Abstract
Stimulation of the immune response after vaccination can occasionally result in adverse effects, including demyelination of the central nervous system. The most common presentation of postvaccination demyelination is acute disseminated encephalomyelitis, but cases of optic neuritis, transverse myelitis, and multiple sclerosis relapses have been reported. More recently, an increasing number of postvaccination neuromyelitis optica spectrum disorder (NMOSD) cases have surfaced in the literature, especially in patients with aquaporin-4 antibodies. In this article, we report an unusual case of myelin oligodendrocyte glycoprotein antibody–related NMOSD after the receipt of multiple vaccines in a first-trimester pregnant woman from Africa. We review the reported cases of postvaccination demyelination in the past decade, with a focus on the relationship between NMOSD and vaccination in patients with aquaporin-4 or myelin oligodendrocyte glycoprotein antibodies. Finally, we discuss the clinical relevance of the present case and similar reported cases as it relates to patient care in the neuroimmunology clinic and identify potential areas for future research.
Keywords: Anti-myelin oligodendrocyte glycoprotein (MOG), Multiple sclerosis (MS), Neuromyelitis optica
Acute disseminated encephalomyelitis is the most common form of postvaccination demyelination, however, an increasing number of neuromyelitis optica spectrum disorders (NMOSDs) have recently been reported with certain vaccines. Most of these cases were positive for aquaporin-4 (AQP-4) antibody. In this article, we report on a patient who developed myelin oligodendrocyte glycoprotein (MOG) antibody–related NMOSD after receiving several vaccines. We review reported cases of postvaccination demyelination over the past decade and discuss the clinical implications of the present case and similar cases in view of the recent advances in demyelinating antibody testing.
Case Description
A 37-year-old woman originally from Ghana, Africa, and living in North America since 2015 with a remote history of ectopic pregnancy presented in the spring with viral prodromal symptoms 3 weeks after receiving the tetanus vaccine and 2 weeks after receiving the measles, mumps, and rubella and the varicella vaccines. She previously had the measles, mumps, and rubella vaccine as a child in Ghana without any known postvaccination adverse events. She had recently been visited by family from Ghana, including a relative with an upper respiratory tract infection. She was originally thought to have sinusitis, but she presented again 3 days later with new-onset bladder and bowel retention. She was found to be 5 weeks pregnant and was thought to have concomitant urinary tract infection and was discharged on nitrofurantoin. She finally presented to our institution 3 days later with bilateral lower extremity weakness/numbness, severe bilateral upper extremity tremor, vertigo, and intractable vomiting. On examination, she exhibited high-frequency irregular bilateral upper extremity tremor more severe on the left present at rest, posture, and with action resembling Holmes tremor but smaller in amplitude and without a significant proximal component (Video S1 (11.4MB, mp4) , which is published in the online version of this article at ijmsc.org). The patient provided informed consent for video recording and for publication of this case report.
Magnetic resonance imaging (MRI) of the brain (done without contrast due to her pregnancy) showed abnormal increased T2-weighted and fluid-attenuated inversion recovery signal involving the cortex, subcortical white matter, bilateral thalami, and brainstem (Figure 1). There were no abnormal findings on T1- or diffusion-weighted images.
Figure 1.
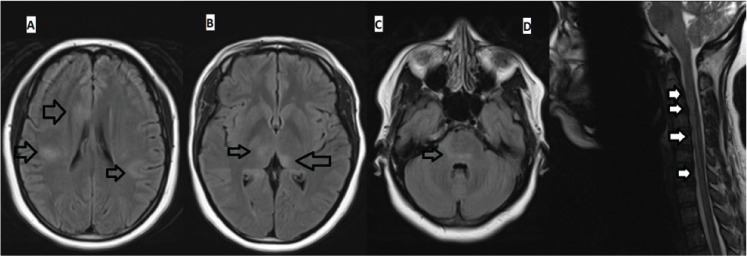
Axial fluid-attenuated inversion recovery magnetic resonance images (MRIs) of brain and sagittal T2-weighted MRI of cervical spine before treatment
Brain MRI shows multifocal large hyperintense lesions involving cortex and subcortical white matter (A), thalami (B), and pons (C) and cervical spine MRI shows a vague longitudinally extensive hyperintense lesion extending from the cervicomedullary junction (continuous with medullary/pontine lesion) to C7 level (D). Arrows point to the abnormal signals in the brain and cervical spinal cord.
