Abstract
Background:
African Americans are consistently found to have a lower prevalence of clinically-detected atrial fibrillation (AF) than whites, despite a higher prevalence of major AF risk factors and higher risk of ischemic stroke. Long-term ambulatory electrocardiographic (ECG) monitors provide the opportunity for unbiased AF detection. We determined differences by race/ethnicity in the prevalence of clinically-detected AF and in the proportion with monitor-detected AF.
Methods:
We conducted a cross-sectional analysis in the Multi-Ethnic Study of Atherosclerosis (MESA), a community-based cohort study that enrolled 6814 Americans free of clinically-recognized cardiovascular disease in 2000–2002. At the 2016–2018 examination, 1556 individuals participated in an ancillary study involving ambulatory ECG monitoring and had follow-up for clinically-detected AF since cohort entry.
Results:
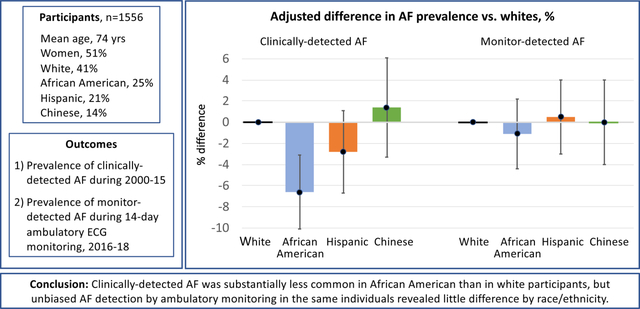
Among 1556 participants, 41% were white, 25% African American, 21% Hispanic, and 14% Chinese; 51% were women; and the mean age was 74 years. The prevalence of clinically-detected AF after 14.4 years’ follow-up was 11.3% in whites, 6.6% in African Americans, 7.8% in Hispanics, and 9.9% in Chinese, and was significantly lower in African Americans than in whites, in both unadjusted and risk factor-adjusted analyses (adjusted rate difference, −6.6%, 95% CI −10.1, −3.1%, P < 0.001). By contrast, in the same individuals, the proportion with monitor-detected AF using a 14-day ambulatory ECG monitor was similar in the four race/ethnic groups: 7.1%, 6.4%, 6.9%, and 5.2%, respectively (compared with whites, all P > 0.5).
Conclusions:
The prevalence of clinically-detected AF was substantially lower in African American than in white participants, without or with adjustment for AF risk factors. However, unbiased AF detection by ambulatory monitoring in the same individuals revealed little difference in the proportion with AF by race/ethnicity. These findings provide support for the hypothesis of differential detection by race/ethnicity in the clinical recognition of AF, which may have important implications for stroke prevention.
Keywords: atrial fibrillation, race and ethnicity, epidemiology, electrocardiography
Graphical Abstract
Introduction
Clinical and community-based studies report a 20–50% lower age- and sex-adjusted risk of clinically-detected atrial fibrillation and flutter (hereafter “AF”) in African Americans than in whites.1–6 Yet paradoxically, African Americans have a higher prevalence of AF risk factors including hypertension, obesity, and diabetes,7–9 and higher stroke risk.10 Less information is available about AF risk in Hispanics and Asian Americans.4–6 Differences in AF risk by race/ethnicity may be real or may be related to differences in symptom perception, clinical recognition, or health care access. Long-term rhythm monitoring with a wearable ambulatory electrocardiographic (ECG) monitor provides a sensitive and objective method of detecting AF in a community-based population. We contrasted the prevalence of clinically-detected AF during an average of 14.4 years of follow-up and the proportion with monitor-detected AF by race/ethnicity in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure due to participant privacy issues. Investigators interested in obtaining MESA data may contact the MESA Coordinating Center at the University of Washington or utilize the NHLBI BioLINCC repository.
