Abstract
Background:
No published study, to our knowledge, has systematically reviewed the prevalence estimates of lower urinary tract symptoms (LUTSs) and LUTS types in the general multiple sclerosis (MS) population. Therefore, a systematic review and a meta-analysis were conducted to determine the totality of literature investigating the prevalence of LUTSs and LUTS types in the general MS population according to International Continence Society definitions.
Methods:
Various electronic databases were searched between January 4, 2018, and February 12, 2018. This review included observational studies involving adults (18 years or older) with a confirmed diagnosis of MS recruited from the general MS population using self-report and/or objective outcome measures for LUTSs.
Results:
Twelve studies were included in the meta-analysis. The results showed that LUTSs were prevalent in people with MS, with a pooled prevalence of 68.41% using self-report and 63.95% using the objective measure of urodynamics. When considering LUTS types, urinary frequency was the predominant symptom, with a pooled prevalence estimate of 73.45%, followed by urgency assessed using self-report measures at 63.87%. Detrusor overactivity was found to be the most prevalent urodynamic symptom, with a pooled prevalence estimate of 42.9%, followed by detrusor sphincter dyssynergia at 35.44%.
Conclusions:
This systematic review revealed that LUTSs are highly prevalent in MS. There is a need for improvement in the conduct and reporting of prevalence studies of LUTSs in MS and for the use of validated self-report outcome measures to enable pooling of data in the future.
Keywords: Multiple sclerosis (MS), Neurogenic bladder, Overactive bladder, Urinary tract symptoms
Lower urinary tract symptoms (LUTSs) are common in people with multiple sclerosis (MS) and result primarily from demyelinating lesions that affect the spinal cord, disturbing neural connections from the pontine micturition center to the parasympathetic sacral micturition center.1,2 Many studies have demonstrated that worsening of urinary symptoms is correlated with increasing spinal cord involvement in people with MS.2–4
Definitions and terminology in lower urinary tract function recommended by the International Continence Society (ICS) that include signs, symptoms, and urodynamic observations5 were followed in this review. Symptoms are defined as subjective indicators perceived by the patient or caregiver that motivate him or her to seek medical advice. They are possibly suggestive of a health problem or a disease. Signs are the self-report measures used to verify and quantify symptoms, including validated questionnaires, frequency-volume charts, pad tests, or bladder diaries, that are usually stated by the patient or caregiver and observed by the physician as an indication of lower urinary tract dysfunction. Urodynamic observations, including urodynamic studies (UDSs), are performed in urodynamic laboratories and are used as objective outcome measures of lower urinary tract function.5,6
The ICS categorizes LUTSs into 1) storage symptoms, 2) voiding symptoms, or 3) postmicturition symptoms.5 Storage symptoms include failure to store urine, which can lead to overactive bladder (OAB), a symptom-based condition defined as a “[u]rinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology.”7 Urinary urgency is a storage symptom with the complaint of a sudden desire to pass urine that is difficult to defer. Similarly, urgency urinary incontinence is a storage symptom that results in an involuntary loss of urine that is accompanied by urgency; frequency is defined as voiding too often throughout the day, and nocturia is the need to wake up at night one or more times to void.5 In UDSs these symptoms present as detrusor overactivity and/or increased or decreased bladder sensation.
Voiding symptoms include failure to empty urine and may present with a variety of clinical manifestations, including hesitancy, poor stream, intermittency, straining, terminal dribble, dysuria, and need to immediately void again, that contribute to reducing the normal functional capacity of the bladder. Common urodynamic findings include detrusor-sphincter dyssynergia, which involves loss of coordination between detrusor muscles and the external sphincter during the storage phase2 and/or detrusor underactivity. Postmicturition symptoms include postmicturition dribble and a feeling of incomplete emptying. The latter is often associated with postvoid residue on UDS or ultrasound.
Early diagnosis of LUTSs in patients with MS is important to prevent possible complications and guide the management pathway. This could be obtained through specific diagnostic measures, including self-report and/or objective outcome measures. There is debate among experts for undertaking UDSs in people with MS. The European Association of Urology and UK National Institute for Care and Clinical Excellence guidelines recommend using UDSs with symptomatic neurologic patients when conservative treatment has failed, whereas the American Urological Association guidelines recommend using UDSs for all neurologic patients with or without LUTSs.8 According to the ICS, UDSs are defined as measurements of all physiologic function and dysfunction of the lower urinary tract that help in diagnosing the cause and nature of the lower urinary tract abnormalities. It involves two principal methods of investigation: conventional UDSs and ambulatory UDSs. It generally requires an individual to have a full bladder for uroflowmetry and postvoid residual measurement before filling cystometry and pressure-flow study.5,6
Several studies have shown that LUTSs in MS can be the source of a significant reduction in health-related quality of life.9–11 A qualitative study was conducted of people with MS to explore and discuss participants’ experiences of living with LUTSs and how these symptoms affect their quality of life. This study revealed that bladder dysfunction in MS results in major disturbances to activities of daily living, which can affect their lifestyle.12 Fear of leaking urine in public among people with MS was identified as a barrier to engaging in physical activities, which might reduce their health-related quality of life.13 Also, bladder incontinence has been found to be associated with increased risk of falling in people with MS aged 45 to 90 years.14 Therefore, increased focus on understanding the prevalence of LUTSs and LUTS types in the MS population may help improve therapeutic interventions that could potentially improve the health-related quality of life of people with MS.
