Abstract
Today, binaural and monaural beats are offered over the Internet or by mental health institutes to improve wellbeing or cognitive functioning. This improvement is explained by the assumption that the brain adapts its brainwave frequency to the frequency of the auditory beat. The present study examined the effects of binaural and monaural beat stimulation on attention and working memory in high and low emotional participants. A group of 24 participants (16 females, 8 males) between 19 and 31 years old (M = 22.33, SD = 3.42) performed a Flanker task to measure attention and a Klingberg task to measure working memory while listening to white noise (WN), 40 Hz gamma binaural beat (BB) and 40 Hz gamma monaural beat (MB). Speed of performance on all three levels of difficulty of the Flanker attention task was faster under the BB and MB condition than under WN. No differences were found between BB and MB conditions. With respect to the quality of performance on the Flanker attention task and the Klingberg working memory task no significant differences under the WN, MB, and BB condition were found. Finally, as participants with low or high emotionality did not respond differently to BB and MB under any of the conditions, effects of BB and MB seem similar in high and low emotional participants. The present study supports the notion that faster attention processing may equally be attributed to the influence of BB and MB. Further research is recommended to gain more insight in the role of factors such as duration of stimulation of BB and MB, frequency range, most appropriate carrier tones, and the role of personality traits.
Keywords: binaural beats, monaural beats, attention, auditory beat stimulation, emotionality, memory, Flanker task, Klingberg task
Introduction
Nowadays, society is characterized by a lot of distraction due to the use of electronic devices like smartphones. As a consequence, people are searching for methods to help them focus. One frequently used method for increasing focus is the creation of a nondistractive environment, for example listening to specific audio files which can create a focused or relaxed state of mind. These audio files usually contain music or nature sounds, accompanied by tones that aim to influence brainwave activity in such a way that listeners are better able to focus. This method, called auditory beat stimulation (ABS), uses pulsating auditory stimuli to induce a frequency-following brainwave response. Many years ago, it was already suggested that ABS can elicit entrainment of brainwave activity (Lane, Kasian, Owens, & Marsh, 1998). The assumption is that the brain can adapt its brainwave frequency to the frequency of the auditory beat by a synchronization process between neural activity and the auditory stimuli (Wahbeh, Calabrese, & Zwickey, 2007). For example, when listening to a 20 Hz binaural beat, the frequency of brainwave activity should change to 20 Hz.
Long before the application of ABS, dynamic attending theory (DAT) proposed synchronization of endogenous perceptual rhythms with temporally structured sequences of external stimuli, generating expectancies for future events (Jones, 1976). This theory further addressed the issue of how neural rhythms may be exploited by an organism to enable attentional coordination with the dynamic external world (Large & Jones, 1999).
Later, electroencephalography (EEG) and magnetoencephalography (MEG) studies have directly examined the role of endogenous oscillation. For instance, MEG and EEG studies revealed that fluctuation in induced beta- and gamma-band power synchronized with periodic and metrical rhythms, revealing sensory and anticipatory responses to tones (Fujioka, Trainor, Large, & Ross, 2009; Snyder & Large, 2005). Additional EEG studies, using the steady-state evoked potential (SSEP) found that a periodic rhythm produced a sustained response in the delta band and meter imagery stimulated an additional subharmonic resonance corresponding to the metric interpretation (Nozaradan, Peretz, & Mouraux , 2012a). In addition, complex rhythms were found to elicit multiple SS-EPs in the EEG spectrum at frequencies corresponding to the rhythmic pattern envelope. Moreover, the amplitude of the SS-EPs at pulse and meter frequencies was selectively enhanced, suggesting a role for neural oscillations in pulse and meter induction (Nozaradan, Peretz, & Mouraux, 2012b). These studies of MEG- and EEG- recorded delta, beta and gamma band responses to auditory rhythms confirm predictions of the DAT. Thus, it can be concluded that the hypothesis of neural resonance to musical rhythms originally proposed by Jones (1976) has been supported by the results of subsequent behavioral and electrophysiological studies (Jones, 2009; Large, Herrera, & Velasco, 2015).
It has been suggested that entrainment of ongoing neural oscillations may be a potent mechanism for the brain to generate temporal predictions and to aid active perception (Obleser, Henry, & Lakatos, 2017). Entrainment to music is shared by humans of all cultures and involves a large network of brain structures. It is a highly complex activity, which involves auditory, visual, proprioceptive and vestibular perception, also requiring attention, motor synchronization, performance, and coordination. (Nozaradan, 2014). However, the concept of rhythm is not well defined yet. Although rhythm is frequently assumed to refer to isochrony, that is, strict regularity, many studies on neural entrainment did not use isochronous sequences but allowed for substantial jitter between distinct stimuli (Obleser et al., 2017).
Because of the hypothesized capacity of the brain to synchronize its brainwave frequencies with the rhythm of periodic external stimuli, ABS has been suggested to elicit particular states of awareness, resulting in an increase of people’s cognitive functioning or a change in state of mind (Marsh, Worden, & Smith, 1970; Mathewson et al., 2011; Will & Berg, 2007). Three types of ABS can be distinguished: isochronic tones, monaural beats (MBs) and binaural beats (BBs). Isochronic tones are tones that turn on and off with evenly-spaced intervals, creating a beat with a frequency depending on the length of the intervals. Isochronic tones are similar to monaural tones in that they are combined into a cohesive listening experience before they reach the ear. Because of their predictable patterns, isochronic tones are supposed to be a hallmark of rhythm making them an effective type of beat in brainwave entrainment (Obleser et al., 2017).
