Abstract
Success of transplantation is not limited to initial receipt of a donor organ. Many kidney transplant recipients experience graft loss following initial transplantation and the benefits of expedited placement on the waiting list and retransplantation extend to this population. Factors associated with access to repeat transplantation may be unique given experience with the transplant process and prior viability as a candidate. We examined the incidence, risk factors, secular changes, and center-level variation of preemptive relisting or transplantation (PRLT) for kidney transplant recipients in the United States with graft failure (not due to death) using Scientific Registry of Transplant Recipients data from 2007 to 2018 (n = 39 557). Overall incidence of PRLT was 15% and rates of relisting declined over time. Significantly lower PRLT was evident among patients who were African American and Hispanic, males, older, obese, publicly insured, had lower educational attainment, were diabetic, had longer dialysis time prior to initial transplant, shorter graft survival, longer distance to transplant center, and resided in distressed communities. There was significant variation in PRLT by center, median = 13%, 10th percentile = 6%, 90th percentile = 24%. Cumulatively, results indicate that despite prior access to transplantation, incidence of PRLT is modest with pronounced clinical, social, and center-level sources of variation suggesting opportunities to improve preemptive care among patients with failing grafts.
Keywords: clinical research/practice, epidemiology, ethnicity/race, gender, health services and outcomes research, kidney failure/injury, kidney transplantation/nephrology, retransplantation
1 |. INTRODUCTION
In 2018, 10.3% of all kidney transplant recipients and 8.3% of new kidney transplant waitlist additions in the United States comprised patients who experienced graft failure from a prior kidney transplant.1 Despite an increased risk of graft failure among these patients, retransplantation is efficacious, improving survival relative to maintenance dialysis, and timely access to care is important.2–4 Preemptive listing (prior to dialysis initiation) is advantageous for transplant candidates under both the former and current Kidney Allocation Policy (KAS), given that there is an increased priority to receive a deceased donor offer.5,6
Numerous studies have evaluated disparities in care and identified barriers to timely access to care to kidney transplantation for patients with end-stage renal disease (ESRD). Studies have identified race and ethnicity, body mass index, health insurance, gender, age, community risk factors, and primary cause of kidney disease as factors associated with time to placement on a waiting list for transplantation.7–12 Many of these factors are also associated with preemptive transplantation, which continues to occur in a small minority of transplants in the United States.13,14 Factors that are associated with access to repeat transplantation may be unique as compared to patients without a prior transplant, given prior navigation of processes to acquire a transplant and prior qualification as a viable transplant candidate. In addition, the duration, complications, and reasons for graft failure from initial transplantation may predispose patients’ willingness and viability for a repeat transplant. Patients with a prior transplant may also have relatively reduced barriers to care given prior or ongoing care at a transplant center. Although transplant centers may have varying levels and duration of posttransplant care for patients following transplantation, the type and frequency of follow-up in the posttransplantation period may influence rates of timely care for retransplantation if applicable.15 There are currently limited studies evaluating the incidence and sources of variation associated with access to repeat transplantation among patients with failing kidney transplants.
The aims of this study were to evaluate the incidence of preemptive placement on the waiting list or retransplantation (PRLT) for patients with graft failure following initial kidney transplantation in the United States. In addition, we sought to evaluate variation in PRLT by patient characteristics and individual transplant center. Finally, we sought to evaluate whether incidence and risk factors for PRLT may have changed over time. Cumulatively the intent of the study was to characterize patients with differential rates of PRLT and assess variation in practice that may identify opportunities for improved care delivery among patients with failing kidney transplant grafts in the United States.
2 |. METHODS
The data source for this study was the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere.16 The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
The study population consisted of adult (age 18+ years) primary kidney transplant recipients in the United States who experienced graft failure, return to dialysis, or repeat transplantation between 2007 and 2018. We excluded patients with death dates within 90 days of graft failure, considering these patients as likely ineligible for relisting based on clinical prognoses. The primary exposure variable was PRLT, defined by a waitlist placement date or retransplant date prior to date of reinitiation of dialysis following primary transplantation. However, as the 90-day period was a relatively arbitrary time period, as a sensitivity analysis, we described the incidence of PRLT with different time frame definitions as well as eliminating the provision that death could not be a cause of graft failure. In addition, we examined trends in the cumulative incidences of relisting and transplantation using competing risks survival models with death considered a competing risk.17 As the time “at risk” for relisting or transplantation prior to graft failure (eg, as patients’ renal function declines to a level applicable for retransplantation such as an estimated glomerular filtration rate <20 mL/min per 1.73 m2) was not available in the database, these models were limited to patients who were not relisted or transplanted preemptively and the inception time of the model was the data of graft failure. Thus, we evaluated both the incidence of preemptively relisting or transplantation and the cumulative incidence over time of relisting or transplantation following graft failure as applicable.
We used descriptive statistics to evaluate the proportion of patients with PRLT and multivariable logistic models based on patient and donor characteristics. In addition to patient demographic and clinical conditions available at the time of transplantation, we also included time of graft survival, distance to the transplant center (based on centroid of residential and transplant center zip codes), and distressed communities index based on patients’ primary residence.18 The distressed community index incorporates multiple factors associated with residential communities including poverty and vacancy rates, educational attainment, employment, income, and changes in business establishments. The association of these factors with health outcomes for residents of communities with these characteristics has been demonstrated in numerous contexts including among transplant patients.19–21 We categorized missing data as a level for applicable variables and included these levels in statistical models. We also examined potential interactions based on a priori hypotheses between age of candidates and other patient characteristics for the incidence of PRLT.
In order to assess continuity of care at concordant transplant centers and/or patient mobility, we described the proportion of patients who were placed on the waiting list or transplanted at the same center (based on de-identified transplant center code) among those who were relisted or transplanted during the study period. We evaluated adjusted probabilities of PRLT by transplant center as well as generated Standardized Incident Ratios (SIR) based on multivariable logistic models and applicable confidence intervals based on observed and expected incidence. We described the proportion of centers with statistically higher and lower adjusted incident ratios of PRLT over the study period. The study was approved by the Cleveland Clinic Institutional Review Board. All analyses were performed in SAS (v. 9.4., SAS Institute, Inc., Cary, NC).
