Abstract
Background
Decreased tumor content (TC) in resection specimens after neoadjuvant therapy is used to predict prognosis. We investigated whether TC assessed in biopsy specimens or the shift in TC from baseline to on-treatment can be used accordingly to predict response in patients with rare tumors who were treated with pembrolizumab.
Methods
A total of 57 tumors (represented by 173 baseline and 179 on-treatment biopsies) from 57 patients with rare tumors participating in an ongoing phase II clinical trial of pembrolizumab were evaluated. TC was estimated on H&E-stained slides and tumors were dichotomized into low and high TC according to a cut-off of 10%. Necrosis, proliferative fibrosis (PF) and normal tissue were assessed in on-treatment biopsies. TC at baseline and on-treatment, as well as the shift in TC from baseline to on-treatment, was correlated with clinical response defined according to Response Evaluation Criteria in Solid Tumors.
Results
A decrease in TC was seen in 14% (n=8); no change in TC was seen in 75% (n=43); and an increase in TC from baseline to on-treatment was seen in 11% (n=6). Objective response was significantly associated with decrease in TC from baseline to on-treatment (38%, 3/8) compared with no change/increase in TC (6%, 3/49) (p=0.031). Patients with a decrease in TC had a significantly increased time to progression (TTP) (75% probability) compared with patients with an increase (20% probability) or no change in TC (19% probability) (p=0.0042). Low TC was seen in 23% (13/57) of the tumors at baseline and in 26% (15/57) on-treatment. High TC was seen in 77% (44/57) of tumors at baseline and in 74% (42/57) on-treatment. No significant associations with response were seen for necrosis, PF or normal tissue in on-treatment biopsies.
Conclusion
Patients with a decrease in TC from baseline to on-treatment had a significant improvement in objective response and a longer TTP. Our data suggest that the shift in TC might be used to predict response to pembrolizumab in rare tumors. However, further investigations in larger cohorts are needed to determine the clinical value of TC, the shift in TC and the cut-off of 10% assessed in biopsies.
Trial registration number
Keywords: tumor biomarkers, immunotherapy, translational medical research
Introduction
Predicting immunotherapy response, resistance, side effects, and pharmacodynamics has become an important component of clinical trials. Correlative studies are used to investigate these variables by integrating tumor biopsies into the clinical trial design to understand the effect of treatment on the tumor tissue.1 Sequential biopsies are performed at different time points, such as prior to treatment and during treatment to capture pharmacodynamic or biomarker changes.1–3 These research biopsies are intended to be used for sophisticated and expensive analysis, for example, sequencing, multiplex immunofluorescence, and other assays; therefore, quality control (QC) is routinely performed to determine which biopsy specimen is most suitable for a certain analysis. One of the most important parameters during the QC is to determine how much tumor was captured in the biopsy.2 To ensure that the biopsy specimen contains tumor, some clinical trials have a cytopathologist on site during the biopsy procedure who evaluates touch preps of the biopsies. For other trials, tumor assessment is performed on an H&E-stained tissue sample after formalin fixation and paraffin embedding. The biopsy specimen with the most tumor content (TC) is the preferred sample to be used for subsequent molecular analysis.
We investigated whether the TC recorded during the QC process might be of clinical value. To the best of our knowledge, no correlative study has looked at treatment response in correlation with the data obtained during the biopsy QC of rare tumors. Hence, the purpose of this study was to determine whether the assessment of TC at baseline or on-treatment, or the shift in TC from baseline to on-treatment, can be used as a predictor of response. According to the TC assessment in resection specimens after neoadjuvant treatment, we assessed TC on an H&E stain only.4–6 To answer the question of what is the clinical value of TC assessment in biopsies from a target lesion, using a cut-off derived from evaluation of the literature on neoadjuvant treatments in different tumor types, we leveraged our ongoing correlative study for a phase II clinical trial of immune checkpoint inhibitor pembrolizumab in patients with rare tumors.