Noncontrast MRI of the cervical spine showed vague longitudinally extensive hyperintense signal on T2-weighted and short-T1 inversion recovery images overlying the upper cervical cord extending to the medulla. There were no abnormal findings on T1-weighted images. No MRI of the thoracic and lumbar spine was performed.
Normal blood test results included antinuclear antibodies, anti-SSA and B, human immunodeficiency virus screen, immunoglobulin (Ig) levels, immunodeficiency profile, vitamin B12, copper, tuberculosis (QuantiFERON-TB Gold; Qiagen Inc, Germantown, MD), human T-cell lymphoma virus types 1 and 2, rubella IgM, and varicella-zoster virus polymerase chain reaction (PCR). Stool study findings were negative for ova and parasites, Clostridium difficile, and bacterial PCR panel. Results of nasopharyngeal studies were negative for influenza A and B PCR and respiratory syncytial virus PCR. Cerebrospinal fluid (CSF) analysis showed a red blood cell count of 2/μL, mixed pleocytosis (204 cells/μL, 35% lymphocytes, 35% neutrophils, and 30% monocytes), mild hyperproteinemia (protein level, 79 mg/dL), normal glucose level, normal IgG index and synthesis rate, and negative oligoclonal bands. The CSF viral PCR was negative for herpes simplex virus types 1 and 2, varicellazoster virus, cytomegalovirus, Epstein-Barr virus, human herpesvirus 6, West Nile virus, and adenovirus. Results of extensive bacterial and fungal studies were likewise negative, and findings from cytologic testing were normal.
The differential diagnosis included postvaccination acute disseminated encephalomyelitis (ADEM)–like multifocal demyelination and NMOSD. The patient was treated with five cycles of plasma exchange followed immediately by 2 days of intravenous immunoglobulin (IVIG), 1 g/kg per day, with subsequent initial improvement of tremor and leg weakness followed by resolution of vertigo, vomiting, and sphincteric dysfunction. Corticosteroids were not used to avoid their potential teratogenic effect in the first trimester and to avoid premature rupture of membranes.1,2 Treatment with plasma exchange was favored given its superior efficacy in NMOSD,3 and IVIG was added given the potential value in postvaccination demyelination.4 Repeated MRI after treatment showed complete resolution of the cervical lesion and near resolution of the brain abnormalities (Figure 2).
Figure 2.
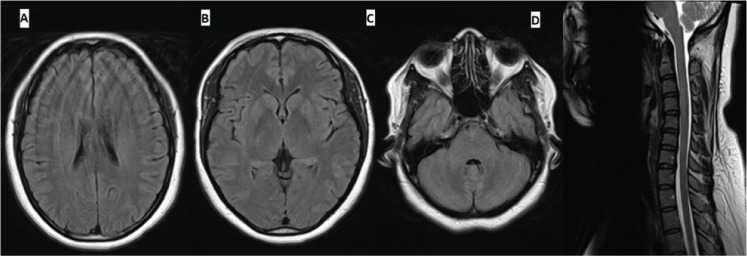
Axial fluid-attenuated inversion recovery magnetic resonance images (MRIs) of brain (A-C) and sagittal T2-weighted MRI of cervical spine (D) after treatment
After treatment (47 days after initial MRI), MRIs show resolution of abnormal signals.
The patient developed vaginal bleeding associated with reduction in beta human chorionic gonadotropin during her hospital admission, and at approximately 6 weeks of gestation she was determined to have a failing early pregnancy. No fetal testing was completed, and the exact cause of the miscarriage is unknown but was thought to be likely spontaneous in nature due to advanced maternal age and possible ectopic pregnancy. Intrauterine pregnancy was never confirmed because four repeated obstetric ultrasounds during admission did not demonstrate an intrauterine fetal sac and instead showed multiple fibromas in the uterus.
