Abstract
Immune activity is variable within and among vertebrates despite the potentially large fitness costs of pathogens to their hosts. From the perspective of life history theory, immunological variability may be the consequence of counterbalancing investments in immune defense against other expensive physiological processes, namely, reproduction. In the present study, we tested the hypothesis that immune defense among captive-bred, disease-free Peromyscus mice would be influenced by their reproductive life history strategies. Specifically, we expected that small species that reproduce prolifically and mature rapidly (i.e., fast pace of life) would favor inexpensive, nonspecific immune defenses to promote reproductive proclivity. Alternatively, we expected that large species that mature slowly and invest modestly in reproduction over multiple events (i.e., slow pace of life) would favor developmentally expensive, specific immune defenses and avoid cheap, nonspecific ones because such defenses are predisposed to self-damage. We found that species exhibited either strong ability to kill (gram-negative) bacteria, a developmentally inexpensive defense, or strong ability to produce antibodies against a novel protein, a developmentally expensive defense, but not both. Cell-mediated inflammation also varied significantly among species, but in a unique fashion relative to bacteria killing or antibody production; wound healing was comparatively similar among species. These results indicate that Peromyscus species use immune strategies that are constrained to a dominant axis, but this axis is not determined solely by reproductive pace of life. Further comparisons, ideally with broader phylogenetic coverage, could identify what ecological and evolutionary forces produce the pattern we detected. Importantly, our study indicates that species may not be differentially immunocompetent; rather, they use unique defense strategies to prevent infection.
Keywords: immunocompetence, life history, Peromyscus spp, rodent, trade-off
Introduction
Reductionist studies of the immune system (e.g., cellular or molecular) have been remarkably fruitful (Janeway 2004), but they have yet to illuminate why immune defenses are so labile when selection against immune incompetence should have been strong over evolutionary time (Williams and Nesse 1991). Counter-balancing of investments between immune defense and other expensive physiological processes may be important (Sheldon and Verhulst 1996, Lochmiller and Deerenberg 2000, Martin et al. 2006b). If resources and time were unlimited, then all organisms might maximize investments in immune defense, at least to the point that autoimmune pathology remained avoidable (Rå berg et al. 1998). Because resource availability and pathogen threats fluctuate over time and space (Nelson et al. 2002) and because immune defense is costly (Lochmiller and Deerenberg 2000, Bonneaud et al. 2003, Martin et al. 2003, Demas 2004), animals must adaptively allocate resources among physiological systems to maximize fitness in their environments (Williams 1966, Stearns 1992, Ricklefs and Wikelski 2002).
A recurrent observation in life history studies has been that species tend either to breed at a young age and reproduce prolifically (i.e., live a fast-paced reproductive life) or mature slowly and parse reproductive efforts over long periods of time (i.e., live a slow-paced reproductive life) (Stearns 1992, Martin 1996, Ghalambor and Martin 2001). Classically, these pace-of-life categorizations are similar to the r- vs. K-selected dichotomy developed years ago by population ecologists (MacArthur and Wilson 1967). Because species living fast-paced reproductive lives invest heavily in rapid reproductive maturation and breeding output per unit time, they should have few resources to invest in immune defense. Species living slow-paced lives, however, should be able to invest heavily in immune defenses because they develop slowly and reproduce modestly at each breeding event.
Meta-analyses of passerine species have detected life history–immune activity covariation across broad phylogenetic scales (Martin et al. 2001, Tella et al. 2002, Palacios and Martin 2006). Comparisons of slow vs. fast pace-of-life populations of House Sparrows, Passer domesticus (Martin et al. 2004, 2005, 2006b) and Tree Swallows, Tachycineta bicolor (Ardia 2005) have documented similar results. A shortcoming of most of these studies was that only one or two components of immune defense were measured. Because the vertebrate immune system is multifaceted and redundant (Schmid-Hempel and Ebert 2003, Janeway 2004), this approach to characterizing species’ immunocompetence is much like characterizing intelligence by measuring head size. If one seeks to understand the immune system in an ecological context, then its complexity must be considered. A recent comparative study highlighted the difficulty of this task, demonstrating only marginal concordance among 10 immunological variables among and within waterfowl species (Matson et al. 2006a). By selectively choosing assays amenable to the hypothesis being tested, however, rigorous tests may be possible (Martin et al. 2006d).
Indeed, it is important to consider the benefits and costs of specific immune defense variants when conducting ecoimmunological studies (Schmid-Hempel and Ebert 2003, Lee 2006; Martin et al., in press). In the waterfowl study cited above (Matson et al. 2006a), only constitutive, nonspecific defenses were measured. Overall, these defenses are thought to impose small costs, but provide generic protection (Lee 2006). Other defenses require large expenditures either during development or during the response itself, but provide targeted, specific defenses and memory of prior exposure. In the life history context described previously, one would predict that cheap defenses would be favored by fast-lived species and all types of defenses favored by the slow-lived ones, as long as no defense variant induces self-damage (Klasing 2004, Martin et al. 2006b).
To test this hypothesis, four measures of immune activity were compared among six species of Peromyscus mice: ex vivo killing of E. coli bacteria (a nonspecific immune defense predominantly mediated by plasma proteins [Tieleman et al. 2005]); cutaneous wound healing (an integrative measure of inflammation and tissue regeneration [Padgett et al. 1998]), antibody production against a novel protein (a specific, induced immune response indicative of efficacy of extra-cellular pathogen control by B lymphocytes [Demas et al. 1997]), and T cell-mediated cutaneous inflammation (a specific, induced immune response indicative of the capacity of T lymphocytes to orchestrate inflammation [Dhabhar and McEwen 1997]). At present, direct estimates of the costs of some of these defenses are not available, but several lines of evidence suggest that they are differentially costly (Klasing 2004, Lee 2006). For example, the random process by which T and B cells diversity is generated requires substantial time and resources (Klasing 2004) and thus makes these defenses relatively expensive. In contrast, E. coli killing capacity is predominantly mediated by soluble proteins (Tieleman et al. 2005), the production of which exacts modest caloric and amino acids costs (Klasing 2004). However, as they are relatively nonspecifiic, these defenses are predisposed to self-destruction via oxidative damage (Klasing 2004, Tieleman et al. 2005), making them costly in a different way. Wound healing is accomplished by multiple immunological and growth processes, so its costs are hard to generalize. Wound healing does not require the large investment during development that B and T cell-mediated defenses do. However, inflammatory processes are engaged early in the healing process, which may damage self-tissues but also provide protection against (bacterial) infection.
Given this potential variation in costliness of different immune responses, we expected that antibody production and T cell-mediated inflammation would be more robust in slow-lived species, whereas bacterial killing capacity and wound healing would be more robust in fast-lived ones. To minimize confounding environmental influences inherent to field studies (Martin et al. 2001, Tella et al. 2002, Ardia 2005, Martin et al. 2006b), we utilized captive-bred mice maintained in disease-free laboratory conditions for several generations. These six species (Table 1) were chosen in particular because they were the only commercially available species that exhibited extensive reproductive life history variation while being amenable to standard husbandry protocols (Modi 1984, Bronson 1988, Millar 1989).
Table 1.
