Abstract
The aim of the present study was to compare the efficacy of microwave ablation (MWA) and surgical resection (RES) for the treatment of hepatocellular carcinoma (HCC) conforming to the Milan criteria and the associated short- and long-term survival rates. The baseline characteristics were obtained from 231 patients with HCC who met the Milan criteria. To compare the mortality rates between groups, survival analysis was conducted using the Kaplan-Meier method and the log-rank test. The factors associated with the survival rate were analyzed using Cox proportional hazard models. A total of 115 patients underwent RES, and 116 were treated with MWA. No significant differences were observed in the 1-, 3- and 5-year OS rates and the 1-year DFS rate between the two groups. The 7- and 10-year OS rates and the 3-, 5-, 7- and 10-year DFS rates of the RES group were significantly higher compared with those in the MWA group (P=0.004, P=0.002, P=0.003 and P=0.002, respectively). In addition, no marked differences were observed in the OS and DFS rates between the two groups of patients with solitary HCC lesions ≤3 cm (P=0.066 and P=0.056) and in the OS of those with solitary lesions of 3–5 cm (P=0.133); however the DFS of patients with single 3–5 cm HCC lesions in the RES group was notably higher compared with the MWA group (P=0.027). The Cox proportional hazard model revealed that age, hepatitis B and C virus infection, tumor size, number, platelet count and the type of treatment intervention were risk factors affecting the survival and recurrence in patients with HCC. These results suggested that RES may provide superior survival benefits compared with MWA for patients with HCC who meet the Milan criteria.
Keywords: hepatocellular carcinoma, surgical resection, microwave ablation, survival analysis
Introduction
Primary liver cancer (PLC) refers to malignancies originating from hepatocytes and the bile duct epithelium (1). In 2018, PLC was the sixth most common type of cancer worldwide, after lung, breast, colorectal, prostate and gastric cancer (2). However, the poor prognosis of patients with PLC makes it the second leading cause of cancer-associated death worldwide (3). Contrary to the steady or declining trend of malignancies such as lung, breast and colon cancer, the incidence and mortality rates of PLC have increased rapidly in the past decade, and the Chinese population accounts for ~50% of all global cases and deaths (4). In China, the incidence and mortality rates of PLC are 2,871/100,000 and 2,604/100,000 individuals, respectively, making it the fourth most common cancer type and the second leading cause of cancer-related death (5). The three different pathological types of PLC, hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC) and mixed type HCC-ICC, differ in their pathogenesis, biological behavior, histological morphology, treatment methods and prognosis; >85% of PLC cases are patients with HCC (6). HCC is responsible for 5% of all malignant tumors in humans and is the third leading cause of cancer-related death, second only to lung cancer and gastric cancer (1). HCC primarily occurs in chronic inflammatory environments (7). The majority of cases of HCC develop in the presence of advanced chronic liver disease associated with chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and alcoholism (8). According to the European Association for the Study of the Liver (EASL), the European Organization for Research and Treatment of Cancer and the American Association for the Study of Liver Diseases Guidelines, liver transplantation, radiofrequency ablation (RFA) and hepatectomy are the recommended treatments for HCC (9–11). Compared with other types of cancer, which are primarily treated by surgery, radiotherapy and chemotherapy, local treatment of HCC is widely used for therapeutic (ablation or surgery) and palliative (arterial chemoembolization) intentions (10). Microwave ablation (MWA) is a type of therapy that uses imaging technology to guide a microwave needle, which directly destroys tumor cells in the local area (12). It has advantages including reduced damage, significant short-term effects and wide indications compared with surgery, and is widely used in the clinic (13). However, a limited number of studies have been conducted on the comparison between the efficacy of MWA and surgical resection (RES). Our previous study found that the DFS rate of MWA under 3 years is lower compared with RES for HCC conforming to Milan criteria (14). The purpose of the present study was to compare the efficacy of MWA and RES treatments in patients with HCC within the Milan criteria, to analyze the impact of these two treatment types on overall survival (OS), DFS and recurrence, and to compare the effects of the two treatments on short-, medium- and long-term survival.
Materials and methods
Patients and sample collection
The records of the patients with HCC admitted to the Tianjin Third Central Hospital (Tianjin, China) between January 2004 and December 2012 were retrospectively analyzed. These patients were diagnosed based on cytohistological evidence or the diagnostic criteria of the EASL (10). In total, 231 patients initially treated with MWA or RES were selected.
The inclusion criteria were as follows: i) Meeting the Milan criteria, which are a single HCC ≤5 cm or ≤3 nodules of <3 cm each; ii) no extrahepatic metastasis or notable vascular invasion; iii) liver function of Child-Pugh Class A or B; iv) no previous or simultaneous malignancies; and v) no previous treatment for HCC. The exclusion criteria were: i) Patients at Child-Pugh Class C or evidence of hepatic decompensation, including refractory ascites, esophageal or gastric variceal bleeding, or hepatic encephalopathy; ii) patients with severe coagulation disorders (platelet count <50×109 cells/l or prothrombin time prolongation >5 sec); and iii) patients who preferred liver transplantation (9,14). The present study was approved by the Ethics Committee of Tianjin Third Central Hospital, and informed consent was obtained from each participant.
Study design
The 231 patients were categorized into MWA (n=116) and RES (n=115) groups based on the therapeutic method. The modified Response Evaluation Criteria in Solid Tumors (15) was used to assess the treatment response of the patients following MWA or RES.