3 |. RESULTS
The study population was 39 557 patients with a failed primary transplant graft (not due to death) between 2007 and 2018 following primary kidney transplantation. We excluded 2969 patients based on death within 90 days after graft loss. The overall incidence of PRLT was 15.3%, including 3.0% with a preemptive retransplant and 15.1% preemptively relisted (some of whom were also retransplanted). Varying the 90-day threshold altered the estimated proportion of PRLT from 14.6% (with no survival requirement) to 16.0% with minimum 1-year survival following graft loss (Supplementary Table S1). Including patients with graft loss due to death reduced the proportion of PRLT to 8.8%. Table 1 provides clinical and demographic characteristics of the study population, the proportion of patients with PRLT, and the adjusted odds ratios for likelihood of PRLT by patient characteristics. The proportion of patients with PRLT was statistically significantly lower among older patients, African American, Hispanic, and Other race/ethnicities and males. Lower PRLT was also evident among patients who were obese, with a failed graft in more recent years, publicly insured, diabetic, highly sensitized, longer pretransplant dialysis prior to initial transplant, and initially received a deceased donor transplant. Recipients with the shortest initial time to graft failure had the lowest incidence of PRLT, with corresponding higher incidence among patients with longer graft survival. Finally, recipients who resided in more distressed communities, had longer travel distance to the transplant center, and had lower educational attainment had significantly reduced incidence of PRLT.
TABLE 1.
Proportion and adjusted odds of patients relisted or retransplanted among patients with graft failure (n = 39 557)
| Patient characteristic | Level, number of patients (%) | Proportion of preemptively listed or transplanted patients, % (n)a | Adjusted odds ratio (95% CI) |
|---|---|---|---|
| Age at transplant (y) | 0–11 | 25.7% (358) | 1.66 (1.40–1.97) |
| 12–17 | 14.6% (301) | 0.83 (0.71–0.98) | |
| 18–34 | 17.3% (1601) | (Reference) | |
| 35–54 | 16.8% (2809) | 0.97 (0.89–1.05) | |
| 55–64 | 11.8% (836) | 0.68 (0.61–0.76) | |
| 65+ | 5.1% (159) | 0.31 (0.26–0.37) | |
| Race/ethnicity | White | 18.3% (3317) | (Reference) |
| African American | 12.6% (1721) | 0.88 (0.81–0.94) | |
| Hispanic | 12.0% (654) | 0.83 (0.75–0.91) | |
| Asian | 17.8% (292) | 1.14 (0.99–1.31) | |
| Other | 11.6% (80) | 0.84 (0.66–1.08) | |
| Gender | Female | 16.0% (2570) | 1.10 (1.03–1.17) |
| Male | 14.9% (3494) | (Reference) | |
| Body mass index (kg/m2)b | 13–20 | 18.6% (711) | 0.98 (0.87–1.09) |
| 21–25 | 16.2% (1643) | (reference) | |
| 26–30 | 14.9% (1628) | 1.00 (0.92–1.08) | |
| 31–35 | 12.9% (939) | 0.91 (0.83–0.99) | |
| 36+ | 11.6% (462) | 0.82 (0.73–0.92) | |
| Year of graft failure | 2007–2009 | 15.4% (1483) | (Reference) |
| 2010–2012 | 16.4% (1725) | 1.01 (0.93–1.09) | |
| 2013–2014 | 15.5% (1120) | 0.91 (0.83–0.99) | |
| 2015–2018 | 14.3% (1736) | 0.80 (0.74–0.88) | |
| Primary insurance type | Private | 20.9% (3192) | (Reference) |
| Medicare | 11.4% (2366) | 0.73 (0.68–0.79) | |
| Medicaid | 13.4% (374) | 0.71 (0.62–0.80) | |
| Other | 18.6% (132) | 0.99 (0.81–1.21) | |
| Primary diagnosis | Glomerulonephritis | 17.8% (2086) | (Reference) |
| Diabetes | 10.3% (835) | 0.64 (0.58–0.70) | |
| Polycystic kidney disease | 20.2% (515) | 1.00 (0.89–1.12) | |
| Hypertension | 12.8% (1046) | 0.92 (0.85–1.01) | |
| Other | 17.7% (1582) | 0.97 (0.90–1.05) | |
| Peak PRA prior to transplantb | 0% | 16.0% (3075) | (Reference) |
| 1%−10% | 17.3% (1406) | 1.12 (1.04–1.20) | |
| 11%−80% | 14.5% (1117) | 1.06 (0.98–1.16) | |
| 81%+ | 13.4% (301) | 1.02 (0.89–1.17) | |
| Dialysis time prior to transplant | None | 24.7% (1564) | (Reference) |
| 1–12 mo | 19.1% (1449) | 0.77 (0.71–0.84) | |
| 13–24 mo | 14.5% (979) | 0.62 (0.56–0.68) | |
| 25–48 mo | 12.2% (1117) | 0.57 (0.52–0.63) | |
| 49–72 mo | 11.0% (577) | 0.56 (0.50–0.64) | |
| 73+ mo | 8.6% (378) | 0.47 (0.41–0.54) | |
| Donor type | Living | 19.7% (2813) | (Reference) |
| Deceased | 12.9% (3251) | 1.08 (1.01–1.16) | |
| Time to graft failure from primary transplant | 0–24 mo | 5.5% (414) | (Reference) |
| 25–48 mo | 12.0% (710) | 2.17 (1.91–2.47) | |
| 49–72 mo | 16.1% (926) | 3.00 (2.64–3.41) | |
| 73+ mo | 19.7% (4014) | 3.43 (3.03–3.88) | |
| Residential distress indexb | 0–20 | 16.9% (614) | (Reference) |
| 21–40 | 14.1% (444) | 0.87 (0.76–1.00) | |
| 41–60 | 13.4% (473) | 0.90 (0.79–1.03) | |
| 61–80 | 12.0% (441) | 0.85 (0.74–0.98) | |
| 81–100 | 11.0% (561) | 0.81 (0.71–0.93) | |
| Distance to transplant center (miles)b | 0–30 miles | 13.3% (1834) | (Reference) |
| 31–60 miles | 12.4% (440) | 0.87 (0.77–0.97) | |
| 61–120 miles | 10.7% (348) | 0.80 (0.70–0.91) | |
| 121–300 miles | 12.0% (237) | 0.93 (0.80–1.08) | |
| 301+ miles | 10.3% (48) | 0.71 (0.52–0.98) | |
| Educational attainmentc | <High school | 9.5% (154) | 0.67 (0.54–0.84) |
| High school | 12.4% (1618) | 0.70 (0.60–0.81) | |
| At least some college | 15.9% (1865) | 0.82 (0.71–0.95) | |
| Postgraduate school | 18.6% (295) | (Reference) | |
| Total study population | 15.3% (6064) |
All group comparisons P < .05.
Missing levels not shown.
Among patients 25 years of age or more, missing levels not shown.
