIRS is the complement cascade, where C1 and C5/C5a proteins have been targeted. While C5 has been targeted with limited success on limiting IRI size [108, 109], C1 inhibition with monoclonal antibodies was able to reduce injury on several clinical trials [110–112], so that complement-blocking antibodies, like Cetor or Berinert, may be used concomitantly to reduce IRI extension. Additionally, corticosteroids have been used to regulate complement-gene expression and activation [113, 114] (Figure 3) (Table 1).
Finally, the potentiation of cardiomyocyte survival should be considered as a valuable alternative to be coopted in the treatment of MIRS. An interesting approach is the modulation of the low-density lipoprotein receptor-related protein 1 (LRP1), which is able to both downregulate the NF-κB-related inflammation during MIRS and enhance cardiomyocyte survival through the activation of the PI3K/Akt and ERK1/2 pathways in such cells, as thoroughly reviewed in [115]. As an example of this approach, a clinical trial using plasma-derived alpha-1 antitrypsin, an agonist of the LRP1 receptor, showed shorter time-to-peak creatine kinase myocardial band (CK-MB) values [116] in relation to a significant reduction on CRP [117] (Table 1).
4.1. As Paracelsus Said: The Dose Makes the Potion
In the 70s, a word of caution was emitted against the use of corticosteroids to treat MIRS as it was observed that in some studies, it caused myocardial thinning and delayed healing [119–121]. Nonetheless, in all these studies, high doses of such hormones were administered and for prolonged times. In this way, even a decade later, this dosing was questioned by studies comparing MI size and healing pace between high and low corticosteroid dose groups [118], finding that as Paracelsus said, “the dose makes the potion.”
It can be speculated that the high doses used in such studies blocked the proliferative and remodeling stages of MIRS, along with the inflammatory phase that was initially the intended target. Nowadays, a protective role for corticosteroids in MIRS has been described in both experimental [122, 123] and clinical settings. Concordantly, a meta-analysis by Giugliano et al. [124] showed this cardioprotective effect for corticosteroids in MIRS in patients. On the other hand, corticosteroids have been successfully used to reduce IRI in kidneys [125], liver [126], and brain [127], with the added benefit of attenuating pathogenic fibrosis during the reparative phase [128].
In this way, the current understanding on the pathophysiology of MIRS and a brief review about the use of such drugs in MIRS-reduction allow us to think that a low dose of corticosteroids administered prior to reperfusion may help to reduce the inflammatory damage of such a syndrome, while allowing the healing phases of the syndrome.
5. Conclusions
MIRS is an unavoidable consequence of MI, with the potential to duplicate the damage made by the ischemic condition. As such, it represents a serious complication to one of the most prevalent diseases worldwide. Designing an effective therapy for such a condition has been challenging because the inflammatory phenomenon behind its pathophysiology is very complex. First, it involves a Th1 response that greatly contributes to tissue damage, which is relatively easy to dampen, but a chronic Th2-type immune response that contributes to the resolution of the inflammatory damage, and tissue remodeling comes later, and its suppression has been associated with increased damage.
As such, a therapy that downregulates the acute Th1 tissue-damaging response, but promotes the later Th2 tissue-repairing phase of the disease, appears to be a good choice. Some well-known, widely used drugs, like rosuvastatin, azithromycin, corticosteroids, Cetor, or Berinert, have been purported as candidates to treat MIRS in the experimental setting, producing good results. Nonetheless, much research is needed in order to confirm such findings as they have not been used concomitantly, and a correct dose may be challenging to find, as too much Th1 undermining may result in a weak reparative stage, but too little may not properly limit the damage.
Innate immune cells, like M/M and neutrophils, appear to be good targets, because they are effector mediators of the damage and because they can regulate the adaptive immune response, both in potency and in profile, so that drugs like azithromycin, which can induce an M2 phenotype in macrophages, or corticosteroids that can reduce ROS production in both cell types could have a positive effect on MIRS management. Also, rosuvastatin may be cardioprotective beyond its effects on dyslipidemia, as it can recruit Treg cells at the injured heart. Such lymphocyte population has been associated to the resolution of both the Th1- and Th2-type responses, thus allowing a healthy scar maturation.
Another point to be considered is the rational use for corticosteroids, as they can limit the extent of MIRI and induce protective leukocyte populations, but overdoses with such a drug have produced myocardial thinning and delayed healing.
Finally, complement-blocking antibodies have been used successfully in the clinical setting, so that they may be coopted with the aforementioned drugs to design a more complete treatment.
