Abstract
The presence of circulating tumor cells (CTCs) in the blood of patients with metastatic breast, colorectal and prostate cancer have been widely investigated; however, few studies have examined CTCs in patients with laryngeal cancer. The present pilot study aimed to detect pre- and postoperative CTCs in the blood of patients with laryngeal cancer and evaluate the association with prognosis. Eight patients with laryngeal squamous cell carcinoma (LSCC) at stage III were included in the present study and underwent total or subtotal laryngectomy and radical bilateral neck lymph node dissection. Blood samples were collected from all patients before and after surgery at different time-points. The following processing steps were followed; preoperative blood sampling, surgery, postoperative blood sampling at 3, 6 and 12 month follow-ups, and prognostic association analysis. CTCs were retained on ScreenCell filters for cytological characterization. The presence of CTCs was associated with a less favorable prognosis, whereas a decrease of CTCs in the postoperative sampling was observed in patients who exhibited an improved therapeutic response. The results of the present pilot study revealed a possible association between the presence of CTCs and a less favorable prognosis in patients with LSCC; therefore, these preliminary findings may encourage further research into the incorporation of a liquid biopsy in the management of LSCC, as this may help identify patients with occult metastatic disease earlier and in a non-invasive manner. In addition, this approach may represent novel independent prognostic factor for use in the clinical evaluation of patients with LSCC.
Keywords: circulating tumor cells, laryngeal cancer, liquid biopsy, epithelial-mesenchymal transition, ScreenCell
Introduction
Laryngeal squamous cell carcinoma (LSCC) is one of the most commonly diagnosed malignancies in the head and neck, with an increased incidence rate in middle-aged and elderly males worldwide in the last few decades (1–3). LSCC originates from laryngeal epithelial tissue and may spread directly to adjacent structures, or through lymphatic and blood vessels to lymph nodes and more distant sites (4). Despite considerable improvements in laryngeal carcinoma treatment, which have improved patient quality of life, the global survival rates have remained unchanged throughout the last 3 decades (5–7).
Circulating tumor cells (CTCs) are rare cells that derive from both primitive cancer and metastases, pass through the blood vessels and circulate together with the red and white blood cells. CTCs are absent in healthy patients (8). Several studies have investigated the presence of CTCs associated with solid types of cancer, including head and neck squamous cell carcinoma (9,10), and proposed the use of liquid biopsy in clinical assessment of patients with cancer (11–19). The presence of CTCs has been validated as a prognostic factor in metastatic breast, colorectal and prostate cancers (15,17), and confirmed in previous meta-analyses (20,21); however, only a few studies have examined CTCs in patients with head and neck and laryngeal cancer (9–11,14,18).
Several techniques for the detection and enumeration of CTCs have been developed during the last decade. For example, epithelial antigenic properties of cancer cells are used to detect and isolate cancer cells from blood using immunomagnetic or microfluidic-based enrichment methods (22). The current techniques allow the isolation of CTCs as: epithelial cells (cytokeratin positive), cells in the epithelial to mesenchymal transition (EMT) phase (cytokeratin negative), stem cells and clusters (two or more CTCs together) (23). However, a number of these detection systems are not commercially available and/or economically accessible (23). Previously, it has been demonstrated that the epithelial antigen-based detection of CTCs may underestimate the real number of CTC (24). This may be due to EMT, which represents an integral component of the metastatic process in which cancer cells downregulate the expression of epithelial markers in favor of mesenchymal markers, inducing the increased stemness of cancer cells and facilitating the development of chemoresistance (25–28). The ScreenCell system, a filtration-based size and antigen-independent technology, has been developed to identify CTCs (29,30), and the rationale of this device is based on the larger size of CTCs compared with hematological cells (31).
The present pilot study aimed to detect CTCs in the blood of patients with laryngeal squamous carcinoma pre- and post-operatively using the ScreenCell system, and to evaluate the association between CTC presence and patient prognosis.
Materials and methods
Patient enrollment
The Ethics Committee of the Sapienza University of Rome approved the present pilot study (approval no. 32/2017). The experimental protocol met the guidelines and the precepts established by the Declaration of Helsinki; experiments were undertaken with the understanding and written consent of each subject and according to the aforementioned principles.
A total of 8/32 patients diagnosed with laryngeal cancer were included in the present study, according to the following sequential inclusion criteria: i) Biopsy specimen positive squamous cell carcinoma (n=32); ii) absence of synchronous and metachronous cancer (n=26); iii) Tumor-Node-Metastasis (TNM)-stage III/IV (n=13); iv) candidates for total or subtotal laryngectomy with neck dissection (n=12); v) no candidates for neoadjuvant therapy (n=10); and vi) patients that provided written informed consent (n=8). In order to increase the uniformity of the population, only patients with homogeneous characteristics for clinical and histopathological parameters were included. All participants were males, smokers, non-alcoholics, age >65 years (age range=61-83; mean age=69 years) with a diagnosis of LSCC. The included patients were waiting for primary laryngeal surgery and were classified, according to the TNM classification (32), as T3N+M0. In the total cohort, 1 patient underwent total laryngectomy and 7 underwent subtotal laryngectomy, according to cancer staging. All patients underwent radical bilateral neck lymph node dissection.