MWA was performed at 2,450 MHz using a Forsea MTC-3 microwave therapeutic apparatus (Qinghai Microwave & Electronic Research Institute). Lidocaine (2%) was used for local anesthesia (Hubei Tianyao Pharmaceutical Co. Ltd.) and intravenous anesthesia included propofol (CordenPharma International) and fentanyl (Yichang Renfu Pharmaceuticals Co., Ltd.). After anesthesia was achieved, a 15-cm 14-gauge unipolar cooled-shaft antenna with an output power of 60–80 W was inserted into the center of the tumor. The ablation process was continuously guided and monitored using the Philips IU-22 (Philips Medical Systems, Inc.) and the Aloka SSD 5000 (Hitachi-Aloka Medical, Ltd.) ultrasound systems with 1–5 MHz convex array probes. The number of ablation repetitions depended on the number, location, shape and coagulation function of the tumor. Ablation was completed when the tumor and a surrounding 1-cm safety margin were filled with hyperechoic microbubbles. MWA was used again as salvage treatment in patients with incomplete tumor ablation. To prevent bleeding and needle track implantation, the needle track was coagulated after completion of MWA. All MWA procedures were completed by ultrasound interventional doctors with >5 years of experience. All complications and adverse reactions were appropriately treated before the patient was discharged. Since the complications and adverse reactions occurred in only a few patients, the conditions of statistical analysis were not met and the analysis for the correlation between adverse reactions and survival was not conducted.
For RES, the patients were decubitus for general anesthesia and suitable rooftop incision. All surgeries were performed at the Department of Hepatobiliary Surgery of Tianjin Third Central Hospital by doctors with >10 years of experience. A margin of ≥1 cm was reserved for tumor resection. To avoid non-R0 resection, all surgeries were routinely performed with intraoperative ultrasonography, including estimating the number, size, location and blood supply of the tumor. Liver anatomy was assessed using a CUSA® surgical system (Integra Life Sciences). Hepatic portal occlusion (Pringle Technologies, Inc.) was routinely applied, with blocking for 15 min and releasing for 5 min. All surgical specimens were pathologically examined, and resection of the tumor without a margin was considered as R0 resection (7). All patients were appropriately treated after surgery, and their liver functions were close to normal prior to discharge.
Follow-up
To assess the effectiveness of treatment, an enhanced CT scan was routinely performed 1 month after the patient was treated; all patients were followed up. Serum α-fetoprotein (AFP) levels were assessed and ultrasonography was performed every 3 months, and an enhanced CT scan was performed every 6 months. Local recurrence was defined as a CT result that revealed an abnormally enhanced area around the ablation lesion or local margin during follow-up. Intrahepatic recurrence was defined as neoplastic foci that occurred in the liver, but away from the ablation site or in the resected liver segment. Extrahepatic recurrence was defined as extrahepatic metastasis (13,16). The primary endpoints were OS and DFS. The overall recurrence rate was also compared, and the study was completed by January 2018. No value was censored, all data were included in this study.
Statistical analysis
The data were statistically analyzed using SPSS software 21.0 (IBM Corp.), and the Student's t-test was applied to continuous variables. The data were presented as mean ± SD. For categorical variables, the χ2 and Fisher's exact tests were used. The OS, DFS and overall recurrence curves were generated using the Kaplan-Meier method and compared with the log-rank test. Univariate and multivariate analyses of OS or DFS were performed using the Cox risk scale model. P<0.05 was considered to indicate a statistically significant difference.
Results
Patients
No significant differences were observed in the baseline characteristics of patients between the two groups (Table I). Following treatment, the ablation rate of the MWA group was 99.14% (115/116), and incomplete ablation of the tumor was rectified by salvage MWA. In the RES group, all patients exhibited tumor-free resection margins of ≥1 cm, which was determined during surgery by the naked eye or using ultrasonic guidance. All patients in the RES group received R0 resection (no residual tumor tissue was observed under the resection microscope); among them, the histological diagnoses were 35 well-differentiated, 62 moderately differentiated and 18 poorly differentiated cases of HCC.
Table I.
Baseline clinical characteristics of patients with HCC conforming to the Milan Criteria.
| Variable | MWA (n=116) | RES (n=115) | P-value |
|---|---|---|---|
| Age, years mean ± SD | 57.474±9.614 | 54.461±10.366 | 0.647 |
| Sex (M/F), n | 92/24 | 93/22 | 0.767 |
| HBV/HCV/NBNC, n | 90/16/10 | 104/9/2 | 0.016a |
| Cirrhosis (yes/no), n | 108/8 | 108/7 | 0.803 |
| BCLC (0/A/B), n | 11/104/1 | 7/108/0 | 0.375 |
| ALT, IU/l, median (range) | 35 (9.0–99.0) | 40 (14.0–93.0) | 0.145 |
| AST, IU/l, median (range) | 28 (4.0–109.0) | 32 (5.0–95.0) | 0.581 |
| Prothrombin time, sec mean ± SD | 14.387±1.334 | 14.215±1.390 | 0.345 |
| Total bilirubin, µmol/l, median (range) | 18.6 (5.8–59.6) | 15.8 (5.6–54.0) | 0.095 |
| ALB, g/l, median (range) | 41.1 (24.6–48.7) | 40.5 (27.7–52.7) | 0.060 |
| Ascites (absent/present), n | 100/16 | 103/12 | 0.434 |
| Solitary tumor (≤3/>3 cm), n | 51/64 | 41/73 | 0.196 |
| Tumor number (1/2/3), n | 94/17/5 | 97/15/3 | 0.716 |
| AFP (<400/≥400 ng/ml), n | 86/23 | 91/24 | 0.966 |
| Child-Pugh (A/B), n | 90/19 | 99/16 | 0.581 |
P<0.05. HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer.
Survival analysis
In the MWA group, the median follow-up time was 43.34±19.63 months, and 82 patients died during the follow-up period. The causes of death included tumor progression (51/82), liver failure (11/82), gastrointestinal hemorrhage (6/82) and others not related to liver function/cancer (14/82). In the RES group, the median follow-up time was 50.36±24.28 months; during this period, 65 patients died of tumor progression (40/65), liver failure (10/65), gastrointestinal hemorrhage (3/65) and other causes that were not related to liver function/cancer (12/65).