Acknowledgments
All the authors wish to thank Sociedad Española de Beneficencia (Pachuca, Hidalgo) for funding the publication of this article. Moreover, César Daniel Sánchez-Hernández, Lucero Torres-Alarcón, and Ariadna González-Cortés wish to give their thanks for the scholarship they receive from such institution.
Conflicts of Interest
The authors declare that no potential conflicts of interest exist both in the writing and in the publication of this paper.
References
- 1.Kosuge M., Kimura K., Ishikawa T., et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circulation Journal. 2006;70(3):222–226. doi: 10.1253/circj.70.222. [DOI] [PubMed] [Google Scholar]
- 2.Antman E. M., Anbe D. T., Armstrong P. W., et al. ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction) Journal of the American College of Cardiology. 2004;44(3):E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Valensi P., Lorgis L., Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: A review of the literature. Archives of Cardiovascular Diseases. 2011;104(3):178–188. doi: 10.1016/j.acvd.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Graham I., Atar D., Borch-Johnsen K., et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194(1):1–45. doi: 10.1016/j.atherosclerosis.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 5.Harrington D. H., Stueben F., Lenahan C. M. ST-elevation myocardial infarction and non-ST-elevation myocardial infarction: medical and surgical interventions. Critical Care Nursing Clinics of North America. 2019;31(1):49–64. doi: 10.1016/j.cnc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Anderson J. L., Karagounis L. A., Califf R. M. Metaanalysis of five reported studies on the relation of early coronary patency grades witk mortality and outcomes after acute myocardial infarction. The American Journal of Cardiology. 1996;78(1):1–8. doi: 10.1016/S0002-9149(96)00217-2. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Sanchez C., Arias-Mendoza A., Gonzalez-Pacheco H., et al. Reperfusion therapy of myocardial infarction in Mexico: a challenge for modern cardiology. Archivos de Cardiología de México. 2017;87(2):144–150. doi: 10.1016/j.acmx.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Seeger J. P., Benda N. M., Riksen N. P., et al. Heart failure is associated with exaggerated endothelial ischaemia-reperfusion injury and attenuated effect of ischaemic preconditioning. European Journal of Preventive Cardiology. 2016;23(1):33–40. doi: 10.1177/2047487314558377. [DOI] [PubMed] [Google Scholar]
- 9.Yellon D. M., Hausenloy D. J. Myocardial reperfusion injury. The New England Journal of Medicine. 2007;357(11):1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 10.Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schonbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. The American Journal of Physiology. 1986;251:H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- 11.Monassier J. P. Reperfusion injury in acute myocardial infarction. From bench to cath lab. Part I: basic considerations. Archives of Cardiovascular Diseases. 2008;101(7-8):491–500. doi: 10.1016/j.acvd.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Gurewich V. Thrombolysis: a critical first-line therapy with an unfulfilled potential. The American Journal of Medicine. 2016;129(6):573–575. doi: 10.1016/j.amjmed.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Frangogiannis N. G. The inflammatory response in myocardial injury, repair, and remodelling. Nature Reviews. Cardiology. 2014;11(5):255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minutoli L., Puzzolo D., Rinaldi M., et al. ROS-mediated NLRP3 inflammasome activation in brain, heart, kidney, and testis ischemia/reperfusion injury. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/2183026.2183026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Werf F., Bax J., Betriu A., et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation. European Heart Journal. 2008;29(23):2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 16.Zahn R., Koch A., Rustige J., et al. Primary angioplasty versus thrombolysis in the treatment of acute myocardial infarction. The American Journal of Cardiology. 1997;79(3):264–269. doi: 10.1016/S0002-9149(96)00745-X. [DOI] [PubMed] [Google Scholar]