Life history traits of six Peromyscus species.
| Trait | Units | P. polionotus (old-field) | P. maniculatus (deer) | P. leucopus (white-footed) | P. eremicus (cactus) | P. aztecus (Aztec) | P. melanophrys (plateau) |
|---|---|---|---|---|---|---|---|
| Adult mass | g | 14.3 | 18.1 | 18.4 | 19.3 | 38.9 | 39.3 |
| Newborn mass | g | 1.6 | 1.6 | 1.9 | 2.4 | 3.8 | 3.9 |
| Litter size | no. | 3.8 | 4.0 | 3.6 | 2.4 | 2.2 | 3.0 |
| Conceptus mass | g | 5.4 | 5.7 | 5.5 | 4.8 | 6.0 | 6.9 |
| Gestation duration | months | 0.79 | 0 88 | 0.83 | 0.85 | 0.80 | 0.82 |
| Nestling period | months | 0.76 | 0.84 | 0.77 | 0.82 | 0.83 | 1.05 |
| Days between litters | d | 27.3 | 26.3 | 28.8 | 30.4 | 41.9 | 32.5 |
Methods
Animal care
All mice were purchased from the Peromyscus Genetic Stock Center (University of South Carolina, Columbia, South Carolina, USA; see Plate 1). Each species was captured from the wild and bred continuously under standard conditions. The Peromyscus Genetic Stock Center screens its colonies every four months for pathogens including: murine pneumonia virus, mouse minute virus, mouse parvovirus, lymphocyte choriomeningitis, Theiler’s murine encephalomyelitis virus, murine hepatitis, Mycoplasma pulmonis, cilia-associated respiratory bacillus, and hantavirus. No mice have ever tested positive for these pathogens. Peromyscus are also screened for endoparasites and ectoparasites. Only Giardia sp. has been found, and only in a very few individuals. In addition to these screenings, sentinel mice (Mus musculus) are maintained in our animal rooms throughout experiments and tested quarterly.
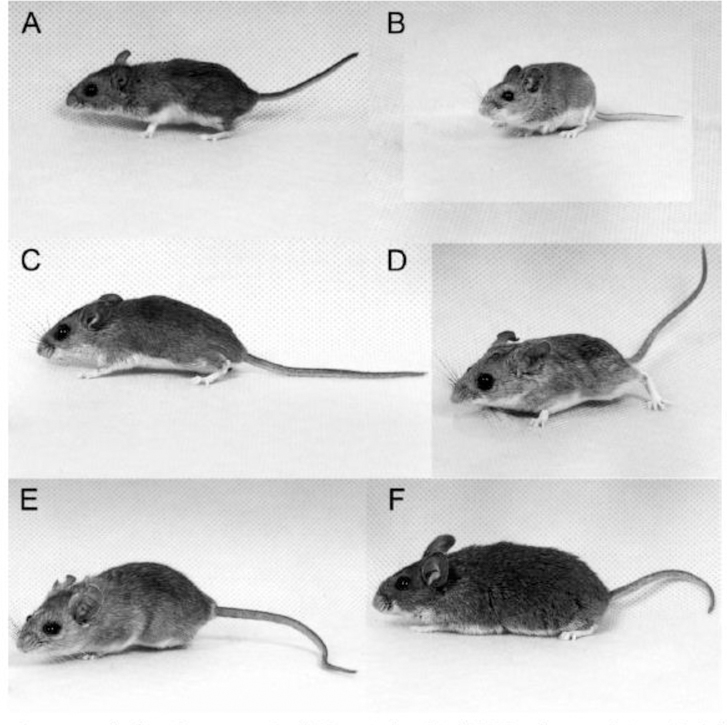
Plate 1.
Peromyscus mice from the present study: (A) P. maniculatus bairdii, (B) P. polionotus subgriseus, (C) P. leucopus, (D) P. eremicus, (E) P. melanophrys, (F) P. aztecus. Sizes of mice were normalized to reference objects during photography so the present image reliably depicts size variation among species. Photo credit: Clint Cook, University of South Carolina Peromyscus Genetic Stock Center.
Prior to and during experiments, mice (all virgin and approximately eight months old) were housed singly in rodent cages (27 3 7.5 3 13 cm), provided with bedding material, food (product 8620, Harlan TekLad, Madison, Wisconsin, USA), and filtered, chlorinated tap water ad libitum, and housed at 22.58 6 38C in either long (16 h light; half of mice) or short (8 h light; remaining half of mice) photoperiods. Mice remained in these conditions for eight weeks prior to measurement of immune activity. Three separate groups of mice (one for each immune assay: n = 20 individuals per species [five males and five females per photoperiod] per assay) were used to ensure that prior immune activity did not influence subsequent immune responses (Martin et al. 2006c). Plasma for the bacterial killing assay, however, came from the antibody production group at the time of necropsy to minimize the number of mice used. These samples were collected four weeks postsecondary challenge to minimize effects of prior experience. All procedures were conducted following NIH guidelines and were approved by Ohio State University’s Institutional Animal Care and Use Committee.
Reproductive data
Reproductive life history data were obtained from primary literature (Modi 1984, Morgan Ernst 2003) or the Peromyscus Genetic Stock Center (Table 1). Additional data (e.g., lifespan or reproductive periodicity) were not available for all species, except P. leucopus, P. maniculatus, and P. polionotus, so further influences on immunity in these species could not be assessed. Reproductive life history characters of captive Peromyscus do not vary substantially from their wild-caught conspecifics (Millar 1989, Botten et al. 2001), although relaxed selection may have affected the immune systems of some species more than others (Lyles and Dobson 1993). This possibility remains to be tested.
Bacterial killing capacity
Blood from the retro-orbital sinus of anesthetized (isoflurane in O2-enriched air) mice was collected into sterile, heparanized microcapillary tubes and kept on ice until centrifugation (Matson et al. 2006b). Plasma was removed and stored at -80°C until bacterial killing capacity was assayed ~1.5 months). Just prior to assays, plasma samples were diluted 1:50 (in CO2-independent media; Gibco no. 18045, Carlsbad, California, USA) under a laminar flow hood. A standard number (;900) of colony-forming units (CFUs) of E. coli (EPower Microorganisms no. 0483E7, MicroBio-Logics, St. Cloud, Minnesota, USA; lot no. 485402; ATCC no. 8739) was added to each sample (ratio, 1:10). Plasma–bacteria mixtures were then incubated for 30 min at 378C, and plated in triplicate onto tryptic-soy agar. To serve as positive controls, three agar-filled petri dishes were inoculated with diluted bacteria alone, and to serve as negative controls, three agar-filled plates were swabbed with a sterile bacteria spreader (under laminarflow conditions), then all plates were incubated at 37°C overnight. To quantify killing capacity, total CFUs were quantified the following morning, then the average of the three replicates was calculated for each mouse and that value divided by the average of the positive control replicates for that assay. Plasma samples were randomly distributed among six assay runs, and plated and scored blind to identity. Mean inter-assay variation was 5.2%; mean intra-assay variation was 4.0%. No negative controls contained CFUs.