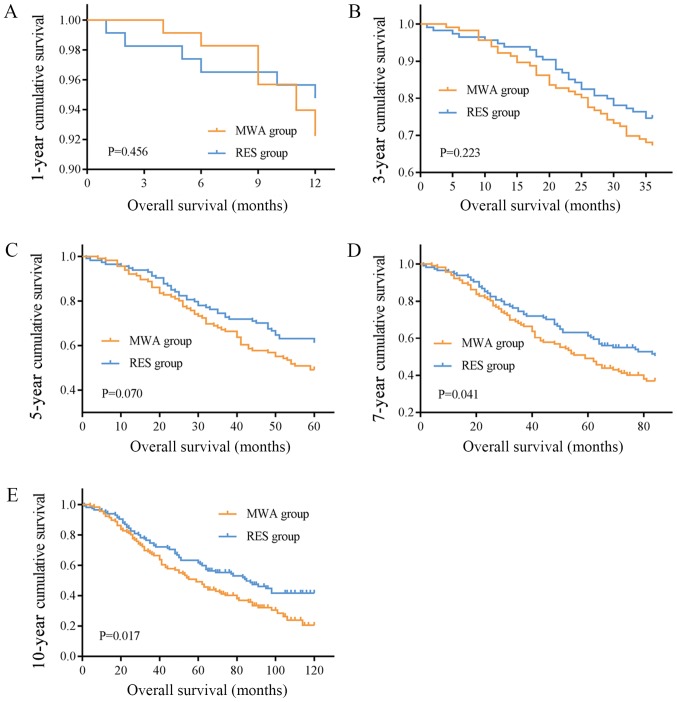
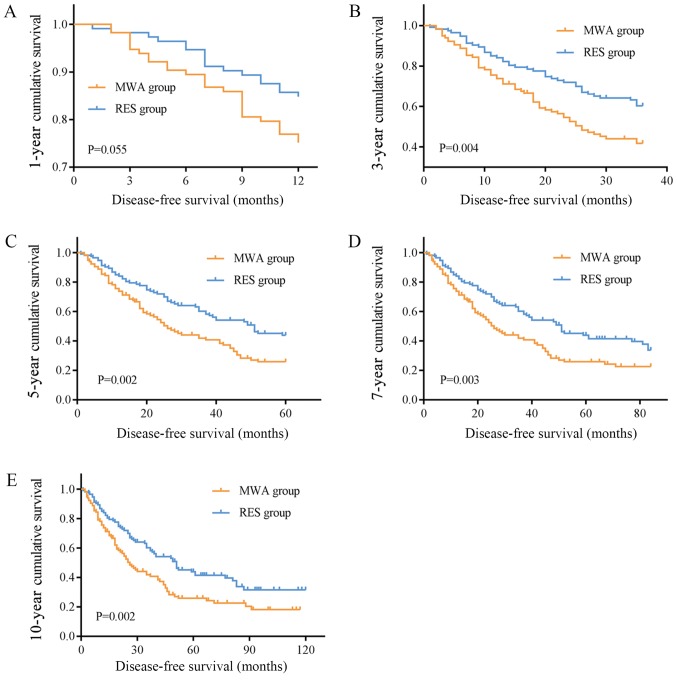
The mean OS times were 67.22 (95% CI, 59.23–75.21) and 93.897 (95% CI, 82.32–105.48) months, and the median OS times were 59.00 (95% CI, 46.89–71.11) and 85.00 (95% CI, 60.94–109.07) months in the MWA and RES groups, respectively. The OS rates for the MWA and RES groups were as follows: 1-year, 92.2 and 94.8%; 3-year, 67.2 and 74.6%; 5-year, 49.1 and 61.3%; 7-year, 36.9 and 50.4%; and 10-year, 20.5 and 41.1% (Table II). No significant differences were observed in the 1-, 3- and 5-year OS rates (Fig. 1A-C), although the 7- and 10-year OS rates were markedly different between the two groups (P=0.041 and P=0.017, respectively; Fig. 1D and E). The mean and median DFS times were 43.40 (95% CI, 35.42–51.38) and 26.00 (95% CI, 19.89–32.12) months in the MWA group, and 74.19 (95% CI, 61.56–86.82) and 51.00 (95% CI, 35.06–66.95) months in the RES group. The DFS rates for the MWA and RES groups were: 1-year, 73.8 and 90.4%; 3-year, 41.8 and 60.2%; 5-year, 25.8 and 43.9%; 7-year, 22.6 and 41.4%; and 10-year, 18.1 and 31.6% (Table II). Among these, no difference was observed in the 1-year DFS rate (Fig. 2A), but the 3-, 5-, 7- and 10-year DFS rates were significantly different between the MWA and RES groups (P=0.004, P=0.002, P=0.003 and P=0.002, respectively; Fig. 2B-E).
Table II.