- 17.Dhalla N. S., Elmoselhi A. B., Hata T., Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovascular Research. 2000;47(3):446–456. doi: 10.1016/S0008-6363(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 18.Brevoord D., Kranke P., Kuijpers M., Weber N., Hollmann M., Preckel B. Remote ischemic conditioning to protect against ischemia-reperfusion injury: a systematic review and meta-analysis. PLoS One. 2012;7(7, article e42179) doi: 10.1371/journal.pone.0042179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ong S. B., Hernandez-Resendiz S., Crespo-Avilan G. E., et al. Inflammation following acute myocardial infarction: multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacology & Therapeutics. 2018;186:73–87. doi: 10.1016/j.pharmthera.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang X. M., Cui L., White J., et al. Mitochondrially targeted endonuclease III has a powerful anti-infarct effect in an in vivo rat model of myocardial ischemia/reperfusion. Basic Research in Cardiology. 2015;110(2):p. 3. doi: 10.1007/s00395-014-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mariathasan S., Weiss D. S., Newton K., et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 22.Cabrera-Fuentes H. A., Ruiz-Meana M., Simsekyilmaz S., et al. RNase1 prevents the damaging interplay between extracellular RNA and tumour necrosis factor-α in cardiac ischaemia/reperfusion injury. Thrombosis and Haemostasis. 2014;112(6):1110–1119. doi: 10.1160/TH14-08-0703. [DOI] [PubMed] [Google Scholar]
- 23.van Hout G. P., Arslan F., Pasterkamp G., Hoefer I. E. Targeting danger-associated molecular patterns after myocardial infarction. Expert Opinion on Therapeutic Targets. 2016;20(2):223–239. doi: 10.1517/14728222.2016.1088005. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y., Yabluchanskiy A., Iyer R. P., et al. Temporal neutrophil polarization following myocardial infarction. Cardiovascular Research. 2016;110(1):51–61. doi: 10.1093/cvr/cvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews. Immunology. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 26.Ma Y., Yabluchanskiy A., Lindsey M. L. Neutrophil roles in left ventricular remodeling following myocardial infarction. Fibrogenesis & Tissue Repair. 2013;6(1):p. 11. doi: 10.1186/1755-1536-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Z. Q., Velez D. A., Wang N. P., et al. Progressively developed myocardial apoptotic cell death during late phase of reperfusion. Apoptosis. 2001;6(4):279–290. doi: 10.1023/a:1011335525219. [DOI] [PubMed] [Google Scholar]
- 28.Timmers L., Pasterkamp G., de Hoog V. C., Arslan F., Appelman Y., de Kleijn D. P. The innate immune response in reperfused myocardium. Cardiovascular Research. 2012;94(2):276–283. doi: 10.1093/cvr/cvs018. [DOI] [PubMed] [Google Scholar]
- 29.Dewald O., Zymek P., Winkelmann K., et al. CCL2/monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circulation Research. 2005;96(8):881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 30.Song P., Zhang J., Zhang Y., et al. Hepatic recruitment of CD11b+Ly6C+ inflammatory monocytes promotes hepatic ischemia/reperfusion injury. International Journal of Molecular Medicine. 2018;41(2):935–945. doi: 10.3892/ijmm.2017.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Italiani P., Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Frontiers in Immunology. 2014;5:p. 514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ter Horst E. N., Hakimzadeh N., van der Laan A. M., Krijnen P. A., Niessen H. W., Piek J. J. Modulators of macrophage polarization influence healing of the infarcted myocardium. International Journal of Molecular Sciences. 2015;16(12):29583–29591. doi: 10.3390/ijms161226187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zouggari Y., Ait-Oufella H., Bonnin P., et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nature Medicine. 2013;19(10):1273–1280. doi: 10.1038/nm.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao J., Lu L., Zhai Y. T cells in organ ischemia reperfusion injury. Current Opinion in Organ Transplantation. 2014;19(2):115–120. doi: 10.1097/MOT.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Crispin J. C., Tedder T. F., Dalle Lucca J., Tsokos G. C. B cells contribute to ischemia/reperfusion-mediated tissue injury. Journal of Autoimmunity. 2009;32(3-4):195–200. doi: 10.1016/j.jaut.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dewald O., Ren G., Duerr G. D., et al. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. The American Journal of Pathology. 2004;164(2):665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang P., Li Y., Xie Y., Liu Y. Different faces for different places: heterogeneity of neutrophil phenotype and function. Journal of Immunology Research. 2019;2019:18. doi: 10.1155/2019/8016254.8016254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega-Gomez A., Perretti M., Soehnlein O. Resolution of inflammation: an integrated view. EMBO Molecular Medicine. 2013;5(5):661–674. doi: 10.1002/emmm.201202382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nahrendorf M., Pittet M. J., Swirski F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation. 2010;121(22):2437–2445. doi: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu L., Zhao Q., Yang T., Ding W., Zhao Y. Cellular metabolism and macrophage functional polarization. International Reviews of Immunology. 2015;34(1):82–100. doi: 10.3109/08830185.2014.969421. [DOI] [PubMed] [Google Scholar]