Previous reviews of LUTSs in people with MS have tended to use samples of people with bladder problems and investigate the prevalence of each type of LUTS. One such systematic review aimed to investigate the incidence and prevalence of urinary incontinence and detrusor overactivity in people with neurogenic OAB. That review found that urinary incontinence in people with MS ranged from 6.9% in an Italian single-center study to 95% in a Japanese study. The review revealed detrusor overactivity prevalence estimates of 27% to 91%.15 Other reviews16–19 reported that the storage symptoms of urgency, frequency, and urgency urinary incontinence were the predominant symptoms in people with MS and ranged from 32% to 86%, 32% to 83%, and 19% to 83%, respectively. The lack of studies sampling from a general MS population, together with the small sample sizes and lack of reporting of detailed methods, affects the reliability of these results.
In 2002, the ICS subcommittee issued standardized terminology and definitions of LUTSs that are recommended to be followed.5 However, the lack of consistency in terminology weakens previous studies’ results and limits the ability to draw strong conclusions from their results. To investigate the burden of LUTSs in people with MS, there is a need for researchers to apply the standardized terminology of LUTSs that has been established by the ICS.
To our knowledge, no study has been conducted to systematically review and summarize existing data to obtain a summary estimate of the prevalence of LUTSs and LUTS types in the general MS population using only the standardized ICS terminology. Therefore, the purpose of this study was to systematically review the literature to determine the totality of literature investigating the prevalence of LUTSs and LUTS types in a general MS population according to ICS recommendations.
Methods
The conduct and reporting of this study are in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.20
Search Strategy
The search strategy was discussed with the faculty librarian to optimize retrieval of relevant studies. The searches were started January 4, 2018, and concluded February 12, 2018, and were not limited by date. The literature was searched for peer-reviewed original articles using the following individual electronic databases: AMED (Allied and Complementary Medicine), CINAHL (Cumulative Index to Nursing and Allied Health Literature), MEDLINE, PsycARTICLES, Embase, Scopus, PubMed, and The Cochrane Library database. The search terms used were multiple sclerosis AND neurogenic bladder OR urinary dysfunction OR urinary bladder OR overactive bladder OR incontinence OR bladder dysfunction OR bladder OR detrusor OR lower urinary tract OR urinary OR catheter* OR enuresis OR nocturia. All identified studies were imported into bibliography management software (EndNote X8; Clarivate Analytics, Philadelphia, PA). The reference lists of full-text screened articles and other systematic reviews on the prevalence of LUTSs in people with MS were hand searched for potentially relevant articles. We contacted authors to clarify missing data and ordered interlibrary loans for any remaining articles unobtainable through author contact.
Eligibility Criteria
The inclusion criteria for the studies were as follows: 1) population—people with a confirmed diagnosis of MS, 18 years and older, recruited from the general MS population in a random/consecutive way that suggests they would be representative of the population; 2) the study provided an estimate of the prevalence of LUTS categories according to ICS definitions5; 3) outcome measures—any means of establishing the presence of LUTSs, including self-report and/or objective measures; and 4) study design—baseline data that are first estimate values of a longitudinal study design or observational study design, including cross-sectional studies, baseline data of prospective or retrospective cohort studies, or case-control studies. Studies were excluded if the population of interest was limited to pregnant women or if studies with mixed neurologic populations did not separate results for people with MS. Two reviewers (H.B.A.D., N.O’M.) independently screened abstracts and full texts to determine the eligibility of the included studies. Any disagreements between reviewers were resolved by consulting with a third reviewer (D.M.) until consensus was achieved.
Data Extraction and Quality Assessment
Each study identified was screened in detail to extract author(s) name, year, title, study design, settings, study population, age, sex, type(s) of MS, severity of MS, disease duration, outcome measure(s), and key findings. Two independent reviewers reviewed the data extraction table to confirm the accuracy and clarity of the extracted information.
Methodological quality and risk of bias were assessed using the Newcastle-Ottawa Scale adapted for cross-sectional studies.21 We extracted cross-sectional data from one case-control study and one longitudinal study, and thus they were considered as cross-sectional studies for quality appraisal. This tool comprises three key domains: selection, comparability, and outcome. A star system was used to allow a semiquantitative assessment of study quality, with a maximum of ten stars awarded depending on the criteria level that the study meets in each section. The process was performed independently by two reviewers (H.B.A.D., N.O’M.), and disagreements were resolved by discussion.