Patients and methods
Patients
All patients had undergone prior treatment and had disease progression under that therapy. Pembrolizumab was administered as a single intravenous dose of 200 mg on the first day of every 21-day dosing cycle until disease progression or until patients developed side effects and needed to withdraw from the study. Image-guided (ultrasound, CT or MRI) biopsies were obtained from lesions (primary or metastasis) that were determined to be accessible with low risk based on preprocedure imaging. A coaxial technique was used to obtain both fine-needle aspirates (FNAs) for cytology assessment and Tru-cut core biopsy specimens when possible. No touch preps were performed from the FNAs for this study. The same target lesion was biopsied on both baseline and between cycle 1, days 15 and 21. On-treatment biopsies were taken in the first cycle between days 15 and 21 from a lesion that was not previously irradiated and was amenable to ultrasound guidance. The patients were evaluated every three cycles (ie, every 9 weeks) with radiographical imaging to assess response to treatment.
Assessment of TC, necrosis, proliferative fibrosis (PF) and normal tissue
All biopsy specimens underwent QC using an H&E stain only. Assessment of TC, defined as the area occupied by viable tumor divided by the entire biopsy area (xx%/100%=result in %), was performed by three pathologists (CT, SR-C, and PA), who were all blinded to the clinical outcome. TC was estimated as the percentage of viable TC in the entire biopsy. If more than one biopsy specimen was taken per tumor, the average TC was calculated; all specimens were taken into account since they together represent the tumor. After TC assessment of all the biopsy specimens, the tumors were dichotomized into high and low TCs. In cases of disagreement about whether a case belonged in the low or high TC category, the pathologists re-evaluated all the biopsy specimens together on the microscope and reached a consensus regarding TC. Biopsies with no TC, no clear invasion, or a possible few tumor cell captures that would have needed IHC to prove the presence of tumor cells were categorized as low TC. Necrosis was assessed as the area occupied by avital tissue divided by the entire biopsy area and was estimated as a percentage (xx%/100%=result in %). Tumors were dichotomized into low and high necrotic content (NC). No NC was categorized into the low NC category. Additionally, the area occupied by PF divided by the entire biopsy area (xx%/100%=result in %), and the area taken by normal tissue divided by the divided entire biopsy area (xx%/100%=result in %) were estimated for this study. PF was regarded as fibrosis with a high fibroblast-to-collagen ratio as previously described.7
Cut-off and statistical considerations
Currently, there is no cut-off to determine a major tumor regression in on-treatment biopsies for rare tumors. Additionally, our study included different tumor types, and therefore, we would need to determine a cut-off suitable for different histologies. According to the literature, across different solid tumor types, a cut-off of around 10% residual tumor in resection specimens after therapy is considered major tumor regression,4–6 8 9 and it is associated with better clinical outcome, for example, in osteosarcomas or lung cancer.6 9 Further, in a recent study in rare tumors (p16 positive oropharyngeal or unknown head and neck squamous cell carcinomas) treated with immune checkpoint inhibitor and radiation therapy, a major response was defined as <10% residual viable tumor.8 Therefore, we chose the cut-off of 10% for our study baseline and on-treatment biopsy specimens and dichotomized the tumors with <10% representing low TC and ≥10% representing high TC. The shift in TC from baseline to on-treatment was recorded according to the cut-off of 10%; a decline represented a shift from high TC to low TC, no change in TC represented TC content in the same category as at baseline, and an increase represented a shift from low TC to high TC. NC was also dichotomized into high (≥10%) and low (<10%) using the same cut-off as for TC.
Treatment efficacy was categorized per Response Evaluation Criteria in Solid Tumors V.1.1.10
To assess the association between TC and objective response and between TC and clinical benefit, we used logistic regression analysis, and to assess the association between TC and time to progression (TTP), we used Cox proportional hazards regression analysis. TTP was defined as the time from start of treatment until progression of disease either radiologically or clinically. Patients who were progression-free at the time of evaluation were censored at the time of evaluation. TTP estimates were generated using the Kaplan-Meier method. A p value of <0.05 was considered statistically significant. All the analyses were carried out using Spotfire S+ V.8.2 for Windows software (TIBCO Software).