In addition to the first-order associations, we tested several interactions based on a priori hypotheses. We examined these associations and displayed the risk-adjusted proportion of PRLT in Figure 1A–D. Figure 1A indicates a statistically significant interaction (P = .04) between age and gender such that females had higher rates of PRLT in younger years, but this relatively higher proportion diminished with age. There was no statistically significant association between recipients’ primary insurance type and age (P = .47, Figure 1B). Rather, recipients with private insurance had higher adjusted PRLT across all age groups. Figure 1C indicates no statistically significant interaction (P = .28) between era and recipient age, suggesting that lower incidence of PRLT in more recent years was consistent across age groups. There was also no statistically significant (P = .17) interaction between race/ethnicity and age for the adjusted incidence of PRLT. Whites and Asians generally had higher adjusted PRLT across all age groups.
FIGURE 1.
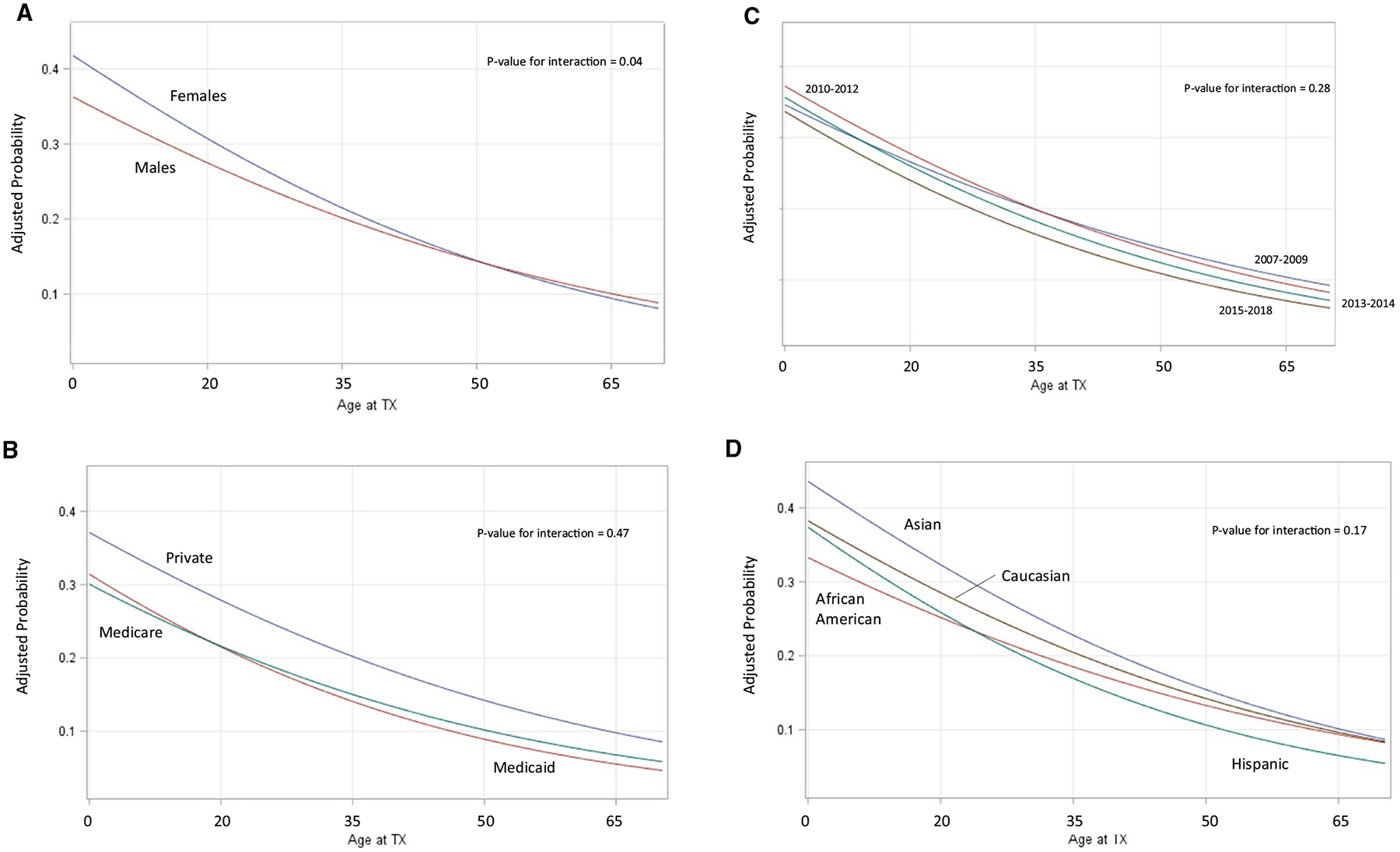
A-D, Adjusted probabilities of relisting or retransplant by interaction of recipient age and transplant and patient characteristics. A, Recipient gender by age: model adjusted for patient age, race/ethnicity, gender, body mass index, year of graft failure, primary insurance type, primary diagnosis, peak panel reactive antibody prior to transplant, dialysis time prior to initial transplant, donor type of initial transplant, time to graft failure from primary transplant, residential distress index, distance to transplant center, and educational attainment. B, Recipient primary insurance by age: model adjusted for patient age, race/ethnicity, gender, body mass index, year of graft failure, primary insurance type, primary diagnosis, peak panel reactive antibody prior to transplant, dialysis time prior to initial transplant, donor type of initial transplant, time to graft failure from primary transplant, residential distress index, distance to transplant center, and educational attainment. C, Recipient era of transplant by age: model adjusted for patient age, race/ethnicity, gender, body mass index, year of graft failure, primary insurance type, primary diagnosis, peak panel reactive antibody prior to transplant, dialysis time prior to initial transplant, donor type of initial transplant, time to graft failure from primary transplant, residential distress index, distance to transplant center, and educational attainment. D, Recipient race/ethnicity by age: model adjusted for patient age, race/ethnicity, gender, body mass index, year of graft failure, primary insurance type, primary diagnosis, peak panel reactive antibody prior to transplant, dialysis time prior to initial transplant, donor type of initial transplant, time to graft failure from primary transplant, residential distress index, distance to transplant center and educational attainment. Tx, transplant
Among patients who were relisted or transplanted after initial transplantation at any point (57% of the original study population), 73% were placed on the waiting list or transplanted at the same center as their primary transplant (Table 2). This proportion was significantly higher (86%) among patients who were relisted preemptively for a repeat transplant as compared to those relisted following graft loss (69%). The proportion of patients placed on the waiting list or retransplanted at any time following primary transplantation varied significantly by race/ethnicity, insurance, age, education, and distance to the center. However, among preemptively listed patients, the proportion listed at concordant centers was higher across all patient characteristics.