The stages of the study were: i) biopsy by microlaryngoscopy and staging by computed tomography (CT) scan, fibro- and micro-laryngoscopy; ii) preoperative blood sampling; iii) surgery; iv) postoperative short- and medium-term follow-up at 3, 6 and 12 months with clinical evaluation and blood sampling for CTC detection; and v) data analysis. The association between CTC detection and prognosis was studied via the comparison between CTC presence with recurrence/lymph node metastasis/death and adjuvant therapy/secondary surgery.
Blood sample collection
A total of 6 ml peripheral blood was drawn from the median cubital vein in K2-EDTA tube (Thermo Fisher Scientific, Inc.) stored at 4°C and processed within 3 h of sampling. Four different blood samples were collected from each patient; the first sample was drawn 1 day prior to surgery and the next 3 time-points of collection were at 3, 6 and 12 months after tumor removal.
Depletion of leukocytes
Leukocyte depletion was performed using Dynabeads CD45 (Invitrogen; Thermo Fisher Scientific, Inc.) in order to enrich CTCs from whole blood, following the manufacturer's instructions, as previously described (33). Each blood sample was incubated with Dynabeads (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at 2°C with gentle tilting rotation. The tube was then removed from the mixer and placed on a magnet for 10 min at 20–25°C. The supernatant was transferred into a new tube and immediately processed for downstream analysis using the ScreenCell device (ScreenCell Ltd.).
CTC detection using ScreenCell
To isolate fixed cells for cytological studies, a ScreenCell Cytokit was used according to the manufacturer's protocol (Caltag Medsystems, Ltd.). A 3 ml leukocyte-depleted blood sample was diluted using 4 ml filtration buffer ScreenCell fixed cells (FC2) dilution buffer (ScreenCell) to fix cells and lyse red blood cells (RBCs). Before use, 30% NaOH was added to the FC buffer until a pH ~7 was reached. After 8 min of incubation at room temperature, 7 ml diluted sample was added into the device tank and filtered under a pressure gradient (determined between the atmosphere pressure and vacuum tube) using a vacutainer tube. Filtration was completed within 3 min. After washing with PBS to remove RBC debris, the filter was left on absorbing paper to dry at room temperature and then stored at −20°C until Giemsa and immunofluorescence staining were performed. For each patient, the filtration was carried out in duplicate.
Giemsa staining and double immunofluorescence analysis
The filters were stained for 30 min at room temperature with Giemsa stain (1:10; cat. no. 453616; Carlo Erba) and examined using a light microscope (magnification, ×400) (Leitz DMRB Camera; Leica Microsystems Inc.) to evaluate the presence of cells adhered to the filter membrane. The double immunofluorescence analysis, using anti-Pan-Cytokeratin (CK) and anti-epithelial cell adhesion molecule (EpCAM) antibodies, was used to identify the adherent cells as CTCs. For this scope, the filters were fixed using 4% paraformaldehyde solution for 5 min at 4°C and subsequently permeabilized using PBS and 0.01% Tween-20 (Merck KGaA) for 20 min at room temperature. Then, both the filters were incubated with monoclonal mouse anti-Pan-Cytokeratin antibody 2A4 (1:100; cat. no. ab118855; Abcam) for 1 h at room temperature. The filters were incubated with secondary antibody Alexa Fluor 488 goat anti-mouse IgG (H + L) (1:300; cat. no. A11001; Thermo Fisher Scientific, Inc.; green staining). Successively, the filters were incubated with monoclonal mouse anti-EpCAM antibody (clone C-10; 1:50; cat. no. sc-25308, Santa Cruz Biotechnology) for 1 h at room temperature followed by incubation with secondary antibody Alexa Fluor 594 goat anti-mouse IgG (H + L) (1:300; cat. no. A11005; Thermo Fisher Scientific, Inc.; red staining). . The nuclei were counterstained for 10 min at room temperature using DAPI (1:1,000; cat. no. D1306; Thermo Fisher Scientific, Inc.). Immunostaining was examined under a fluorescence microscope (Olympus Corporation) at magnification, ×400. For each sample, five randomly selected microscopic fields were evaluated and cells positive for anti-Pan-Cytokeratin (cytoplasmatic green stain) and anti-EpCAM (cytoplasmatic red stain) immunostaining, were counted. The cells positive for anti-Pan-Cytokeratin and anti-EpCAM were considered to be CTCs.