In 2000–2002, 6814 participants 45–84 years of age and free of clinically-recognized cardiovascular disease, including AF, were enrolled in MESA in six US communities.11 Participants self-identified with one of four investigator-specified race/ethnic groups: white, African American, Hispanic, and Chinese. At baseline and at five follow-up visits, height, weight, blood pressure, fasting serum glucose, smoking, current medications, and physician diagnoses of hypertension and diabetes were ascertained; educational attainment was self-reported at baseline. At telephone contacts every 9–12 months during follow-up, participants were asked to identify new hospitalizations and diagnoses, and medical records were obtained. Clinically-recognized AF was identified by an International Classification of Disease (ICD) hospital discharge diagnosis code (version 9: 427.31 or 427.32; version 10: I48) in any position; and for those enrolled in fee-for-service Medicare, by an inpatient, outpatient, or physician claim with an AF code.12
During the 2016–2018 study visit, 1942 MESA participants were invited to participate in an ancillary study involving ambulatory ECG monitoring;13 1557 participated (80% of those invited). One participant had no follow-up for clinically-detected AF after the baseline exam, leaving 1556 available for analysis. Compared with those included in the analysis, the 386 participants not included were on average 2 years older, but otherwise their demographic and clinical characteristics were similar. The ambulatory ECG monitoring was conducted with a patch monitor that detects and stores up to 14 days of rhythm (Zio Patch XT, iRhythm Technologies, Inc, San Francisco, CA). The manufacturer processed and analyzed the ECG data, and all reported arrhythmias were independently verified by the Epidemiological Cardiology Research Center at Wake Forest University School of Medicine, Winston-Salem, NC; all readers were blinded to the race/ethnicity of participants.13 Participants were not asked to record symptoms. Atrial fibrillation was defined as an irregularly irregular rhythm with absent P waves lasting at least 30 seconds; AF as the presence of either atrial fibrillation or atrial flutter; and monitoring duration as the total time during which the ECG tracing was adequate to determine rhythm. Participant characteristics were ascertained at the most recent study exam.
With white participants as the reference group, we calculated unadjusted and adjusted rate differences by race/ethnic group. Multivariable linear regression models with robust standard errors were adjusted for traditional AF risk factors included in an AF risk score;14 models analyzing monitor-detected AF were additionally adjusted for monitoring duration. Linear regression with a binary outcome provides an estimate of the quantity of clinical interest, the adjusted prevalence difference. Among those with no history of clinically-detected AF, we reported by race/ethnic group the proportion with an episode of monitor-detected AF lasting more than 24 hours. In the ASSERT study, device-detected AF duration of more than 24 hours, but not shorter durations, was associated with significantly increased stroke risk, compared with no AF.15
The study was approved by the institutional review board at each participating institution and all participants provided written informed consent. The ECG monitoring devices were purchased for the study and the device manufacturer had no role in the study design, statistical analysis, or interpretation of results.
Results
Among the 1556 participants, the mean age was 74 years and 51% were women (Table 1). African American and Hispanic participants generally had the highest prevalence of AF risk factors; few participants had a history of myocardial infarction or heart failure. The ECG monitors provided a median monitoring duration of 13.8 (interquartile range, 12.8 – 14.0) days.
Table 1.
Characteristics of 1556 Participants with Ambulatory Electrocardiographic Monitoring, by Race/ethnicity
| Characteristic | White N=631 |
African American N=392 |
Hispanic N=321 |
Chinese N=212 |
|---|---|---|---|---|
| Age, mean (SD), y | 74 (8) | 74 (9) | 73 (8) | 73 (8) |
| Female, N (%) | 319 (50) | 223 (57) | 150 (47) | 107 (50) |
| Current smoking, N (%) | 35 (6) | 37 (9) | 17 (5) | 5 (2) |
| Treated hypertension,* N (%) | 325 (51) | 276 (70) | 185 (58) | 116 (55) |
| Systolic blood pressure, mean (SD), mm Hg | 126 (20) | 133 (21) | 125 (18) | 124 (20) |
| Height, mean (SD), cm | 167 (10) | 167 (10) | 162 (9) | 161 (9) |
| Weight, mean (SD), kg | 78 (17) | 83 (17) | 79 (16) | 64 (12) |
| Diabetes,* N (%) | 94 (15) | 108 (28) | 102 (32) | 53 (25) |
| History of myocardial infarction,† N (%) | 17 (3) | 5 (1) | 13 (4) | 4 (2) |
| History of heart failure,† N (%) | 9 (1) | 8 (2) | 9 (3) | 3 (1) |
Treated hypertension was defined as use of an antihypertensive medication in combination with a physician diagnosis of hypertension, and diabetes as use of a diabetes medication or fasting glucose ≥ 126 mg/dL.
Myocardial infarction and heart failure during follow-up were adjudicated.