TABLE 2.
Proportion of relisted patients placed on the waiting list at concordant transplant centers
| Patient group | Proportion of patients relisted at any time (%) (n = 39 557) | Patients relisted at any time (n = 22 706) | ||
|---|---|---|---|---|
| Proportion listed at concordant center among preemptive listings (%) (n = 6064) | Proportion listed at concordant center among patients on dialysis after graft failure (%) (n = 10 511) | Overall proportion listed at concordant center among relisted patients (%) (n = 16 575) | ||
| All patients | 57% | 86% | 69% | 73% |
| Female patients | 57% | 85% | 69% | 73% |
| Male patients | 57% | 87% | 69% | 73% |
| White patients | 60% | 84% | 67% | 72% |
| African American patients | 53% | 91% | 72% | 76% |
| Hispanic patients | 57% | 88% | 68% | 72% |
| Asian patients | 63% | 85% | 68% | 72% |
| Private primary insured patients | 68% | 84% | 67% | 72% |
| Medicare primary insurance patients | 49% | 88% | 71% | 75% |
| Age 60+ y at transplant | 29% | 94% | 85% | 87% |
| Age 40–59 y at transplant | 53% | 88% | 75% | 79% |
| Age 18–39 y at transplant | 71% | 85% | 64% | 69% |
| Educational attainment <high school | 40% | 95% | 78% | 82% |
| Educational attainment high school | 49% | 89% | 74% | 78% |
| Educational attainment at least some college | 58% | 86% | 72% | 75% |
| Educational attainment graduate school | 61% | 87% | 71% | 76% |
| Distance to initial transplant center <120 miles | 52% | 91% | 75% | 79% |
| Distance to initial transplant center ≥120 miles | 54% | 83% | 64% | 68% |
CI, confidence interval; PRA, panel reactive antibody.
Among patients who were not placed on the waiting list or transplanted preemptively, the cumulative incidences of relisting or retransplantation and mortality over time are displayed in Figure 2. As indicated, rates of relisting and mortality increased most rapidly following initial graft failure. As displayed in Figure 3, both preemptive listing or transplantation and the incidences over time following graft failure declined over the study period, including significantly lower rates among patients with graft failure between 2015 and 2018 (P < .001).
FIGURE 2.
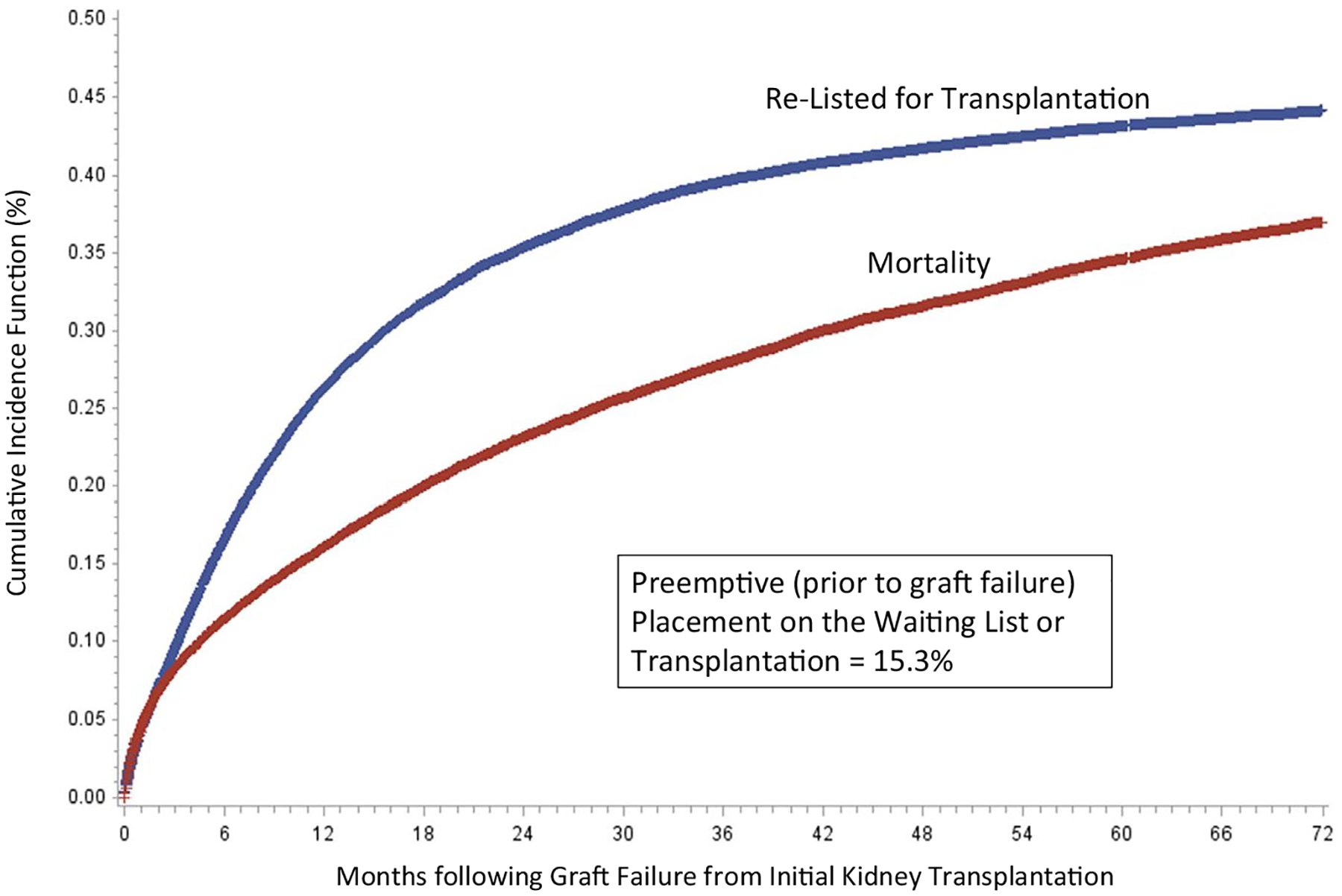
Cumulative incidence of waitlist placement and mortality following graft failure (preemptive placements and deaths prior to graft failure are not included in cumulative incidence models; cumulative incidence function based on Fine and Gray competing risk models)
FIGURE 3.