Results
Our patient cohort (n=57) comprised 57 tumors that were biopsied at baseline and on-treatment. The tumors were represented by 352 biopsy specimens, including 173 baseline and 179 on-treatment biopsy specimens (average: 3 baseline and 3.1 on-treatment biopsy specimens per tumor) from 26 women (46%) and 31 men (54%) who were enrolled in the trial from August 2016 to August 2017 and for whom follow-up data were available. The average age at the time of enrollment was 54 years (range: 24–86 years). The trial had the following patient cohorts defined: carcinoma of unknown primary (n=9), squamous cell carcinoma of the skin (n=9), germ cell tumor/testicular tumor (n=6), adrenocortical carcinoma (n=5), paraganglioma/pheochromocytoma (n=5), small cell malignancies of non-pulmonary origin (n=4), medullary renal cell carcinoma (n=2), penile carcinoma (n=1), vascular sarcoma (n=1), and other rare tumors (n=15). The cohort of other rare tumors consisted of granulosa cell tumor, adult type (n=3), and one case each of the following histology: alveolar rhabdomyosarcoma; alveolar soft part sarcoma; clear cell adenocarcinoma of the cervix uteri; epithelioid hemangioendothelioma; granulosa cell tumor, juvenile type; mesothelioma of the testis; pituitary carcinoma; spindle cell rhabdomyosarcoma; squamous cell carcinoma of the vagina; squamous cell carcinoma of the cervix uteri; uterine inflammatory myofibroblastic tumor; and Wilms tumor. See table 1 for patient characteristics.
Table 1.
Summary of patient characteristics, cohorts, response data, and TC (N=57)
| Feature | Category | Baseline biopsy specimen | On-treatment biopsy specimen | ||
| Low TC (n=13, 23%) |
High TC (n=44, 77%) |
Low TC (n=15, 26%) |
High TC (n=42, 74%) | ||
| Sex | Female | 2 (15) | 24 (55) | 3 (20) | 23 (55) |
| Male | 11 (85) | 20 (45 | 12 (80) | 19 (45) | |
| Age (years) | Mean Range |
51 26–80 |
56 24–86 |
53 24–78 |
55.2 24–86 |
| Trial cohorts | Carcinoma of unknown primary | 1 (8) | 8 (18) | 2 (13) | 7 (17) |
| Skin squamous cell carcinoma | 3 (25) | 6 (14) | 5 (33) | 4 (10) | |
| Germ cell tumor/testicular tumor | 2 (17) | 4 (9) | 1 (6) | 5 (12) | |
| Adrenocortical carcinoma | 0 (0) | 5 (11) | 0 (0) | 5 (12) | |
| Paraganglioma/pheochromocytoma | 1 (8) | 4 (9) | 2 (13) | 3 (7) | |
| Small cell malignancies of non-pulmonary origin | 1 (8) | 3 (7) | 1 (6) | 3 (7) | |
| Medullary renal cell carcinoma | 1 (8) | 1 (2) | 1 (6) | 1 (2) | |
| Penile carcinoma | 0 (0) | 1 (2) | 0 (0) | 1 (2) | |
| Vascular sarcoma | 1 (8) | 0 (0) | 1 (6) | 0 (0) | |
| Other rare tumors | 3 (25) | 12 (27) | 2 (6) | 13 (31) | |
| Response | CR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PR | 0 (0) | 6 (14) | 3 (2) | 3 (7) | |
| SD (≥6 months) | 0 (0) | 7 (16) | 3 (2) | 5 (12) | |
| SD (<6 months) | 3 (25) | 2 (5) | 2 (1) | 2 (5) | |
| PD | 10 (83) | 29 (66) | 7 (47) | 32 (76) | |
| Clinical benefit rate (PR or SD >6 months) | 1 (8) | 13 (30) | 6 (40) | 8 (19) | |
CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; TC, tumor content.