During an average of 14.4 (standard deviation, 0.8) years of follow-up, AF was detected clinically in more whites (11.3%) than African Americans (6.6%), with intermediate proportions for Hispanics (7.8%) and Chinese (9.9%; Supplemental Table 1). With adjustment for AF risk factors, the AF prevalence was 6.6% lower in African Americans than in whites (Figure 1 and Supplemental Table 1, P < 0.001). The prevalence of clinically-detected AF did not differ significantly between Hispanics or Chinese and whites (Figure 1, P > 0.5). Findings were unchanged with use of an alternative definition of clinically-detected AF that included self-report of a physician diagnosis of AF, after further adjustment for educational attainment or enrollment in fee-for-service Medicare, and in analyses limited to those enrolled in fee-for-service Medicare.
Figure 1.
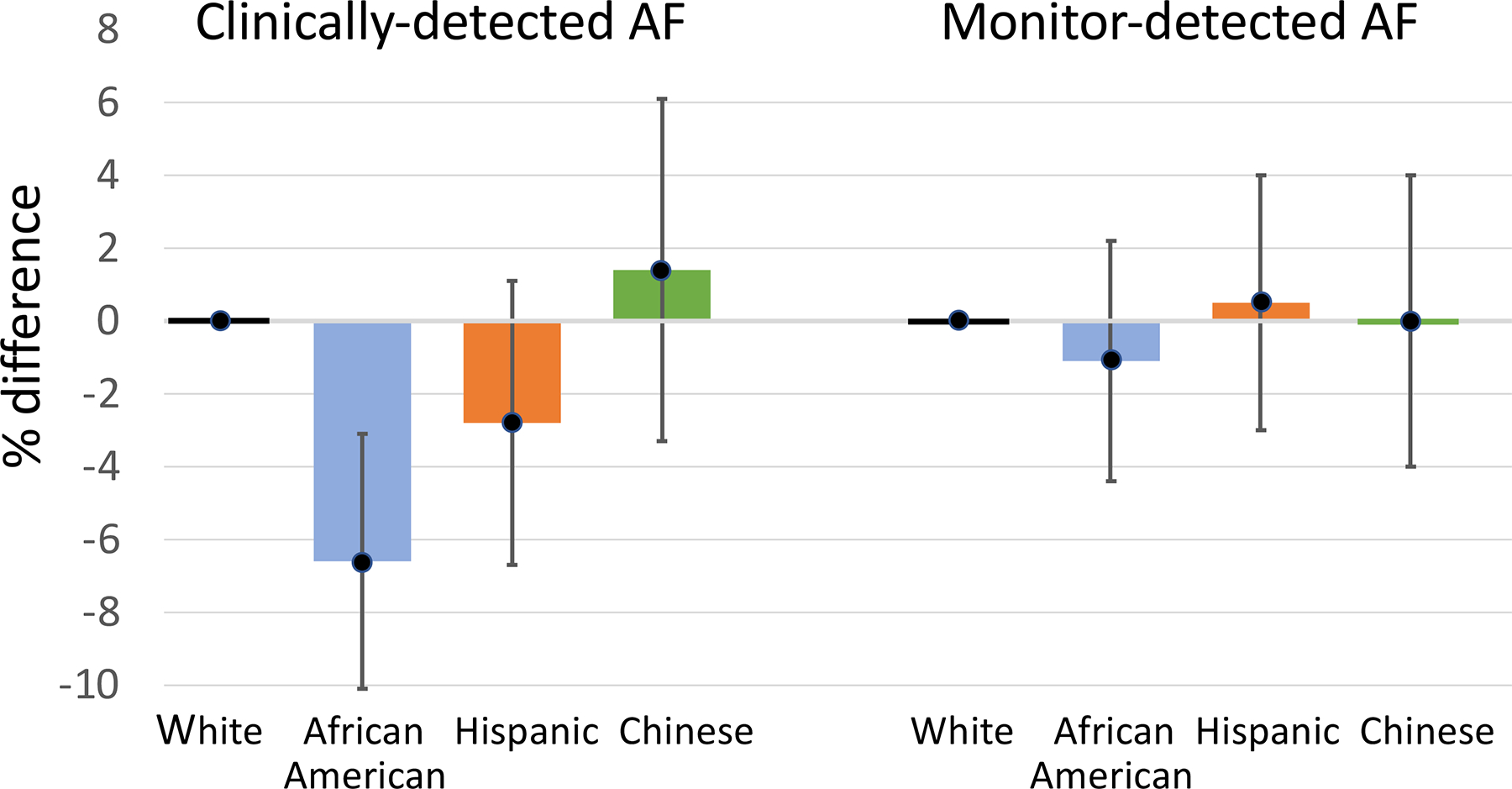
Adjusted % difference in AF prevalence vs whites for clinically-detected AF and monitor-detected AF. All estimates are adjusted for age, sex, height, weight, treated hypertension, current smoking, diabetes, systolic blood pressure, history of heart failure, history of myocardial infarction; estimates for monitor-detected AF are also adjusted for monitoring duration. 95% confidence intervals are shown.
