Abstract
A recent report identified significant reductions or disappearance of viral load in COVID-19 patients given a combination of hydroxychloroquine and azithromycin. The present communication discusses some common pharmacokinetic properties of these two drugs that may be linked to a potential underlying mechanism of action for these antiviral effects. The physicochemical properties of both hydroxychloroquine and azithromycin are consistent with particularly high affinity for the intracellular lysosomal space, which has been implicated as a target site for antiviral activity. The properties of both drugs predict dramatic accumulation in lysosomes, with calculated lysosomal drug concentrations that exceed cytosolic and extracellular concentrations by more than 50 000-fold. These predictions are consistent with previously reported experimentally measured cellular and extracellular concentrations of azithromycin. This is also reflected in the very large volumes of distribution of these drugs, which are among the highest of all drugs currently in use. The combination of hydroxychloroquine and azithromycin produces very high local concentrations in lysosomes. The clinical significance of this observation is unclear; however, the magnitude of this mechanism of drug accumulation via ion-trapping in lysosomes could be an important factor for the pharmacodynamic effects of this drug combination.
Recent reports identified significant reduction or disappearance of viral load in COVID-19 patients given a combination of hydroxychloroquine and azithromycin [1,2]. However, other clinical studies could not confirm these early findings [3] and there are considerable concerns about the safety of this drug combination [4]. The present communication discusses some common pharmacokinetic properties of these two drugs that may be linked to a potential underlying mechanism of action for their pharmacological activity.
1. Chemical structures
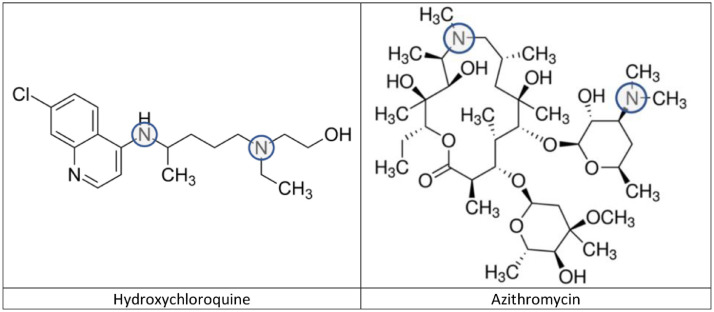
Fig. 1 shows the chemical structures of hydroxychloroquine and azithromycin. Although the two compounds are from two chemically distinct classes, they have a structural similarity that is pharmacokinetically relevant. Both compounds are multibasic amines with pKa values that are susceptible to protonation in the physiological pH range. Azithromycin has two nitrogens with pKa values of 8.1 and 8.8 [5]. Hydroxychloroquine has three nitrogens with pKa values of 4.0, 8.3 and 9.7 [6]. However, only the two nitrogens with the higher values (shown in circles in Fig. 1) are protonated under physiological conditions.
Fig. 1.
Chemical structures of hydroxychloroquine and azithromycin. The circles indicate the pH-sensitive basic nitrogens.
2. Lysosomal ion-trapping
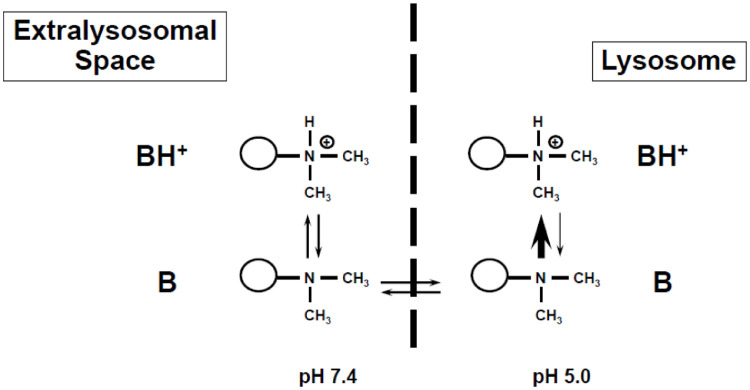
If the pH of the molecular environment is lower (more acidic), more nitrogens are protonated, which in turn hinders the now-charged moieties from crossing membranes. This is particularly relevant for the intracellular distribution of basic drugs crossing between the cytosol (pH approx. 7.4) and the acidic lysosomal space (pH approx. 5.0). Fig. 2 shows this distribution conceptually. Basic compounds are in an equilibrium of a less polar unionized form (B) that can easily cross membranes, and a polar protonated form (BH+) that cannot easily cross membranes. As the unionized drug enters the acidic environment of the lysosome, it will be protonated and ‘trapped’ in the lysosome as the protonated form BH+ cannot easily diffuse back into the cytosol. As a result, high concentrations of the compound can accumulate in lysosomes. This concept of “ion-trapping” has been described and reviewed [7,8]. The magnitude of this accumulation depends on the mathematical relationship of the pKa of the compound of interest, its permeability, and the pH gradient between the two environments (e.g. cytosol [pH 7.4] and lysosome [pH 5.0]). If there is no permeability limitation, the expected ratio or concentration gradient can be calculated based on the well-known Henderson-Hasselbalch equation [8]. If the compound of interest has only one basic group, the ratio between the concentrations will be:
| (1) |
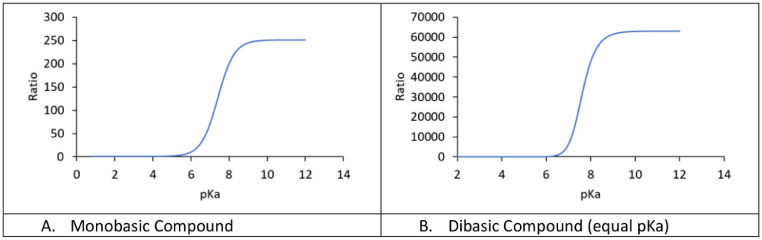
where H1 and H2 are the respective proton concentrations (=10−pH) of the two environments (pH 5 and 7.4) and Ka is the dissociation constant (=10−pKa). Fig. 3 A shows the magnitude of the resulting accumulation as a function of the pKa value of the compound. Accumulation of up to 250-fold higher concentrations in lysosomes can be explained by the described mechanism.