- 41.Liu H., Gao W., Yuan J., et al. Exosomes derived from dendritic cells improve cardiac function via activation of CD4+ T lymphocytes after myocardial infarction. Journal of Molecular and Cellular Cardiology. 2016;91:123–133. doi: 10.1016/j.yjmcc.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 42.Marek-Trzonkowska N., Mysliwiec M., Dobyszuk A., et al. Therapy of type 1 diabetes with CD4(+)CD25(high)CD127-regulatory T cells prolongs survival of pancreatic islets - results of one year follow-up. Clinical Immunology. 2014;153(1):23–30. doi: 10.1016/j.clim.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Hofmann U., Beyersdorf N., Weirather J., et al. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125(13):1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 44.Barron L., Wynn T. A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2011;300(5):G723–G728. doi: 10.1152/ajpgi.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gieseck R. L., 3rd, Wilson M. S., Wynn T. A. Type 2 immunity in tissue repair and fibrosis. Nature Reviews Immunology. 2018;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 46.Rashid S. T., Alexander G. J. Induced pluripotent stem cells: from Nobel prizes to clinical applications. Journal of Hepatology. 2013;58(3):625–629. doi: 10.1016/j.jhep.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 47.Littman D. R., Rudensky A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140(6):845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q., Lee K. Regulatory T-cell therapy for transplantation: how many cells do we need? Current Opinion in Organ Transplantation. 2012;17(4):349–354. doi: 10.1097/MOT.0b013e328355a992. [DOI] [PubMed] [Google Scholar]
- 49.Weirather J., Hofmann U. D., Beyersdorf N., et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circulation Research. 2014;115(1):55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 50.Saxena A., Dobaczewski M., Rai V., et al. Regulatory T cells are recruited in the infarcted mouse myocardium and may modulate fibroblast phenotype and function. American Journal of Physiology. Heart and Circulatory Physiology. 2014;307(8):H1233–H1242. doi: 10.1152/ajpheart.00328.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Homma T., Kinugawa S., Takahashi M., et al. Activation of invariant natural killer T cells by α-galactosylceramide ameliorates myocardial ischemia/reperfusion injury in mice. Journal of Molecular and Cellular Cardiology. 2013;62:179–188. doi: 10.1016/j.yjmcc.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Frangogiannis N. G., Michael L. H., Entman M. L. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovascular Research. 2000;48(1):89–100. doi: 10.1016/S0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz-Villalba A., Simon A. M., Pogontke C., et al. Interacting resident epicardium-derived fibroblasts and recruited bone marrow cells form myocardial infarction scar. Journal of the American College of Cardiology. 2015;65(19):2057–2066. doi: 10.1016/j.jacc.2015.03.520. [DOI] [PubMed] [Google Scholar]
- 54.Kohan M., Muro A. F., White E. S., Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. The FASEB Journal. 2010;24(11):4503–4512. doi: 10.1096/fj.10-154435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shinde A. V., Frangogiannis N. G. Fibroblasts in myocardial infarction: a role in inflammation and repair. Journal of Molecular and Cellular Cardiology. 2014;70:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santiago J. J., Dangerfield A. L., Rattan S. G., et al. Cardiac fibroblast to myofibroblast differentiation in vivo and in vitro: expression of focal adhesion components in neonatal and adult rat ventricular myofibroblasts. Developmental Dynamics. 2010;239(6):1573–1584. doi: 10.1002/dvdy.22280. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez A. C., Costa T. F., Andrade Z. A., Medrado A. R. Wound healing - a literature review. Anais Brasileiros de Dermatologia. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eming S. A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine. 2014;6(265):p. 265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savonitto S., Azzarone M., Salsi R., Tortorella G. The new ESC guidelines for non-ST-elevation acute coronary syndromes: one direction, many ways, clinical wisdom. Giornale Italiano Di Cardiologia. 2012;13(3):157–168. doi: 10.1714/1038.11320. [DOI] [PubMed] [Google Scholar]