In contrast, MBs are generated by simultaneously presenting sine waves of two different, neighbouring frequencies to both ears and BBs are generated by presenting the sine waves of neighbouring frequencies to each ear separately. Unlike with BBs, MBs also use two different frequencies (e.g., 400 Hz and 440 Hz, but they are merged together within one headphone speaker and presented to one or both ears at the same time (Becher et al., 2015; Chaieb, Wilpert, Reber, & Fell, 2015; Oster, 1973). The presentation of two frequencies results in a perceived single beat in a frequency that equals the difference between the two beats. For example, when a 380 Hz beat and a 420 Hz beat are presented at the same time, a beat of 40 Hz is perceived (Colzato, Barone, Sellaro, & Hommel, 2017; Schwarz & Taylor, 2005).
There is a slight difference in neurophysiological processing of BBs and MBs: BBs are referred to as central beats because the interaction of the auditory stimuli most likely occurs in the superior olivary nuclei in the brainstem (Draganova, Ross, Wollbrink, & Pantev, 2008). Neurons in the brainstem are sensitive to phase shifts between both ears. When these phase shifts occur, brainstem neurons fire action potentials which correspond in rate to the phase difference between both ears (Chaieb et al., 2015). In MBs, the auditory stimuli interact in the cochlear and are therefore called peripheral beats (Draganova et al., 2008). From the cochlear, the auditory stimuli are relayed to the cochlear nucleus on the brainstem and to the auditory cortex.
Currently, it is still unclear whether presenting BBs leads to a frequency following response of the presented frequencies or whether it evokes different responses in the brain. Gao et al. (2014) presented BBs in delta (1 Hz), theta (5 Hz), alpha (10 Hz), and beta (20 Hz) band frequencies to 13 healthy participants. Each type of BB was presented for 5 min at a time, with 2-min breaks in between. Participants were asked to keep their eyes closed while listening to the BB, without performing any task; EEG was recorded during the whole experiment. Gao et al. found an increase in relative power (RP) of theta and alpha band EEG and a decrease in RP of beta band EEG after presenting delta and alpha band BB. Furthermore, they found a decrease in RP of beta band EEG after presenting theta band BB and they found a decrease in RP of theta band EEG after presenting beta band BB. These results indicate that there was no frequency following response of the presented BB. In addition, connectivity changes in the brain were examined.I Increased anterior-posterior intra-cerebral connectivity in the theta band was observed under delta, alpha, and beta BBs. These effects suggest that BBs could affect functional brain connectivity, but not necessarily by inducing a frequency-following response.
A small number of studies examined the relationship ABS and attention in healthy people. Results of these studies are conflicting. For instance, Lane et al. (1998) studied whether BBs affected performance on a vigilance task. On three different days, 29 volunteers listened to pink noise, beta (16 and 24 Hz) and theta/delta (1.5 and 4 Hz) for 30 min while performing a vigilance task. Results showed that in the delta BB condition, more targets were detected and fewer errors were made, compared to the theta/delta BB condition. This indicates that high frequency BBs seem to affect vigilance more than low frequency BBs. Additionally, the monotonous task was found to lead to negative changes in mood in all conditions, but these were less pronounced when beta range BBs were presented, as compared to theta or delta range BBs. The authors suggest that beta range BBs may in particular reduce the negative mood effects when performing an unexciting task (Lane et al., 1998). In a more recent study, high frequency (40 Hz) BBs were presented to 36 students (22 female, 14 male; aged 18–28 years old) and it was shown that the presentation of these BBs increased attentional focusing (Colzato et al., 2017). In contrast, in a placebo controlled study in healthy adults, no significant differences were found between the BB group and control group on any of the presented attention tasks (Crespo, Recuero, Galvez, & Begona, 2013).
In addition, the relationship between ABS and memory has been studied, with contrasting results. In one study, 4 healthy participants listened to a 7 Hz (theta) BB for 30 min with an overlay of rain sound, or to rain sounds only (Wahbeh, Calabrese, Zwickey, & Zajdel, 2007). Results showed that immediate recall memory was significantly decreased in the experimental condition compared to the control condition, which suggests a negative effect of BB on memory. In contrast, participants undergoing a 12-minute BB stimulation of 9.55 Hz achieved a significant increase in the capacity of their working memory (Kraus & Porubanová, 2015) and a series of 5 Hz BBs twice a day for 15 days resulted in improvement of immediate word recall (Ortiz et al., 2008). Another controlled study showed that listening to 15 Hz BBs increased response accuracy on a visual-spatial working memory task, while the three control conditions and 5 Hz and 10 Hz BBs all decreased accuracy (Beauchene, Abaid, Moran, Diana, & Leonessa, 2016).
With respect to the effects of BB on cognition, most evidence available concerns the effects of BB on attention and memory. Present meta-analytic evidence indicates that alpha, beta, gamma, and theta BB exposure improves the performance on attention and memory tasks (Garcia-Argibay, Santed, & Reales, 2018).
In addition, a number of studies exploring the effectiveness of BBs on anxiety levels were included in the meta-analysis of Garcia et al. (2018). The included studies of Padmanabhan, Hildreth, and Laws (2005), Wahbeh, Calabrese, Zwickey et al. (2007) revealed that thetafrequency BB reduced anxiety scores compared to the control group, and lead to an increase in quality of life scores. McConnell, Froeliger, Garland, Ives, & Sforzo (2014), who recorded heart rate variability when participants were exposed to theta-frequency BBs, found differences in the sympathetic and parasympathetic activities compared to the control group, which, in turn, indicated a greater self-reported relaxation. Isik, Esen, Buyukerkmen, Kilinc, & Menziletoglu (2017) demonstrated that a 10 min exposure to theta-frequency BBs reduced anxiety levels prior to a dental operation compared to the control group that listened to a blank tape. The results of the meta-analysis on these studies confirmed the efficacy of BBs in the reduction of anxiety scores after delta/theta exposure. Other studies, which focused primarily on brain activity during high levels of anxiety, mention relatively reduced theta activity and relatively increased beta activity (Engelbregt, Keeser, Promes, Verhagen-Schouten, & Deijen, 2012; Guevara et al., 2018; Kara & Polo, 2014; Schicho & Pogarell, 2014). It is therefore important that while studying BB, researchers pay attention to possible side effects. For example, researchers studying the effects of 40 Hz frequency BB should be attentive of possible increases in anxiety levels of participants.