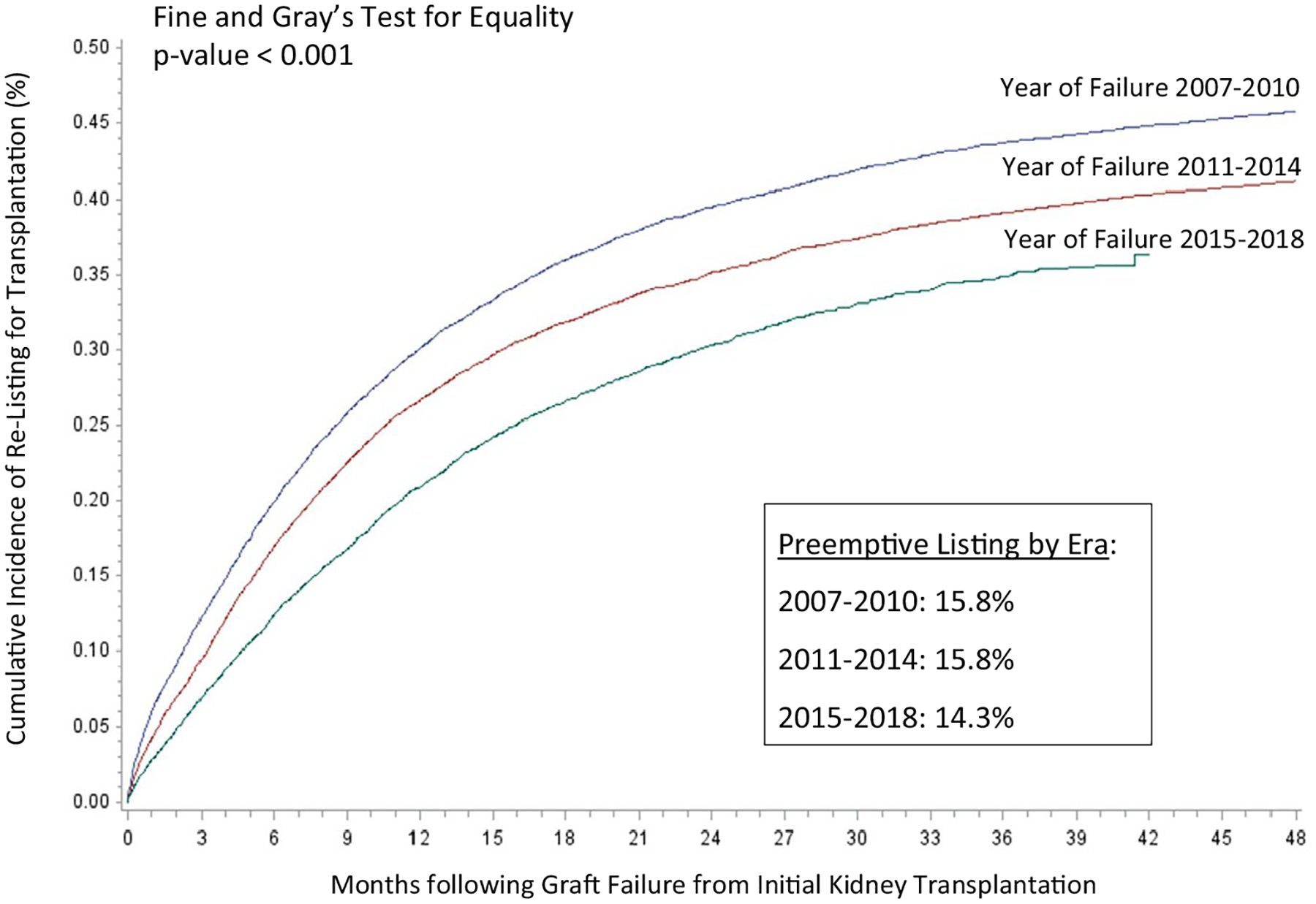
Cumulative incidence of placement on the waiting list for kidney transplantation following graft failure from primary transplant by era (preemptive placements and deaths prior to graft failure are not included in cumulative incidence models; cumulative incidence function is based on Fine and Gray competing risk models)
Figure 4 displays the distribution of the standardized incidence of PRLT among US transplant centers with at least 40 patients over the study period. Among these transplant centers (n = 200), the median center-level PRLT was 13.4% (10th percentile = 6.4% and 90th percentile 24.4%). There was a modest positive correlation (Pearson correlation = 0.28) between the number of patients with failed grafts by center and the center-level incidence of PRLT. Based on the SIR, 71 (34%) centers had a statistically higher than expected incidence of PRLT, including 26 centers with SIR for PRLT >1.5. In contrast, 105 (53%) of centers had a statistically lower than expected incidence of PRLT, including 23 centers with a SIR <0.50 (50% lower incidence than expected).
FIGURE 4.
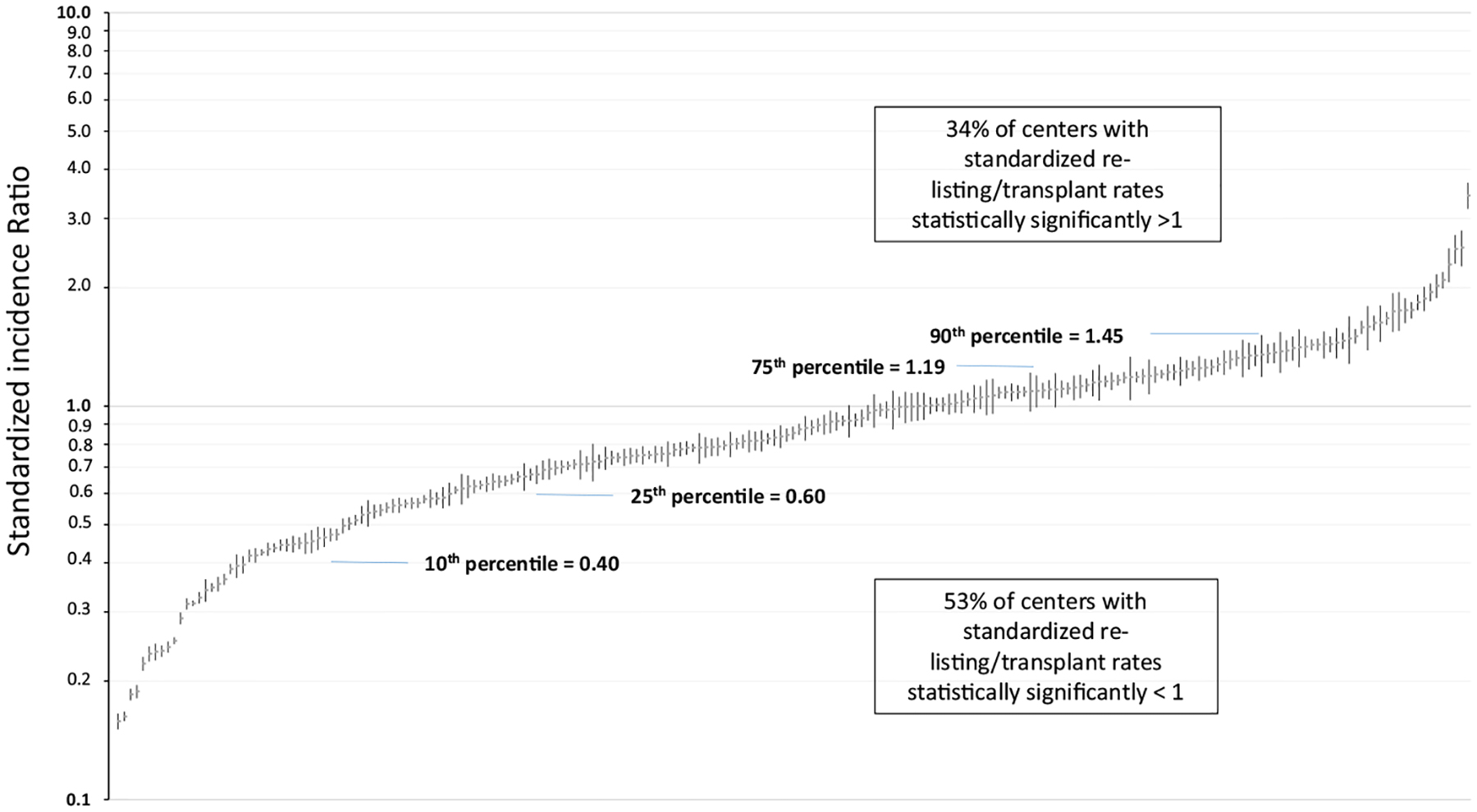
Standardized incidence rate of preemptive listings by US transplant center (among centers with at least 40 patients with failed grafts over the study period; model adjusted for patient age, race/ethnicity, gender, body mass index, year of graft failure, primary insurance type, primary diagnosis, peak panel reactive antibody prior to transplant, dialysis time prior to initial transplant, donor type of initial transplant, time to graft failure from primary transplant, residential distress index, distance to transplant center, and educational attainment)
4 |. DISCUSSION
The primary findings of the study indicated that ≈15% of patients with a failing kidney transplant undergo preemptive placement on a waiting list or are retransplanted in the United States. The proportion of those relisted or transplanted preemptively are also highly variable by patient demographic, clinical, and social factors. In addition, the proportion of patients listed or transplanted preemptively varied significantly by patients’ initial transplant center. Moreover, rates of preemptive and relisting following graft failure have declined over time. Cumulatively, the study results suggest despite the fact that preemptive placement on the waiting list and receiving a retransplant provides a marked benefit to patients, only a minority are placed on the waiting list expeditiously. Based on these results, barriers to timely access to repeat transplantation appear to be prominent, and interventions to improve care delivery should be examined for this population.
The association of demographic characteristics with differential rates of access to primary kidney transplantation has been well characterized among ESRD patients. In general, the current study results confirmed that many of the associations that have been identified as barriers to initial kidney transplantation persist for evaluating access to repeat transplantation. Race and ethnicity were associated with preemptive listing for repeat transplantation, as has been demonstrated in prior studies evaluating access to primary transplantation.9,22,23 These results persisted with adjustment for demographic and clinical factors as well as indicators for socioeconomic status and social factors based on insurance type, educational attainment, and residential distress index. The degree to which these differences are based on timely referral and identification of failing graft function and are amenable to interventions targeting access to care among ESRD patients is important to evaluate.9,23–27 Similar to improving care for the general ESRD population, these factors likely involve earlier recognition of the need for repeat transplant as well as care coordination with community caregivers.28,29 Interestingly, the current findings indicated higher incidence of preemptive care among women with graft failure, which is contrary to results demonstrating lower access to primary kidney transplantation among women.11,30,31 Results further highlighted that the increased PRLT rates were apparent in younger but not older women. The explanation for these differences requires further study, including whether rates of renal function decline during graft failure and causes of graft failure differ by gender.