Statistical Analysis
The prevalence estimates of LUTSs and LUTS types are reported as percentages extracted from each study. We pooled the prevalence estimates for self-report measures and UDSs. Statistical analysis was performed using Review Manager software, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for meta-analysis, and a random effect model with 95% CIs was applied. Standard error was obtained using the following formula: SQRT(p*(1–p)/n), where p indicates prevalence; n, sample size. Heterogeneity was assessed using I2 statistics. We used the Cochrane interpretation of these values where an I2 value of 30% to 60% indicates moderate heterogeneity; 50% to 90%, substantial heterogeneity; and 75% to 100%, considerable heterogeneity.22,23
Results
Figure S1 (464.9KB, pdf) (published in the online version of this article at ijmsc.org) shows the flow diagram of the literature search and results. After screening of 15,274 abstracts, 22 studies met the inclusion criteria, and 12 studies were included in the meta-analysis: 11 cross-sectional studies and one case-control study.
Study and Patient Characteristics
Of the 22 studies that met the inclusion criteria, nine were not included in the meta-analysis because the methods of data collection were not described in sufficient detail to allow accurate interpretation of the findings,24–32 and one additional study provided prevalence estimates of OAB with no details given for single symptoms to enable pooling of the data for meta-analysis.33 Some urinary symptoms in two studies were not included in the final analysis because the terminology was inconsistent with the ICS classification or the urinary symptoms were combined, making prevalence estimates for individual ICS categories not possible.34,35
Descriptive characteristics of included studies are provided in Table S1 (464.9KB, pdf) . The 12 studies34–45 in the final analysis included 2507 MS participants, including 1799 women (71.8%), with sample sizes ranging from 21 to 1047 and age ranging from 18 to 89 years. There were 1554 participants (62.0%) with relapsing-remitting MS, 108 (4.3%) with primary progressive MS, and 155 (6.2%) with secondary progressive MS; 690 participants (27.5%) did not have descriptive characteristics presented in the included studies. Outcome measures used to estimate the prevalence of LUTSs in the MS population are presented in Table 1. Nine studies administered nine different self-report measures, and six studies investigated the prevalence based on objective measures (five using UDSs and one using portable ultrasound).
Table 1.
Outcome measures used in included studies
| Measure type and study | Outcome measure |
|---|---|
| Self-report measures | |
| Akkoç et al, 201636 | Overactive Bladder Symptom Score |
| de Almeida et al, 201334 | Kurtzke Functional System Scale for Bladder |
| Khalaf et al, 201538 | Lower Urinary Tract Symptoms tool |
| Nortvedt et al, 200741 | International Prostate Symptom Score–Norwegian |
| Murphy et al, 201239 | Urogenital Distress Inventory-6 |
| Zecca et al,201643 | International Consultation of Incontinence Questionnaire |
| Hall et al, 201233 | Boston Area Community Health Survey |
| Kale et al, 200937 | Self-report measure (developed by author) |
| Vieira et al, 201542 | Composite Autonomic Symptom Score (Portuguese) |
| Objective measures | |
| Akkoç et al, 201636 | Ultrasound, catheterization (postvoid residue) |
| de Almeida et al, 201334 | Urodynamic studies |
| Nakipoglu et al, 200940 | Urodynamic studies |
| Porru et al, 199735 | Urodynamic studies |
| Weinstein et al, 198844 | Urodynamic studies |
| Bemelmans et al, 199145 | Urodynamic studies |
Urodynamic studies were performed according to ICS standards46 in four studies, with three using liquid cystometry35,40,45 and one using gas cystometry including carbon dioxide.44 The UDS procedure in one study was not described in sufficient detail.34
Prevalence
The pooled prevalence of general LUTSs using self-report measures from four studies34,37,38,42 was 68.41% (95% CI, 45.24%-91.58%) (Figure 1A) and detected by UDSs from three studies34,40,45 was 63.95% (95% CI, 57.78%-70.12%), with significant heterogeneity (I2 = 100%) (Figure 1B).
Figure 1.
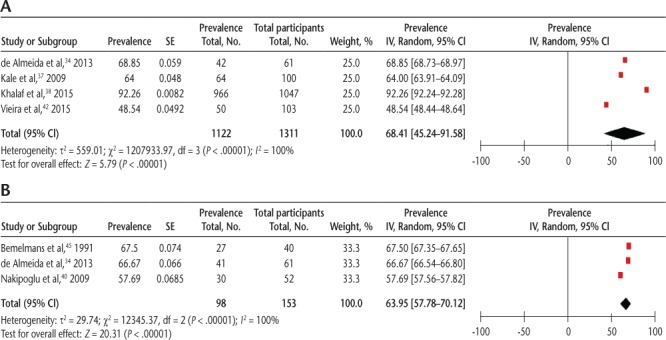
Pooled prevalence of general lower urinary tract symptoms using (A) self-report measures and (B) urodynamic studies
IV, inverse-variance approach that applied in RevMan.