Results
Patient follow-up
CTCs were characterized by positivity for CK and EpCAM. Fig. 1 shows Giemsa staining of circulating tumor cells in patients with laryngeal squamous cell carcinoma. Fig. 2 shows immunofluorescence analysis with anti-Pan-Cytokeratin antibody used to characterize tumor circulating cells of patients with laryngeal squamous cell carcinoma before and after surgery. Preoperatively, 6 patients had evidence of CTCs and 2 patients were negative. At the 3-month follow-up, all participants were disease-free and CTC+. At the 6-month follow-up, 3 patients were disease-free (1 CTC+ and 2 CTC−), two patients had died (CTC+), 1 patient had recurrence and 2 had metastasis (1 CTC+, 1 underwent secondary laryngectomy and 2 were false negatives (due to chemo/radiotherapy for liver/pulmonary metastasis). At 12-months follow-up, 4 patients were disease-free (1 CTC+ and 3 CTC-) and 4 had died (CTC+) (Table I).
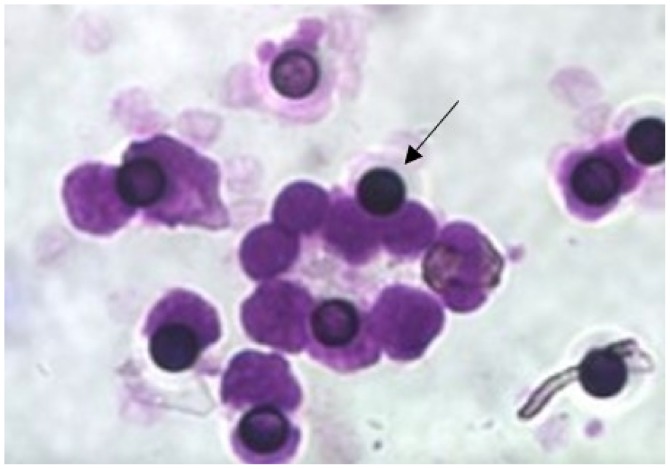
Figure 1.
Circulating tumor cells in patients with laryngeal cancer. Giemsa staining of circulating tumor cells in a patient (patient #4) with laryngeal squamous cell carcinoma. ScreenCell filters were stained with Giemsa and examined using a light microscope to evaluate the presence of cells adhered to the filter membrane. Arrow shows the micropore of the filter. Magnification, ×400.
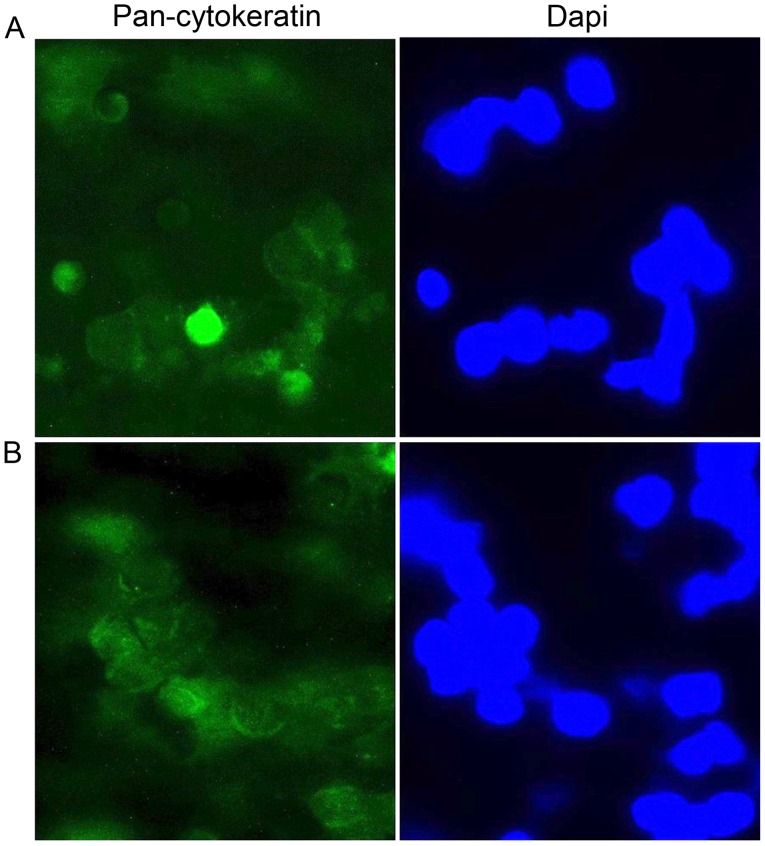
Figure 2.
Immunofluorescence analysis. Immunofluorescence analysis with anti-Pan-Cytokeratin antibody used to characterize tumor circulating cells of patients with laryngeal squamous cell carcinoma adherent to the filter membrane (A) pre- and (B) post-surgery. The nuclei were counterstained using DAPI. Magnification, ×400.
Table I.