Response data
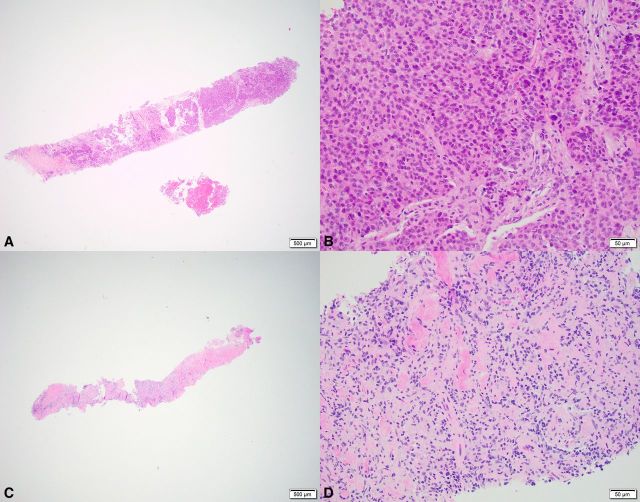
Objective response (all partial response (PR)) was seen in six patients (11%, 95% CI 5% to 21%); and clinical benefit (PR or SD >6 months) was seen in 14 patients (25%, 95% CI 16% to 38%). See example of biopsy specimen from a patient with PR (figure 1).
Figure 1.
Example of a patient with partial response and decrease in TC from baseline to on-treatment. Shown are overviews (A+C: ×2 magnification) and detail (B+D: ×20 magnification) of H&E-stained biopsy specimens. (A+B) Baseline biopsy specimen with ≥10% TC (category: high TC). (C+D) On-treatment biopsy specimen does not contain any tumor, but fibrosis and inflammatory cells are visible (category: low TC). TC, tumor content.
Frequencies of TC at baseline and on-treatment, and TTP
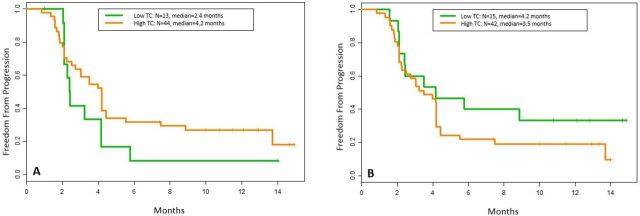
Low TC was seen in 23% (13/57) of the tumors at baseline and in 26% (15/57) on-treatment. High TC was seen in 77% (44/57) of tumors at baseline and in 74% (42/57) on-treatment. Patients with low TC at baseline had a median TTP of 2.4 months compared with 4.2 months in patient with high TC (p=0.19; HR 0.62, 95% CI 0.31 to 1.23) (figure 2A). Patients with low TC on-treatment had a median TTP of 4.2 months compared with 3.5 months in patients with high TC (p=0.22; HR 1.5, 95% CI 0.8 to 3.1) (figure 2B).
Figure 2.
Kaplan-Meier plots for patients with low versus high TC at baseline and on-treatment. Kaplan-Meier plots showing time to progression for tumors with low TC (green) versus high TC (orange). (A) Only baseline biopsy specimens have been taken into account. (B) Only on-treatment biopsy specimens have been taken into account. TC, tumor content
Frequencies in shift of TC from baseline to on-treatment, response and estimated probability of remaining progression-free
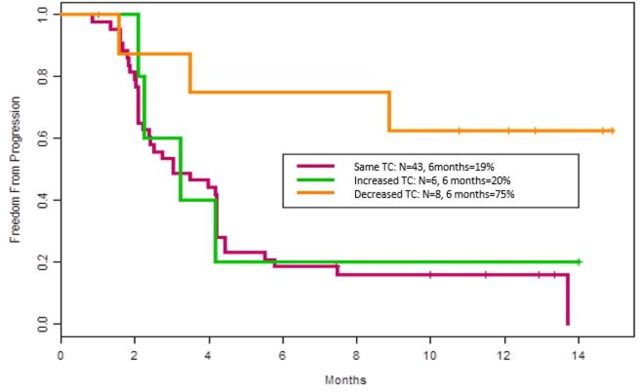
A decrease in TC was seen in 14% (n=8); no change in TC was seen in 75% (n=43); and an increase in TC from baseline to on-treatment was seen in 11% (n=6). Objective response (PR) was significantly associated with a decrease in TC from baseline to on-treatment (38%, 3/8) compared with no change or increase in TC combined (6%, 3/49) (p=0.031). Patients with a decrease in TC from baseline to on-treatment had a significantly higher probability (75%) to remain progression-free at 6 months compared with the probability in patients with no change (19%) and increase in TC (20%)(p=0.0042; HR 0.24, 95% CI 0.07 to 0.79) (figure 3).