- 60.Task Force on the management of STseamiotESoC, Steg P. G., James S. K., et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European Heart Journal. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs215. [DOI] [PubMed] [Google Scholar]
- 61.Hobl E. L., Stimpfl T., Ebner J., et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. Journal of the American College of Cardiology. 2014;63(7):630–635. doi: 10.1016/j.jacc.2013.10.068. [DOI] [PubMed] [Google Scholar]
- 62.Hobl E. L., Reiter B., Schoergenhofer C., et al. Morphine decreases ticagrelor concentrations but not its antiplatelet effects: a randomized trial in healthy volunteers. European Journal of Clinical Investigation. 2016;46(1):7–14. doi: 10.1111/eci.12550. [DOI] [PubMed] [Google Scholar]
- 63.Barstow C., Rice M., McDivitt J. D. Acute coronary syndrome: diagnostic evaluation. American Family Physician. 2017;95(3):170–177. [PubMed] [Google Scholar]
- 64.Fernandez-Jimenez R., Garcia-Prieto J., Sanchez-Gonzalez J., et al. Pathophysiology underlying the bimodal edema phenomenon after myocardial ischemia/reperfusion. Journal of the American College of Cardiology. 2015;66(7):816–828. doi: 10.1016/j.jacc.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 65.Ge L., Zhou X., Ji W. J., et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. American Journal of Physiology. Heart and Circulatory Physiology. 2015;308(5):H500–H509. doi: 10.1152/ajpheart.00381.2014. [DOI] [PubMed] [Google Scholar]
- 66.Wallert M., Ziegler M., Wang X., et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biology. 2019;26, article 101292 doi: 10.1016/j.redox.2019.101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Arai M., Lefer D. J., So T., DiPaula A., Aversano T., Becker L. C. An anti-CD18 antibody limits infarct size and preserves left ventricular function in dogs with ischemia and 48-hour reperfusion. Journal of the American College of Cardiology. 1996;27(5):1278–1285. doi: 10.1016/0735-1097(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 68.Faxon D. P., Gibbons R. J., Chronos N. A., Gurbel P. A., Sheehan F., Investigators H-M The effect of blockade of the CD11/CD18 integrin receptor on infarct size in patients with acute myocardial infarction treated with direct angioplasty: the results of the HALT-MI study. Journal of the American College of Cardiology. 2002;40(7):1199–1204. doi: 10.1016/s0735-1097(02)02136-8. [DOI] [PubMed] [Google Scholar]
- 69.Baran K. W., Nguyen M., McKendall G. R., et al. Double-blind, randomized trial of an anti-CD18 antibody in conjunction with recombinant tissue plasminogen activator for acute myocardial infarction: limitation of myocardial infarction following thrombolysis in acute myocardial infarction (LIMIT AMI) study. Circulation. 2001;104(23):2778–2783. doi: 10.1161/hc4801.100236. [DOI] [PubMed] [Google Scholar]
- 70.Puhl S. L., Steffens S. Neutrophils in post-myocardial infarction inflammation: damage vs. resolution? Frontiers in Cardiovascular Medicine. 2019;6:p. 25. doi: 10.3389/fcvm.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frangogiannis N. G. Regulation of the inflammatory response in cardiac repair. Circulation Research. 2012;110(1):159–173. doi: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horckmans M., Ring L., Duchene J., et al. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. European Heart Journal. 2017;38(3):187–197. doi: 10.1093/eurheartj/ehw002. [DOI] [PubMed] [Google Scholar]
- 73.Fan F., Huang X., Yuan K., et al. Glucocorticoids may exacerbate fungal keratitis by increasing fungal aggressivity and inhibiting the formation of neutrophil extracellular traps. Current Eye Research. 2020;45(2):124–133. doi: 10.1080/02713683.2019.1657464. [DOI] [PubMed] [Google Scholar]
- 74.Dey R., Bishayi B. Dexamethasone exhibits its anti-inflammatory effects in S. aureus induced microglial inflammation via modulating TLR-2 and glucocorticoid receptor expression. International Immunopharmacology. 2019;75, article 105806 doi: 10.1016/j.intimp.2019.105806. [DOI] [PubMed] [Google Scholar]
- 75.Hiemstra I. H., van Hamme J. L., Janssen M. H., van den Berg T. K., Kuijpers T. W. Dexamethasone promotes granulocyte mobilization by prolonging the half-life of granulocyte-colony-stimulating factor in healthy donors for granulocyte transfusions. Transfusion. 2017;57(3):674–684. doi: 10.1111/trf.13941. [DOI] [PubMed] [Google Scholar]