By contrast, the proportions with monitor-detected AF were similar in the four race/ethnic groups (Figure 1 and Supplemental Table 1). Compared with white participants, no statistically-significant differences in the proportion with monitor-detected AF were detected by race/ethnic group in either unadjusted or multiply-adjusted analyses (compared with whites, all P > 0.5).
In analyses limited to those with no history of clinically-detected AF, the proportion with monitor-detected AF was again similar in the four race/ethnic groups (Table 2). Among those with monitor-detected AF, there was little difference by race/ethnicity in the proportion with longest AF episodes lasting more than 24 hours. But in those with a history of clinically-detected AF, the proportion with monitor-detected AF was higher in African American and Hispanic participants (42% and 40%) than in white and Chinese participants (28% and 19%).
Table 2.
Characteristics of Monitor-Detected Atrial Fibrillation by Race/Ethnicity in Participants Without and With a History of Clinically-Detected Atrial Fibrillation
| White | African American | Hispanic | Chinese | |
|---|---|---|---|---|
| Participants with no history of clinically-detected AF, N | 560 | 366 | 296 | 191 |
| With monitor-detected AF, N (%) | 25 (4.5) | 14 (3.8) | 12 (4.1) | 7 (3.7) |
| Longest AF episode >24 hr, % | 32 | 43 | 42 | 43 |
| Participants with a history of clinically-detected AF, N | 71 | 26 | 25 | 21 |
| With monitor-detected AF, N (%) | 20 (28) | 11 (42) | 10 (40) | 4 (19) |
AF = atrial fibrillation or atrial flutter
Discussion
In MESA, the prevalence of clinically-detected AF was substantially lower in African Americans than in whites. Hispanics had a lower prevalence of clinically-detected AF than whites, but the difference did not reach statistical significance, and no difference vs whites was demonstrated for Chinese participants. However, unbiased AF detection by ambulatory ECG monitoring in the same individuals revealed little difference by race/ethnicity in the proportion with AF detected. In participants with no history of clinically-detected AF, the proportions with monitor-detected AF and with an episode of AF longer than 24 hours were also similar across race/ethnic groups.
Differences by race/ethnic group in clinically-detected AF may be real; may reflect differences in symptom perception, clinical AF recognition, or health care access; or may be due to differences in the completeness of clinical event ascertainment. Our findings for differences in clinically-detected AF in the four race/ethnic groups are in agreement with a large number of population- and community-based studies,1–6 with the exception that we observed little difference for Chinese vs white individuals, unlike a claims-based analysis from California which found lower AF prevalence in Asians.5
Existing studies of implanted device-detected AF have reported more AF in white than in African American patients, but the ASSERT study analysis was based on a small number of African American patients (n=73),16 and the difference in device-detected AF between white and African American participants was no longer significant after adjustment for AF risk factors. In an analysis relying on administrative claims data,17 implanted device-detected AF was identified only when a physician assigned an AF diagnosis code, and was therefore subject to the same potential bias as for clinically-detected AF. Finally, patients with implanted devices included in these studies had considerably more underlying cardiovascular disease than MESA participants, in whom the prevalence of past myocardial infarction and heart failure was low. Associations of AF with race/ethnicity may differ in individuals with different risk profiles.
Our findings should be interpreted in the context of several considerations. Clinical recognition of AF during 14 years of follow-up is not measuring the same quantity as AF detected by ambulatory monitoring over 14 days. Both methods of AF detection are imperfect: not all AF is recognized by either the patient or the physician, and ECG monitoring for 14 days misses paroxysmal AF that occurs infrequently. Despite the careful hospitalization follow-up in MESA and the inclusion of Medicare claims data, the study may have failed to identify some clinical encounters where an AF diagnosis was made. Finally, power to detect differences in the prevalence of monitor-detected AF was limited, particularly for the small group of Chinese participants.