1-, 3-, 5-, 7- and 10-year overall survival rate and disease-free survival rate of MWA, RES and their subgroups.
| Overall survival rate (%) | Disease-free survival rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 year | 3 year | 5 year | 7 year | 10 year | 1 year | 3 year | 5 year | 7 year | 10 year |
| MWA | 92.2 | 67.2 | 49.1 | 36.9 | 20.5 | 73.8 | 41.8 | 25.8 | 22.6 | 18.1 |
| RES | 94.8 | 74.6 | 61.3 | 50.4 | 41.1 | 90.4 | 60.2 | 43.9 | 41.4 | 31.6 |
| Single HCC ≤3 cm in MWA | 97.4 | 76.9 | 64.1 | 50.3 | 26.7 | 82.1 | 51.1 | 41.5 | 31.6 | 25.3 |
| Single HCC ≤3 cm in RES | 100 | 83.3 | 75.0 | 62.7 | 59.0 | 88.6 | 72.7 | 57.6 | 46.5 | 46.5 |
| Single HCC between 3–5 cm in MWA | 90.9 | 65.5 | 47.3 | 34.6 | 21.1 | 68.4 | 41.1 | 23.1 | 23.1 | 18.5 |
| Single HCC between 3–5 cm in RES | 93.5 | 74.2 | 61.0 | 48.2 | 37.7 | 83.6 | 58.8 | 41.7 | 32.8 | 28.7 |
MWA, microwave ablation; RES, surgical resection; HCC, hepatocellular carcinoma.
Figure 1.
OS curves of patients who underwent MWA or RES. (A) 1-, (B) 3- and (C) 5-year OS rates were not different between the two groups. (D) 7- and (E) 10-year OS rates were higher in the RES group compared with those in the MWA group. OS, overall survival; MWA, microwave ablation; RES, surgical resection.
Figure 2.
DFS curves of patients treated with MWA or RES. (A) 1-year DFS rates were not different between two groups. (B) 3-, (C) 5-, (D) 7- and (E) 10-year DFS rates in the RES group were significantly higher compared with those in the MWA group. DFS, disease-free survival; MWA, microwave ablation; RES, surgical resection.
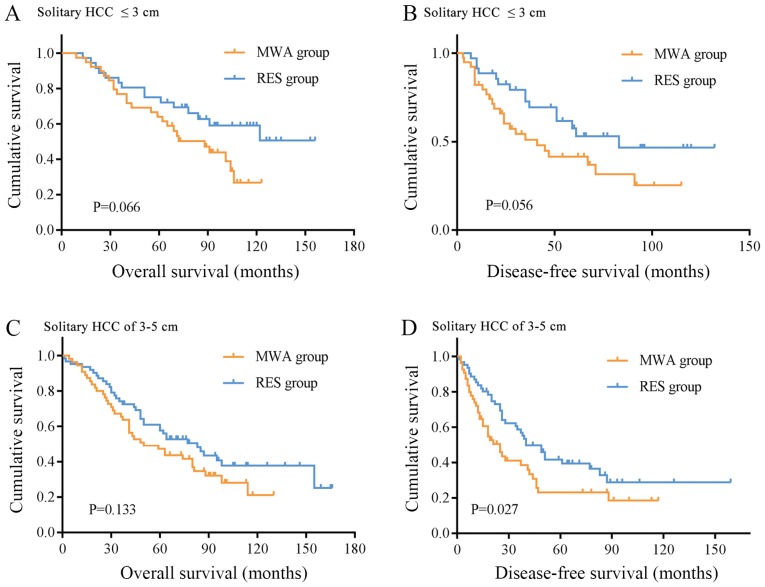
Subgroup analysis was performed on patients with solitary HCC nodules ≤3 and 3–5 cm. There were 39 and 36 cases of solitary HCC lesions ≤3 cm in the MWA and RES groups, respectively. The OS rates were: 1-year, 97.4 and 100.0%; 3-year, 76.9 and 83.3%; 5-year, 64.1 and 75.0%; 7-year, 50.3 and 62.7%; and 10-year, 26.7 and 59.0% (Table II). The DFS rates were: 1-year, 82.1 and 88.6%; 3-year, 51.1 and 72.7%; 5-year, 41.5 and 57.6%, 7-year, 31.6 and 46.5%; and 10-year, 25.3 and 46.5% (Table II). No significant differences were observed in the OS and DFS rates between the two groups (Fig. 3A and B). For solitary HCC lesions between 3 and 5 cm, there were 55 patients in the MWA group and 62 patients in the RES group. The 1-, 3-, 5-, 7-and 10-year OS rates in the MWA and RES groups were: 1-year, 90.9 and 93.5%; 3-year, 65.5 and 74.2%; 5-year, 47.3 and 61.0; 7-year, 34.6 and 48.2%; and 10-year, 21.1 and 37.7% (Table II); no significant differences were observed between the two groups (Fig. 3C). The DFS rates of the MWA and RES groups were: 1-year, 68.4 and 83.6%; 3-year, 41.1 and 58.8%; 5-year, 23.1 and 41.7%; 7-year, 23.1 and 32.8%; and 10-year, 18.5 and 28.7% (Table II), and the RES group exhibited a superior outcome compared with the MWA group (P=0.027, Fig. 3D).
Figure 3.
Subgroup analysis of OS and DFS of patients treated with MWA or RES. (A) OS time of patients with solitary HCC lesions ≤3 cm. (B) DFS time of patients with solitary HCC lesions ≤3 cm and (C) OS time of patients with solitary HCC lesions of 3–5 cm were not significantly different between two groups. (D) DFS time of patients with solitary HCC lesions of 3–5 cm was longer in the RES group compared with that in the MWA group. OS, overall survival; DFS, disease-free survival; MWA, microwave ablation; RES, surgical resection; HCC, hepatocellular carcinoma.
Univariate and multivariate analysis
Among all variables, age (P=0.007), HBV (P=0.032) and HCV (P=0.010) infection, platelet count (P=0.041), tumor number (P=0.001) and intervention type (P=0.019) were considered significant risk factors for OS. Following univariate analysis, variables with statistically significant differences were included in the Cox regression model. To avoid missing some important factors, the P-value was relaxed to 0.1. A total of nine variables with P<0.1 in the univariate analysis were included in Cox multivariate analysis, and tumor size (P=0.012), tumor number (P=0.028) and intervention type (P=0.034) were considered to be significant risk factors for OS (Table III).