- 76.Marinkovic G., Grauen Larsen H., Yndigegn T., et al. Inhibition of pro-inflammatory myeloid cell responses by short-term S100A9 blockade improves cardiac function after myocardial infarction. European Heart Journal. 2019;40(32):2713–2723. doi: 10.1093/eurheartj/ehz461. [DOI] [PubMed] [Google Scholar]
- 77.Hilgendorf I., Gerhardt L. M., Tan T. C., et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circulation Research. 2014;114(10):1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kumar A. G., Ballantyne C. M., Michael L. H., et al. Induction of monocyte chemoattractant protein-1 in the small veins of the ischemic and reperfused canine myocardium. Circulation. 1997;95(3):693–700. doi: 10.1161/01.cir.95.3.693. [DOI] [PubMed] [Google Scholar]
- 79.Tarzami S. T., Cheng R., Miao W., Kitsis R. N., Berman J. W. Chemokine expression in myocardial ischemia: MIP-2 dependent MCP-1 expression protects cardiomyocytes from cell death. Journal of Molecular and Cellular Cardiology. 2002;34(2):209–221. doi: 10.1006/jmcc.2001.1503. [DOI] [PubMed] [Google Scholar]
- 80.Maekawa Y., Anzai T., Yoshikawa T., et al. Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. Journal of the American College of Cardiology. 2002;39(2):241–246. doi: 10.1016/S0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 81.Mariani M., Fetiveau R., Rossetti E., et al. Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. European Heart Journal. 2006;27(21):2511–2515. doi: 10.1093/eurheartj/ehl191. [DOI] [PubMed] [Google Scholar]
- 82.Swirski F. K., Libby P., Aikawa E., et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of Clinical Investigation. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andreadou I., Cabrera-Fuentes H. A., Devaux Y., et al. Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovascular Research. 2019;115(7):1117–1130. doi: 10.1093/cvr/cvz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yue Y., Yang X., Feng K., et al. M2b macrophages reduce early reperfusion injury after myocardial ischemia in mice: a predominant role of inhibiting apoptosis via A20. International Journal of Cardiology. 2017;245:228–235. doi: 10.1016/j.ijcard.2017.07.085. [DOI] [PubMed] [Google Scholar]
- 85.Chang C., Ji Q., Wu B., et al. Chemerin15-Ameliorated Cardiac Ischemia-Reperfusion Injury Is Associated with the Induction of Alternatively Activated Macrophages. Mediators of Inflammation. 2015;2015:9. doi: 10.1155/2015/563951.563951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mao X., Xing H., Mao A., et al. Netrin-1 attenuates cardiac ischemia reperfusion injury and generates alternatively activated macrophages. Inflammation. 2014;37(2):573–580. doi: 10.1007/s10753-013-9771-3. [DOI] [PubMed] [Google Scholar]
- 87.Abbate A., Kontos M. C., Grizzard J. D., et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] pilot study) The American Journal of Cardiology. 2010;105(10):1371–1377.e1. doi: 10.1016/j.amjcard.2009.12.059. [DOI] [PubMed] [Google Scholar]
- 88.Abbate A., Van Tassell B. W., Biondi-Zoccai G., et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study] The American Journal of Cardiology. 2013;111(10):1394–1400. doi: 10.1016/j.amjcard.2013.01.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morton A. C., Rothman A. M., Greenwood J. P., et al. The effect of interleukin-1 receptor antagonist therapy on markers of inflammation in non-ST elevation acute coronary syndromes: the MRC-ILA Heart Study. European Heart Journal. 2015;36(6):377–384. doi: 10.1093/eurheartj/ehu272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Toldo S., Van Tassell B. W., Abbate A. Interleukin-1 blockade in acute myocardial infarction and heart failure: getting closer and closer. JACC: Basic to Translational Science. 2017;2(4):431–433. doi: 10.1016/j.jacbts.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harouki N., Nicol L., Remy-Jouet I., et al. The IL-1β Antibody Gevokizumab Limits Cardiac Remodeling and Coronary Dysfunction in Rats With Heart Failure. JACC: Basic to Translational Science. 2017;2(4):418–430. doi: 10.1016/j.jacbts.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun S. C. The non-canonical NF-κB pathway in immunity and inflammation. Nature Reviews. Immunology. 2017;17(9):545–558. doi: 10.1038/nri.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Napetschnig J., Wu H. Molecular basis of NF-κB signaling. Annual Review of Biophysics. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jimenez-Sainz M. C., Fast B., Mayor F., Jr., Aragay A. M. Signaling pathways for monocyte chemoattractant protein 1-mediated extracellular signal-regulated kinase activation. Molecular Pharmacology. 2003;64(3):773–782. doi: 10.1124/mol.64.3.773. [DOI] [PubMed] [Google Scholar]