The prevalence estimate of each type of LUTS was detected through self-report measures in seven studies and by UDSs in three studies. Prevalence ranges and meta-analyses are presented in Table S2 (464.9KB, pdf) . The 95% CIs for most estimates were wide. Sensitivity analysis was not appropriate in this review owing to the small number of studies included in the meta-analyses.
The pooled prevalence of storage symptoms detected by self-report measures showed that frequency was the predominant symptom, followed by urgency and nocturia (Figure S2 (464.9KB, pdf) ). The pooled prevalence estimates using objectively measured UDSs showed that detrusor overactivity is the predominant symptom, followed by reduced bladder sensation (Figure S3 (464.9KB, pdf) ).
Some types of voiding symptoms were not measured subjectively in any studies (hesitancy and dysuria), and others were measured in only one study (weak stream, intermittency, straining, terminal dribble). Therefore, the pooled prevalence estimates using self-report outcome measures of voiding symptoms in people with MS could not be reported in this review. Pooled prevalence of voiding symptoms using UDSs showed that detrusorsphincter dyssynergia is the predominant symptom, followed by detrusor underactivity (Figure S4 (464.9KB, pdf) ).
For postmicturition symptoms, the pooled prevalence estimates for the feeling of incomplete bladder emptying using self-report outcome measures was 60.56% (95% CI, 60.26%-60.85%) (Figure S5 (464.9KB, pdf) ). Using objective outcome measures, one study found prevalence estimates of the feeling of incomplete bladder emptying of 13.3% using ultrasound and 16.2% using a catheter. Postmicturition dribble was not investigated in the studies.
Only one study used a longitudinal study design25 and was not included in the meta-analysis due to insufficient details provided for the self-report outcome measure. The study investigated the prevalence of LUTSs and LUTS types at three time points (baseline and 3- and 6-year follow-up). The prevalence estimate of general LUTSs at baseline was 46.23%. The prevalence of each type of LUTS estimated at baseline was as follows: urgency, 41.93%; hesitancy, 30.1%; urge incontinence, 31.18%; nocturia, 24.73%; and incomplete bladder emptying, 33.3%.
Methodological Quality
Results of the quality assessment are presented in Table S3 (464.9KB, pdf) . Study ratings ranged from one to eight of ten stars. Five studies were identified as having high risk of bias for selection, attrition, information, and detection bias.26,28,29,35,40 Two studies were found to be at high risk for bias in all areas.31,44 All the studies included in meta-analyses were at high risk for attrition bias, which may indicate overestimation of the prevalence of LUTSs in people with MS. Differences in sampling methods and outcome assessments lead to considerable heterogeneity, with wide CI ranges.
Discussion
To our knowledge, no published study to date has systematically reviewed the prevalence estimates of LUTSs and LUTS types in the general MS population. Therefore, this study was conducted to systematically review and summarize existing data to obtain a summary estimate of the prevalence of LUTSs and LUTS types in the general MS population according to ICS definitions. In people with MS, LUTSs are highly prevalent, with an estimated prevalence of 68.4% for general LUTSs using self-report outcome measures and a prevalence of 63.95% using UDSs. This review showed that the most common symptom when using self-report measures is frequency, with a pooled prevalence estimate of 73.45%, followed by urgency (63.87%), incomplete bladder emptying (60.56%), nocturia (58.95%), and urinary incontinence (42.9%). The most common objectively measured symptoms were detrusor overactivity, with a pooled prevalence estimate of 42.9%, followed by detrusor-sphincter dyssynergia at 35.44%. Voiding symptoms were infrequently reported, and meta-analysis was not possible.
Compared with healthy individuals, LUTSs are predominant in the MS population. The present findings showed that the prevalence estimates of LUTSs and some types of storage symptoms were up to four times higher than those in the general population. A worldwide prevalence estimate47 and other population-based surveys among healthy individuals48,49 reported the prevalence estimate of general LUTSs at 45.2%, far less than the pooled prevalence from this review. Some storage symptoms were reported in healthy individuals: 45% to 57.7% for frequency and 36.6% for urgency,47–49 which is much less than the pooled prevalence from the present review. A study of 30,000 healthy individuals49 found that nocturia was prevalent, with an estimate of 72.2%, which is greater than the estimates of prevalence for people with MS. This difference could be attributed to methodological issues, including use of the Epidemiology of LUTS (EpiLUTS) survey. The participants were asked to respond to each question related to LUTSs by choosing either sometimes or often. Different interpretations of each question among participants might lead to variety in reported responses that results in overestimates of the prevalence.