Wound healing
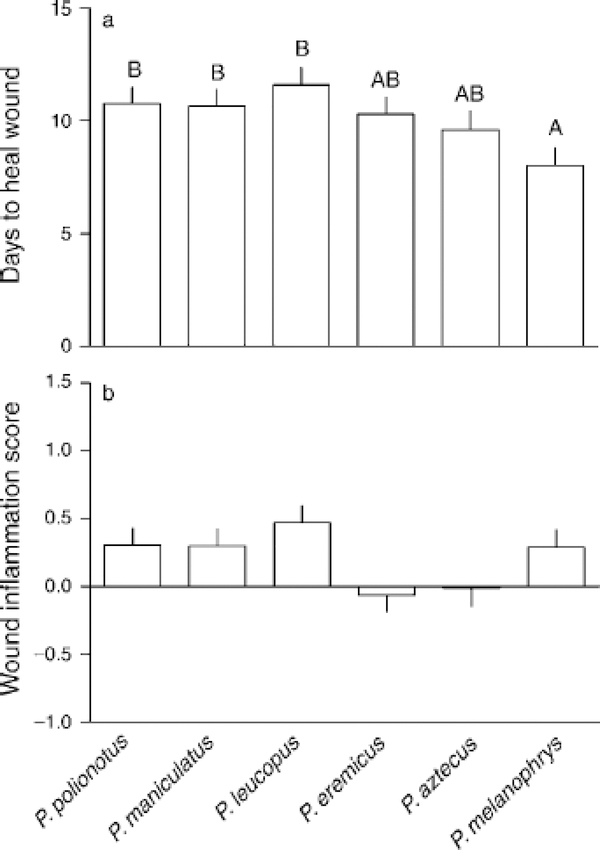
Mice were anesthetized (isoflurane in O2-enriched air) and a ~90-mm2 patch of fur was shaved between the scapulae and sterilized with 70% EtOH (Glasper and Devries 2005, Martin et al. 2006a). Two 3.5 mm diameter circular wounds were made in the dorsal skin with a sterile biopsy punch (Miltex Instrument, Bethpage, New York, USA), and wounds were photographed under standard conditions immediately postwounding and for the next seven days using a digital camera (Coolpix 775, Nikon, Tokyo, Japan). A reference standard was included in every photograph. Entrance wounds and reference standards were traced in each photo, and the area of each wound and reference was calculated (Canvas 6, Deneba Systems, Miami, Florida, USA). The ratio of the wound area to the standard was determined, then relative wound size calculated by dividing standardized wound area each day by the standardized area on day 0. These data were used to calculate two values: (1) total days to complete healing and (2) swelling at the wound site in the first three days post-wounding. To estimate total days necessary to heal the wound, the slope of the healing curve over the seven days post-wounding was determined for each individual, then days to heal wound extrapolated by setting y (wound size) = 0 and solving for x (days post-wounding). Swelling at the wound site was determined by dividing wound size on days 1–3 by initial wound size then summing these three values; inflamed wounds increase in size relative to initial wound size in some Peromyscus. A few outliers (>2 SD from mean; n = 6 for days to heal wound, n = 5 for swelling at wound site) were not included in statistical analyses.
Antibody production
Mice were anesthetized (isoflurane in O2) then an 100-lL blood sample was drawn from the retro-orbital sinus. Mice were injected (intraperitoneally) with150-lg keyhole limpet hemocyanin (KLH; in aluminum phosphate; Calbiochem, La Jolla, California, USA, catalog no. 374811, lot no. B30450) in 100 μL sterile 0.9% saline, then returned to their home cages (Demas et al. 1997). Blood samples were kept on ice until centrifugation (88 632 m/s2 for 20 min), then plasma was removed and stored at -80°C until assays (~3 months later). Seven and 14 days post-injection, mice were anesthetized again (between 10:00 and 14:00), another blood sample taken, and plasma collected and stored. Two weeks after the last blood sample, a fourth blood sample was collected, plasma harvested and stored, then mice were injected with 30 μg KLH in 100 μL sterile 0.9% saline to initiate a secondary antibody response; one additional plasma sample was then collected four days post injection.
Once all plasma samples were obtained, immunoglobulin (IgG) specific to KLH was measured using an enzyme-linked immunosorbent assay for Peromyscus (Demas and Nelson 2003). Briefly, 96-well plates were coated with KLH then diluted plasma samples (in PBS-Tween, 1:40; Sigma, St. Louis, Missouri, USA) were plated in duplicate. Positive (plasma from Peromyscus predetermined to possess anti-KLH IgG) and negative (plasma from KLH naÏve mice) controls were also added to each plate in triplicate. Plates were incubated at 378C for 3 h, washed (PBS-Tween), then a secondary antibody (alkaline-phosphatase conjugated anti-mouse IgG; MP Biomedicals, Aurora, Ohio, USA, code 59296, lot no. 04325) was added to each well (1:750). Plates were again incubated (378C for 1 h), washed, then each well was treated with p-nitrophenyl phosphate. Exactly 20 min later, optical density (OD) of samples was assessed (405-nm filter, BioRad Benchmark microplate reader, Richmond, California, USA). Data analysis was performed on sample OD readings expressed as a percentage of the mean of the plate positive controls.
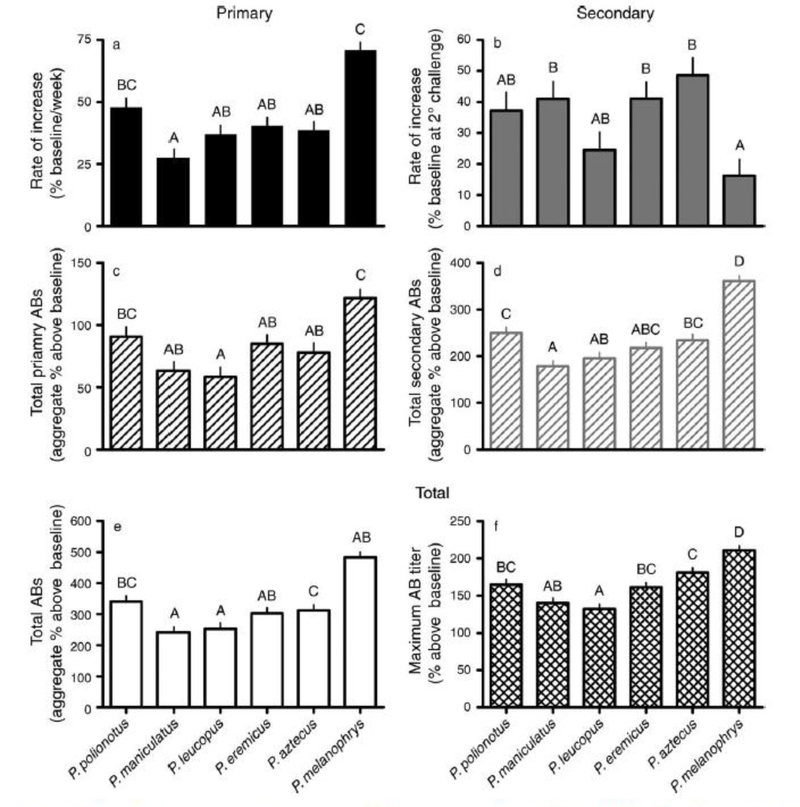
To compare antibody production to KLH among species, six values were calculated: rate of primary antibody production, rate of secondary antibody production, total primary IgG, total secondary IgG, maximum IgG (across both primary and secondary responses), and total IgG (over both primary and secondary responses). The slope of the line describing the increase in anti-KLH IgG for each individual represented the IgG production rates. The highest titer measured at any time point represented maximum IgG; in all but 11 cases (9.2% of mice), this maximum occurred at d32. The total area under the primary and secondary antibody production curve (integration of titers across all time points; day 0–32) represented total IgG.