Serum AQP-4 IgG results by cell-based assay were negative, but serum MOG IgG values were elevated at a titer of 1:20, supporting the diagnosis of anti-MOG NMOSD or ADEM-like disease (but without encephalopathy). The patient was completely back to baseline with normal neurologic examination findings when she was seen in follow-up 3 months after discharge (Video S2 (4.8MB, mp4) ). Repeated MOG IgG values 6 months after presentation were negative, supporting low likelihood of recurrence and probable postvaccination monophasic disease.5 Visual evoked potentials and optical coherence tomography were not ordered given the absence of visual symptoms and the low likelihood of multiple sclerosis (MS) and recurrent NMOSD in view of transient MOG-IgG positivity.
Review Methods
To obtain current and relevant data, including detailed clinical information for each case, we searched the PubMed database from January 2008 through December 2018 for full-text case reports and case series in English. We queried the terms vaccination, measles mumps rubella/MMR, hepatitis B, influenza, human papillomavirus/HPV, with central demyelination, optic neuritis, transverse myelitis, neuromyelitis optica, NMO, NMOSD, MOG, acute disseminated encephalomyelitis/ADEM, encephalitis, and multiple sclerosis. The initial search yielded 169 results. Based on the criteria mentioned previously herein, we excluded 94 results (three were review articles or articles studying large populations without detailed clinical information on each individual case, and 91 articles were focused on peripheral rather than central demyelination or on basic science). The remaining 75 articles were filtered for repeats. Of the articles then left, we had 58 articles featuring 72 unrepeated cases to examine.
Literature Review
The possible relationship between vaccination and demyelinating diseases, including MS, has been frequently cited in the literature. It is important to note, and is beyond the scope of this article, that there is no clear evidence for a causal link between vaccination and development/relapse of MS, only a temporal association. The influenza and human papillomavirus vaccines are among the most commonly reported vaccinations linked to central nervous system demyelination.6,7 The incidence of postvaccination central demyelination is low: approximately 0.1 to 0.2 per 100,000 vaccinated individuals eventually show signs of and are diagnosed as having ADEM or ADEM-like conditions, so clearly the benefit of preventing serious infections and infection-triggered autoimmune attacks outweighs the risk of the rare postvaccination events.8
There have been several reports raising concerns that vaccines may trigger demyelinating events or cause or exacerbate MS.9 The pathophysiology behind this potential connection is not fully known, but several theories have been proposed. One theory suggests that molecular mimicry (cross-reaction between vaccine antigens and myelin proteins) could trigger autoimmune demyelination.10 Another theory proposes that because upper respiratory tract and other infections are known risk factors for MS relapses, vaccines could heighten the risk of central nervous system demyelination through a similar mechanism induced by infection.11 Some of the specific mechanisms involved in the pathogenesis include expansion and stimulation of autoreactive T-cell clones, enhanced antigen presentation, and epitope spreading.11 Vaccinations can also trigger peripheral demyelination and other autoimmune neurologic conditions, such as chronic inflammatory demyelinating polyneuropathy and myopathies, and exacerbate preexisting conditions such as myasthenia gravis.12,13
There have been several case reports in the literature depicting a temporal relationship between vaccination and central demyelination. Table S1 (366.4KB, pdf) presents complete clinical data for patients from these case reports in the past 10 years (2008–2018) regarding their demographic features, imaging findings, CSF results, treatment, and prognosis. See the list under Table S1 (366.4KB, pdf) for full citations of these case reports.
There were 58 studies encompassing 72 patients. See Table 1 for a clinical summary of the reported cases. Some patients received more than one vaccine before the demyelinating event, and some patients displayed multiple demyelinating syndromes. The mean time to event after vaccination was 25.8 days. The overall prognosis was excellent, including spontaneous improvement in seven patients without treatment and partial or complete response to corticosteroids in most of the remaining patients.
Table 1.