Figure 3.
Kaplan-Meier plot showing probability estimates to remain progression-free for 6 months: Patients with decrease in TC from baseline to on-treatment (orange), increase in TC (green), and no change in TC (red).
Frequencies of necrosis, PF and normal tissue in on-treatment biopsies, and TTP
Low NC (<10%) was seen in 79% (45/57) of the patients and high NC (≥10%) in 21% (12/57). Patients with low NC had a median TTP of 4.2 months, compared with 2.1 months in patients with high NC (p=not significant (n.s.)). In 54% (31/57), no PF was present and in 46% (26/57), PF was seen. Patients with no PF had a median TTP of 3.2 months compared with 4.2 months in patients with PF (p=n .s.). Normal tissue was not present in 54% (31/57) and present in 46% (26/57). The relative proportion of PF and NC compared with normal tissue was not associated with any response.
Association between TC shift and response in different tumor subgroups
PR was observed in 40% (2/5) of carcinomas that had a decrease in TC from baseline to on-treatment, compared with 11% (3/28) of carcinomas with the same TC and an increase in TC combined (p=0.15). PR was observed in 100% of sarcomas (1/1) that had a decrease in TC from baseline to on-treatment, compared to 0% (0/3) of sarcomas with the same TC and an increase in TC combined (p=0.25). No PR (0%) was observed in (0/2) other tumor types that had a decrease in TC from baseline to on-treatment, and also no PR (0%) was seen in other tumor types with the same TC and an increase in TC combined (0/18) (p=n/a).
Discussion
Certain carcinomas and other malignancies can respond to immune checkpoint inhibitors, such as renal cell carcinomas,11 small cell lung cancer,12 non-small-cell lung cancer (NSCLC),13 hepatocellular carcinoma,14 metastatic cutaneous squamous cell carcinoma,15 gastric and esophageal cancer,16 head and neck squamous cell carcinoma,17 melanoma,7 18 and others. Among rare tumors, Merkel cell carcinomas showed response to immune checkpoint inhibitors.19 In sarcomas, it seems that immune checkpoint inhibitors only show some efficacy in certain subtypes, such as alveolar soft part sarcoma.20 21
It is well known that chemotherapies can induce morphological changes within tumor tissue, including decreased tumor cellularity.22 Morphological changes also occur after immunotherapy, for example, increased inflammatory/immune cell infiltrates, necrosis, or fibrosis.2 7 13 23 24 The evaluation of TC in resection specimens after therapy represents the residual tumor cells surviving the attacks/pressure of the drug. Microscopic shrinkage of the tumor is regarded as tumor regression, and several scoring systems include the assessment of TC in surgical resection specimens after neoadjuvant therapy to determine prognosis. In several of these tumor-regression scoring systems, a cut-off of <10% residual tumor after therapy is considered significant microscopic tumor shrinkage4–6 8 9 and is reported to be associated with better prognosis in osteosarcoma6 or survival in NSCLC.9 However, whether the assessment of TC assessed in pretreatment and post-treatment biopsy specimens in rare tumors can be used to predict response is unknown. TC is routinely assessed during the QC of research biopsies, and this parameter is generally regarded as only useful for determining whether a certain biopsy can be used for additional analysis. However, we believe that TC obtained during the QC process should be regarded as a valuable parameter that might predict early responsiveness. Such data could be especially valuable when an early on-treatment biopsy is available prior to the first radiological image analysis assessment and especially in trials evaluating immune checkpoint inhibitors, where pseudo-progression has been described as a challenge for radiologists.25
We applied the cut-off of <10%, as previously described in resection specimens for different tumor types,4–6 8 9 to dichotomize TC in our biopsy specimens. We observed that a decrease in TC from baseline to on-treatment was significantly (p=0.031) associated with response to pembrolizumab. Additionally, patients with a decrease of TC had a significantly higher overall chance to remain progression-free at 6 months (p=0.0042). Further, patients with low TC in on-treatment biopsies had a longer median TTP of around 3 weeks (0.7 months, p=0.22). However, the results from baseline biopsies showed the opposite with a nearly 2 months of longer median TTP in patients with high TC (p=0.19). These results imply the importance of capturing the tumor dynamic by longitudinal tumor sampling to predict response rather than to look at a static picture at baseline or on-treatment alone using