T cell-mediated inflammation (delayed-type hypersensitivity)
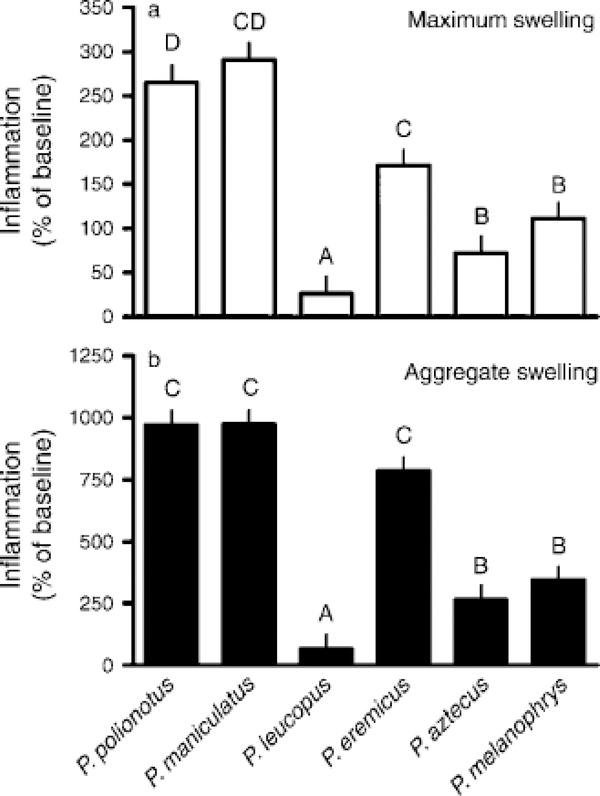
Delayed-type hypersensitivity (DTH) was induced by sensitizing a small shaved patch on the dorsum of anesthetized (isoflurane in O2) mice to 2,4-dinitro-1-fluorobenzene (DNFB; Sigma, St. Louis, Missouri, USA; 50 lL of 0.5% DNFB in 4:1 acetone/olive oil vehicle) on two consecutive days (Dhabhar and McEwen 1997). Seven days later, thickness of the right and left pinnae were measured with a constant-loading dial micrometer (Mitutoyo America, Aurora, Illinois, USA), then DTH was elicited (20 lL of 0.7% DNFB in 4:1 acetone/olive oil) on the dorsal surface of the right pinna. Left pinnae were treated with an equal volume of vehicle. For the next six days, pinnae thicknesses were measured in all individuals while mice were lightly anesthetized. No swelling occurred in vehicle-treated pinnae. To quantify DTH, baseline pinnae thickness was subtracted from right pinnae thickness each day, and this value divided by baseline thickness multiplied by 100. Two values were then extracted from these data: maximum swelling across all measurements and total swelling over the entire measurement period.
Data analysis
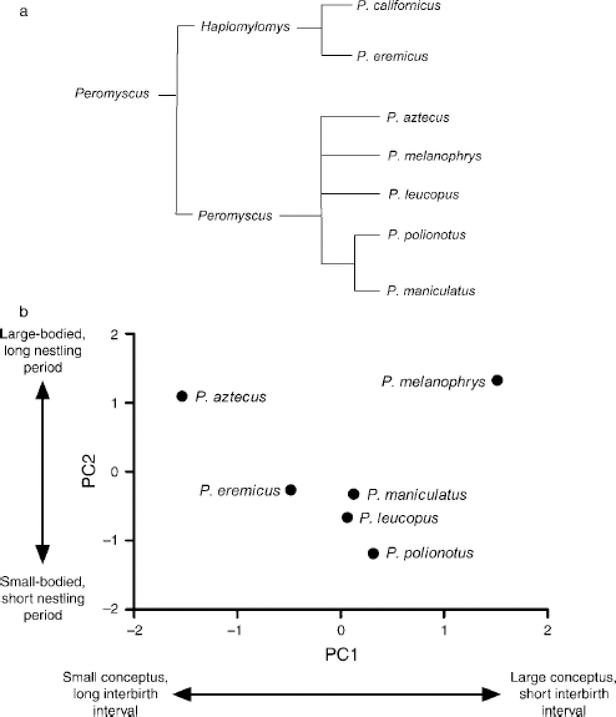
Data distributions were tested for variance homogeneity and normality prior to analysis. Transformations were used when necessary, and when used, were successful at improving normality and/or variance homogeneity. To determine whether phylogenetic relatedness affected data, tests for serial independence (Abouheif 1999) were conducted. Neither raw data nor principal component (PC) scores of life history or immune data were significantly influenced by phylogeny (Fig. 1a), so phylogeny was not considered further. To summarize life history variation among species, principal components analysis (PCA) was performed on the correlation matrix of the life history data in Table 1.
Fig. 1.
(a) Phylogeny (Carleton 1980) and (b) reproductive pace of life of Peromyscus in the present study. Points are scores from principal components analysis of data in Table 1.
Allometric effects were first controlled by using residuals from linear regressions between body mass and other life history data (see Table 2). Varimax rotation was implemented and the Kaiser criterion (eigen values >1) was used to identify important components. Factors affecting immune measures were then determined using ANOVA with species, sex, and photoperiod as factors followed by Tukey-Kramer post hoc tests. Because photoperiod and/or sex influenced some immune responses, estimated marginal means of species’ immune measures were used in later multivariate analyses (when appropriate). To compare variability in immune responses among species, pairwise Fmax tests were conducted on the variances of raw immune data (the six antibody response variables were first reduced to two using principal components analysis due to collinearity of these variables); a was adjusted to P = 0.01 to minimize Type I error in pairwise comparisons. To identify potential covariation between immune and life history parameters, canonical correlation analysis was employed on life history PC scores and scores from a PCA of the raw immune data matrix. Scores from the antibody production PCA and sex and photoperiod were then used as independent variables in backwards linear regression models (F value to remove factor from model λ=0.10) to predict bacterial killing capacity separately for each species and thus identify within-species relationships between immune measures. Additional analyses were impossible as these were the only two immune measures obtained from the same individuals. Finally, bivariate Spearman rank correlations were conducted between body mass and individual immune measures, as plots of PC scores indicated that immune measures were loosely related to body mass (Figs. 2–6). Differences were considered statistically significant when P < 0.05.
Table 2.
Principal components analysis results for Peromyscus life histoiy data.
| Measure | Correlation coefficients with PCs† |
|
|---|---|---|
| PCI | PC2 | |
| Adult mass | −0.03 | 0.98 |
| Birth mass‡ | −0.10 | 0.00 |
| Litter size | 0.51 | −0.64 |
| Conceptus mass‡ | 0.99 | 0.01 |
| Gestation | 0.09 | −0.12 |
| Nestling | 0.58 | 0.79 |
| Days between litters‡ | −0.95 | −0.08 |
Note: Values in boldface indicated strongest correlates with PC scores.
For principal components 1 and 2 the eigenvalue, percentage of variance explained, and cumulative percentage explained were 2.87 and 2.24, 41.1% and 32.1%, and 41.1% and 73.1%, respectively.
Linear regression indicated significant effect of body mass; residuals used in PC A.
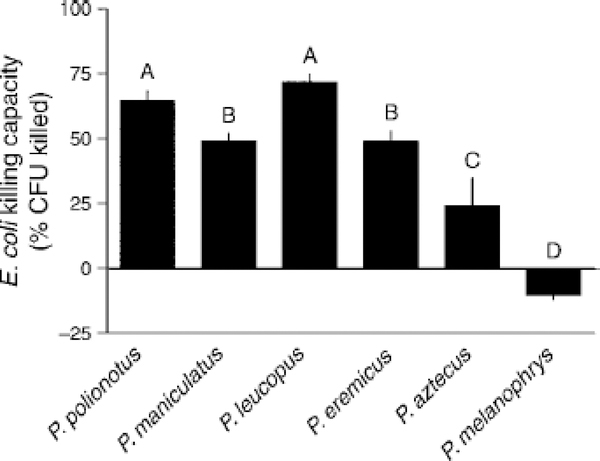
Fig. 2.
Ex vivo E. coli killing capacity of six Peromyscus spedcs ordered by increasing body mass. Letters represent group membership by Tukey-Kramer post hoc tests; bars are means with SE. “CFU” stands for colony-forming units.
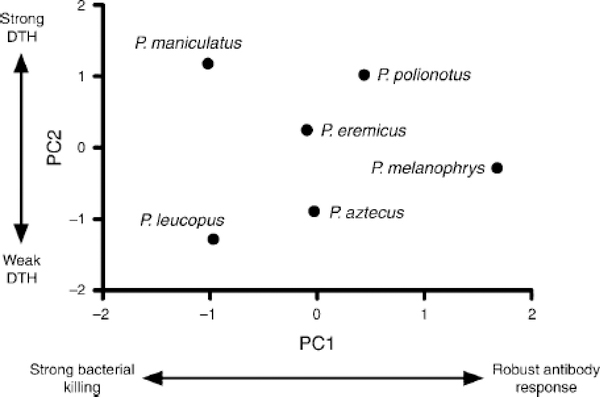
Fig. 6.