Public insurance and lower educational attainment were associated with lower rates of preemptive care during graft failure across all age groups, which is consistent with studies demonstrating lower rates of primary transplantation.24,31 These associations may be explained by factors including health literacy, quantity and quality of care in the posttransplant period, coordinating insurance coverage, as well as other social factors that may be perceived as barriers to repeat candidacy.32,33 These results strongly imply that factors beyond clinical factors have a strong association with timely preemptive care. The impact of broader accountable care or universal care models for attenuating racial disparities is not clear, but it is notable that inequities in income-level and racial/ethnic disparities among kidney disease patients persist in countries with less fragmented organizational structure.34–39 In addition, patients who listed preemptively or early after ESRD initiation for primary transplantation had higher rates of PRLT, suggesting that factors such as seeking care proactively, access to providers, and disinclination to dialysis persist for access to repeat transplantation.
In addition to results depicting patient-level factors associated with differential rates of access to retransplantation, findings also indicate variation associated with patients’ initial transplant center. As compared to studies that depict regional and neighborhood-level differences in access to primary transplantation, the current study illustrated significant variation specifically associated with patients’ primary transplant center.40–43 This pronounced variation by transplant center (almost 4-fold differences in adjusted preemptive relisting rates between the 10th and 90th percentiles), which was adjusted for residential distress level and distance to the center, suggests that processes of care, follow-up protocols, and patient selection for retransplantation vary markedly between centers. Importantly, Israni et al demonstrated that only 69% of patients visited their transplant center in posttransplant months 25–36 and that the rate of ongoing care of recipients by transplant centers was lower among certain minority groups.15 Thus, the caregivers most responsible for early referral for repeat transplant may vary widely by transplant center. However, given the reduced rate of preemptive placement on the waiting list for repeat transplantation (particularly among minorities, publicly insured, and less educated patients), it is likely that facilitating more coordinated care with the initial transplant center during follow-up may attenuate these disparities. This may include the degree to which centers continue to follow patients’ posttransplantation in a rigorous manner as well as coordination between other primary care providers and nephrologists. The study results also indicated that preemptive listings were more common among patients listed at the same center as their original transplant, furthering the suggestion that ongoing coordination with the center may improve timely care. Interestingly, there was a positive correlation between transplant center size and preemptive listing, suggesting that the “burden” of following a larger posttransplant population does not systematically inhibit timely care. Perhaps this suggests that larger centers have relatively greater resources to manage their posttransplant populations on average and to facilitate timely care. Further understanding of best care practices that identify patients who have failing grafts and that facilitate preemptive care is important to evaluate in future studies.