The aim of the current study was to establish the effects of a 40 Hz gamma frequency MB and BB on attention and memory. We focused on attention and memory in healthy participants because the effects of BBs on these cognitive skills have been previously studied mainly in healthy participants, which allows for obtaining more conclusive evidence. Moreover, as BBs have been found to decrease anxiety levels, it may well be true that the effects of BBs on cognition may be different for participants with different levels of anxiety. Therefore, we included measurements of the Emotionality scale of the HEXACO-SPI, high scores indicating fear of physical dangers, anxiety in response to life's stresses, need for emotional support from others, empathy, and sentimental attachments with others (De Vries, Ashton, & Lee, 2009).
In our study, we used white noise as the control condition because, by definition, its power spectral density is constant and does not depend on frequency (Garcia-Argibay et al., 2018). However, white noise has been found to differentially affect cognitive performance. For instance, white noise improved the performance of executive function tasks in subattentive children and worsened the memory and executive performance in superattentive children, while the performance of normal-attentive children was unaffected (Helps, Bamford, Sonuga-Barke, & Soderlund, 2014). With respect to the pleasantness of the auditory environment, it was found that task performance was closely related to the participants’ feelings of pleasantness of the noise. In healthy participants, short-term memory task performance increased when they felt the white noise was pleasant while performance decreased in the group that felt the noise was unpleasant (Hiwa, Katayama, & Hiroyasu, 2018).
To control for these unpredictable effects of white noise in the control condition, we masked the MB and BB conditions with white noise. In this way, the possible positive or negative cognitive effect of white noise could be assumed to be present in all three conditions. However, as a BB masked in pink noise or white noise was found to have similar effects on cognition as an unmasked BB (Garcia-Argibay et al., 2018), the present masking of ABS could be considered not to alter the potential cognitive effects.
All in all, by comparing MBs and BBs against white noise, we aimed to compare two conditions with active auditory stimulation in which auditory beats supposedly enhance the performance and white noise acts as a neutral stimulus (Goodin et al., 2012). Notably, a number of studies in this field have also used white noise as a control condition (Dabu-Bondoc, Vadivelu, Benson, Perret, & Kain, 2010).
We chose to examine the effects of this frequency band because (a) 40 Hz is related to the highest level of consciousness (Kaiser & Lutzenberger, 2005), (b) it seems to have maximal steady state responses (Schwarz & Taylor, 2005), (c) it may enhance information transmission from one brain region to another (Fell & Axmacher, 2011), and (d) it has been shown to increase attentional focusing and visual working memory (Colzato et al., 2017).
Additionally, we hypothesized that participants with high emotionality scores would benefit more from ABS based on the findings cited above that the level of anxiety declined during or after ABS.
Methods
Participants
Twenty-four healthy participants (8 males, 16 females) with higher education, aged between 18 and 35 years (M = 22.3; SD = 3.42), participated in the study. Participants were recruited by using the social networks of the experimenters. All had normal or corrected-to-normal sight and none of the participants reported a history of hearing problems or psychiatric disorders. They were not allowed to use medication or drugs before or during the experiment.
Instruments
The Emotionality subscale of the HEXACO-SPI personality questionnaire (De Vries et al., 2009) was used. This self-reported personality questionnaire is a Dutch adaptation of the HEXACO model (Lee & Ashton, 2004).
Two cognitive tests were presented on an tablet, a Flanker task and a Klingberg test. The Flanker task is an attention and inhibition test based on the Eriksen Flanker task (Eriksen & Eriksen, 1974). Instead of using letters, as in the original Flanker Task, arrows were used as target stimuli. The test consisted of three stages. Before each stage started, written instructions appeared on the screen, followed by practice trials. In the first stage, five green arrows appeared on the screen. Participants were instructed to press one of the two arrow-shaped buttons on the bottom of the screen that pointed in the same direction as the middle arrow. In the second stage, five red arrows appeared. This time, the participants were instructed to press the button that pointed in the opposite direction as the middle arrow. In the third stage, five arrows appeared again, this time either green or red. Participants were instructed to press the button that pointed in the same direction as the middle arrow if the arrows were green, or the button that pointed in the opposite direction as the middle arrow if the arrows were red. In all three stages, the participants were instructed to press the correct button as fast as possible. The reaction times (RTs) and number of errors were taken as output variables.
The Klingberg task is a visuospatial working memory test, based on the Corsi block-tapping test (Bouma, Mulder, Lindeboom, & Schmand, 2012) and consisted of two series. In the first series, participants were presented with a 4 × 4 square grid. A yellow dot appeared for 2250 ms in random order in two different squares, with an interstimuli interval of 750 ms. Participants were then asked to follow the pattern by tapping the same squares in the same order. If the pattern was replicated correctly, the yellow dot appeared two times again. The pattern increased in length and difficulty in subsequent stages after correctly replicating the sequences of at least three out of four trials within one stage. The task ended when two mistakes were made within the same stage. The maximum reached stage was taken as the output variable. In the second series of the test, squares needed to be tapped in a reversed order to the one they were presented in. Again, by tapping the squares in the correct order, higher, more difficult stages could be achieved, but the test ended when two mistakes were made in the same stages. The maximum reached stage was taken as the output variable again.