TC as a parameter in rare tumor types. This seems also to be true when using biomarkers2 and underlines the value of both baseline and on-treatment evaluation. However, a recent study by Stein et al,7 assessing 14 histological parameters and generating from these parameters a semiquantitative scale of immune-related pathological response (irPR) ranging from 0 to 3, showed that patients with advanced melanoma and an irPR score of 3 in on-treatment biopsies had better overall survival.7 Hence, the study by Stein et al7 and our study showed that morphological parameters are important and should not be disregarded. However, comparing TC from baseline and on-treatment biopsies that are available from QC data is very time efficient since these data are already collected, whereas the assessment of several morphological parameters in on-treatment biopsies can be time-consuming but may be valuable in cases where only an on-treatment biopsy is available.
We would argue that the phenomenon of decrease in TC represents a therapy effect. It is known that tumor necrosis can occur after treatment with immune checkpoint inhibitors,7 13 23 24 and we know from the wound-healing process that remodeling of tissue occurs within hours. In myocardial infarction, for example, inflammation occurs within hours; myofibroblasts appear slightly later to construct new collagen; fibrosis can be seen within days; and solid scar formation occurs within weeks.26 We assume that the healing processes after drug-induced tumor necrosis occur quickly as well and that we see this phenomenon in on-treatment biopsies with low TC. Patients with high NC (≥10%) in on-treatment biopsies had a shorter median TTP by around 9 weeks compared with patients with low NC (2.1 months vs 4.2 months, p=n.s.), which is in line with the observation in on-treatment biopsies from melanoma, where necrosis was not associated with response.7 This is an interesting observation since we would think that more necrosis would be associated with better response. It may be that assessment of vital tumor is a better readout of the tumor dynamic under therapy than NC. Patients with no PF in their tumor had a shorter median TTP by around 4 weeks compared with patients with PF (3.2 months vs 4.2 months, p=n .s.). In melanoma, the presence of PF was significantly associated with response.7 Hence, more research is needed to evaluate whether the presence of PF correlates with response to immune checkpoint inhibitors across different tumor types.
We cannot be sure that the low TC we observed in some biopsies was not due to sampling bias, for example, that the same area of tissue was sampled as in the previous baseline biopsy; however, a TC of <10% is a very low tumor content, more likely representing general therapy-induced microscopic tumor shrinkage that was captured in the biopsy rather than a very local healing process due to a prior biopsy. In cases with no tumor content, no clear invasion, or a possible few tumor cells in the biopsy, the lesion could have been missed even though it was an image-guided biopsy and the radiologist assessed the needle as in the lesion. It is known that lesions can be missed even under image guidance.27 28 We included all biopsies/cases in our study since we wanted to assess whether prediction of response is possible using QC data without performing selection of any cases. To include all biopsies and to use a cut-off point for the shift in tumor content can be regarded as a weakness. However, selection of cases could also lead to biases, and using a certain cut-off point can always be challenged. Therefore, more investigations on this topic are needed. We would conclude that biopsies taken during early cycles of treatment are generally suitable for assessing TC to investigate response but also for investigating tumor biology and biomarker dynamics. This might be especially useful in clinical trials to obtain a signal of response even prior to the first radiological assessment. This might give the opportunity to plan an expansion cohort in cases where a response signal can be observed, or to look into prescreening for other possible treatment options for the patient in cases where no signal of response is seen. Whether QC TC can be used for further therapy decision making needs to be evaluated in additional studies across different tumor types. One could argue that sampling of only one tumor site is not representative for the systemic disease process. This might be true, but it was shown that indeed biopsy specimens from one site can capture important tumor biology for the disease.2 Further, TC assessment using an H&E slide can be easily performed by a pathologist.