Several of the findings suggest that comorbid conditions and clinical factors may affect differential rates of PRLT. Older age, obesity, and diabetes were associated with lower rates of listing for repeat transplantation, consistent with findings associated with access to primary transplantation.11,31,44 These results suggest there are a number of clinical factors, including potential noncodified factors that may render patients nonviable for a repeat transplant procedure.45–48 Interestingly, results demonstrated a strong association between longer graft survival and likelihood of PRLT. This association may be indicative of clinical complications for those with short graft survival that reduce viability for a repeat procedure as well as a complicated transplant experience that may dissuade patients’ desire for a repeat transplant.49 Because these data do not capture the cause of graft failure consistently, this relationship may be partially explained by clinical contraindications due to the nature of the failing graft.
The overall proportion of patients with PRLT in this study was 15% (13% in 2018). While this proportion is modest, there may be a significant proportion of patients who were not viable or interested in a repeat procedure. However, it is notable that more than half (57%) of patients who experienced graft failure and survived were eventually relisted over the study period. Therefore, as a conservative estimate, only ≈26% of patients who were eventually re-evaluated and considered candidates for repeat transplantation were listed preemptively. This suggests that the processes to consider reevaluation for transplant do not commonly occur in a timely manner concurrent with graft failure. Rates of relisting or transplantation following graft failure were also markedly reduced with increasing age at the time of graft failure. It is also notable that beyond preemptive listing, the incidence of repeat listing across all follow-up periods were reduced over the study period. Whether these findings are suggestive of overall reduced acceptance of candidates following graft failure, more complex sources of graft failure that do not result in clinical acceptability or other systematic factors such as regulatory oversight and risk aversion for more complex patients is not clear from the current findings but also warrants further study.50–53 The reduced rates may also be a product of KAS in more recent years, because timely listing is less beneficial following dialysis initiation given the algorithm for prioritization based on dialysis starting time rather than time of placement on the waiting list.
There are several limitations of the study that should be considered for appropriate inferences of the current findings. Based on the observational study design, associations cannot be considered causal and there may be confounding factors not codified that may explain certain associations. Causes of graft loss are not well captured in these data, and although the data are informative to explain certain associations, they may need to be evaluated with other data sources. Data regarding referral patterns and evaluations for candidacy are also not available with the data source for this study and as such, ascertainment of steps to placement on the waiting list for repeat transplantation and patients considered inappropriate for a repeat procedure were not evaluated. Finally, the timing of viability for a repeat transplant based on renal function level was not available in the data used for the analysis to specify the rate of preemptive waitlist placement relative to the timing of eligibility for waitlist prioritization.
Cumulatively, the study results depict relatively modest rates of timely preemptive listing for repeat transplantation prior to graft failure following kidney transplantation. Rates of listing were highly variable by patient demographic and clinical characteristics as well as indicators of social risk factors. There was wide variation in preemptive relisting rates by transplant center, suggesting potential important processes of care that may affect access to repeat transplantation. These findings may inform prospective research studies and interventions and improve access to care and clinical outcomes for patients experiencing graft failure.
Supplementary Material
Abbreviations:
- ESRD
end-stage renal disease
- KAS
Kidney Allocation Policy
- OPTN
Organ Procurement and Transplantation Network
- PRLT
preemptive relisting or transplantation
- SIR
Standardized Incident Ratios
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
Data are openly available in a public repository that issues datasets with DOIs (https://www.srtr.org/about-the-data/the-srtr-database/).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Organ Procurement and Transplantation Network. Transplant: Previous Transplant (Same Organ). 2019. https://optn.transplant.hrsa.gov/data/view-data-reports/build-advanced/
- 2.Clark S, Kadatz M, Gill J, Gill JS. Access to kidney transplantation after a failed first kidney transplant and associations with patient and allograft survival: an analysis of national data to inform allocation policy. Clin J Am Soc Nephrol. 2019;14:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharnidharka VR, Cherikh WS, Neff R, Cheng Y, Abbott KC. Retransplantation after BK virus nephropathy in prior kidney transplant: an OPTN database analysis. Am J Transplant. 2010;10:1312–1315. [DOI] [PubMed] [Google Scholar]
- 4.Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant. 2007;7:1140–1147. [DOI] [PubMed] [Google Scholar]
- 5.Formica RN Jr. Perspectives on the strengths and weaknesses of the national kidney allocation system. Clin J Am Soc Nephrol. 2017;12:2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93:1395–1406. [DOI] [PubMed] [Google Scholar]
- 7.Gill JS, Hendren E, Dong J, Johnston O, Gill J. Differential association of body mass index with access to kidney transplantation in men and women. Clin J Am Soc Nephrol. 2014;9:951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan S, Mutell R, Patzer RE, Holt J, Cohen D, McClellan W. Kidney transplantation and the intensity of poverty in the contiguous United States. Transplantation. 2014;98:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314:582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salter ML, McAdams-Demarco MA, Law A, et al. Age and sex disparities in discussions about kidney transplantation in adults undergoing dialysis. J Am Geriatr Soc. 2014;62:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol. 2013;8:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jay CL, Dean PG, Helmick RA, Stegall MD. Reassessing preemptive kidney transplantation in the United States: are we making progress? Transplantation. 2016;100:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King KL, Husain SA, Jin Z, Brennan C, Mohan S. Trends in disparities in preemptive kidney transplantation in the United States. Clin J Am Soc Nephrol. 2019;14(10):1500–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israni AK, Snyder JJ, Skeans MA, Tuomari AV, Maclean JR, Kasiske BL. Who is caring for kidney transplant patients? Variation by region, transplant center, and patient characteristics. Am J Nephrol. 2009;30:430–439. [DOI] [PubMed] [Google Scholar]
- 16.Leppke S, Leighton T, Zaun D, et al. Scientific registry of transplant recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando). 2013;27:50–56. [DOI] [PubMed] [Google Scholar]
- 17.Fine JP, Gray R. Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Economic Innovation Group. Distressed Communities Index. https://eig.org/dci. Accessed May 20, 2019.