ECG monitoring provides an unbiased assessment of cardiac arrhythmias. Our finding of substantially lower prevalence of clinical AF detection in African Americans than in whites, but little difference in the proportion with monitor-detected AF, provides support for the hypothesis of differential detection by race/ethnicity in the clinical recognition of AF. Additional study is needed to confirm these findings and to increase understanding of the reasons for the observed differences, which may have important implications for stroke prevention.
Supplementary Material
What Is Known?
Atrial fibrillation (AF) risk factors, including hypertension, diabetes, and obesity, are more common in African Americans than in whites.
Paradoxically, African Americans are consistently found to have lower rates of physician-diagnosed AF than whites.
What the Study Adds?
In an epidemiologic study including 1556 Americans, average age 74, the prevalence of clinically-detected AF during 14 years of follow-up was substantially lower in African Americans than in whites.
Yet in the same individuals, the proportion with AF detected by 14-day ambulatory ECG monitoring did not differ between African Americans and whites.
These results provides support for the hypothesis of differential detection by race/ethnicity in the clinical recognition of AF.
Acknowledgments:
Dr. Heckbert had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Sources of Funding: This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grant R01 HL127659 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The research reported here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agencies had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Non-standard Abbreviations and Acronyms:
- MESA
Multi-Ethnic Study of Atherosclerosis
- ICD
International Classification of Disease
Footnotes
Disclosures: Dr. Bruce Psaty serves on the DSMB of a clinical trial funded by Zoll LifeCor and on the Yale Open Data Access Project funded by Johnson & Johnson.
References:
- 1.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen PN, Thacker EL, Dublin S, Psaty BM, Heckbert SR. Racial differences in the incidence of and risk factors for atrial fibrillation in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2013;61:276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez MV, Wang PJ, Larson JC, Soliman EZ, Limacher M, Rodriguez B, Klein L, Manson JE, Martin LW, Prineas R, et al. Risk factors for atrial fibrillation and their population burden in postmenopausal women: the Women’s Health Initiative Observational Study. Heart. 2013;99:1173–1178. [DOI] [PubMed] [Google Scholar]
- 5.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC Jr., Heckbert SR. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol. 2015;25:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett DK, Rautaharju P, Crow R, Folsom AR, Ekelund LG, Hutchinson R, Tyroler HA, Heiss G. Black-white differences in electrocardiographic left ventricular mass and its association with blood pressure (the ARIC study). Atherosclerosis Risk in Communities. Am J Cardiol. 1994;74:247–252. [DOI] [PubMed] [Google Scholar]
- 8.Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Hypertens. 2004;17:963–970. [DOI] [PubMed] [Google Scholar]
- 9.O’Neal WT, Judd SE, Limdi NA, McIntyre WF, Kleindorfer DO, Cushman M, Howard VJ, Howard G, Soliman EZ. Differential impact of risk factors in blacks and whites in the development of atrial fibrillation: the Reasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Racial Ethn Health Disparities. 2017;4:718–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinstein M, Ning H, Kang J, Bertoni A, Carnethon M, Lloyd-Jones DM. Racial differences in risks for first cardiovascular events and noncardiovascular death: the Atherosclerosis Risk in Communities study, the Cardiovascular Health Study, and the Multi-Ethnic Study of Atherosclerosis. Circulation. 2012;126:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 12.Heckbert SR, Wiggins KL, Blackshear C, Yang Y, Ding J, Liu J, McKnight B, Alonso A, Austin TR, Benjamin EJ, et al. Pericardial fat volume and incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis and Jackson Heart Study. Obesity (Silver Spring). 2017;25:1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: The Multi-Ethnic Study of Atherosclerosis. J Electrocardiol. 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 16.Lau CP, Gbadebo TD, Connolly SJ, Van Gelder IC, Capucci A, Gold MR, Israel CW, Morillo CA, Siu CW, Abe H, et al. Ethnic differences in atrial fibrillation identified using implanted cardiac devices. J Cardiovasc Electrophysiol. 2013;24:381–387. [DOI] [PubMed] [Google Scholar]
- 17.Kamel H, Kleindorfer DO, Bhave PD, Cushman M, Levitan EB, Howard G, Soliman EZ. Rates of atrial fibrillation in black versus white patients with pacemakers. J Am Heart Assoc. 2016;5:e002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