Auditory Beats
The white noise, MBs and BBs used in the experiment were created by the IT department of the Vrije Universiteit. The sounds were presented on a tablet, through wired headphones with separate channels for right and left ear. Auditory volume was adjusted to a comfortable listening level (speech volume). The selected frequencies for the MB and BB were 440 Hz and 480 Hz. As a consequence, the perceived frequency was 40 Hz. To create the MB, both frequencies were broadcast through both channels. To create the BB, 440 Hz was broadcast through one channel and 480 Hz was broadcast through the other channel. The BB and MB were masked with white noise.
Procedure
Participants were instructed not to drink any coffee or tea or other drinks that contain caffeine at least 2 hr prior to testing. Testing took place in a quiet environment in a home setting. Participants were informed about a sham condition, but not about the presence of BBs and MBs. First, they were asked to fill out a paper version of the HEXACO-SPI. Subsequently, participants put on the headphones and their age and gender were recorded on the tablet. Auditory volume was adjusted to a comfortable listening level (speech volume). We used a within-subject cross over design, that is, all participants performed under all three conditions: white noise (WN), BB (40 Hz; 440 and 480 Hz), and MB (40 Hz; 440 and 480 Hz).
Participants started with listening to white noise. Then, the BB and MB condition were presented. The order of BB and MB was randomly counterbalanced across participants; one half started with the BB, the other half with the MB. All conditions started with the Flanker task, followed by the Klingberg task. Conditions were separated by a 2 min break. To minimize learning effects, there were three parallel versions of the Klingberg task. The Flanker task was randomly predefined. When participants had finished the tasks, they were asked to report ow they had experienced the tasks and the sounds. Testing took about one hour.
Written informed consent was obtained from all participants. This study was positively assessed by the Scientific and Ethical Review Committee of the Faculty of Behavioral and Movement Sciences of the Vrije Universiteit.
Statistical Analysis
Before testing the hypotheses, we assessed the normality of the dependent variables with the Kolmogorov-Smirnov test. Its results were significant for almost all variables, indicating that normality was not achieved. Therefore, we decided to apply the nonparametric Friedman test of differences among repeated measures to compare the total group scores under each condition (WN, MB, and BB). In the cases the Friedman test was significant, pairwise comparisons between conditions were made by means of the Wilcoxon signed ranks test and effect sizes were calculated (r = z / sqrt(N)).
Thereafter, we used the median split to make a distinction between low- and high-emotional people. To avoid large differences in gender in these two groups, we made this distinction between low and high emotionality separately for men and women. We merged high-emotional men and women and low-emotional men and women, establishing groups of high- and low-emotional participants. Friedman tests (and, if appropriate, Wilcoxon signed ranks tests) were ran separately for the low- and high-emotional groups. The dependent variables were: (a) the number of errors and mean RTs for each condition (WN, MB, BB) and for each level of the Flanker Task, and (b) the maximum reached stage of the Klingberg task for each of the three conditions and the two series. Data were analysed using SPSS version 22.0.
Results
First, to determine the presence of order effects (the BB or MB condition), Wilcoxon signed ranks tests were applied on all dependent variables separately for the BB-MB and the MB-BB order. There were no significant effects between MB and BB in orders of presentation (p > .05), excluding the presence of learning effects between these conditions. Non-parametric Friedman tests of differences among repeated measures regarding the RTs of the Flanker task yielded significant effects for the lowest stage, χ2(2) = 16.08, p < .001, the medium stage, χ2(2) = 18.08, p < .001, and the highest stage of difficulty, χ2(2) = 21.70, p < .001). Regarding all stages of difficulty, the Wilcoxon signed ranks tests indicated significant differences between MB and WN as well as BB and WN conditions (MB vs. WN: Z = −3.86, p < .001, r = −0.79; BB vs. WN: Z = −3.74, p < .001, r = −0.76 for the lowest stage, MB vs. WN: Z = −3.09, p = .002, r = −0.62; BB vs WN: Z = −3.49, p < .001, r = −0.71 for the medium stage, and MB vs. WN: Z = −3.73, p < .001, r = −0.76; BB vs. WN: Z = −3.85, p < .001, r = −0.78 for the highest stage). All significant differences indicated a slower RT under the WN condition as compared to the MB and BB conditions. No significant differences between the MB and the BB were found. Results are shown in Figure 1. We performed the same analyses regarding the RTs of the Flanker task separately for the low- (n = 12) and high- (n = 12) emotional groups. In the low-emotional group, Friedman tests yielded significant effects for the lowest stage, χ2(2) = 6.17, p = .046, the medium stage, χ2(2) = 8.67, p = .013, and the highest stage of difficulty, χ2(2) = 12.20, p = .002). The Wilcoxon signed ranks tests indicated significant differences on all levels of difficulty between MB and WN as well as BB and WN conditions (MB vs. WN: Z = −2.35, p = .019, r = −0.68; BB vs. WN: Z = −2.43, p = .070, r = −0.76 for the lowest stage, MB vs. WN: Z = −1.25, p = .21, r = −0.36; BB vs. WN: Z = −2.20, p = .028, r = −0.63 for the medium stage, and MB vs. WN: Z = −2.60, p = .009 r = −0.75; BB vs. WN: Z = −2.93, p = .003, r = −0.85). Notably, for the medium stage, no significant difference was found between the MB and WN. All significant differences indicated a slower RT under the WN condition as compared to the MB and the BB conditions. No significant differences between the MB and the BB were found.
Figure 1.