Detection of CTCs pre- and postoperatively in patients with laryngeal squamous cell carcinoma.
| Postoperative follow up time, months | |||||||
|---|---|---|---|---|---|---|---|
| 3 | 6 | 12 | |||||
| Case | Preoperative CTC detection | Follow-up status | CTC detection | Follow-up status | CTC detection | Follow-up status | CTC detection |
| #1 | [+] | Disease-free | (+) | CH-RT for metastasis | False negative for CH | Dead | (−) |
| #2 | [+] | Disease-free | (+) | Dead | (−) | (−) | (−) |
| #3 | [+] | Disease-free | (+) | Dead | (−) | (−) | (−) |
| #4 | [+] | Disease-free | (+) | Disease-free | (+) | Disease-free | (−) |
| #5 | [+] | Disease-free | (+) | CH for metastasis | False negative for CH | Dead | (−) |
| #6 | [-] | Disease-free | (+) | Disease-free | (−) | Disease-free | (−) |
| #7 | [+] | Disease-free | (+) | Secondary surgery for recurrence | (+) | Disease-free | (+) |
| #8 | (−) | Disease-free | (+) | Disease-free | (−) | Disease-free | (−) |
CTC, circulating tumor cells; (+), positive for CTCs; (−), negative for CTCs; NA, not available; CH, chemotherapy; RT, radiotherapy.
Association between pre-operative CTC detection and prognosis
Preoperatively, 6 patients had evidence of CTCs and 2 patients were negative. The data revealed that the presence of CTC before the surgery may be associated with a less favorable prognosis, whereas a negative result for CTCs preoperatively was associated with a favorable prognosis. The majority of patients who were CTC+ (83.3%) exhibited a poor prognosis (4 died and 1 underwent secondary surgery), while all patients who were CTC− preoperatively were disease-free at all follow-up visits (Table I).
Association between post-operative CTC detection and prognosis
Three months after surgery, all patients were CTC+ and disease-free. Patients exhibiting increased or overlapping values of CTCs, had a poor prognosis (4 deceased and 1 relapsed). Conversely, if there was a reduction or zeroing of CTC value, this was associated with a more positive prognosis (Table I).
The analysis of the data at medium-term (6 months after surgery) revealed that: i) Negative matching of CTC in the pre- and post-operative at 6 months may be a positive prognostic factor (25% of patients CTC− in the pre- and post-6-months were disease-free at all follow-ups); ii) patients who underwent chemotherapy were negative for CTCs; and iii) 50% of patients with detectable CTC both in pre- and post-6-months follow up experienced a progression of disease (Table I).
Data collected 12 months after surgery indicated that: i) CTC negativity preoperatively and at the 12-month follow up were associated with a more favorable prognosis; and ii) CTC positivity preoperatively and at the 12-month follow up was associated with a less favorable prognosis. In fact, all patients that were CTC+ both pre- and post-operative died, except the patient that exhibited a decrease in CTC levels in the long-term (Table I).
Discussion
In the last two decades, comprehensive treatment measures, such as surgery, radiotherapy, chemotherapy and gene therapy, have resulted in a higher 5-year survival rate globally for patients with laryngeal cancer; however, 30–40% of patients still succumbed to the disease due to tumor recurrence or metastasis (34). An improved understanding of the metastatic processes underlying LSCC is needed to identify novel prognostic factors and treatment methods.
The present pilot study evaluated whether the presence of CTCs in patients with laryngeal cancer may represent a novel independent prognostic factor and may be quantified in a liquid biopsy to aid clinical evaluation. The techniques used for CTC detection in the present study were immunological and morphological; CTCs were isolated from the blood of patients with LSCC using the ScreenCell system, a technology based on polycarbonate filter with 8 µm diameter pores able to isolate CTCs from other blood cells. An advantage of this technology is the possibility to detect CTCs using Giemsa histochemical staining and to identify the presence of CTC markers by immunofluorescence staining (29). Leukocyte depletion was performed prior to the CTC isolation in order to improve the CTC detection (35).
To the best of our knowledge, the present study is the first to focus exclusively on CTCs in laryngeal cancer using the ScreenCell system. No other studies have been published on the use of ScreenCell in laryngeal cancer and these preliminary results may present an incentive for further studies on this topic. The ScreenCell system differs from other systems such as the CellCollector system, used by Zhang et al (36) in which CTCs from laryngeal cancer were evaluated as it is a filtration-based size and antigen-independent technology (29). CTC isolation using the ScreenCell system is promising due to its simplicity, speed and the benefit that it eliminates any antibody bias that may be introduced by other techniques (37,38). Although no complex instruments or training are needed to use this system, the costs are high.
The development of semiautomatic technologies, such as the CellSearch system, has allowed evaluation of the prognostic role of CTC status in patients with other types of cancer, such as lung and breast cancer, with promising results (37–49). A recent study from Chudasama et al (38) evaluated the efficacy of the ScreenCell filtration system, to capture, isolate and propagate CTCs from patients with primary lung cancer. The results suggested that the ScreenCell system had the potential to be used as a CTC isolation tool following further work, adaptations and improvements to the technology and validation of results. Another study from Hashimoto et al (48) concluded that there was an increase in the CTC count of pulmonary vein blood following surgical manipulation of a tumor. Hou et al (49) identified an association between an increased CTC count and less favorable patient survival in small cell lung cancer.