Table III.
Univariate and multivariate analysis of relative factors for overall survival.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Subgroup | HR | P-value | HR | P-value |
| Sex | Male vs. female | 0.897 | 0.612 | ||
| Age, years | ≤65 vs. >65 | 1.747 | 0.007a | 1.515 | 0.071 |
| HBV | Yes vs. no | 0.642 | 0.032a | 0.769 | 0.582 |
| HCV | Yes vs. no | 1.812 | 0.010a | 0.922 | 0.876 |
| Liver cirrhosis | Yes vs. no | 0.799 | 0.248 | ||
| ALT, IU/l | ≤40 vs. >40 | 0.896 | 0.514 | ||
| AST, IU/l | ≤40 vs. >40 | 0.804 | 0.219 | ||
| Total bilirubin, µmol/l | ≤19 vs. >19 | 1.222 | 0.234 | ||
| Serum albumin, g/l | ≤35 vs. >35 | 1.296 | 0.172 | ||
| Prothrombin time, sec | ≤15 vs. >15 | 0.731 | 0.082 | 0.948 | 0.795 |
| Platelet count 109 cells/l | ≤100 vs. >100 | 1.408 | 0.041a | 1.216 | 0.333 |
| Ascites | Absent vs. present | 1.201 | 0.458 | ||
| Child-Pugh | A vs. B | 1.473 | 0.070 | 1.28 | 0.277 |
| AFP, ng/ml | ≤400 vs. >400 | 1.105 | 0.636 | ||
| Tumor size, cm | ≤3 vs. >3 | 1.404 | 0.051 | 1.589 | 0.012a |
| Tumor number | Single vs. multiple | 0.509 | 0.001a | 0.607 | 0.028a |
| Intervention | RES vs. MWA | 0.673 | 0.019a | 0.687 | 0.034a |
P<0.05. HR, hazard ratio; HBV, hepatitis B virus; HCV, hepatitis C virus.
For univariate analysis, six variables [age (P=0.031), HBV infection (P=0.039), HCV infection (P=0.015), tumor size (P=0.048), tumor number (P=0.004) and intervention type (P=0.003)] were associated with DFS. Taking P<0.1 in the univariate analysis as the standard, 10 variables were introduced into Cox multivariate analysis, the results of which revealed that tumor size (P=0.010) and intervention type (P=0.007) were associated with DFS (Table IV).
Table IV.
Univariate and multivariate analysis of relative factors for disease-free survival.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Variable | Subgroup | HR | P-value | HR | P-value |
| Sex | Male vs. female | 0.804 | 0.308 | ||
| Age, years | ≤65 vs. >65 | 1.553 | 0.031a | 1.308 | 0.243 |
| HBV | Yes vs. no | 0.655 | 0.039a | 0.641 | 0.355 |
| HCV | Yes vs. no | 1.761 | 0.015a | 0.766 | 0.614 |
| Liver cirrhosis | Yes vs. no | 0.582 | 0.162 | ||
| ALT, IU/l | ≤40 vs. >40 | 0.994 | 0.972 | ||
| AST, IU/l | ≤40 vs. >40 | 0.786 | 0.174 | ||
| Total bilirubin, µmol/l | ≤19 vs. >19 | 1.164 | 0.367 | ||
| Serum albumin, g/l | ≤35 vs. >35 | 1.398 | 0.078 | 1.083 | 0.705 |
| Prothrombin time, sec | ≤15 vs. >15 | 0.720 | 0.069 | 0.838 | 0.395 |
| Platelet count 109 cells/l | ≤100 vs. >100 | 1.325 | 0.093 | 1.101 | 0.640 |
| Ascites | Absent vs. present | 1.171 | 0.522 | ||
| Child-Pugh | A vs. B | 1.446 | 0.084 | 1.296 | 0.257 |
| AFP, ng/ml | ≤400 vs. >400 | 1.167 | 0.464 | ||
| Tumor size, cm | ≤3 vs. >3 | 1.409 | 0.048a | 1.621 | 0.010a |
| Tumor number | Single vs. multiple | 0.557 | 0.004a | 0.650 | 0.061 |
| Intervention | RES vs. MWA | 0.607 | 0.003a | 0.607 | 0.007a |
P<0.05. HR, hazard ratio; HBV, hepatitis B virus; HCV, hepatitis C virus.
Recurrence analysis
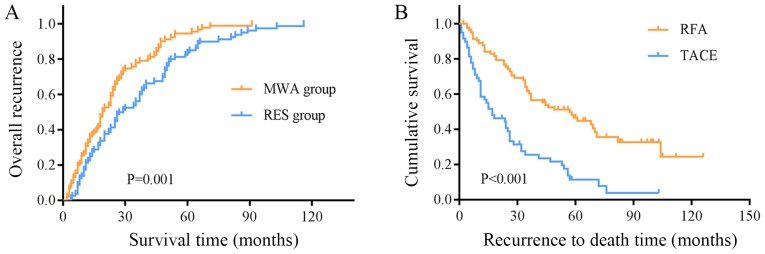
At the end of the follow-up, recurrence had occurred in 89 patients in the MWA group and 80 patients in the RES group. The overall recurrence rates in the two groups were: 1-year, 5.3 and 1.8%; 3-year, 27.5 and 20.3%; 5-year, 43.7 and 30.8%; 7-year, 63.5 and 48.1%; and 10-year, 93.7 and 72.9% (Fig. 4A). The overall recurrence rate in the MWA group was significantly higher compared with that in the RES group (P=0.001; Fig. 4A). The recurrence rate in the early stage (recurrence within 2 years) in the MWA group was higher compared with that in the RES group (57/89 vs. 34/80; P=0.003; Table V). No significant difference was observed in the recurrence location between the two groups (Table V); however, the local recurrence rate in the MWA group was significantly higher compared with that in the RES group (12/116 vs. 4/115; P=0.026).