Clinical summary of all 72 reported cases of postvaccination demyelination, 2008–2018
| Clinical data | Patients, No. (%) |
|---|---|
| Vaccine type | |
| Influenza | 29 (40.3) |
| HPV | 20 (27.8) |
| DTAP/TDAP | 4 (5.5) |
| MMR | 4 (5.5) |
| Hepatitis B | 3 (4.2) |
| Yellow fever | 3 (4.2) |
| Hepatitis A | 2 (2.8) |
| Meningococcal | 2 (2.8) |
| Japanese encephalitis | 2 (2.8) |
| Varicella-zoster | 2 (2.8) |
| Oral polio | 1 (1.4) |
| Rabies | 1 (1.4) |
| Typhoid | 1 (1.4) |
| Pneumococcal | 1 (1.4) |
| Clinical presentation | |
| ADEM | 32 (44.4) |
| Optic neuritis | 19 (26.4) |
| Transverse myelitis | 10 (13.9) |
| NMOSD | 9 (12.5) |
| Other CIS | 4 (5.5) |
| MS relapse | 3 (4.2) |
| Treatment | |
| Corticosteroids alone | 48 (66.7) |
| Corticosteroids + IVIG | 8 (11.1) |
| No treatment | 7 (9.7) |
| Corticosteroids + PLEX | 6 (8.3) |
| Corticosteroids + rituximab | 2 (2.8) |
| Corticosteroids + PLEX + rituximab | 1 (1.4) |
| Prognosis | |
| Any improvement | 65 (90.3) |
| Complete resolution | 33 (45.8) |
| No improvement or unknown outcome | 5 (6.9) |
Abbreviations: ADEM, acute disseminated encephalomyelitis; CIS, clinically isolated syndrome; DTAP/TDAP, diphtheria, tetanus, and pertussis; HPV, human papillomavirus; IVIG, intravenous immunoglobulin; MMR, measles, mumps, and rubella; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorder; PLEX, plasma exchange.
Ten patients (13.9%) had clinical or radiologic recurrence spontaneously or after repeated exposure to the vaccine or tapering of the corticosteroids. Of the patients with recurrent disease, three were eventually diagnosed as having new NMOSD with AQP-4, three with multiphasic ADEM or ADEM flare (one was anti-MOG positive), two with new relapsing MS, one with recurrent optic neuritis, and one with optic neuritis followed by ADEM 2 months later. In total there were five patients with MS in the reviewed papers: three with preexisting clinically isolated syndrome who met the criteria for MS after a postvaccination second relapse and two whose first relapse started after the vaccine then went on to develop MS later based on clinical or radiologic criteria. Interestingly, there were more postvaccination NMOSD cases than MS cases: nine patients, including four whose first relapse started after vaccination then went on to develop de novo AQP-4–positive disease (one of the four patients did not have documented relapses but tested positive for AQP-4 antibody), four with monophasic opticospinal attack with negative or unknown AQP-4, and one with preexisting NMOSD with AQP-4 who relapsed after pneumococcal vaccination.
Discussion
There are several interesting clinical aspects to the present case. In this patient, pregnancy state might have contributed to disease onset because pregnancy is known to trigger NMOSD relapses, especially in anti-MOG–positive patients.14,15 The combination of pregnancy and recent vaccination likely resulted in a heightened immune state culminating in an autoimmune attack on the central nervous system possibly facilitated by underlying predisposition to autoimmunity. The fact that the patient was an immigrant from Africa necessitated more extensive infectious evaluation, especially in view of the high CSF cellularity. Presentation with Holmes-like tremor secondary to MOG-related brainstem encephalitis has not been reported previously, although a patient with nonspecific tremor type has been reported by Ramanathan and colleagues.16 This adds to the growing literature of autoimmune movement disorders. Most previous anti-MOG patients and patients with postvaccination demyelination were treated with corticosteroids with or without additional rescue therapy. The patient responded fully to plasma exchange and IVIG without corticosteroids, indicating that alternative rescue therapy is feasible in postvaccination demyelination if corticosteroids are contraindicated or not tolerated. It is unclear whether the hormonal changes related to miscarriage have influenced the relapse outcome or contributed to the dramatic improvement seen in this patient.