The LUTSs are more prevalent in people with MS than in those with other neurologic diseases. These prevalence estimates suggest that some types of storage symptoms are five times higher in people with MS than in stroke survivors. In a large sample of people with stroke, frequency was estimated to be 15% and urgency 19%,50 which are significantly lower than for people with MS. Similarly, a systematic review investigating the estimates of LUTSs in patients with Parkinson disease51 reported that the prevalence of general LUTSs ranged from 27% to 85%, which is lower than the range of the prevalence estimates reported in the present review. In addition, the ranges of prevalence estimates for frequency, urgency, and incomplete bladder emptying in patients with Parkinson disease were found to be 32% to 71%, 32% to 68%, and 8% to 28%, respectively, which is far less than the prevalence estimate ranges in people with MS in the present review. In contrast, nocturia in patients with Parkinson disease was the most prevalent storage symptom, with prevalence estimates ranging from 57% to 86%. This prevalence may be attributed to the age-related pathophysiological factors that contribute to Parkinson disease. Age-related physiological, hormonal, and structural factors play an important role in increasing the prevalence of nocturia.52–54
This systematic review raises issues about the methodological quality of the included studies, and there are learning points for future research in the area. Notably, most of the studies included in this review were conducted using either small groups of patients or larger cross-sectional populations recruited by online survey. Online surveys are typically prone to selection bias,55 and this method of data collection may not obtain data from a representative MS sample. Recruitment through neurology clinics or rehabilitation services should be considered. Moreover, all the included studies were affected by attrition bias, and the number of respondents versus nonrespondents was not addressed by statistical analyses and needs to be considered in future studies.
In addition to issues of bias, there is a lack of consistency in self-report outcome measures used, with each study using a different measure. Interestingly, none of the studies that investigated frequency symptoms were consistent with the ICS recommendation of using a bladder diary. These factors led to considerable variability in study results, including wide CIs and considerable heterogeneity. The European Association of Urology guidelines recommend using validated questionnaires in future studies,8 which, in turn, will enable data synthesis and allow more detailed comparison of results from different studies.
There are limited studies in the literature comparing self-report measures against the reference standard of UDS-based diagnosis.56–58 A correlation has been shown between International Consultation on Incontinence Questionnaire–Short Form (ICIQ-SF) and urodynamic parameters56 and between one question dealing with urgency urinary incontinence on the Urogenital Distress Inventory-6 (UDI-6) and UDSs.57 These findings would help reduce UDSs without negatively influencing patient condition. On the other hand, one study found that scores on the symptom scales of the Incontinence Impact Questionnaire-7 (IIQ-7) and UDI-6 were inadequate predictors of eventual urodynamic diagnoses.58 Hence, no conclusion could be drawn for the correlation of self-report outcome measures and UDSs due to limited studies in the literature. Further studies are needed in this regard.
The strengths of this study are that the prevalence estimates are derived from studies of the general MS population and not solely from people with MS who had LUTSs. In addition, this review is seemingly the first to classify symptoms according to ICS definitions and to meta-analyze LUTSs across the storage, voiding, and micturition symptoms. Moreover, we used extensive search techniques and made every effort to ensure that the totality of literature was included in this review. A limitation of this review is that we limited it to studies published in the English language. Also, the generalizability of the results is poor because almost all the included studies were of poor quality. A further limitation is that the prevalence of LUTSs in the general MS population was not reported by sex in the included studies. Hence, there is a need for future study to establish the prevalence of LUTSs by sex in people with MS. Also, there is a lack of information in the literature related to the prevalence of LUTSs accompanying types of MS. Therefore, we could not consider the prevalence of LUTSs among types of MS in the general MS population. This limitation should be considered in future studies. An additional consideration is whether the prevalence of LUTSs is in a treated or untreated population; it is possible that the prevalence would be reduced if the population included had recently undergone treatment for the condition, and this factor needs to be considered in future studies.
In conclusion, this systematic review revealed that LUTSs are highly prevalent in people with MS when using either self-report or UDSs. Frequency is the predominant symptom, followed by urgency, detected by self-report outcome measures; detrusor overactivity is the predominant symptom, followed by detrusor sphincter dyssynergia, detected by UDSs. There is a need for improved conduct and reporting of prevalence studies of LUTSs, including ICS classification, and for the use of validated self-report outcome measures to enable pooling of data in the future.
PRACTICE POINTS
This review found that lower urinary tract symptoms are prevalent in people with MS using self-report and objective outcome measures.
The most prevalent symptom using self-report measures was urinary frequency, with a pooled prevalence estimate of 73.45%, followed by urgency at 63.87%.
Using the objective measure of urodynamic studies, detrusor overactivity was the most prevalent symptom, with a pooled prevalence estimate of 42.9%, followed by detrusor sphincter dyssynergia at 35.44%.
There is a lack of consistency in self-report measures and methodological issues, such as attrition bias, among included studies. Therefore, there is a need for larger, high-quality studies using validated self-report outcome measures to estimate the prevalence of lower urinary tract symptoms in MS.