Scatterplot of principal components scores for immune data among Peromyscus species. “DTH” stands for delayed-type hypersensitivity.
Results
Life history variation among species
PCA of data in Table 1 produced two PCs capturing 73.1% of the variation in the life history data matrix (Table 2). PC1 represented a continuum of species producing large, heavy litters rapidly at one end and species producing small, lighter litters slowly at the other (Fig. 1b). PC2 represented a continuum of body size and nestling duration with large-bodied species with long nest periods at one end and small-bodied species with short nest periods at the other.
Immunological variation among species
Ability to kill E. coli bacteria varied among species (F5, 115 = 34.1, P< 0.001; Fig. 2). Photoperiod affected killing capacity differently among species, as indicated by a significant species and photoperiod interaction (F5, 115 =4.05, P <0.002). This effect was a consequence of enhanced killing capacity in long vs. short days in P. eremicus (t18 =4.7, P <0.001). Sex and other interactions did not otherwise influence bacterial killing. Healing cutaneous wounds also varied among species (F5, 103 = 3.2, P < 0.01; Fig. 3a), but less so than other immune measures. Unlike bacterial killing, neither photoperiod, sex, nor any interaction affected the duration of wound healing. Similar interspecific variation was reflected in terms of physical inflammation at the wound site (F5, 103 = 2.63, P < 0.03; Fig. 3b), but no pairwise differences were detected. Photoperiod, sex, and interactions did not affect wound site swelling.
Fig. 3.
(a) Duration of wound healing and (b) inflammation at the wound site during the first three days post-wounding among Peromyscus species ordered by increasing body mass. Letters represent group membership by Tukey-Kramer post hoc tests; bars are means with SE.
Primary antibody production against KLH differed among species (F10, 190 ¼ 4.9, P < 0.001); marginally significant effects of sex and photoperiod on the primary response were also detected (F10, 190 =1.9, P = 0.06). Secondary antibody responses also varied among species (F5,95 =7.1, P<0.001), but sex, photoperiod, and interactions did not influence secondary responses. Rate of primary antibody response varied among species (F5, 119 =12.9, P<0.01; Fig. 4a), as did rate of the secondary response (F5, 119 =4.2, P < 0.02; Fig. 4b); photoperiod, sex, and interactions did not affect 0.001; Fig. 5a) and total (F5, 113 =72.8, P< 0.001; Fig. 5b) T cell-mediated inflammation. Two statistically significant interactions, species and sex (F5, 113 =3.7, P<0.004) and species and photoperiod (F5, 113 ¼ 3.1, P ¼ 0.014) were also indicated; sex alone did not affect maximum T cell-mediated inflammation. Differential effects of photoperiod on the maximum swelling responses within species were not statistically significant, but both P. leucopus and P. maniculatus tended to show higher DTH responses in short vs. long days (P< 0.1), as in previous studies (Bilbo et al. 2002, Pyter et al. 2005). The differential effects of sex on DTH within species stemmed from female P. leucopus (t17 =2.3, P < 0.003) and P. aztecus (t17 =−2.4, P < 0.03) having greater swelling responses than conspecific males. In terms of total swelling over the measured period, there were also significant interactions between species and sex (F5, 113 = 3.3, P < 0.009) and between species and photoperiod (F5, 113 = 3.41, P < 0.007). As with maximum swelling, the differential effects of sex on total swelling were due to elevated measures in female vs. male P. aztecus (t17 = −3.4, P < 0.004). Similarly, differential effects of photoperiod on total swelling among species stemmed from greater values in short vs. long days in P. maniculatus (t17 =−2.3, P < 0.03) and P. leucopus (t17 = −2.1, P< 0.05).
Fig. 4.
(a) Rate of primary response, (b) rate of secondary response, (c) total antibodies (AB) produced during primary response, (d) total antibodies produced during secondary response, (e) total antibody titer produced over primary and secondary responses, and (f) maximum antibodies among Peromyscus species ordered by increasing body mass. Letters represent group membership by Tukey-Kramer post hoc tests; bars are means + SE.
Fig. 5.
(a) Maximum and (b) aggregate T-cell-mediated inflammation among Peromyscus species ordered by increasing body mass. Letters represent group membership by Tukey-Kramer post hoc tests; bars are means + SE.
Intraspecies variation in immune defenses
PCA of the six antibody response variables produced two principal components (PC1 eigenvalue, 3.7, percentage variance explained 62%; PC2 eigenvalue, 1.6, variance explained 27%). PC1 was most strongly correlated to the primary antibody response (rate of production and primary IgG produced), and PC2 most strongly correlated to the secondary antibody response (secondary IgG produced; Table 3). Fmax tests indicated that immune measures were more variable in some species than others (all P< 0.01). Variance for bacterial killing capacity was greater in P. aztecus than the other five species; P. polionotus also exhibited greater variance than P. melanophrys. The only significant pairwise difference for either antibody PC was the greater variance in the primary response (PC1) in P. eremicus vs. P. polionotus. Wound healing and DTH, on the other hand, were substantially more variable in some species than others. Variance in swelling at the wound site in P. eremicus was significantly greater than P. maniculatus and P. leucopus. Variance in P. leucopus was also significantly greater than P. melanophrys and P. polionotus. In terms of duration until wound closure, P. melanophrys was more uniform than all other species. Maximum swelling during DTH was more variable in P. maniculatus and P. polionotus than the other four species; the two species were not different from each other however. As with maximum swelling, P. maniculatus and P. polionotus were more variable than all other species in terms of total swelling, but not each other; P. aztecus was more variable than all species but P. melanophrys, and P. eremicus was significantly more variable than P. leucopus.
Table 3.
Principal components analysis results for Peromyscus immune data.
| Measure | Correlation coefficients with PCs† |
||
|---|---|---|---|
| PCI | PC2 | PC3 | |
| Bacteria killing | −0.76 | 0.17 | 0.13 |
| Rate primary | 0.96 | −0.14 | 0.23 |
| Total primary | 0.99 | 0.09 | −0.01 |
| Rate secondary | −0.46 | 0.29 | −0.76 |
| Total secondary | 0.97 | −0.13 | 0.15 |
| Total ABs | 0.99 | −0.07 | 0.1 1 |
| Maximum titer | 0.94 | −0.11 | −0.20 |
| Maximum DTH swell | −0.07 | 1.00 | 0.00 |
| Total DTH swell | −0.07 | 0.98 | −0.16 |
| Da vs to heal | −0.84 | 0.03 | 0.16 |
| Wound swell | −0.13 | −0.02 | 0.98 |
Note: Abbreviations are: DTH, delayed-type hypersensitivity; AB, antibody.
For principal components 1, 2, and 3 the eigenvalue, percentage of variance explained, and cumulative percentage explained were 6.73, 2.23, and 1.56; 61.18%, 20.31%, and 14.18%; and 61.18%, 81.5%, and 95.7%, respectively.
Interrelationships between immune variables
Backward linear regression was used to identify relationships between immune variables, but as only bacterial killing capacity and antibody production were measured in the same individuals, only these variables were considered. Backward regression analysis of antibody response PC scores and sex and photoperiod predicted bacterial killing capacity in some species. In P. melanophrys (p=-0.052; P < 0.046), PC1 was negatively related to bacterial killing; a similar but nonsignificant trend was detected in P. aztecus (p =−0.25; P < 0.07). In P. polionotus (p = −0.11; P < 0.04), PC2 was negatively related to bacterial killing; in P. maniculatus, a significant positive relationship was detected (p= 0.28; P< 0.004). No significant relationships were detected in the other two species.