Conclusion
In summary, to the best of our knowledge, this is the first study looking at TC in biopsy specimens of rare tumors and its utility for predicting clinical outcome using QC data. In the past, such a study would have been difficult to conduct because biopsies were not taken as often, but with the increase of research biopsies being performed during clinical trials, such analyses are now feasible. In clinical trials with longitudinal research biopsies, monitoring TC can be easily performed since TC will be assessed routinely during biopsy QC. Whether the cut-off of <10% and the assessment of the shift in TC from baseline to on-treatment will be suitable to predict response or prognosis to immune checkpoint inhibitors needs to be investigated in future studies. The extent to which TC assessment can be used for therapy decision making, such as switching treatment or changing combination regimens, needs to be determined in large cohorts. Our study had the limitation of a cohort that was too small to answer all of these questions, and sampling bias as mentioned previously could not be excluded. However, we showed that TC assessment is easy and cost-effective and worth investigating further if it can predict treatment outcomes to immune checkpoint inhibitors.
Acknowledgments
We thank Sunita C Patterson from MD Anderson’s Department of Scientific Publications for providing editorial assistance, Denái R Milton for assisting with responding to statistical questions from the reviewers and Dr Naing’s research laboratory team for all the support we received for this study.
Footnotes
Twitter: @AnaingMD
Deceased: Dr. Hess is deceased
Contributors: CT developed this study design, oversaw the correlation study, performed tumor content assessment and wrote the manuscript; SR-C and PPA performed tumor content assessment; MX performed data entry, put together the final table for statistical analysis and was involved in tissue logistics; FO and LC were involved in obtaining patient consent for the clinical trial, data entry and tissue logistics; AA was involved in obtaining patient consent for the clinical trial, data entry and putting together the clinical data; JH gave intellectual input to the study; GS was involved in sample collection and processing; VY was involved in putting together tables (data) for further statistical use; HL created the database for this study; RM performed the biopsies; BS helped in putting together the clinical data; KRH performed the statistical analysis; IW reviewed the manuscript and gave intellectual input, AN is the principal investigator (PI) of the clinical study, performed the clinical study design and gave intellectual input for this study design. All authors approved the final manuscript.
Funding: Merck was the sponsor of the drug pembrolizumab. This work was supported in part by the National Cancer Institute at the National Institutes of Health (award number P30CA016672; used the Clinical Trials Support Resource, Biostatistics Resource Group, and Research Histology Core Laboratory).
Competing interests: Merck was the sponsor of the drug pembrolizumab. CT had salary support on this study.
Patient consent for publication: Not required.
Ethics approval: Patients with rare tumors who consented to the phase II clinical trial to receive pembrolizumab were included in this study that was approved by MD Anderson’s Institutional Review Board (2015-0948).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. None.