In addition to the single pediatric patient with anti-MOG multiphasic ADEM included in the literature review, two additional adults with vaccine-related anti-MOG NMOSD were described by Jairus and colleagues15 in their anti-MOG case series. Both patients had their first attack shortly after vaccination and then went on to develop recurrent disease, including recurrent transverse myelitis, optic neuritis, and brainstem encephalitis. One of the two patients had an excellent response to intravenous methylprednisolone and plasma exchange, and the other had only a partial response despite additional treatment with rituximab and cyclophosphamide. Both patients had received the tetanus, diphtheria, and pertussis vaccine, but one had also received the influenza and polio vaccines. The fact that all previous postvaccination MOG-positive cases were recurrent suggested that vaccination might result in unmasking of a predetermined disease, but the present case shows that NMOSD-like disorder after vaccination can be associated with a monophasic course and transient anti-MOG positivity. Mealy and colleagues17 studied the link between vaccination and NMOSD relapses in a multicenter retrospective study of primarily AQP-4–positive patients (11% seronegative, no MOG-positive cases). They found that patients with NMOSD who were not maintained on immunosuppressive therapy were more likely to relapse within 3 months of vaccination, whereas patients receiving long-term immunosuppression were protected against postvaccine relapses. Indeed, patients on immunosuppressive therapy who received routine vaccination had lower relapse rates than those who did not receive vaccination, likely secondary to reduction in infection-related relapses. This led the authors to suggest that vaccination with inactivated vaccines in patients with NMOSD who are receiving immunosuppressive therapy should be encouraged. The use of live attenuated vaccines in immunosuppressed patients is not safe and should generally be avoided.18 Patients with untreated NMOSD should consider starting immunosuppressive therapy before receiving killed or inactivated vaccines to reduce the likelihood of postvaccination relapses.17 Note, however, that patients receiving immunosuppressive therapy may not mount an immune response against inactivated vaccines.19 The value of vaccination in treated patients with anti-MOG NMOSD could be inferred based on the reviewed case reports and the studies in AQP-4–positive patients. This is opposite to MS, in which the link between relapses and vaccination is thought to be less strong according to a recent review.20 An update of the American Academy of Neurology guidelines on immunization in patients with MS has recently been published.21 In conclusion, untreated patients with anti-MOG NMOSD, similar to AQP-4–positive patients, may be at increased risk of relapse after vaccination based on a few case reports. Systematic research is needed to confirm these preliminary findings and to explore the value of vaccination in treated patients.
One limitation of this study is only case reports and case series being included. The reason for this was that we wanted to break down specific factors in each case (demographic characteristics, timing of vaccination from first dose and last dose before symptoms, the specific clinical syndrome, imaging and CSF findings, prognosis, treatment, etc), and we would not have had all this detailed information available for each individual in population-based studies. Another limitation is that there are several articles that we did not include in the review because of lack of complete clinical information or overlapping cases. We believe that, despite this, the review provides relevant clinical data and the case reports we examined were representative of the wide spectrum of postvaccination demyelination.
PRACTICE POINTS
There have been frequent instances recorded in which vaccination was followed by monophasic demyelinating events or unmasking of a chronic relapsing disease.
Neuromyelitis optica spectrum disorder (NMOSD) attacks have been reported to occur in the setting of pregnancy and after vaccinations.
Both aquaporin-4–positive and myelin oligodendrocyte glycoprotein–positive cases have been reported after vaccination.
Patients with NMOSD receiving immunosuppressive therapy may be protected against vaccine-related relapses.
Supplementary Material
Financial Disclosures
Dr Abboud is a consultant for Biogen, Genentech, Alexion, Viela Bio, and Genzyme and receives research support from Novartis and Roche. The other authors declare no conflicts of interest.
Funding/Support
None.