The preliminary results of the present study indicate that, in laryngeal cancer, the absence of CTCs may predict a more favorable prognosis, while high levels of CTCs in the peripheral blood may be associated with a less favorable prognosis. A decrease of CTCs in postoperative sampling may suggest an improved response to surgical treatment, and the early detection of CTCs may predict recurrence or metastasis.
The results of the present study are in accordance with other studies investigating CTCs in solid cancers, including the head and neck, which revealed that the presence of CTCs may influence prognosis (11–17,44–57). Zhang et al (36) and He et al (58) revealed that CTCs have a role in the progression and metastasis of head and neck squamous cell carcinoma. Nichols et al (59) isolated CTCs in 6/15 patients with advanced head and neck carcinoma using CellSearch and demonstrated an association with lung nodules >1 cm. Winter et al (60) tested 16 patients with advanced head and neck squamous cell carcinoma and demonstrated that almost all (15/17) patients had circulating cells at the time of surgery, similar to what was observed in the patients in the present study (6/8 were positive to CTC preoperatively). A recent meta-analysis comprised of 17 studies confirmed the significant prognostic value of CTCs in patients with head and neck cancer, wherein positive CTCs were significantly associated with poor overall, disease-free and progression-free survival (61). Patients who were CTC+ tended to have higher recurrence and regional lymph node metastasis rate and a more advanced tumor stage. The authors concluded that the presence of CTCs may be used as a monitoring tool for tumor status of head and neck cancer, especially for the early detection of tumor recurrence and progression, advanced disease and node metastasis.
The primary limitation of the present study is the small number of patients included. Such small sample did not allow reliable statistical analyses to be performed. Further studies aimed at investigating CTCs in laryngeal cancer using the ScreenCell system in a larger cohort of patients are necessary to improve the definition of the sensitivity and specificity of the ScreenCell filtration system and to confirm the preliminary results.
In conclusion, the results of the present study revealed a possible association between the presence of CTCs and a less favorable prognosis in patients with LSCC. The current preliminary findings may encourage more research into the incorporation of a liquid biopsy test in the management of LSCC, as it may help identify patients with occult metastatic disease earlier and in a non-invasive manner. This may also represent an independent prognostic factor which may help in clinical evaluation. Further studies aimed at investigating the role of CTC using the ScreenCell system in a larger number of patients with laryngeal cancer are necessary to confirm these preliminary results.
Acknowledgements
The authors wish to thank Mr Jay Joseph Abedin for his thoughtful revision of the English language and grammar of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MIR and MR conceived and designed the study. CN and AGra collected and analyzed the samples and interpreted the data. CN, CDG and RC prepared the first draft of the manuscript. CDG, AGre and RC made substantial contributions to acquisition and analysis and interpretation of data. MR and MDV performed the clinical evaluations and surgical procedures. AGre and CDG reviewed and revised the manuscript critically for important intellectual content. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The Ethics Committee of the Sapienza University of Rome approved this pilot study (approval no. 32/2017). All patients provided informed written consent.
Patient consent for publication
Signed informed consent for publication has been obtained from the patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, Pagliuca G, Martellucci S, Turchetta R, De Vincentiis M. Cancer stem cells in laryngeal cancer: What we know. Eur Arch Otorhinolaryngol. 2016;273:3487–3495. doi: 10.1007/s00405-015-3837-9. [DOI] [PubMed] [Google Scholar]
- 2.Yu D, Liu Y, Yang J, Jin C, Zhao X, Cheng J, Liu X, Qi X. Clinical implications of BMI-1 in cancer stem cells of laryngeal carcinoma. Cell Biochem Biophys. 2015;71:261–269. doi: 10.1007/s12013-014-0194-z. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Mäkitie AA, Monni O. Molecular profiling of laryngeal cancer. Expert Rev Anticancer Ther. 2009;9:1251–1260. doi: 10.1586/era.09.102. [DOI] [PubMed] [Google Scholar]
- 5.Wan G, Zhou L, Xie M, Chen H, Tian J. Characterization of side population cells from laryngeal cancer cell lines. Head Neck. 2010;32:1302–1309. doi: 10.1002/hed.21325. [DOI] [PubMed] [Google Scholar]