- 19.Akwo EA, Kabagambe EK, Harrell FE, et al. Neighborhood deprivation predicts heart failure risk in a low-income population of blacks and whites in the southeastern United States. Circ Cardiovasc Qual Outcomes. 2018;11:e004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey VM, Enos CW, Chen JT, Galadima H, Eschbach K. The role of neighborhood characteristics in late stage melanoma diagnosis among hispanic men in California, Texas, and Florida, 1996–2012. J Cancer Epidemiol. 2017;2017:8418904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schold JD, Heaphy E, Buccini LD, et al. Prominent impact of community risk factors on kidney transplant candidate processes and outcomes. Am J Transplant. 2013;13:2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gander JC, Zhang X, Plantinga L, et al. Racial disparities in preemptive referral for kidney transplantation in Georgia. Clin Transplant. 2018;32:e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi S, Gaynor JJ, Bayers S, et al. Disparities among Blacks, Hispanics, and Whites in time from starting dialysis to kidney transplant waitlisting. Transplantation. 2013;95:309–318. [DOI] [PubMed] [Google Scholar]
- 24.Johansen KL, Zhang R, Huang Y, Patzer RE, Kutner NG. Association of race and insurance type with delayed assessment for kidney transplantation among patients initiating dialysis in the United States. Clin J Am Soc Nephrol. 2012;7:1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche H-U. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760–1767. [DOI] [PubMed] [Google Scholar]
- 26.Wedd J, Basu M, Curtis LM, et al. Racial, ethnic, and socioeconomic disparities in web-based patient portal usage among kidney and liver transplant recipients: cross-sectional study. J Med Internet Res. 2019;21:e11864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkelmayer WC, Glynn RJ, Levin R, Owen WF, Avorn J. Determinants of delayed nephrologist referral in patients with chronic kidney disease. Am J Kidney Dis. 2001;38:1178–1184. [DOI] [PubMed] [Google Scholar]
- 28.Fishbane S, Nair V. Opportunities for increasing the rate of preemptive kidney transplantation. Clin J Am Soc Nephrol. 2018;13:1280–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helmick RA, Jay CL, Price BA, Dean PG, Stegall MD. Identifying barriers to preemptive kidney transplantation in a living donor transplant cohort. Transplant Direct. 2018;4:e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14:151–164. [DOI] [PubMed] [Google Scholar]
- 31.Schold JD, Srinivas TR, Kayler LK, et al. The overlapping risk profile between dialysis patients listed and not listed for renal transplantation. Am J Transplant. 2008;8:58–68. [DOI] [PubMed] [Google Scholar]
- 32.Taylor DM, Bradley JA, Bradley C, et al. Limited health literacy is associated with reduced access to kidney transplantation. Kidney Int. 2019;95:1244–1252. [DOI] [PubMed] [Google Scholar]
- 33.Thong M, Kaptein AA, Krediet RT, Boeschoten EW, Dekker FW. Social support predicts survival in dialysis patients. Nephrol Dial Transplant. 2007;22:845–850. [DOI] [PubMed] [Google Scholar]
- 34.Harris D, Davies SJ, Finkelstein FO, et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95:S1–S33. [DOI] [PubMed] [Google Scholar]
- 35.Keddis MT, Sharma A, Ilyas M, et al. Transplant center assessment of the inequity in the kidney transplant process and outcomes for the Indigenous American patients. PLoS ONE. 2018;13:e0207819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Hare AM, Armistead N, Schrag W, Diamond L, Moss AH. Patient-centered care: an opportunity to accomplish the “Three Aims” of the National Quality Strategy in the Medicare ESRD program. Clin J Am Soc Nephrol. 2014;9:2189–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pauly MV. Accountable care organizations and kidney disease care: health reform innovation or more same-old, same-old? Am J Kidney Dis. 2012;60:524–529. [DOI] [PubMed] [Google Scholar]
- 38.Schold JD, Meier-Kriesche HU. Comparable barriers to access to kidney transplantation across national lines. Transplantation. 2009;88:21–22. [DOI] [PubMed] [Google Scholar]
- 39.Sypek MP, Clayton PA, Lim W, et al. Access to waitlisting for deceased donor kidney transplantation in Australia. Nephrology (Carlton). 2019;24(7):758–766. [DOI] [PubMed] [Google Scholar]
- 40.Axelrod DA, Guidinger MK, Finlayson S, et al. Rates of solid-organ wait-listing, transplantation, and survival among residents of rural and urban areas. JAMA. 2008;299:202–207. [DOI] [PubMed] [Google Scholar]
- 41.Kimmel PL, Fwu CW, Eggers PW. Segregation, income disparities, and survival in hemodialysis patients. J Am Soc Nephrol. 2013;24:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schold JD, Buccini LD, Kattan MW, et al. The association of community health indicators with outcomes for kidney transplant recipients in the United States. Arch Surg. 2012;147:520–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou S, Massie AB, Luo X, et al. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacLaughlin HL, Campbell KL. Obesity as a barrier to kidney transplantation: time to eliminate the body weight bias? Semin Dial. 2019;32:219–222. [DOI] [PubMed] [Google Scholar]
- 45.Haugen CE, Agoons D, Chu NM, et al. Physical impairment and access to kidney transplantation [published online ahead of print 2019]. Transplantation. 10.1097/TP.0000000000002778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickson L, El-Zoghby ZM, Lorenz EC, Stegall MD, Jaffe AS, Cosio FG. Patient survival after kidney transplantation: relationship to pretransplant cardiac troponin T levels. Am J Transplant. 2009;9:1354–1361. [DOI] [PubMed] [Google Scholar]
- 47.Schold JD, Flechner SM, Poggio ED, et al. Residential area life expectancy: association with outcomes and processes of care for patients with ESRD in the United States. Am J Kidney Dis. 2018;72:19–29. [DOI] [PubMed] [Google Scholar]
- 48.Weinhandl ED, Snyder JJ, Israni AK, Kasiske BL. Effect of comorbidity adjustment on CMS criteria for kidney transplant center performance. Am J Transplant. 2009;9:506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heaphy E, Poggio ED, Flechner SM, et al. Risk factors for retransplant kidney recipients: relisting and outcomes from patients’ primary transplant. Am J Transplant. 2014;14:1356–1367. [DOI] [PubMed] [Google Scholar]
- 50.Bowring MG, Massie AB, Craig-Schapiro R, Segev DL, Nicholas LH. Kidney offer acceptance at programs undergoing a Systems Improvement Agreement. Am J Transplant. 2018;18:2182–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schold JD, Arrigain S, Flechner SM, et al. Dramatic secular changes in prognosis for kidney transplant candidates in the United States. Am J Transplant. 2019;19:414–424. [DOI] [PubMed] [Google Scholar]
- 52.Schold JD, Buccini LD, Goldfarb DA, Flechner SM, Poggio ED, Sehgal AR. Association between kidney transplant center performance and the survival benefit of transplantation versus dialysis. Clin J Am Soc Nephrol. 2014;9:1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schold JD, Buccini LD, Poggio ED, Flechner SM, Goldfarb DA. Association of candidate removals from the kidney transplant waiting list and center performance oversight. Am J Transplant. 2016;16:1276–1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