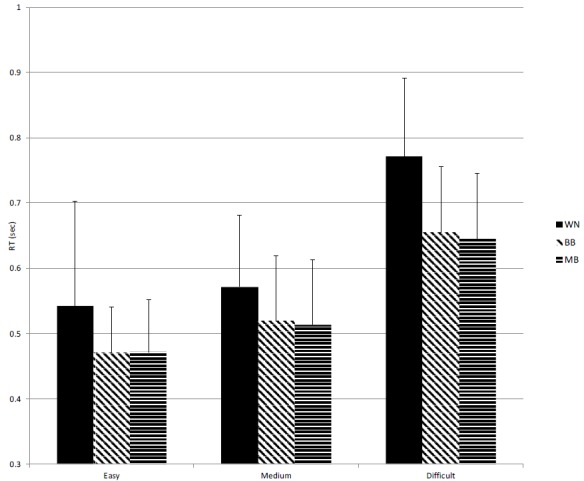
Median RTs and SDs under the white noise (WN), binaural beat (BB), and monaural beat (MB) condition on the three difficulty stages of the Flanker task.
Similarly, in the high-emotional group, Friedman tests yielded significant effects for the lowest stage, χ2(2) = 10.17, p = .046, the medium stage, χ2(2) = 10.50, p = .005, and the highest stage of difficulty, χ2(2) = 9.60, p = .008). The Wilcoxon signed ranks tests indicated significant differences on all stages of difficulty between MB and WN as well as BB and WN conditions (MB vs. WN: Z = -2.30, p = .003, r = −0.86; BB vs. WN: Z = −2.28, p = .005, r = −0.81 for the lowest stage, MB vs. WN: Z = −2.98, p = .003, r = −0.86; BB vs. WN: Z = −2.67, p = .008, r = −0.77 for the medium stage, and MB vs. WN: Z = −2.70, p = .007, r = −0.78; BB vs. WN: Z = −2.67, p = .008, r = −0.77 for the highest level). All significant differences indicated a slower RT under the WN condition as compared to the MB and BB conditions. No significant differences between the MB and the BB were found. Notably, all effect sizes for significant effects were large according to Cohen’s classification of effect sizes, which is 0.1 for a small effect, 0.3 for a moderate effect, and 0.5 and above for a large effect (Cohen, 1992).
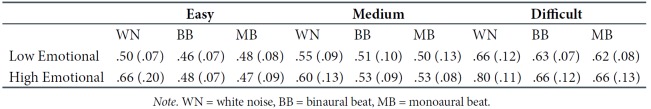
These analyses performed separately for low- (n = 12) and high- (n = 12) emotional participants resulted in similar effects of MB and BB on the RTs in the Flanker task as in the total group of participants (N = 24), except for one nonsignificant effect of MB versus WN for the medium stage of difficulty in the low-emotional group. Mann-Whitney U tests indicated that the RTs in the Flanker task for the low- and highemotional groups were not significantly different under the WN, MB, and BB conditions for any difficulty stage (ps > .05). The median RTs of the low- and high-emotional groups are shown in Table 1.
With respect to the quality of performance on the Flanker attention task and the Klingberg working memory task, no significant differences under the WN, MB, and BB conditions were found.
Discussion
The aim of the present study was to examine the effects of MB and BB stimulation on cognitive functioning, in particular attention and working memory. The expectation was that participants would perform better under MB and/or BB as compared to WN. In addition, the potential differential effects of MB versus BB were examined. With respect to personality characteristics, we expected that participants with higher levels of emotionality would benefit the most from MB and/or BB stimulation.
The present results indicate that with respect to attention, as measured by the Flanker task, speed of performance was higher in the MB and BB conditions, relative to WN. The calculated effect sizes indicate that all significant effects can be considered large.
In contrast to reduced RTs, the number of errors on the Flanker task was similar in all conditions. As a consequence, the increased speed of attention processes can be assumed not to be at the expense of an increase of the number of errors. This finding makes it plausible that the enhanced speed of performance under MB and BB reflects a higher cognitive efficacy.
Although the presence of learning effects cannot be excluded, it is unlikely. The Flanker task was presented in three blocks of progressively increasing difficulty stages. Each difficulty stage was preceded by practice trials. After completing the highest stage of difficulty, the participants had to start with the easiest stage in the next condition. The alternation of stages can be assumed to interfere with the occurrence of transfer of learning. Moreover, no significant order effects were present between MB and BB conditions.
The absence of effects of MB and BB on the quality of attention in the present study is also in line with previous studies which examined the relationship between ABS and attention (Crespo et al., 2013; Kennel, Taylor, Lyon, & Bourguignon, 2010). In these studies, no significant improvement was found in attention performance with BB stimulation. Kennel et al. (2010) suggested that the small sample size (10 participants in the experimental group) might be an explanation for not finding significant results. Crespo et al. (2013) attributed the results to the small sample size as well (20 participants in the experimental group) and to the short duration of stimulation (20 min). In the present study, a small sample size (24 participants) may have played a part as well, but the extent to which duration of stimulation plays a role is still unclear. Other authors, who did find a significant improvement in performance while listening to BBs, presented ABS for a relatively long duration (30 min on three different days, Lane et al., 1998). However, a long stimulation period (20 min, three times a week for three weeks) has been found not to yield significant results (Kennel et al., 2010), while listening to BBs for only 12 min improved cognitive performance (Kraus & Porubanová, 2015). Thus, it seems that the duration of ABS might not be the key factor to improve performance, and other, still unknown factors should be examined.
Table 1.
Median RT and SDs for Low- and High-Emotional Groups Under White Noise, Binaural Beat, and Monaural Beat Condition on Three Difficulty Stages of the Flanker Task.
However, the finding that only speed and not the quality of performance on the Flanker attention task improved under the MB and BB condition may be explained by a ceiling effect, that is, the low degree of difficulty of the Flanker task. The very few errors that were made in the entire task might indicate that the task was quite easy, making a reduction of errors unlikely.