- 6.De Virgilio A, Ralli M, Longo L, Mancini P, Attanasio G, Atturo F, De Vincentiis M, Greco A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment. Oncol Lett. 2018;16:3415–3423. doi: 10.3892/ol.2018.9140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bussu F, Paludetti G, Almadori G, De Virgilio A, Galli J, Miccichè F, Tombolini M, Rizzo D, Gallo A, Giglia V, et al. Comparison of total laryngectomy with surgical (cricohyoidopexy) and nonsurgical organ-preservation modalities in advanced laryngeal squamous cell carcinomas: A multicenter retrospective analysis. Head Neck. 2013;35:554–561. doi: 10.1002/hed.22994. [DOI] [PubMed] [Google Scholar]
- 8.García SA, Weitz J, Schölch S. Circulating tumor cells. Methods Mol Biol. 2018;1692:213–219. doi: 10.1007/978-1-4939-7401-6_18. [DOI] [PubMed] [Google Scholar]
- 9.Wu XL, Tu Q, Faure G, Gallet P, Kohler C, Bittencourt MC. Diagnostic and prognostic value of circulating tumor cells in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Sci Rep. 2016;6:20210. doi: 10.1038/srep20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z, Cui K, Xue Y, Tong F, Li S. Prognostic value of circulating tumor cells in patients with squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Med Oncol. 2015;32:164. doi: 10.1007/s12032-015-0579-x. [DOI] [PubMed] [Google Scholar]
- 11.Grisanti S, Almici C, Consoli F, Buglione M, Verardi R, Bolzoni-Villaret A, Bianchetti A, Ciccarese C, Mangoni M, Ferrari L, et al. Circulating tumor cells in patients with recurrent or metastatic head and neck carcinoma: Prognostic and predictive significance. PLoS One. 2014;9:e103918. doi: 10.1371/journal.pone.0103918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alix-Panabieres C, Pantel K. The circulating tumor cells: Liquid biopsy of cancer. Klin Lab Diagn. 2014;4:60–64. (In Russian) [PubMed] [Google Scholar]
- 13.Heitzer E, Auer M, Ulz P, Geigl JB, Speicher MR. Circulating tumor cells and DNA as liquid biopsies. Genome Med. 2013;5:73. doi: 10.1186/gm477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hristozova T, Konschak R, Stromberger C, Fusi A, Liu Z, Weichert W, Stenzinger A, Budach V, Keilholz U, Tinhofer I. The presence of circulating tumor cells (CTCs) correlates with lymph node metastasis in nonresectable squamous cell carcinoma of the head and neck region (SCCHN) Ann Oncol. 2011;22:1878–1885. doi: 10.1093/annonc/mdr130. [DOI] [PubMed] [Google Scholar]
- 15.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 16.Danila DC, Heller G, Gignac GA, Gonzalez-Espinoza R, Anand A, Tanaka E, Lilja H, Schwartz L, Larson S, Fleisher M, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol. 2005;23:1420–1430. doi: 10.1200/JCO.2005.08.140. [DOI] [PubMed] [Google Scholar]
- 18.Nonaka T, Wong DT. Liquid biopsy in head and neck cancer: Promises and Challenges. J Dent Res. 2018;97:701–708. doi: 10.1177/0022034518762071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Economopoulou P, Kotsantis I, Kyrodimos E, Lianidou ES, Psyrri A. Liquid biopsy: An emerging prognostic and predictive tool in Head and Neck Squamous Cell Carcinoma (HNSCC). Focus on Circulating Tumor Cells (CTCs) Oral Oncol. 2017;74:83–89. doi: 10.1016/j.oraloncology.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S, Liu Y, Zhang Q, Li H, Zhang M, Ma W, Zhao W, Wang J, Yang M. The prognostic role of circulating tumor cells (CTCs) detected by RT-PCR in breast cancer: A meta-analysis of published literature. Breast Cancer Res Treat. 2011;130:809–816. doi: 10.1007/s10549-011-1659-z. [DOI] [PubMed] [Google Scholar]
- 21.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Parkinson DR, Dracopoli N, Petty BG, Compton C, Cristofanilli M, Deisseroth A, Hayes DF, Kapke G, Kumar P, Lee JS, et al. Considerations in the development of circulating tumor cell technology for clinical use. J Transl Med. 2012;10:138. doi: 10.1186/1479-5876-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol. 2016;10:374–394. doi: 10.1016/j.molonc.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Heliebi A, Kroneis T, Zöhrer E, Haybaeck J, Fischereder K, Kampel-Kettner K, Zigeuner R, Pock H, Riedl R, Stauber R, et al. Are morphological criteria sufficient for the identification of circulating tumor cells in renal cancer? J Transl Med. 2013;11:214. doi: 10.1186/1479-5876-11-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papadaki MA, Stoupis G, Theodoropoulos PA, Mavroudis D, Georgoulias V, Agelaki S. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther. 2019;18:437–447. doi: 10.1158/1535-7163.MCT-18-0584. [DOI] [PubMed] [Google Scholar]
- 26.Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res. 2013;73:8–11. doi: 10.1158/0008-5472.CAN-12-3422. [DOI] [PubMed] [Google Scholar]
- 27.Krebs MG, Hou JM, Sloane R, Lancashire L, Priest L, Nonaka D, Ward TH, Backen A, Clack G, Hughes A, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7:306–315. doi: 10.1097/JTO.0b013e31823c5c16. [DOI] [PubMed] [Google Scholar]