- 95.O'Shea J. J., Schwartz D. M., Villarino A. V., Gadina M., McInnes I. B., Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annual Review of Medicine. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tu G. W., Shi Y., Zheng Y. J., et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. Journal of Translational Medicine. 2017;15(1):p. 181. doi: 10.1186/s12967-017-1284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amantea D., Certo M., Petrelli F., et al. Azithromycin protects mice against ischemic stroke injury by promoting macrophage transition towards M2 phenotype. Experimental Neurology. 2016;275(Part 1):116–125. doi: 10.1016/j.expneurol.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 98.Wang J., Xie L., Wang S., Lin J., Liang J., Xu J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death & Disease. 2018;9(11):p. 1080. doi: 10.1038/s41419-018-1097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seropian I. M., Gonzalez G. E., Maller S. M., Berrocal D. H., Abbate A., Rabinovich G. A. Galectin-1 as an emerging mediator of cardiovascular inflammation: mechanisms and therapeutic opportunities. Mediators of Inflammation. 2018;2018:11. doi: 10.1155/2018/8696543.8696543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seropian I. M., Cerliani J. P., Toldo S., et al. Galectin-1 controls cardiac inflammation and ventricular remodeling during acute myocardial infarction. The American Journal of Pathology. 2013;182(1):29–40. doi: 10.1016/j.ajpath.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Boag S. E., Das R., Shmeleva E. V., et al. T lymphocytes and fractalkine contribute to myocardial ischemia/reperfusion injury in patients. The Journal of Clinical Investigation. 2015;125(8):3063–3076. doi: 10.1172/JCI80055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Azouz A., Razzaque M. S., El-Hallak M., Taguchi T. Immunoinflammatory responses and fibrogenesis. Medical Electron Microscopy. 2004;37(3):141–148. doi: 10.1007/s00795-004-0255-2. [DOI] [PubMed] [Google Scholar]
- 103.Sharir R., Semo J., Shimoni S., et al. Experimental myocardial infarction induces altered regulatory T cell hemostasis, and adoptive transfer attenuates subsequent remodeling. PLoS One. 2014;9(12, article e113653) doi: 10.1371/journal.pone.0113653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Frenkel D., Pachori A. S., Zhang L., et al. Nasal vaccination with troponin reduces troponin specific T-cell responses and improves heart function in myocardial ischemia-reperfusion injury. International Immunology. 2009;21(7):817–829. doi: 10.1093/intimm/dxp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Forero-Pena D. A., Gutierrez F. R. Statins as modulators of regulatory T-cell biology. Mediators of Inflammation. 2013;2013:10. doi: 10.1155/2013/167086.167086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ke D., Fang J., Fan L., Chen Z., Chen L. Regulatory T cells contribute to rosuvastatin-induced cardioprotection against ischemia-reperfusion injury. Coronary Artery Disease. 2013;24(4):334–341. doi: 10.1097/MCA.0b013e3283608c12. [DOI] [PubMed] [Google Scholar]
- 107.Sorathia N., Al-Rubaye H., Zal B. The effect of statins on the functionality of CD4+CD25+FOXP3+ regulatory T-cells in acute coronary syndrome: a systematic review and meta-analysis of randomised controlled trials in Asian populations. European Cardiology Review. 2019;14(2):123–129. doi: 10.15420/ecr.2019.9.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Granger C. B., Mahaffey K. W., Weaver W. D., et al. Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction. Circulation. 2003;108(10):1184–1190. doi: 10.1161/01.CIR.0000087447.12918.85. [DOI] [PubMed] [Google Scholar]
- 109.Verrier E. D., Shernan S. K., Taylor K. M., et al. Terminal complement blockade with pexelizumab during coronary artery bypass graft surgery requiring cardiopulmonary bypass: a randomized trial. JAMA. 2004;291(19):2319–2327. doi: 10.1001/jama.291.19.2319. [DOI] [PubMed] [Google Scholar]
- 110.de Zwaan C., Kleine A. H., Diris J. H., et al. Continuous 48-h C1-inhibitor treatment, following reperfusion therapy, in patients with acute myocardial infarction. European Heart Journal. 2002;23(21):1670–1677. doi: 10.1053/euhj.2002.3191. [DOI] [PubMed] [Google Scholar]