The present results can be explained as additional evidence that MBs and BBs improve the performance on an attention task. However, we must consider the possibility that the performance decreased in the WN condition compared to the BB and MB conditions. It may be that participants were more annoyed under the WN condition than under the BB and MB conditions, impairing their performance . Indeed, the choice of the control condition is debatable and WN may be less preferable than a control condition with isochronous stimulation or without any stimulation. However, about 30% of our participants reported that they experienced both WN, BB, and MB as annoying. In spite of this experience, performance under the BB and the MB was better relative to the equally annoying WN condition.
Notably, isochronous stimulation or no stimulation at all may be mind-numbing and thus also impair performance as compared to the performance in natural settings. Therefore, by masking BB and MB in WN, the specific difference between control and experimental condition was the additional BB and MB. As a consequence, we are quite convinced that the present results indicate an improvement of performance under the BB and MB conditions relative to the WN control condition.
The MEG and EEG studies regarding the entrainment of neuronal oscillations to rhythmic stimulation provide evidence of synchronization of beta- and gamma-band power with periodic and metrical rhythms (Fujioka et al., 2009; Snyder & Large, 2005). Also neural oscillations in pulse and meter induction have been suggested as the amplitude of the SS-EPs at pulse and meter frequencies was selectively enhanced (Nozaradan et al., 2012b). Thus, in the present study, neural resonance to 40 Hz MBs and BBs may have occurred. As this particular frequency is associated with a high level of awareness (Kaiser & Lutzenberger, 2005) and enhanced transmission from one brain region to another (Fell & Axmacher, 2011), it is conceivable that neural oscillations have contributed to the observed faster performance on the Flanker attention task.
In many commercial products, BBs are accompanied by music. It is possible that the music mediates the effects described by the users and producers. Especially when BBs are intended for relaxation, it seems plausible that the music partly induces mood changes in the listener. To avoid these kinds of confounding effects and to be able to draw conclusions about the pure nature of the effects of MBs and BBs, the beats in the current study were not accompanied by music. Positive significant results were found in studies using carrier tones with a frequency of respectively 230 and 220.45 Hz, and 240 and 255 Hz (Beauchene et al., 2016;Kraus & Porubanová, 2015). The carrier tones used in the present study were as high as 440 and 480 Hz, and although the BB with a carrier tone around 440 Hz has been shown to be perceived the best (Oster, 1973), these tones might be too high for comfortable listening. Unfortunately, not all studies reported the carrier tones of the BB. It is important that future studies are aware of the importance of carrier tones and further investigation into which tones are best received is needed.
With respect to personality characteristics, we expected that highemotional people would benefit more from ABS than low-emotional people. However, our results show that the whole group of participants was, relative to WN, faster under the BB and MB conditions on all stages of the Flanker task. In addition, exactly the same results were observed in high-emotional and low-emotional participants. Therefore, we have to conclude that the effects of MB and BB are similar in highand low-emotional participants.
Contrary to expectation, we found no evidence of any influence of ABS on working memory performance. This is not in line with results of studies in which improvements in working memory capacity were found after listening to alpha and beta BB stimulation, respectively (Beauchene at al., 2016; Kraus & Porubanová, 2015).
The present findings are in line with those of a recent study which also found faster reaction times in participants who had listened to BBs of 40 Hz (Colzato et al., 2017). In a global-local task, participants who had listened to a BB previous to the task were better able to focus their attention to target stimuli than participants who had not listened to a BB. Similar to the global-local task, in the current study, the participants had to switch between reacting to congruent and incongruent stimuli in the Flanker Task. Thus, the study of Colzato et al. and the present study support the notion that faster attention processing may be attributed to the influence of BBs.
Although MBs and BBs are processed in different ways (Draganova et al., 2008), no differences were found between the effects of MBs and BBs, which was in line with the participants’ evaluation. The participants did not notice any difference between the two sounds. Thus, subjective experience may suggest that the MB and BB of 40 Hz have similar effects.
Many aspects of ABS are still unclear. The importance of duration of stimulation and the time the effect of auditory beats may persist is still unknown. Moreover, it is unclear whether ABS elicits brain entrainment or alters brain connectivity. In addition, it is not clear yet which particular MB or BB frequency works best to enhance cognitive performance and which two carrier tones are most appropriate. With respect to personality traits, not everyone is assumed to be affected in the same way by auditory beats (Reedijk, Bolders, & Hommel, 2013). Moreover, cognitive performance seems to depend on the supply of striatal dopamine (Ashby, Isen, & Turken, 1999). Spontaneous eyeblink rates (EBR), which are a clinical marker of dopamine functioning (Karson, 1983), were measured in participants in the study of Reedijk et al. Results showed that people with low EBRs (low dopamine levels) did benefit from BBs, while participants with high EBRs (high dopamine levels) did not. This was explained by the assumption that low EBRs are associated with less effective cognitive performance and therefore, these individuals might have more opportunity to improve their cognitive performance. Moreover, musicians may be better at processing auditory beats than nonmusicians. Musicians seem not only to have a larger gray matter volume of the auditory cortex, but also the activity in their cortex after hearing sinusoidal tones seems to be larger than normal (Schneider et al., 2002). Indeed, there is increasing evidence that the synchronization process between the auditory beat and neural activity works better in musicians compared to nonmusicians (Ioannou, Pereda, Lindsen, & Bhattacharya, 2015).
Because ABS is a safe and noninvasive method to potentially enhance cognitive functions, it is worth further study. It is important that future studies take individual differences into account.