Figure 4.
Recurrence curves of patients who underwent MWA or RES. (A) The overall recurrence rate in the MWA group was significantly higher compared with that in the RES group. (B) The mean survival time of patients with recurrence treated with RFA was different compared with that of patients treated TACE. MWA, microwave ablation; RES, surgical resection; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization.
Table V.
Recurrence analysis and recurrence therapy of patients treated with MWA or RES.
| Variable | MWA (89/116) | RES (80/115) | P-value |
|---|---|---|---|
| Single HCC ≤3 cm | 29/38 | 20/36 | 0.050a |
| Single HCC between 3–5 cm | 40/55 | 45/62 | 0.57 |
| Early-stage recurrence (<2 years) | 57/89 | 34/80 | 0.003a |
| Recurrence location | |||
| Local | 12 | 4 | 0.062 |
| Intrahepatic | 73 | 71 | |
| Extrahepatic | 4 | 5 | |
| Recurrence location for single HCC ≤3 cm | |||
| Local | 4 | 2 | 0.572 |
| Intrahepatic | 34 | 22 | |
| Recurrence location for single HCC between 3–5 cm | |||
| Local | 8 | 1 | 0.012a |
| Intrahepatic | 38 | 48 | |
| Recurrence treatment | |||
| RFA | 50 | 34 | 0.153 |
| TACE | 30 | 33 | |
| Other | 9 | 13 | |
| Recurrence treatment for single HCC ≤3 cm | |||
| RFA | 17 | 12 | 0.598 |
| TACE | 9 | 4 | |
| Other | 3 | 4 | |
| Recurrence treatment for single HCC between 3–5 cm | |||
| RFA | 25 | 19 | 0.159 |
| TACE | 12 | 19 | |
| Other | 3 | 7 |
P<0.05. MWA, microwave ablation; RES, surgical resection; HCC, hepatocellular carcinoma; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
Subgroup analysis revealed that the recurrence rate for solitary HCC lesions ≤3 cm and those of 3–5 cm did no differ between the two groups. In lesions between 3 and 5 cm, the local recurrence rates of the MWA and the RES groups were markedly different (local/intrahepatic recurrence: 8/38 vs. 1/48; P=0.012; Table V), and the local recurrence rate was higher compared with the intrahepatic recurrence rate. By contrast, no difference was observed in the local recurrence rate between the two groups (4/34 vs. 2/22; Table V) in patients with solitary HCC lesions ≤3 cm.
Among the 89 patients with recurrence in the MWA group, 50 received RFA, 2 received RES, 30 received transcatheter arterial chemoembolization (TACE), 2 received systemic chemotherapy, 2 received symptomatic treatment, 1 received supportive treatment and 2 did not receive treatment. Among the 80 patients with recurrence in the RES group, 37 underwent RFA, 3 underwent RES, 32 underwent TACE, 2 received systemic chemotherapy, 2 received symptomatic treatment, 1 received supportive treatment and 3 did not receive treatment. No significant differences were observed in the results of radical treatment (local ablation or RES) between the two groups (52/89 vs. 36/80), nor between all patients with recurrence receiving RFA and TACE (84/169 vs. 63/169). For all patients with recurrence, the mean survival time of patients with recurrent HCC treated with RFA was 63.55±5.43 months (95% CI, 52.92–74.19) and for those treated with TACE, it was 27.11±3.58 months (95% CI, 20.09–34.12), revealing a distinct difference between the two treatment types (P<0.001, Fig. 4B).
Discussion
HCC is one of the most prevalent types of cancer globally, but progress in the development of effective treatments for advanced disease has been limited (17). Due to its complexity (with early symptoms that are not obvious, usually with cirrhosis, recurrence, metastasis and heterogeneity after surgery), HCC is one of the most lethal malignant tumor types (18). The majority of patients are diagnosed in the late stages of disease and have missed the optimal surgical period; therefore, non-surgical resection has become the treatment of choice for advanced HCC (19,20). Ablation therapy and TACE are common treatments for localized diseases. Ablation using alcohol, radiofrequency, microwave or cryoablation is considered a therapeutic option for surgical excision (21,22). As well as RES and liver transplantation, RFA has been recognized as the first-line treatment for small HCCs (<3 cm) (23). However, multiple retrospective and prospective randomized controlled trials have demonstrated that there is no significant difference in survival between RFA and RES for the treatment of small hepatic lesions (24). By contrast, RES is a more clinically established method, with lower recurrence rates and prolonged DFS compared with RFA, and can be used to remove multiple lesions, satellite occlusions and tumor thrombi in the same liver segment (25). MWA therapy is a treatment method that has been developed in recent years; it is an effective treatment for liver cancer due to its minimal invasiveness, safety and wide range of indications for local tumor treatment (26). Compared with RFA, MWA provides a larger ablation range and higher intratumoral temperature and is less influenced by the heat sink effect (27). Therefore, MWA technology can theoretically achieve ideal local tumor control. Lucchina et al (28) reviewed six studies of MWA and RFA in the treatment of HCC; compared with RFA, the 1- and 3-year survival rates of patients treated with MWA were 89–100 and 49–80%, respectively, with fewer postoperative complications.
In the present study, survival analysis revealed no differences in OS and DFS rates between the MWA and RES groups in the short-term (≤5 years), which was consistent with our previous study (14). For long-term survival (7 and 10 years), both the OS and DFS rates of the RES group were significantly higher compared with those of the MWA group. In addition, the total and early recurrence rates in the MWA group were significantly higher compared with those in the RES group. In theory, RES has the advantage of providing improved local control of HCC, whereas MWA is limited by the location of the lesion. For isolated small liver cancers, ≥13% of cases may have small accessory tumors near the primary tumor that are not detected by imaging (29). In addition, MWA is also limited by the size of the lesion; for tumors >3 cm, MWA may result in insufficient ablation, and residual tumor tissue may potentially result in local recurrence (30). Moreover, RES is able to remove small tumor satellites (30), which may reduce intrahepatic recurrence rates compared with MWA.