References
- 1.Parchment RE, Ferry-Galow K, Makhlouf HR, et al. Suitability factors of core needle biopsies for pharmacodynamic (PD) studies. JCO 2017;35:2540 10.1200/JCO.2017.35.15_suppl.2540 [DOI] [Google Scholar]
- 2.Dowlati A, Haaga J, Remick SC, et al. Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res 2001;7:2971–6. [PubMed] [Google Scholar]
- 3.Chen P-L, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016;6:827–37. 10.1158/2159-8290.CD-15-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521–30. 10.1002/cncr.11660 [DOI] [PubMed] [Google Scholar]
- 5.Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 2003;12:320–7. 10.1016/S0960-9776(03)00106-1 [DOI] [PubMed] [Google Scholar]
- 6.Rejniak KA, Lloyd MC, Reed DR, et al. Diagnostic assessment of osteosarcoma chemoresistance based on virtual clinical trials. Med Hypotheses 2015;85:348–54. 10.1016/j.mehy.2015.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stein JE, Soni A, Danilova L, et al. Major pathologic response on biopsy (MPRbx) in patients with advanced melanoma treated with anti-PD-1: evidence for an early, on-therapy biomarker of response. Ann Oncol 2019;30:589–96. 10.1093/annonc/mdz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leidner R, Bell RB, Yound K, et al. NIRT) in head and neck cancer: phase I/Ib study of combined PD-1/SBRT prior to surgical resection. Cancer Res 2019;79. [Google Scholar]
- 9.Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer : morphology and prognosis. Chest 2001;120:1584–91. 10.1378/chest.120.5.1584 [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 11.Escudier B. Combination therapy as first-line treatment in metastatic renal-cell carcinoma. N Engl J Med 2019;380:1176–8. 10.1056/NEJMe1900887 [DOI] [PubMed] [Google Scholar]
- 12.Horn L, Mansfield AS, Szczęsna A, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018;379:2220–9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 13.Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol 2018;29:1853–60. 10.1093/annonc/mdy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 15.Migden MR, Rischin D, Schmults CD, et al. Pd-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018;379:341–51. 10.1056/NEJMoa1805131 [DOI] [PubMed] [Google Scholar]
- 16.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol 2018;4:e180013 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rischin D, Harrington KJ, Greil R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (r/m HNSCC). JCO 2019;37:6000 10.1200/JCO.2019.37.15_suppl.6000 [DOI] [Google Scholar]
- 18.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 2018;378:1789–801. 10.1056/NEJMoa1802357 [DOI] [PubMed] [Google Scholar]
- 19.Nghiem P, Bhatia S, Lipson EJ, et al. Durable tumor regression and overall survival in patients with advanced Merkel cell carcinoma receiving pembrolizumab as first-line therapy. J Clin Oncol 2019;37:693–702. 10.1200/JCO.18.01896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groisberg R, Hong DS, Behrang A, et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer 2017;5:100 10.1186/s40425-017-0301-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Angelo SP, Mahoney MR, Van Tine BA, et al. Streicher H. a non-comparative multi-center randomized phase II study of nivolumab +/− ipilimumab for patients with metastatic sarcoma (alliance A091401). Lancet Oncol 2018;19:416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thies S, Langer R. Tumor regression grading of gastrointestinal carcinomas after neoadjuvant treatment. Front Oncol 2013;3:1–7. 10.3389/fonc.2013.00262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koelzer VH, Rothschild SI, Zihler D, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors—an autopsy study. j. immunotherapy cancer 2016;4:1–8. 10.1186/s40425-016-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaria RN, Reddy SM, Tawbi HA, et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat Med 2018;24:1649–54. 10.1038/s41591-018-0197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katz SI, Hammer M, Bagley SJ, et al. Radiologic pseudoprogression during anti-PD-1 therapy for advanced non-small cell lung cancer. J Thorac Oncol 2018;13:978–86. 10.1016/j.jtho.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 26.Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res 2005;66:22–32. 10.1016/j.cardiores.2005.01.011 [DOI] [PubMed] [Google Scholar]
- 27.Shah VI, Raju U, Chitale D, et al. False-Negative core needle biopsies of the breast: an analysis of clinical, radiologic, and pathologic findings in 27 concecutive cases of missed breast cancer. Cancer 2003;97:1824–31. 10.1002/cncr.11278 [DOI] [PubMed] [Google Scholar]
- 28.Gold SA, Hale GR, Bloom JB, et al. Follow-Up of negative MRI-targeted prostate biopsies: when are we missing cancer? World J Urol 2019;37:235–41. 10.1007/s00345-018-2337-0 [DOI] [PubMed] [Google Scholar]