Acknowledgments
The authors thank Ms Liz Dore, librarian, University of Limerick, for her assistance with the search strategy; Dr Rose Galvin, senior lecturer, School of Allied Health, University of Limerick, for her assistance with the statistical analysis; and Nicola O’Malley, PhD student, School of Allied Health, University of Limerick, for her contribution to the study as an independent reviewer.
Financial Disclosures
Dr Coote has disclosed a relationship with Novartis (fees for non-CME/CE services received directly from a commercial interest [contractor to deliver telerehabilitation as part of patient support program]). The other authors declare no conflicts of interest.
Funding/Support
Ms Al Dandan is a PhD student at the University of Limerick funded by Imam Abdulrahman Bin Faisal University (tuition paid by the Saudi cultural commission as part of higher education program); Ms O’Malley is an Embark Scholar funded by the Irish Research Council.
References
- 1.Thompson AJ, Toosy AT, Ciccarelli O. Pharmacological management of symptoms in multiple sclerosis: current approaches and future directions. Lancet Neurol. 2010;9:1182–1199. doi: 10.1016/S1474-4422(10)70249-0. [DOI] [PubMed] [Google Scholar]
- 2.Betts CD, D’Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1993;56:245–250. doi: 10.1136/jnnp.56.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koldewijn EL, Hommes OR, Lemmens WA, Debruyne FM, van Kerrebroeck PE. Relationship between lower urinary tract abnormalities and disease-related parameters in multiple sclerosis. J Urol. 1995;154:169–173. [PubMed] [Google Scholar]
- 4.Awad SA, Gajewski JB, Sogbein SK, Murray TJ, Field CA. Relationship between neurological and urological status in patients with multiple sclerosis. J Urol. 1984;132:499–502. doi: 10.1016/s0022-5347(17)49710-2. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 6.D’Ancona C, Haylen B, Oelke M et al. The International Continence Society (ICS) report on the terminology for adult male lower urinary tract and pelvic floor symptoms and dysfunction. Neurourol Urodyn. 2019;38:433–477. doi: 10.1002/nau.23897. [DOI] [PubMed] [Google Scholar]
- 7.Haylen BT, Ridder D, Freeman RM et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21:5–26. doi: 10.1007/s00192-009-0976-9. [DOI] [PubMed] [Google Scholar]
- 8.Nambiar A, Lucas M. Chapter 4: guidelines for the diagnosis and treatment of overactive bladder (OAB) and neurogenic detrusor overactivity (NDO) Neurourol Urodyn. 2014;33(S3):S21–S25. doi: 10.1002/nau.22631. [DOI] [PubMed] [Google Scholar]
- 9.Nortvedt MW, Riise T, Myhr KM, Landtblom AM, Bakke A, Nyland HI. Reduced quality of life among multiple sclerosis patients with sexual disturbance and bladder dysfunction. Mult Scler. 2001;7:231–235. doi: 10.1177/135245850100700404. [DOI] [PubMed] [Google Scholar]
- 10.Vitkova M, Rosenberger J, Krokavcova M et al. Health-related quality of life in multiple sclerosis patients with bladder, bowel and sexual dysfunction. Disabil Rehabil. 2014;36:987–992. doi: 10.3109/09638288.2013.825332. [DOI] [PubMed] [Google Scholar]
- 11.Khalaf KM, Coyne KS, Globe DR et al. The impact of lower urinary tract symptoms on health-related quality of life among patients with multiple sclerosis. Neurourol Urodyn. 2016;35:48–54. doi: 10.1002/nau.22670. [DOI] [PubMed] [Google Scholar]
- 12.Browne C, Salmon N, Kehoe M. Bladder dysfunction and quality of life for people with multiple sclerosis. Disabil Rehabil. 2015;37:2350–2358. doi: 10.3109/09638288.2015.1027007. [DOI] [PubMed] [Google Scholar]
- 13.Kayes NM, McPherson KM, Schluter P, Taylor D, Leete M, Kolt GS. Exploring the facilitators and barriers to engagement in physical activity for people with multiple sclerosis. Disabil Rehabil. 2011;33:1043–1053. doi: 10.3109/09638288.2010.520801. [DOI] [PubMed] [Google Scholar]
- 14.Finlayson ML, Peterson EW, Cho CC. Risk factors for falling among people aged 45 to 90 years with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:1274–1279. doi: 10.1016/j.apmr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Ruffion A, Castro-Diaz D, Patel H et al. Systematic review of the epidemiology of urinary incontinence and detrusor overactivity among patients with neurogenic overactive bladder. Neuroepidemiology. 2013;41:146–155. doi: 10.1159/000353274. [DOI] [PubMed] [Google Scholar]