Relating immunological to life history variation
To detect covariation between life history and immune data, PCA was first performed on all 11 raw immune variables. PCA of immunological data produced two components capturing 96% of the variation in the immune data matrix (Table 3). The first PC represented a continuum from strong antibody responses and rapid wound healing at one extreme to strong bacterial killing capacity at the other (Fig. 6). The second PC represented differences in T cell-mediated inflammation among species, with high responders at one end and low responders at the other. A third PC separated species that exhibited slow secondary anti-body responses and mild inflammation at the site of wounds from those that exhibited opposite traits.
Canonical correlation analysis did not indicate significant covariation between immune and life history variables (Wilks’ λ = 0.051, F2,6 = 1.1, P < 0.54). However, visual inspection of PC plots (Fig. 6) suggested that this lack of relationship may be due to the small number of species used in the analysis. Indeed, had one species (P. polionotus) occupied a position farther to the left along PC1 for the immune matrix, an influence of body mass and perhaps reproductive pace of life may have been detected. As a conservative way to obviate such patterns, Spearman rank correlations were performed between species’ raw immune parameters and body mass. Other variables were not used to minimize false detection rate. As suggested by Fig. 6, body mass was significantly negatively correlated with bacterial killing capacity (p= -0.83, P <0.04) and days to heal wounds (p=-0.83, P < 0.04); no other correlations were significant.
Discussion
Immunological variability among Peromyscus was extensive, but species were either proficient at killing E. coli or proficient at rapidly generating antibodies against a novel protein, but not both. Species also tended to mount either weak or strong T cell-mediated inflammatory responses; except for one species, T cell inflammation was opposite of antibody production capacity. Wound healing varied modestly among species, with degree of inflammation at the wound site being the most remarkable difference and complementing T cell-mediated inflammation. Species also exhibited differing degrees of intraspecific variability in immune responses, but there was no one immune defense or species consistently more variable than others. Finally, there were no consistent trends between bacterial killing ability and antibody production within species, providing little evidence for antagonism or facilitation of one of these immune responses on another.
These results partially support our initial hypothesis: although all species were captive-bred in the absence of (known) infections, some species favored specific, developmentally expensive immune defenses whereas others favored broadly effective, developmentally cheap ones. In other words, there was little indication that one species was more immunocompetent than the others; species simply use different immunological strategies. As yet, it remains unclear why these particular strategies manifest. Life history orientation was apparently not important, as species producing heavy litters rapidly (the fast pace of life extreme that existed among these species) did not favor bacterial killing and minimize antibody responses as expected. Further, DTH responses did not mirror antibody production, as predicted based on the expense of generating diverse lymphocyte repertoires. We discuss the implications of the existence of an immunological continuum in Peromyscus, address possible mediators of this pattern, and discuss the lack of concordance between life history and immune activity among these Peromyscus.
Immunocompetence or immuno-continuum?
Immunocompetence is usually portrayed as monolithic by ecologists and evolutionary biologists, but the present study suggests that this is inappropriate. In Peromyscus, immunocompetence may be reducible to a few dimensions, but one species was not overall more immunocompetent than others. This suggests that species may use unique immunological strategies, which does not necessarily make some less immunocompetent than others. If broadly valid, many inconsistencies reported in the ecoimmunological literature may simply be the result of incomplete studies (Martin et al. 2006d ). In other words, null or unexpected results may be the consequence of measuring the wrong arm of the immune system in the wrong context. For example, testosterone is not obligately immunosuppressive (Roberts et al. 2004), as the immunocompetence handicap hypothesis (Folstad and Karter 1992), a mechanism for the handicap hypothesis of sexual selection (Hamilton and Zuk 1982), purports it to be. Perhaps this is due to only some immune defenses being sensitive to androgens (Martin et al., in press). Likewise, during stress, immune activity was historically thought to be suppressed. What often occurs during an acute stressor is a redistribution of immune resources out of circulation and into the periphery, perhaps to minimize infection at the site of insult (Dhabhar and McEwen 1997, Viswanathan and Dhabhar 2005). Such heightened appreciation of the immune system as a complex entity in its own right should advance future work in ecoimmunology. Whether the biases from cheap, broadly effective defenses to expensive, specific ones in Peromyscus also hold for other vertebrates is as yet unknown. However, it is intriguing that this pattern was predictable based simply on the costs vs. benefits of different types of immune activity (Leshchinsky and Klasing 2001, Schmid-Hempel and Ebert 2003, Lee 2006, Martin et al. 2006d).
What produces the Peromyscus immune continuum?
Contrary to our prediction, reproductive pace of life did not drive variation in immune defense among these Peromyscus. In part, this outcome may be due to the small number of species used. Additional species would have been valuable, as is indicated by the positioning of certain species within the life history and immune data matrices, and may have changed the overall outcome of the study. In terms of reproductive life history, P. melanophrys produces much heavier litters than would be predicted from its large body mass, so it may not be representative of slow-paced Peromyscus species. The same is true for P. polionotus (along PC1) and P. leucopus (PC2) in regard to immune measures. Addition of other small-bodied species may have revealed that fast-paced species by-and-large favor bacterial killing and cell-mediated inflammation more so than larger, slow-paced species. Indeed, body mass was predictive of some immune measures, including bacterial killing and wound healing rate. Thus, life history orientation may yet influence immunological variation among Peromyscus, but was undetectable using these species here. Indeed, all Peromyscus are fast-living species compared to many other mammals; broader comparative studies may yet find an influence of life history on immune defense. Of course at present, the most conservative perspective is that life history orientation does not affect immune defense in Peromyscus. What else might?
One possibility is the number and types of parasites and pathogens species have encountered in ecological or evolutionary time. At present, the diseases that have shaped the Peromyscus immune system are unknown, but as some species inhabit dry deserts (P. eremicus and P. melanophrys) and others inhabit montane cloud forests (P. aztecus), coastal areas (P. polionotus), or temperate deciduous forests (P. leucopus and P. maniculatus), differential exposure to disease is possible (King 1968). Variation in habitat type and subsequent disease exposure cannot explain the specific immunological pattern that was detected among species even if the diseases that are experienced by wild Peromyscus are identified. It is intriguing, therefore, that the most cosmopolitan species, P. leucopus and P. maniculatus, are quite distinct immunologically. Recent work in passerines suggests that successful avian invaders (Passer domesticus) invest less in immune defense than reproduction compared to less successful invaders (Lee and Klasing 2004, Lee et al. 2005, 2006). Further, the successful colonization of North America by one especially successful invader, the House Sparrow (P. domesticus), is related to a decreased reliance on the expensive immune defenses used by its European ancestors (i.e., systemic inflammatory responses; K. A. Lee, K. C. Klasing, L. B. Martin, L. Fusani, G. Sorci, B. Faivre, and M. Wikelski, unpublished manuscript). Perhaps the reliance of P. leucopus and P. maniculatus on cheap defenses is reflective of similar defense investment strategies in species capable of invading and persisting in a variety of environments. In other words, cosmopolitan species may bias their immune systems toward broadly effective, cheap immune defenses to allow them to succeed in a variety of habitats.
One caveat to this hypothesis is that populations within species may exhibit as much phenotypic variation as species themselves (Bronson 1988). P. leucopus (Virginia) and P. maniculatus (Michigan) in the current study are from a single location, and may not be immunologically representative of other populations. Indeed, immunological variation exists among Peromyscus populations in terms of sensitivity of the immune system to environmental cues (Demas et al. 1996). Day length in particular affects the immune systems of many vertebrates (Nelson and Demas 1996, Nelson 2004), which motivated one component of the present study (the housing of animals in long- and short-day conditions). Generally, photoperiod effects on immune responses were similar to other studies; T cell-mediated inflammation was reduced in long days but elevated in short days in the fast-lived species (Bilbo et al. 2002, Pyter et al. 2005); other measures were not as labile.