References
- Ashby F. G., Isen A. M., Turken A. U. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Beauchene C., Abaid N., Moran R., Diana R. A., Leonessa A. The effect of binaural beats on visuospatial working memory and cortical connectivity. PLoS One. 2016;11: e0166630. doi: 10.1371/journal.pone.0166630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher A. K., Hohne M., Axmacher N., Chaieb L., Elger C. E., Fell J. Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. European Journal of Neuroscience. 2015;41:254–263. doi: 10.1111/ejn.12760. [DOI] [PubMed] [Google Scholar]
- Bouma A., Mulder J., Lindeboom J., Schmand B. Amsterdam, The Netherlands: Pearson; 2012. Handboek neuropsychologische diagnostiek [Handbook of neuropsychological diagnosis] [Google Scholar]
- Chaieb L., Wilpert E. C., Reber T. P., Fell J. Auditory beat stimulation and its effects on cognition and mood states. Frontiers in Psychiatry. 2015;6:70. doi: 10.3389/fpsyt.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Colzato L. S., Barone H., Sellaro R., Hommel B. More attentional focusing through binaural beats: Evidence from the global–local task. Psychological Research. 2017;18:271–277. doi: 10.1007/s00426-015-0727-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo A., Recuero M., Galvez G., Begona A. Effect of binaural stimulation on attention and EEG. Archives of Acoustics. 2013;38:517–528. [Google Scholar]
- Dabu-Bondoc S., Vadivelu N., Benson J., Perret D., Kain Z. N. Hemispheric synchronized sounds and perioperative analgesic requirements. 2010;110:208–210. doi: 10.1213/ANE.0b013e3181bea424. [DOI] [PubMed] [Google Scholar]
- De Vries R. E., Ashton M. C., Lee K. De zes belangrijkste persoonlijkheidsdimensies en de HEXACO Persoonlijkheidsvragenlijst. (The six most important personality dimensions and the HEXACO Personality Inventory) 2009;22: 232–274. [Google Scholar]
- Draganova R., Ross B., Wollbrink A., Pantev C. Cortical steady-state responses to central and peripheral auditory beats. Cerebral Cortex. 2008;18:1193–1200. doi: 10.1093/cercor/bhm153. [DOI] [PubMed] [Google Scholar]
- Engelbregt H. J., Keeser D., Promes V. H., Verhagen-Schouten S., Deijen J. B. In-vivo EEG changes during a panic attack in a patient with specific phobia. Journal of Medical Cases. 2012;3:34–38. [Google Scholar]
- Eriksen B. A., Eriksen C. W. Effects of noise letters upon the identification of a target letter in a nonsearch task. 1974;16:143–149. [Google Scholar]
- Fell J., Axmacher N. The role of phase synchronization in memory processes. Nature Reviews Neuroscience. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- Fujioka T., Trainor L. J., Large E. W., Ross B. Beta and gamma rhythms in human auditory cortex during musical beat processing. Annals of the New York Academy of Sciences. 2009;1169:89–92. doi: 10.1111/j.1749-6632.2009.04779.x. [DOI] [PubMed] [Google Scholar]
- Gao X., Cao H., Ming D., Qi H., Wang X., Chen R., Zhou P. Analysis of EEG activity in response to binaural beats with different frequencies. International Journal of Psychophysiology. 2014;94:399–406. doi: 10.1016/j.ijpsycho.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Garcia-Argibay M., Santed M. A., Reales J. M. Efficacy of binaural auditory beats in cognition, anxiety, and pain perception: a meta-analysis. Psychological Research. 2018;83:1–16. doi: 10.1007/s00426-018-1066-8. [DOI] [PubMed] [Google Scholar]
- Goodin P., Ciorciari J., Baker K., Carrey A.-M., Harper M., Kaufman J. A high-density EEG investigation into steady state binaural beat stimulation. PloS One. 2012;7:e34789. doi: 10.1371/journal.pone.0034789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara H., Keeser D., Verhagen-Schouten S., Stobbe-Meijers M., Promes V., Deijen J., Engelbregt H. Hersenactiviteit tijdens een paniekaanval (Brain activity during a panic attack) GGZ Vaktijdschrift GGZV. 2018;1:2–13. [Google Scholar]
- Helps S. K., Bamford S., Sonuga-Barke E. J., Soderlund G. B. Different effects of adding white noise on cognitive performance of sub-, normal and super-attentive school children. PLoS One. 2014;9:e112768. doi: 10.1371/journal.pone.0112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiwa S., Katayama T., Hiroyasu T. Functional near-infrared spectroscopy study of the neural correlates between auditory environments and intellectual work performance. Brain and Behavior. 2018;8:e01104. doi: 10.1002/brb3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou C. I., Pereda E., Lindsen J. P., Bhattacharya J. Electrical brain responses to an auditory illusion and the impact of musical expertise. PLoS One. 2015;10:e0129486. doi: 10.1371/journal.pone.0129486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isik B. K., Esen A., Buyukerkmen B., Kilinc A., Menziletoglu D. Effectiveness of binaural beats in reducing preoperative dental anxiety. British Journal of Oral and Maxillofacial Surgery. 2017;55:571–574. doi: 10.1016/j.bjoms.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Jones M. R. Time, our lost dimension: Toward a new theory of perception, attention, and memory. Psychological Review. 1976;83:323–255. [PubMed] [Google Scholar]
- Jones M. R. The handbook of music psychology. Oxford, England: Oxford University Press; 2009. Musical time; pp. 89–92. [Google Scholar]
- Kaiser J., Lutzenberger W. Human gamma-band activity: A window to cognitive processing. NeuroReport. 2005;16:207–211. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- Kara O., Polo O. Autonomic and central stress-regulation disintegration in stress-related anxiety disorders. Acta Neuropsychologica. 2014;12:1–25. [Google Scholar]