- 16.Corcos J. A urological challenge: voiding dysfunction in multiple sclerosis. J Can Urol Assoc. 2013;7(suppl 4):S181–S182. doi: 10.5489/cuaj.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Sèze M, Ruffion A, Denys P, Joseph P-A, Perrouin-Verbe B. The neurogenic bladder in multiple sclerosis: review of the literature and proposal of management guidelines. Mult Scler. 2007;13:915–928. doi: 10.1177/1352458506075651. [DOI] [PubMed] [Google Scholar]
- 18.Cohen BL, Leboeuf L, Gousse AE. Neurogenic bladder from multiple sclerosis. Curr Bladder Dysfunct Rep. 2008;3:5–12. [Google Scholar]
- 19.Aharony SM, Lam O, Corcos J. Evaluation of lower urinary tract symptoms in multiple sclerosis patients: review of the literature and current guidelines. Can Urol Assoc J. 2017;11:61–64. doi: 10.5489/cuaj.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Herzog R, Alvarez-Pasquin MAJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health. 2013;13:154. doi: 10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiest KM, Pringsheim T, Patten SB, Svenson LW, Jette N. The role of systematic reviews and meta-analyses of incidence and prevalence studies in neuroepidemiology. Neuroepidemiology. 2014;42:16–24. doi: 10.1159/000355533. [DOI] [PubMed] [Google Scholar]
- 24.Khan F, Pallant JF, Shea TL, Whishaw M. Multiple sclerosis: prevalence and factors impacting bladder and bowel function in an Australian community cohort. Disabil Rehabil. 2009;31:1567–1576. doi: 10.1080/09638280802639566. [DOI] [PubMed] [Google Scholar]
- 25.Kisic Tepavcevic D, Pekmezovic T, Dujmovic Basuroski I, Mesaros S, Drulovic J. Bladder dysfunction in multiple sclerosis: a 6-year follow-up study. Acta Neurol Belg. 2017;117:83–90. doi: 10.1007/s13760-016-0741-z. [DOI] [PubMed] [Google Scholar]
- 26.Patti F, Ventimiglia B, Failla G, Genazzani AA, Reggio A. Micturition disorders in multiple sclerosis patients: neurological, neurourodynamic and magnetic resonance findings. Eur J Neurol. 1997;4:259–265. [Google Scholar]
- 27.Torelli F, Terragni E, Blanco S, Di Bella N, Grasso M, Bonaiuti D. Lower urinary tract symptoms associated with neurological conditions: observations on a clinical sample of outpatients neurorehabilitation service. Arch Ital Urol Androl. 2015;87:154–157. doi: 10.4081/aiua.2015.2.154. [DOI] [PubMed] [Google Scholar]
- 28.Ukkonen M, Elovaara I, Dastidar P, Tammela TLJ. Urodynamic findings in primary progressive multiple sclerosis are associated with increased volumes of plaques and atrophy in the central nervous system. Acta Neurol Scand. 2004;109:100–105. doi: 10.1034/j.1600-0404.2003.00184.x. [DOI] [PubMed] [Google Scholar]
- 29.Ventimiglia B, Patti F, Reggio E et al. Disorders of micturition in neurological patients. a clinical study of 786 patients. J Neurol. 1998;245:173–177. doi: 10.1007/s004150050200. [DOI] [PubMed] [Google Scholar]
- 30.Wollin J, Bennie M, Leech C, Windsor C, Spencer N. Continence: multiple sclerosis and continence issues: an exploratory study. Br J Nurs. 2005;14:439–446. doi: 10.12968/bjon.2005.14.8.17926. [DOI] [PubMed] [Google Scholar]
- 31.Van Poppel H, Vereecken RL, Leruitte A. Neuro-muscular dysfunction of the lower urinary tract in multiple sclerosis. Paraplegia. 1983;21:374–379. doi: 10.1038/sc.1983.61. [DOI] [PubMed] [Google Scholar]
- 32.Hassouna M, Lebel M, Elhilali M. Neurourologic correlation in multiple sclerosis. Neurourol Urodyn. 1984;3:73–77. [Google Scholar]
- 33.Hall SA, Curto TM, Onyenwenyi A et al. Characteristics of persons with overactive bladder of presumed neurologic origin: results from the Boston Area Community Health (BACH) survey. Neurourol Urodyn. 2012;31:1149–1155. doi: 10.1002/nau.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Almeida CR, Carneiro K, Fiorelli R, Orsini M, Alvarenga RMP. Urinary dysfunction in women with multiple sclerosis: analysis of 61 patients from Rio de Janeiro, Brazil. Neurol Int. 2013;5:79–83. doi: 10.4081/ni.2013.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porru D, Campus G, Garau A et al. Urinary tract dysfunction in multiple sclerosis: is there a relation with disease-related parameters? Spinal Cord. 1997;35:33–36. doi: 10.1038/sj.sc.3100337. [DOI] [PubMed] [Google Scholar]