A third possible explanation for the immune orientation we detected involves mating system. In females of polygamous primate species, leukocyte counts in circulation are high (Nunn et al. 2000). The functional interpretation of higher immune cell densities is difficult, but may indicate a reliance on greater surveillance by cheap, nonspecific immune defenses in polygamous species. Polygamous Peromyscus, including P. leucopus and P. maniculatus, may rely on cheap defenses because they tend to mate with multiple individuals, which may impose high reproductive costs or expose them to diseases conducive to nonspecific immune defense control. At present, this hypothesis cannot be tested because the mating system is unknown for the other four species (although P. polionotus is thought to be socially monogamous). Along these lines, some immune parameters differed between sexes. In the cost–benefit context motivating the present study, female mammals would be expected to invest less in immune defense than males as the costs of reproduction are greater for them (Westneat and Birkhead 1998). Immune defenses regardless of type were generally stronger in females than males though, although sex differences were modest compared to interspecific variation. The pattern of female immune activity being greater than male is typical of most mammals (Klein 2000a), perhaps because of pleiotropic effects of androgens and estrogens (Klein 2000b, Klein et al. 2002).
All of the above hypotheses warrant testing, but none rectify the lack of covariance between T-(DTH) and B-cell (antibody production) defenses among species. The high developmental costs of mediators of DTH and antibody responses led us to predict that both defenses would be robust in slow-paced species. Fast-paced species, however, mounted large T cell-mediated inflammatory responses and small antibody responses. As T cells mediate, but do not necessarily cause inflammation (Janeway 2004), it may be that inflammatory mediators (e.g., neutrophils, basophils, macrophages) differed in such a way as to obscure the role of T cells in the DTH response (Sroga et al. 2003). Indeed, a tendency to rely on nonspecific bacterial killing ability and a predilection for more inflammation at wound sites in P. leucopus and P. maniculatus suggests that inflammatory defenses are favored in these species, perhaps because of their low costs. Because B cells are directly responsible for antibody production, antibody production is probably a more reliable surrogate for B-cell mediated immune function than DTH is for T cell-mediated immune function. A related issue is the lack of significant correlations within species between bacterial killing and antibody production. This may also be seen as unexpected, but is difficult to interpret given the small number and inconsistent direction of correlations.
Conclusion
Peromyscus species either favored cheap, nonspecific defenses or developmentally expensive, specific ones. Immune defense strategy was not correlated with reproductive pace of life, thus immunological variation among species may be a consequence of prior selection in different disease environments, geographic distribution, mating system, or other factors. As many studies have identified relationships between reproductive life history and immune defense in vertebrates, further study in Peromyscus and other mammals is warranted. Data on longevity and reproductive periodicity, which are currently unavailable for most Peromyscus, would be valuable. Furthermore, identification of the role of immunopathology in future studies may be insightful (Rå berg et al. 1998, Graham et al. 2005). In sum, we hope that this study provides an impetus to identify whether the continuum of immunological strategies among Peromyscus also exist in other vertebrates, and if so why. As importantly, we hope this study encourages other ecoimmunologists to account for the complex, but intelligible, nature of the immune system in their own work.
Acknowledgments
This research was supported by NIH Grants MH 57535 and MH 66144 and NSF Grant IBN 04-16897. Additional support was received from NIH Grant P30NS045758. The authors thank K. J. Navara, B. C. Trainor, K. C. Klasing, S. L. Klein, K. A. Lee, A. Scheuerlein, and M. Wikelski for feedback; J. R. Kuhlman, S. L. Kidder, J. Maloney, and M. Hamway for help with data collection and analysis; and R. Beattie, W. Dawson, and J. Crossland at the Peromyscus Genetic Stock Center for information on disease screens and life history traits.
Literature Cited
- Abouheif E 1999. A method to test the assumption of phylogenetic independence in comparative data. Evolutionary Ecology Research 1:895–909. [Google Scholar]
- Ardia DR 2005. Tree swallows trade off immune function and reproductive effort differently across their range. Ecology 86:2040–2046. [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, and Nelson RJ 2002. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proceedings of the National Academy of Sciences (USA) 99:4067–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, and Sorci G 2003. Assessing the cost of mounting an immune response. American Naturalist 161:367–379. [DOI] [PubMed] [Google Scholar]
- Botten J, Ricci R, and Hjelle B 2001. Establishment of a deer mouse (Peromyscus maniculatus rufinus) breeding colony from wild-caught founders: comparison of reproductive performance of wild-caught and laboratory-reared pairs. Comparative Medicine 51:314–318. [PubMed] [Google Scholar]
- Bronson FH 1988. Mammalian reproductive strategies: genes, photoperiod and latitude. Reproduction Nutrition Development 28:335–347. [DOI] [PubMed] [Google Scholar]
- Carleton MD 1980. Phylogenetic relationships in neotomine-peromyscine rodents (Muroidea) and a reappraisal of the dichotomy within New World Cricetinae. Miscellaneous Publications, University of Michigan, Museum of Zoology; 157:1–146. [Google Scholar]
- Demas GE 2004. The energetics of immunity: a neuroendocrine link between energy balance and immune function. Hormones and Behavior 45:173–180. [DOI] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, and Nelson RJ 1997. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 42:R1631–R1637. [DOI] [PubMed] [Google Scholar]
- Demas GE, Klein SL, and Nelson RJ 1996. Reproductive and immune responses to photoperiod and melatonin are linked in Peromyscus subspecies. Journal of Comparative Physiology A 179:819–825. [DOI] [PubMed] [Google Scholar]
- Demas GE, and Nelson RJ 2003. Lack of immunological responsiveness to photoperiod in a tropical rodent, Peromyscus aztecus hylocetes. Journal of Comparative Physiology B 173:171–176. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, and McEwen BS 1997. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain, Behavior, and Immunity 11:286–306. [DOI] [PubMed] [Google Scholar]
- Folstad I, and Karter AJ 1992. Parasites, bright males, and the immunocompetence handicap. American Naturalist 139: 603–622. [Google Scholar]
- Ghalambor CK, and Martin TE 2001. Fecundity–survival trade-offs and parental risk-taking in birds. Science 292:494–497. [DOI] [PubMed] [Google Scholar]
- Glasper ER, and Devries AC 2005. Social structure influences effects of pair-housing on wound healing. Brain, Behavior, and Immunity 19:61–68. [DOI] [PubMed] [Google Scholar]
- Graham AL, Allen JE, and Read AF 2005. Evolutionary causes and consequences of immunopathology. Annual Review of Ecology, Evolution, and Systematics 36:373–397. [Google Scholar]
- Hamilton WD, and Zuk M 1982. Heritable true fitness and bright birds: a role for parasites. Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Janeway C 2004. Immunobiology. Garland Science, New York, New York, USA. [Google Scholar]
- King J, editor. 1968. Biology of Peromyscus (Rodentia). Special Publication Number 2. American Society of Mammalogists, Stillwater, Oklahoma, USA. [Google Scholar]
- Klasing K 2004. The costs of immunity. Acta Zoologica Sinica 50:961–969. [Google Scholar]
- Klein SL 2000a. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience and Biobehavioral Reviews 24:627–638. [DOI] [PubMed] [Google Scholar]
- Klein SL 2000b. Hormones and mating system affect sex and species differences in immune function among vertebrates. Behavioural Processes 51:149–166. [DOI] [PubMed] [Google Scholar]