Subgroup analysis in the present study indicated that no differences between the OS and DFS rates in the MWA and RES groups for patients with solitary HCC lesions ≤3 cm, and in the OS rate for solitary HCCs >3 cm. By contrast, there was a significant difference in the DFS rate of patients with solitary HCC lesions >3 cm between the two groups. The size of a tumor is an indication of its age; the longer the time in vivo, the greater the degree of microvascular invasion to the surrounding tissues, and the more the recurrence and survival rates are affected (31,32). Lazzara et al (33) have reported that a safety margin of <1.0 cm is an independent factor for early recurrence of liver cancer. In the present study, RES resulted in a more adequate safety margin. Additionally, MWA produces a necrotic area of ~4.8×4×4 cm, providing a 1.0 cm safety margin for tumors <3 cm in diameter (34), which may explain the similarity in the OS and DFS rates of patients with single HCC lesions ≤3 cm in the two groups in the present study. By contrast, single HCC lesions >3 cm are commonly accompanied by microsatellite foci and vascular invasion, which are risk factors for survival and recurrence in HCC treated by RES, MWA or hepatic artery chemotherapy (35). The most important point in ablation therapy for medium- and large-sized HCC lesions is that the tissue coagulation area formed by a single energy output needs to be sufficiently large. In large tumors, even with multipoint ablation, it is still possible to retain a gap (i.e. residual cancer tissue) due to the small range of the single coagulation area and the incomplete overlap of the ablation foci (36). Therefore, the local recurrence rate is higher after ablation. In the present study, for multiple tumors, no differences were observed in OS and DFS rates between the MWA and RES groups. This was primarily due to the multicentric nature of HCC, and the fact that most patients had a history of chronic HBV infection and cirrhosis. Following surgical resection, these tumorous tissues were still present in the residual liver, resulting in a high risk of recurrence.
In the present study, age, HBV and HCV infection, tumor size, tumor number and intervention type were significantly associated with OS and DFS rates. Pompili et al (37) have reported that elderly patients are at a higher risk of death from extrahepatic diseases and are more likely to suffer from liver failure, as they may suffer a longer course of chronic liver disease. Studies have also suggested that HBV and HCV infections are the primary causes of HCC (38). Active HBV or HCV infection causes liver necrosis that may lead to gene mutations and promote recurrence and metastasis (39). Tumor size and number are the other two major factors that influence the postoperative metastasis and recurrence of HCC; lesions with a diameter >5 cm, multiple tumors and vascular or microvascular infiltration are frequently observed in tumor microsatellite foci far from the primary tumor, which reduces the possibility of radical resection and significantly increases the rate of recurrence (40). These results are in agreement with those of the present study.
There were several limitations to the current study. Since this was a single center retrospective analysis, the selection of patients may have been biased. For further investigation, prospective randomized controlled trials are being considered. The sample size was also limited and did not reveal statistical significance in certain comparisons. In addition, a number of patients were followed-up for >10 years, whereas others were only assessed for ≤5 years; thus, further follow-up is required.
In summary, the present study indicated that for patients with HCC meeting the Milan criteria, MWA resulted in a higher tumor recurrence rate and lower DFS rate compared with those of patients treated with RES. For the short term, the effects of the two therapies were comparable, but the long-term survival rate was higher in the RES group. For patients with solitary HCC lesions ≤3 cm, MWA and RES were equally effective, although for patients with solitary HCC lesions >3 cm, the DFS was longer after RES treatment. A multicenter, large-sample, prospective randomized controlled study is being considered for further investigation.
Acknowledgements
Not applicable.
Funding
This work was supported by the Program of Tianjin Science and Technology Development Plan (grant. no. 17YFZCSY01070).
Availability of data and materials
All data analyzed during this study are included in this published article.