- 28.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 29.Desitter I, Guerrouahen BS, Benali-Furet N, Wechsler J, Jänne PA, Kuang Y, Yanagita M, Wang L, Berkowitz JA, Distel RJ, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31:427–441. [PubMed] [Google Scholar]
- 30.Zheng S, Lin HK, Lu B, Williams A, Datar R, Cote RJ, Tai YC. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices. 2011;13:203–213. doi: 10.1007/s10544-010-9485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schütze K, Capron F, Franco D, Pazzagli M, Vekemans M, et al. Isolation by size of epithelial tumor cells : A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156:57–63. doi: 10.1016/S0002-9440(10)64706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang SH, O'Sullivan B. Overview of the 8th edition TNM classification for head and neck cancer. Curr Treat Options Oncol. 2017;18:40. doi: 10.1007/s11864-017-0484-y. [DOI] [PubMed] [Google Scholar]
- 33.Nicolazzo C, Raimondi C, Francescangeli F, Ceccarelli S, Trenta P, Magri V, Marchese C, Zeuner A, Gradilone A, Gazzaniga P. EpCAM-expressing circulating tumor cells in colorectal cancer. Int J Biol Markers. 2017;32:e415–e420. doi: 10.5301/ijbm.5000284. [DOI] [PubMed] [Google Scholar]
- 34.Yu D, Jin C, Liu Y, Yang J, Zhao Y, Wang H, Zhao X, Cheng J, Liu X, Liu C. Clinical implications of cancer stem cell-like side population cells in human laryngeal cancer. Tumour Biol. 2013;34:3603–3610. doi: 10.1007/s13277-013-0941-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Lang JC, Balasubramanian P, Jatana KR, Schuller D, Agrawal A, Zborowski M, Chalmers JJ. Optimization of an enrichment process for circulating tumor cells from the blood of head and neck cancer patients through depletion of normal cells. Biotechnol Bioeng. 2009;102:521–534. doi: 10.1002/bit.22066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang HD, Gong SC, Liu YQ, Liang LJ, He SB, Zhang QX, Si MY, Yu ZK. The significance of circulating tumor cells in head and neck squamous cell carcinoma: A preliminary study. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:39–44. doi: 10.3760/cma.j.issn.1673-0860.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Chudasama D, Katopodis P, Stone N, Haskell J, Sheridan H, Gardner B, Urnovitz H, Schuetz E, Beck J, Hall M, et al. Liquid biopsies in lung cancer: Four emerging technologies and potential clinical applications. Cancers (Basel) 2019;11:E331. doi: 10.3390/cancers11030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chudasama D, Burnside N, Beeson J, Karteris E, Rice A, Anikin V. Perioperative detection of circulating tumour cells in patients with lung cancer. Oncol Lett. 2017;14:1281–1286. doi: 10.3892/ol.2017.6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amantini C, Morelli MB, Nabissi M, Piva F, Marinelli O, Maggi F, Bianchi F, Bittoni A, Berardi R, Giampieri R, et al. Expression profiling of circulating tumor cells in pancreatic ductal adenocarcinoma patients: Biomarkers predicting overall survival. Front Oncol. 2019;9:874. doi: 10.3389/fonc.2019.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicolazzo C, Raimondi C, Gradilone A, Emiliani A, Zeuner A, Francescangeli F, Belardinilli F, Seminara P, Loreni F, Magri V. Circulating tumor cells in right- and left-sided colorectal cancer. Cancers (Basel) 2019;11:E1042. doi: 10.3390/cancers11081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuvendjiska J, Bronsert P, Martini V, Lang S, Pitman MB, Hoeppner J, Kulemann B, et al. Non-metastatic esophageal adenocarcinoma: Circulating tumor cells in the course of multimodal tumor treatment. Cancers (Basel) 2019;11:E397. doi: 10.3390/cancers11030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolazzo C, Colangelo L, Corsi A, Carpino G, Gradilone A, Sonato C, Raimondi C, Gaudio E, Gazzaniga P, Gianni W. Liquid Biopsy in Rare Cancers: Lessons from Hemangiopericytoma. Anal Cell Pathol (Amst) 2018;2018:9718585. doi: 10.1155/2018/9718585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mascalchi M, Maddau C, Sali L, Bertelli E, Salvianti F, Zuccherelli S, Matucci M, Borgheresi A, Raspanti C, Lanzetta M, et al. Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions. J Cancer. 2017;8:2223–2230. doi: 10.7150/jca.18418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fina E, Necchi A, Bottelli S, Reduzzi C, Pizzamiglio S, Iacona C, Daidone MG, Verderio P, Cappelletti V. Detection of circulating tumour cells in urothelial cancers and clinical correlations: Comparison of two methods. Dis Markers. 2017;2017:3414910. doi: 10.1155/2017/3414910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Awe JA, Saranchuk J, Drachenberg D, Mai S. Filtration-based enrichment of circulating tumor cells from all prostate cancer risk groups. Urol Oncol. 2017;35:300–309. doi: 10.1016/j.urolonc.