- Klein SL, Bird BH, Nelson RJ, and Glass GE 2002. Environmental and physiological factors associated with Seoul virus infection among urban populations of Norway rats. Journal of Mammalogy 83:478–488. [Google Scholar]
- Lee KA 2006. Linking immune defenses and life history at the levels of the individual and species. Integrative and Comparative Biology 46:1000–1015. [DOI] [PubMed] [Google Scholar]
- Lee KA, and Klasing KC 2004. A role for immunology in invasion biology. Trends in Ecology and Evolution 19:523–529. [DOI] [PubMed] [Google Scholar]
- Lee KA, Martin LB, Hasselquist D, Ricklefs RE, and Wikelski M 2006. Contrasting adaptive immune defenses and blood parasite prevalence in closely related Passer species. Oecologia 150:383–392. [DOI] [PubMed] [Google Scholar]
- Lee KA, Martin LB, and Wikelski MC 2005. Responding to inflammatory challenges is less costly for a successful avian invader, the house sparrow (Passer domesticus), than its less-invasive congener. Oecologia 145:244–251. [DOI] [PubMed] [Google Scholar]
- Leshchinsky TV, and Klasing KC 2001. Divergence of the inflammatory response in two types of chickens. Developmental and Comparative Immunology 25:629–638. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, and Deerenberg C 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88:87–98. [Google Scholar]
- Lyles AM, and Dobson AP 1993. Infectious disease and intensive management: population dynamics, threatened hosts, and their parasites. Journal of Zoo and Wildlife Medicine 24:315–326. [Google Scholar]
- MacArthur RH, and Wilson EO 1967. The theory of island biogeography. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Martin LB, Gilliam J, Han P, Lee K, and Wikelski M 2005. Corticosterone suppresses cutaneous immune function in temperate but not tropical House Sparrows, Passer domesticus. General and Comparative Endocrinology 140: 126–135. [DOI] [PubMed] [Google Scholar]
- Martin LB, Glasper ER, Nelson RJ, and DeVries AC 2006a. Prolonged separation delays wound healing in monogamous California mice, Peromyscus californicus, but not in polygynous white-footed mice, P. leucopus. Physiology and Behavior 87:836–841. [DOI] [PubMed] [Google Scholar]
- Martin LB, Hasselquist D, and Wikelski M 2006b. Immune investments are linked to pace of life in house sparrows. Oecologia 147:565–575. [DOI] [PubMed] [Google Scholar]
- Martin LB, Pless M, Svoboda J, and Wikelski M 2004. Immune activity in temperate and tropical house sparrows: a common-garden experiment. Ecology 85:2323–2331. [Google Scholar]
- Martin LB, Scheuerlein A, and Wikelski M 2003. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proceedings of the Royal Society B 270:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Kuhlman JR, and Nelson RJ 2006c. Trade-offs between cutaneous immune responses in female white-footed mice (Peromyscus leucopus). Functional Ecology 20:630–636. [Google Scholar]
- Martin LB, Weil ZM, and Nelson RJ 2006d. Refining approaches and diversifying directions in ecoimmunology. Integrative and Comparative Biology 46:1030–1039. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, and Nelson RJ In press. Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Philosophical Transactions of the Royal Society B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TE 1996. Life history evolution in tropical and south temperate birds: What do we really know? Journal of Avian Biology 27:263–272. [Google Scholar]
- Martin TE, Moller AP, Merino S, and Clobert J 2001. Does clutch size evolve in response to parasites and immunocompetence? Proceedings of the National Academy of Sciences (USA) 98:2071–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson KD, Cohen AA, Klasing KC, Ricklefs RE, and Scheuerlein A 2006a. No simple answers for ecological immunology: relationships among immune indices at the individual level break down at the species level in waterfowl. Proceedings of the Royal Society B 273:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson KD, Tieleman BI, and Klasing KC 2006b. Capture stress and the bactericidal competence of blood and plasma in five species of tropical birds. Physiological and Biochemical Zoology 79:556–564. [DOI] [PubMed] [Google Scholar]
- Millar JS 1989. Reproduction and development Pages 169–232 in Kirkland GL Jr. and Layne JN, editors. Advances in the study of Peromyscus (Rodentia). Texas Tech University Press, Lubbock, Texas, USA. [Google Scholar]
- Modi W 1984. Reproductive tactics among deer mice of the genus Peromyscus. Canadian Journal of Zoology 62:2576–2581. [Google Scholar]
- Morgan Ernst SK 2003. Life history characters of placental non-volant mammals. Ecology 84:3402. [Google Scholar]
- Nelson RJ 2004. Seasonal immune function and sickness responses. Trends in Immunology 25:187–192. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, and Demas GE 1996. Seasonal changes in immune function. Quarterly Review of Biology 71:511–548. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klein SI, and Kriegsfeld LJ 2002. Seasonal patterns of stress, immune function, and disease. Cambridge University Press, New York, New York, USA. [Google Scholar]
- Nunn CL, Gittleman JL, and Antonovics J 2000. Promiscuity and the primate immune system. Science 290: 1168–1170. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, and Sheridan JF 1998. Restraint stress slows cutaneous wound healing in mice. Brain, Behavior, and Immunity 12:64–73. [DOI] [PubMed] [Google Scholar]
- Palacios MG, and Martin TE 2006. Incubation period and immune function: a comparative field study among coexisting birds. Oecologia 146:505–512. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Neigh GN, and Nelson RJ 2005. Social environment modulates photoperiodic immune and reproductive responses in adult male white-footed mice (Peromyscus leucopus). American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 288:R891–R896. [DOI] [PubMed] [Google Scholar]
- Rå berg L, Grahn M, Hasselquist D, and Svensson E 1998. On the adaptive significance of stress-induced immunosuppression. Proceedings of the Royal Society B 265:1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, and Wikelski M 2002. The physiology/life-history nexus. Trends in Ecology and Evolution 17:462–468. [Google Scholar]
- Roberts ML, Buchanan KL, and Evans MR 2004. Testing the immunocompetence handicap hypothesis: a review of the evidence. Animal Behaviour 68:227–239. [Google Scholar]
- Schmid-Hempel P, and Ebert D 2003. On the evolutionary ecology of specific immune defence. Trends in Ecology and Evolution 18:27–32. [Google Scholar]
- Sheldon BC, and Verhulst S 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology and Evolution 11:317–321. [DOI] [PubMed] [Google Scholar]
- Sroga JM, Jones TB, Kigerl KA, McGaughy VM, and Popovich PG 2003. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. Journal of Comparative Neurology 462:223–240. [DOI] [PubMed] [Google Scholar]
- Stearns SC 1992. The evolution of life histories. Oxford University Press, Oxford, UK. [Google Scholar]
- Tella JL, Scheuerlein A, and Ricklefs RE 2002. Is cell-mediated immunity related to the evolution of life-history strategies in birds? Proceedings of the Royal Society B 269: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieleman BI, Williams JB, Ricklefs RE, and Klasing KC 2005. Constitutive innate immunity is a component of the pace-of-life syndrome in tropical birds. Proceedings of the Royal Society B 272:1715–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, and Dhabhar FS 2005. Stress-induced enhancement of leukocyte trafficking into sites of surgery or immune activation. Proceedings of the National Academy of Sciences (USA) 102:5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westneat DF, and Birkhead TR 1998. Alternative hypotheses linking the immune system and mate choice for good genes. Proceedings of the Royal Society B 265:1065–1073. [Google Scholar]
- Williams GC 1966. Adaptation and natural selection. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Williams GC, and Nesse RM 1991. The dawn of Darwinian medicine. Quarterly Review of Biology 66:1–22. [DOI] [PubMed] [Google Scholar]