- 36.Akkoç Y, Ersöz M, Yüceyar N et al. Overactive bladder symptoms in patients with multiple sclerosis: frequency, severity, diagnosis and treatment. J Spinal Cord Med. 2016;39:229–233. doi: 10.1179/2045772315Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kale N, Magana S, Agaoglu J, Tanik O. Assessment of autonomic nervous system dysfunction in multiple sclerosis and association with clinical disability. Neurol Int. 2009;1:e5–e5. doi: 10.4081/ni.2009.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalaf KM, Coyne KS, Globe DR, Armstrong EP, Malone DC, Burks J. Lower urinary tract symptom prevalence and management among patients with multiple sclerosis. Int J MS Care. 2015;17:14–25. doi: 10.7224/1537-2073.2013-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy AM, Bethoux F, Stough D, Goldman HB. Prevalence of stress urinary incontinence in women with multiple sclerosis. Int Neurourol J. 2012;16:86–90. doi: 10.5213/inj.2012.16.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakipoglu GF, Kaya AZ, Orhan G et al. Urinary dysfunction in multiple sclerosis. J Clin Neurosci. 2009;16:1321–1324. doi: 10.1016/j.jocn.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Nortvedt MW, Riise T, Frugard J et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Mult Scler. 2007;13:106–112. doi: 10.1177/1352458506071210. [DOI] [PubMed] [Google Scholar]
- 42.Vieira B, Costa A, Videira G, Sá MJ, Abreu P. Prevalence of autonomic dysfunction in patients with multiple sclerosis. Acta Med Port. 2015;28:51–55. doi: 10.20344/amp.5562. [DOI] [PubMed] [Google Scholar]
- 43.Zecca C, Riccitelli GC, Disanto G et al. Urinary incontinence in multiple sclerosis: prevalence, severity and impact on patients’ quality of life. Eur J Neurol. 2016;23:1228–1234. doi: 10.1111/ene.13010. [DOI] [PubMed] [Google Scholar]
- 44.Weinstein MS, Cardenas DD, O’Shaughnessy EJ, Catanzaro ML. Carbon dioxide cystometry and postural changes in patients with multiple sclerosis. Arch Phys Med Rehabil. 1988;69:923–927. [PubMed] [Google Scholar]
- 45.Bemelmans BL, Hommes OR, Van Kerrebroeck PE, Lemmens WA, Doesburg WH, Debruyne FM. Evidence for early lower urinary tract dysfunction in clinically silent multiple sclerosis. J Urol. 1991;145:1219–1224. doi: 10.1016/s0022-5347(17)38581-6. [DOI] [PubMed] [Google Scholar]
- 46.Abrams P, Blaivas J, Stanton S, Andersen J. The standardisation of terminology of lower urinary tract function. World J Urol. 1989;6:233–245. [PubMed] [Google Scholar]
- 47.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132–1138. doi: 10.1111/j.1464-410X.2010.09993.x. [DOI] [PubMed] [Google Scholar]
- 48.Boyle P, Robertson C, Mazzetta C et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409–414. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 49.Coyne KS, Wein AJ, Tubaro A et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103:4–11. doi: 10.1111/j.1464-410X.2009.08371.x. [DOI] [PubMed] [Google Scholar]
- 50.Brittain RK, Perry IS, Peet MS et al. Prevalence and impact of urinary symptoms among community-dwelling stroke survivors. Stroke. 2000;31:886–891. doi: 10.1161/01.str.31.4.886. [DOI] [PubMed] [Google Scholar]
- 51.McDonald C, Winge K, Burn DJ. Lower urinary tract symptoms in Parkinson’s disease: prevalence, aetiology and management. Parkinsonism Relat Disord. 2017;35:8–16. doi: 10.1016/j.parkreldis.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 52.Davie CA. A review of Parkinson’s disease. Br Med Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 53.Stewart DA. NICE guideline for Parkinson’s disease. Age Ageing. 2007;36:240–242. doi: 10.1093/ageing/afm040. [DOI] [PubMed] [Google Scholar]
- 54.Boongird S, Shah N, Nolin TD, Unruh ML. Nocturia and aging: diagnosis and treatment. Adv Chronic Kidney Dis. 2010;17:e27–e40. doi: 10.1053/j.ackd.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Bethlehem J. Selection bias in Web surveys. Int Stat Rev. 2010;78:161–188. [Google Scholar]
- 56.Seckiner I, Yesilli C, Mungan NA, Aykanat A, Akduman B. Correlations between the ICIQ-SF score and urodynamic findings. Neurourol Urodyn. 2007;26:492–494. doi: 10.1002/nau.20389. [DOI] [PubMed] [Google Scholar]
- 57.Lemack GE, Zimmern PE. Predictability of urodynamic findings based on the Urogenital Distress Inventory-6 questionnaire. Urology. 1999;54:461–466. doi: 10.1016/s0090-4295(99)00246-0. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald MP, Brubaker L. Urinary incontinence symptom scores and urodynamic diagnoses. Neurourol Urodyn. 2002;21:30–35. doi: 10.1002/nau.2116. [DOI] [PubMed] [Google Scholar]