- 111.Thielmann M., Marggraf G., Neuhauser M., et al. Administration of C1-esterase inhibitor during emergency coronary artery bypass surgery in acute ST-elevation myocardial infarction. European Journal of Cardio-Thoracic Surgery. 2006;30(2):285–293. doi: 10.1016/j.ejcts.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 112.Fattouch K., Bianco G., Speziale G., et al. Beneficial effects of C1 esterase inhibitor in ST-elevation myocardial infarction in patients who underwent surgical reperfusion: a randomised double-blind study. European Journal of Cardio-Thoracic Surgery. 2007;32(2):326–332. doi: 10.1016/j.ejcts.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 113.Packard B. D., Weiler J. M. Steroids inhibit activation of the alternative-amplification pathway of complement. Infection and Immunity. 1983;40(3):1011–1014. doi: 10.1128/iai.40.3.1011-1014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lappin D. F., Whaley K. Modulation of complement gene expression by glucocorticoids. The Biochemical Journal. 1991;280(1) Part 1:117–123. doi: 10.1042/bj2800117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Potere N., Del Buono M. G., Niccoli G., Crea F., Toldo S., Abbate A. Developing LRP1 agonists into a therapeutic strategy in acute myocardial infarction. International Journal of Molecular Sciences. 2019;20(3):p. 544. doi: 10.3390/ijms20030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abouzaki N. A., Christopher S., Trankle C., et al. Inhibiting the inflammatory injury after myocardial ischemia reperfusion with plasma-derived alpha-1 antitrypsin: a post hoc analysis of the VCU-α1RT study. Journal of Cardiovascular Pharmacology. 2018;71(6):375–379. doi: 10.1097/FJC.0000000000000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abbate A., Van Tassell B. W., Christopher S., et al. Effects of prolastin C (plasma-derived alpha-1 antitrypsin) on the acute inflammatory response in patients with ST-segment elevation myocardial infarction (from the VCU-alpha 1-RT pilot study) The American Journal of Cardiology. 2015;115(1):8–12. doi: 10.1016/j.amjcard.2014.09.043. [DOI] [PubMed] [Google Scholar]
- 118.Hammerman H., Kloner R. A., Hale S., Schoen F. J., Braunwald E. Dose-dependent effects of short-term methylprednisolone on myocardial infarct extent, scar formation, and ventricular function. Circulation. 1983;68(2):446–452. doi: 10.1161/01.CIR.68.2.446. [DOI] [PubMed] [Google Scholar]
- 119.Kloner R. A., Fishbein M. C., Lew H., Maroko P. R., Braunwald E. Mummification of the infarcted myocardium by high dose corticosteroids. Circulation. 1978;57(1):56–63. doi: 10.1161/01.cir.57.1.56. [DOI] [PubMed] [Google Scholar]
- 120.Bulkley B. H., Roberts W. C. Steroid therapy during acute myocardial infarction: A cause of delayed healing and of ventricular aneurysm. The American Journal of Medicine. 1974;56(2):244–250. doi: 10.1016/0002-9343(74)90603-2. [DOI] [PubMed] [Google Scholar]
- 121.Roberts R., DeMello V., Sobel B. E. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204–I206. [PubMed] [Google Scholar]
- 122.Paulus P., Holfeld J., Urbschat A., et al. Prednisolone as preservation additive prevents from ischemia reperfusion injury in a rat model of orthotopic lung transplantation. PLoS One. 2013;8(8, article e73298) doi: 10.1371/journal.pone.0073298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tokudome S., Sano M., Shinmura K., et al. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. The Journal of Clinical Investigation. 2009;119(6):1477–1488. doi: 10.1172/JCI37413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Giugliano G. R., Giugliano R. P., Gibson C. M., Kuntz R. E. Meta-analysis of corticosteroid treatment in acute myocardial infarction. The American Journal of Cardiology. 2003;91(9):1055–1059. doi: 10.1016/S0002-9149(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 125.Zhang J., Yao Y., Xiao F., et al. Administration of dexamethasone protects mice against ischemia/reperfusion induced renal injury by suppressing PI3K/AKT signaling. International Journal of Clinical and Experimental Pathology. 2013;6(11):2366–2375. [PMC free article] [PubMed] [Google Scholar]
- 126.Taghizadieh M., Hajipour B., Asl N. A., Khodadadi A., Somi M. H., Banei M. Combination effect of melatonin and dexamethasone on liver ischemia/reperfusion injury. Bratislavské Lekárske Listy. 2016;117(1):47–53. doi: 10.4149/bll_2016_010. [DOI] [PubMed] [Google Scholar]
- 127.Sun W. H., He F., Zhang N. N., Zhao Z. A., Chen H. S. Time dependent neuroprotection of dexamethasone in experimental focal cerebral ischemia: the involvement of NF-κB pathways. Brain Research. 2018;1701:237–245. doi: 10.1016/j.brainres.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 128.Moonen L., Geryl H., D'Haese P. C., Vervaet B. A. Short-term dexamethasone treatment transiently, but not permanently, attenuates fibrosis after acute-to-chronic kidney injury. BMC Nephrology. 2018;19(1):p. 343. doi: 10.1186/s12882-018-1151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