- Karson C. N. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- Kennel S., Taylor A. G., Lyon D., Bourguignon C. Pilot feasibility study of binaural auditory beats for reducing symptoms of inattention in children and adolescents with attention-deficit/ hyperactivity disorder. Journal of Pediatric Nursing. 2010;25:3–11. doi: 10.1016/j.pedn.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Kraus J., Porubanová M. The effect of binaural beats on working memory capacity. Studia Psychologica. 2015;57:135–145. [Google Scholar]
- Lane J. D., Kasian S. J., Owens J. E., Marsh G. R. Binaural auditory beats affect vigilance performance and mood. 1998;63:249–252. doi: 10.1016/s0031-9384(97)00436-8. [DOI] [PubMed] [Google Scholar]
- Large E. W., Herrera J. A., Velasco M. J. Neural networks for beat perception in musical rhythm. Frontiers in Systtems Neuroscience. 2015;9:159. doi: 10.3389/fnsys.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large E. W., Jones M. R. The dynamics of attending: How people track time-varying events. Psychological Review. 1999;106:119–159. [Google Scholar]
- Lee K., Ashton M. C. Psychometric properties of the HEXACO personality inventory. Multivariate Behavioral Research. 2004;39:329–358. doi: 10.1207/s15327906mbr3902_8. [DOI] [PubMed] [Google Scholar]
- Marsh J. T., Worden F. G., Smith J. C. Auditory frequency-following response: Neural or artifact? . Science. 1970 Sep;169:1222–1223. doi: 10.1126/science.169.3951.1222. [DOI] [PubMed] [Google Scholar]
- Mathewson K. E., Lleras A., Beck D. M., Fabiani M., Ro T., Gratton G. Pulsed out of awareness: EEG alpha oscillations represent a pulsed-inhibition of ongoing cortical processing. Frontiers in Psychology. 2011;2:99. doi: 10.3389/fpsyg.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell P. A., Froeliger B., Garland E. L., Ives J. C., Sforzo G. A. Auditory driving of the autonomic nervous system: Listening to theta-frequency binaural beats post-exercise increases parasympathetic activation and sympathetic withdrawal. Frontiers in Psychology. 2014;5:1248. doi: 10.3389/fpsyg.2014.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philosophical Transactions of the Royal Society B. 2014;369:20130393. doi: 10.1098/rstb.2013.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S., Peretz I., Mouraux A. Selective neuronal entrainment to the beat and meter embedded in a musical rhythm. Journal of Neuroscience. 2012a;32:17572–17581. doi: 10.1523/JNEUROSCI.3203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaradan S., Peretz I., Mouraux A. Steady-state evoked potentials as an index of multisensory temporal binding. NeuroImage. 2012b;60:21–28. doi: 10.1016/j.neuroimage.2011.11.065. [DOI] [PubMed] [Google Scholar]
- Obleser J., Henry M. J., Lakatos P. What do we talk about when we talk about rhythm? . PLoS Biology. 2017;15:e2002794. doi: 10.1371/journal.pbio.2002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz T., Fernández A., Maestu F., Campo P., Hornero R., Escudero J., Poch J. Impact of auditory stimulation at a frequency of 5 Hz in verbal memory. Actas Españolas de Psiquiatría. 2008;36:307–313. [PubMed] [Google Scholar]
- Oster G. Auditory beats in the brain. Scientific American. 1973;229:94–102. doi: 10.1038/scientificamerican1073-94. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Hildreth A. J., Laws D. A prospective, randomised, controlled study examining binaural beat audio and pre-operative anxiety in patients undergoing general anaesthesia for day case surgery. Anaesthesia. 2005;60:874–877. doi: 10.1111/j.1365-2044.2005.04287.x. [DOI] [PubMed] [Google Scholar]
- Reedijk S. A., Bolders A., Hommel B. The impact of binaural beats on creativity. Frontiers in Human Neuroscience. 2013;7:786. doi: 10.3389/fnhum.2013.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho W., Pogarell O. Electrophysiology and psychophysiology in psychiatry and psychopharmacology. Cham, Switzerland: Springer; 2014. Physiological aberrations in panic disorder; pp. 185–195. [DOI] [PubMed] [Google Scholar]
- Schneider P., Scherg M., Dosch H. G., Specht H. J., Gutschalk A., Rupp A. Morphology of Heschl's gyrus reflects enhanced activation in the auditory cortex of musicians. Nature Neuroscience. 2002;5:688–694. doi: 10.1038/nn871. [DOI] [PubMed] [Google Scholar]
- Schwarz D. W., Taylor P. Human auditory steady state responses to binaural and monaural beats. Clinical Neurophysiology. 2005;116:658–668. doi: 10.1016/j.clinph.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Large E. W. Gamma-band activity reflects the metric structure of rhythmic tone sequences. Cognitive Brain Research. 2005;24:117–126. doi: 10.1016/j.cogbrainres.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Wahbeh H., Calabrese C., Zwickey H. Binaural beat technology in humans: A pilot study to assess psychologic and physiologic effects. Journal of Alternative and Complementary Medicine. 2007;13:25–32. doi: 10.1089/acm.2006.6196. [DOI] [PubMed] [Google Scholar]
- Wahbeh H., Calabrese C., Zwickey H., Zajdel D. Binaural beat technology in humans: a pilot study to assess neuropsychologic, physiologic, and electroencephalographic effects. Journal of Alternative and Complementary Medicine. 2007;13:199–206. doi: 10.1089/acm.2006.6201. [DOI] [PubMed] [Google Scholar]
- Will U., Berg E. Brain wave synchronization and entrainment to periodic acoustic stimuli. Neuroscience Letters. 2007;424:55–60. doi: 10.1016/j.neulet.2007.07.036. [DOI] [PubMed] [Google Scholar]