Authors' contributions
QS made substantial contributions to the conception and design and wrote the manuscript. JS and CR acquired the data. ZD and GS analyzed and interpreted the data. YW contributed to conception and design of this study, reviewed the manuscript and gave final approval of the version to be published and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Informed consent was obtained from the participants. This study was approved by the Ethical Committee of Tianjin Third Central Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Cross TJS, Evans JC, editors. Liver Cancers. Springer International Publishing Corp.; Berlin: 2019. Transarterial embolization therapies in hepatocellular carcinoma: Principles of management: From mechanisms to management; pp. 123–138. [DOI] [Google Scholar]
- 2.Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;2:600–611. doi: 10.1002/hep.29498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, Chen J, Guideline Committee Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22:9279–9287. doi: 10.3748/wjg.v22.i42.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Chen WQ, Zheng RS, Zhang SW. Liver cancer incidence and mortality in China, 2009. Chin J Cancer. 2013;32:162–169. doi: 10.5732/cjc.013.10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bureau of Medical Administration National Health and Family Planning Comission of the People's Republic of China, corp-author. Diagnosis, management, and treatment of hepatocellular carcinoma (V2017) Zhonghua Gan Zang Bing Za Zhi. 2017;25:886–895. doi: 10.3760/cma.j.issn.1007-3418.2017.12.002. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein S, Dufour JF. Nonalcoholic fatty liver disease and hepatocellular carcinoma. Hepat Oncol. 2017;4:83–98. doi: 10.2217/hep-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer TD, Haskal ZJ, American Association for the Study of Liver Diseases The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: Update 2009. Hepatology. 2010;51:306. doi: 10.1002/hep.23383. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver. Electronic address, corp-author. easloffice@easloffice.eu; European Association for the Study of the Liver: EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda H. European organization for research and treatment of cancer. Jpn J Clin Oncol. 2000;30:169. [PubMed] [Google Scholar]
- 12.Zhou Y, Xu X, Ding J, Jing X, Wang F, Wang Y, Wang P. Dynamic changes of T-cell subsets and their relation with tumor recurrence after microwave ablation in patients with hepatocellular carcinoma. J Cancer Res Ther. 2018;14:40–45. doi: 10.4103/jcrt.JCRT_775_17. [DOI] [PubMed] [Google Scholar]
- 13.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: A retrospective comparative study. Radiology. 2012;262:1022–1033. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 14.Shi J, Sun Q, Wang Y, Jing X, Ding J, Yuan Q, Ren C, Shan S, Wang Y, Du Z. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29:1500–1507. doi: 10.1111/jgh.12572. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;1:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, III, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Jr, et al. Image-guided tumor ablation: Standardization of terminology and reporting criteria. Radiology. 2005;235:728–739. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llovet JM, Hernandez-Gea V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin Cancer Res. 2014;20:2072–2079. doi: 10.1158/1078-0432.CCR-13-0547. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Li B, Li B, Guo T, Sun Z, Li X, Chen L, Chen W, Chen P, Mao Y, Zeng Y. ITIH4: Effective serum marker, early warning and diagnosis, hepatocellular carcinoma. Pathol Oncol Res. 2018;24:663–670. doi: 10.1007/s12253-017-0285-4. [DOI] [PubMed] [Google Scholar]
- 19.Bréchot C. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: Old and new paradigms. Gastroenterology. 2004;127(5 Suppl 1):S56–S61. doi: 10.1053/j.gastro.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 20.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lan WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(Suppl 3):S104–S111. doi: 10.1016/j.jceh.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan X, Fu BM, Tang B, et al. Progress in clinical research of radiofrequency ablation for primary liver cancer. J Hepato Surg. 2016;1:87–89. [Google Scholar]
- 25.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: A systematic review. Ann Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 26.Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7:2578–2589. doi: 10.4254/wjh.v7.i25.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT., Jr Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology. 2005;236:132–139. doi: 10.1148/radiol.2361031249. [DOI] [PubMed] [Google Scholar]
- 28.Lucchina N, Tsetis D, Ierardi AM, Giorlando F, Macchi E, Kehagias E, Duka E, Fontana F, Livraghi L, Carrafiello G. Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol. 2016;29:460–465. doi: 10.20524/aog.2016.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Harada N, Shirabe K, Maeda T, Kayashima H, Takaki S, Maehara Y. Comparison of the outcomes of patients with hepatocellular carcinoma and portal hypertension after liver resection versus radiofrequency ablation. World J Surg. 2016;40:1709–1719. doi: 10.1007/s00268-016-3465-6. [DOI] [PubMed] [Google Scholar]
- 31.Kim SJ, Lee KK, Kim DG. Tumor size predicts the biological behavior and influence of operative modalities in hepatocellular carcinoma. Hepatogastroenterology. 2010;57:121–126. [PubMed] [Google Scholar]
- 32.Chen X, Zhang B, Yin X, Ren Z, Qiu S, Zhou J. Lipiodolized transarterial chemoembolization in hepatocellular carcinoma patients after curative resection. J Cancer Res Clin Oncol. 2013;139:773–781. doi: 10.1007/s00432-012-1343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazzara C, Navarra G, Lazzara S, Barbera A, Saitta C, Raimondo G, Latteri S, Curro G. Does the margin width influence recurrence rate in liver surgery for hepatocellular carcinoma smaller than 5 cm? Eur Rev Med Pharmacol Sci. 2017;21:523–529. [PubMed] [Google Scholar]
- 34.Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, Liu GJ, Wu MC. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: Experimental and clinical studies. Eur Radiol. 2012;22:1983–1990. doi: 10.1007/s00330-012-2442-1. [DOI] [PubMed] [Google Scholar]
- 35.Huo TI, Liu WY, Wu JC, Huang YH, King KL, Loong CC, Lee PC, Chang FY, Lee SD. Deterioration of hepatic functional reserve in patient with hepatocellular carcinoma after resection: Incidence risk factors, and association with intrahepatic tumor recurrence. World J Surg. 2004;28:258–262. doi: 10.1007/s00268-003-7182-6. [DOI] [PubMed] [Google Scholar]
- 36.Yang W. Current status and prospective of imaging guided radiofrequency ablation in medium to large sized hepatocellular carcinomas. World Chin J Digestol. 2015;30:4771. doi: 10.11569/wcjd.v23.i30.4771. (In Chinese) [DOI] [Google Scholar]
- 37.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, Brunello F, Pinna AD, Giorgio A, Giulini SM, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhang P, Gu A, Shi DM. Analysis of the status of postoperative recurrence and metastasis of hepatocellular carcinoma and its influencing factors. Oncol Prog. 2017;4 [Google Scholar]
- 39.Li T, Wang SK, Zhou J, Sun HC, Qiu SJ, Ye QH, Wang L, Fan J. Positive HBcAb is associated with higher risk of early recurrence and poorer survival after curative resection of HBV-related HCC. Liver Int. 2016;36:284–292. doi: 10.1111/liv.12898. [DOI] [PubMed] [Google Scholar]
- 40.Huang JQ, Peng MH, Zou YQ, Yang DH, Chen B, Xiao KY. Analysis of risk factors for early recurrence of primary hepatocellular carcinoma after radical hepatectomy. Chinese J Practl Surg. 2009;5:418–420. (In Chinese) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during this study are included in this published article.