References
- 1.Park-Wyllie L, Mazzotta P, Pastuszak A et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62:385–392. doi: 10.1002/1096-9926(200012)62:6<385::AID-TERA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 2.Guller S, Kong L, Wozniak R et al. Reduction of extracellular matrix protein expression in human amnion epithelial cells by glucocorticoids: a potential role in preterm rupture of the fetal membranes. J Clin Endocrinol Metab. 1995;80:2244–2250. doi: 10.1210/jcem.80.7.7608287. [DOI] [PubMed] [Google Scholar]
- 3.Abboud H, Petrak A, Mealy M et al. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2016;22:185–192. doi: 10.1177/1352458515581438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchioni E, Marinou-Aktipi K, Uggetti C et al. Effectiveness of intravenous immunoglobulin treatment in adult patients with steroid-resistant monophasic or recurrent acute disseminated encephalomyelitis. J Neurol. 2002;249:100–104. doi: 10.1007/pl00007836. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira LM, Apóstolos-Pereira SL, Pitombeira MS, Bruel Torretta PH, Callegaro D, Sato DK. Persistent MOG-IgG positivity is a predictor of recurrence in MOG-IgG-associated optic neuritis, encephalitis and myelitis. Mult Scler. 2019;25:1907–1914. doi: 10.1177/1352458518811597. [DOI] [PubMed] [Google Scholar]
- 6.Karussis D, Petrou P. The spectrum of post-vaccination inflammatory CNS demyelinating syndromes. Autoimmun Rev. 2014;13:215–224. doi: 10.1016/j.autrev.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Huynh W, Cordato DJ, Kehdi E et al. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15:1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menge T, Hemmer B, Nessler S et al. Acute disseminated encephalomyelitis: an update. Arch Neurol. 2005;62:1673–1680. doi: 10.1001/archneur.62.11.1673. [DOI] [PubMed] [Google Scholar]
- 9.DeStefano F, Verstraeten T, Jackson LA et al. Vaccinations and risk of central nervous system demyelinating diseases in adults. Arch Neurol. 2003;60:504–509. doi: 10.1001/archneur.60.4.504. [DOI] [PubMed] [Google Scholar]
- 10.Liblau R, Gautam AM. HLA, molecular mimicry and multiple sclerosis. Rev Immunogenet. 2000;2:95–104. [PubMed] [Google Scholar]
- 11.Langer-Gould A, Qian L, Tartof SY et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71:1506–1513. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- 12.Brostoff JM, Beitverda Y, Birns J. Post-influenza vaccine chronic inflammatory demyelinating polyneuropathy. Age Ageing. 2008;37:229–230. doi: 10.1093/ageing/afm151. [DOI] [PubMed] [Google Scholar]
- 13.Seok HY, Shin HY, Kim JK et al. The impacts of influenza infection and vaccination on exacerbation of myasthenia gravis. J Clin Neurol. 2017;13:325–330. doi: 10.3988/jcn.2017.13.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shosha E, Pittock SJ, Flanagan E et al. Neuromyelitis optica spectrum disorders and pregnancy: interactions and management. Mult Scler. 2017;23:1808–1817. doi: 10.1177/1352458517740215. [DOI] [PubMed] [Google Scholar]
- 15.Jarius S, Ruprecht K, Kleiter I et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients, part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanathan S, Mohammad S, Tantsis E et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. 2018;89:127–137. doi: 10.1136/jnnp-2017-316880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mealy MA, Cook LJ, Pache F et al. Vaccines and the association with relapses in patients with neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2018;23:78–82. doi: 10.1016/j.msard.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Lopez A, Mariette X, Bachelez H et al. Vaccination recommendations for the adult immunosuppressed patient: a systematic review and comprehensive field synopsis. J Autoimmun. 2017;80:10–27. doi: 10.1016/j.jaut.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Friedman MA, Winthrop KL. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheum Dis Clin North Am. 2017;43:1–13. doi: 10.1016/j.rdc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2017;264:1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 21.Farez MF, Correale J, Armstrong MJ et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93:584–594. doi: 10.1212/WNL.0000000000008157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