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 46.Mu Z, Benali-Furet N, Uzan G, Znaty A, Ye Z, Paolillo C, Wang C, Austin L, Rossi G, Fortina P, Yang H, et al. Detection and characterization of circulating tumor associated cells in metastatic Breast Cancer. Int J Mol Sci. 2016;17:E1665. doi: 10.3390/ijms17101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulasinghe A, Kenny L, Perry C, Thiery JP, Jovanovic L, Vela I, Nelson C, Punyadeera C. Impact of label-free technologies in head and neck cancer circulating tumour cells. Oncotarget. 2016;7:71223–71234. doi: 10.18632/oncotarget.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashimoto M, Tanaka F, Yoneda K, Takuwa T, Matsumoto S, Okumura Y, Kondo N, Tsubota N, Tsujimura T, Tabata C, et al. Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg. 2014;18:775–783. doi: 10.1093/icvts/ivu048. [DOI] [PubMed] [Google Scholar]
- 49.Hou JM, Greystoke A, Lancashire L, Cummings J, Ward T, Board R, Amir E, Hughes S, Krebs M, Hughes A, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol. 2009;175:808–816. doi: 10.2353/ajpath.2009.090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang HM, Wu MH, Chang PH, Lin HC, Liao CD, Wu SM, Hung TM, Lin CY, Chang TC, Tzu-Tsen Y, et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck. 2019;41:2676–2687. doi: 10.1002/hed.25744. [DOI] [PubMed] [Google Scholar]
- 51.Chikamatsu K, Tada H, Takahashi H, Kuwabara-Yokobori Y, Ishii H, Ida S, Shino M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019;89:34–39. doi: 10.1016/j.oraloncology.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Kulasinghe A, Kapeleris J, Kimberley R, Mattarollo SR, Thompson EW, Thiery JP, Kenny L, O'Byrne K, Punyadeera C. The prognostic significance of circulating tumor cells in head and neck and non-small-cell lung cancer. Cancer Med. 2018;7:5910–5919. doi: 10.1002/cam4.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng SP, Bahig H, Wang J, Cardenas CE, Lucci A, Hall CS, Meas S, Sarli VN, Yuan Y, Urbauer DL, et al. Predicting treatment Response based on Dual assessment of magnetic resonance Imaging kinetics and Circulating Tumor cells in patients with Head and Neck cancer (PREDICT-HN): Matching ‘liquid biopsy’ and quantitative tumor modeling. BMC Cancer. 2018;18:903. doi: 10.1186/s12885-018-4808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan TM, Wang X, Qian X, Switchenko JM, Nie S, Patel KR, Cassidy RJ, Shin DM, Beitler JJ. Measurement of circulating tumor cells in squamous cell carcinoma of the head and neck and patient outcomes. Clin Transl Oncol. 2019;21:342–347. doi: 10.1007/s12094-018-1930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lou JL, Guo L, Zheng WH, Zhao JZ, Zhao JQ, Liang Z, Wang SY, Fang MY. Peripheral blood circulating tumor cells in local advanced head and neck squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2017;52:824–829. doi: 10.3760/cma.j.issn.1673-0860.2017.11.005. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 56.Kawada T, Takahashi H, Sakakura K, Ida S, Mito I, Toyoda M, Chikamatsu K. Circulating tumor cells in patients with head and neck squamous cell carcinoma: Feasibility of detection and quantitation. Head Neck. 2017;39:2180–2186. doi: 10.1002/hed.24893. [DOI] [PubMed] [Google Scholar]
- 57.Fanelli MF, Oliveira TB, Braun AC, Corassa M, Abdallah EA, Nicolau UR, da Silva Alves V, Garcia D, Calsavara VF, Kowalski LP, et al. Evaluation of incidence, significance, and prognostic role of circulating tumor microemboli and transforming growth factor-β receptor I in head and neck cancer. Head Neck. 2017;39:2283–2292. doi: 10.1002/hed.24899. [DOI] [PubMed] [Google Scholar]
- 58.He S, Li P, He S, Long T, Zhang N, Fang J, Yu Z. Detection of circulating tumour cells with the CellSearch system in patients with advanced-stage head and neck cancer: Preliminary results. J Laryngol Otol. 2013;127:788–793. doi: 10.1017/S0022215113001412. [DOI] [PubMed] [Google Scholar]
- 59.Nichols AC, Lowes LE, Szeto CC, Basmaji J, Dhaliwal S, Chapeskie C, Todorovic B, Read N, Venkatesan V, Hammond A, et al. Detection of circulating tumor cells in advanced head and neck cancer using the CellSearch system. Head Neck. 2012;34:1440–1444. doi: 10.1002/hed.21941. [DOI] [PubMed] [Google Scholar]
- 60.Winter SC, Stephenson SA, Subramaniam SK, Paleri V, Ha K, Marnane C, Krishnan S, Rees G. Long term survival following the detection of circulating tumour cells in head and neck squamous cell carcinoma. BMC Cancer. 2009;9:424. doi: 10.1186/1471-2407-9-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun T, Zou K, Yuan Z, Yang C, Lin X, Xiong B. Clinicopathological and prognostic significance of circulating tumor cells in patients with head and neck cancer: A meta-analysis. OncoTargets Ther. 2017;10:3907–3916. doi: 10.2147/OTT.S136530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.