Fig. 2.
Concept of lysosomal ion-trapping. Basic compounds are in an equilibrium of a less polar unionized form (B) that can easily cross membranes, and a polar protonated form (BH+) that cannot easily cross membranes. As the unionized drug enters the acidic environment of a lysosome, it will be protonated and ‘trapped’ in the lysosome as the protonated form BH+ cannot easily diffuse back into the cytosol. As a result, high concentrations of the compound can accumulate in the lysosomes.
Fig. 3.
Magnitude of lysosomal ion-trapping depending on the pKa of the compound of interest. For a monobasic compound (A), up to 250-fold higher concentrations are possible depending on pKa. For a dibasic compound (B), the accumulation can be over 60 000-fold. These simulations assume a pH gradient of 5.0 (lysosomes) and 7.4 (cytosol).
However, in cases where there are two basic centers in the molecule, this effect is considerably potentiated. There are two different monobasic species that are produced by protonation of the respective nitrogens, and neither of these can easily diffuse from the lysosome back into the cytosol. Furthermore, both species are in equilibrium with the biprotonated species, which is also trapped in the lysosome. Therefore, there are three different forms of the molecule (two monoprotonated and one biprotonated) that cannot easily diffuse back to the cytosol. This tremendously magnifies the ion-trapping effect. The expected accumulation ratio under these conditions is calculated as follows:
| (2) |
where H1 and H2 are the respective proton concentrations (=10−pH) of the two environments (pH 5 and 7.4) and Ka1 and Ka2 are the dissociation constant (=10−pKa). Fig. 3B shows the magnitude of the resulting accumulation as a function of the pKa value of the compound, assuming equal values for pKa1 and pKa2. Accumulation of up to 60 000-fold higher concentrations in the lysosomes can be explained by the described mechanism.
3. Hydroxychloroquine
When the above described concepts are applied to hydroxychloroquine, the expected accumulation ratio in lysosomes compared with cytosol is calculated as 56 000-fold; therefore, after drug intake a large fraction of the dose will reside in lysosomes. This is confirmed by the known pharmacokinetic parameters for hydroxychloroquine derived from plasma concentrations. After intravenous administration to healthy volunteers (average weight 63.5 kg), the volume of distribution of hydroxychloroquine was reported to be over 44 000 L, which is equivalent to approximately 700 L/kg [9]. This means that most of the drug in the body resides in the tissues, which is consistent with the predicted extraordinarily high lysosomal accumulation ratio. Furthermore, it explains the very long half-life (approximately 40 days) reported in the same study. In this study, hydroxychloroquine could still be detected in blood more than 6 months after a single dose administration of 310 mg.
4. Azithromycin
When the same ion-trapping concepts are applied to azithromycin, the expected accumulation ratio in lysosomes compared with cytosol is calculated as 52 000-fold, which is very similar to that of hydroxychloroquine. After administration of azithromycin, a large fraction of the dose will reside in lysosomes where it also binds to acidic phospholipids [10], [11], [12], [13]. A clinical study supports azithromycin lysosomal accumulation [14]. In this study, the experimentally measured average total azithromycin concentrations in polymorphonuclear white blood cells (PMLs) was over 14 000 ng/mL. However, the model-predicted unionized azithromycin concentration of 6.0 ng/mL in the cytosol of PMLs was comparable to the measured interstitial concentrations in the muscle (8.7 ng/mL) and subcutis (4.1 ng/mL). Hence, the measured concentrations in white blood cells was approximately 2000-fold higher than the measured unbound concentrations in the interstitial space. Considering the lysosomal volume is estimated to range between 0.5 to 5% of the cellular volume depending on cell type [14], [15], [16], [17], these concentrations are consistent with the expected magnitude of lysosomal accumulation and explain these very high cellular concentrations.
Just as for hydroxychloroquine, the pharmacokinetic parameters of azithromycin confirm these extreme distribution properties. The volume of distribution of azithromycin was reported to be 31 L/kg and the half-life was 68 h, despite a relatively rapid clearance of 630 mL/min [18]. Furthermore, the pharmacokinetics of azithromycin have been shown to be affected by the disease state [19]. In a preclinical investigation, azithromycin was injected into rats and interstitial concentrations were measured in both thighs using microdialysis. After 3 h, an infection was induced in only one leg. Shortly after, the unbound interstitial tissue concentrations increased in the infected leg, without any additional dosing. The results can be explained by macrophages loaded with lysosomal azithromycin migrating to and releasing drug at the infection site.
5. Discussion and conclusion
Although the present paper is focused on the pharmacokinetic properties of hydroxychloroquine and azithromycin, a similar intracellular accumulation in the lysosomal space can be expected and has been reported for chloroquine as early as 1974 [20,21]. The pharmacokinetics of chloroquine and hydroxychloroquine have been described to be similar [9]. The very high accumulation of these drugs in lysosomes produces very high local concentrations of both compounds. Furthermore, the uptake of these basic drugs into lysosomes increases the lysosomal pH [22]. However, it is difficult to estimate the magnitude and time course of this alkalization as there is continuous acidification of the lysosomal space by proton pumps that function to maintain low pH.
The combination of chloroquine and azithromycin is not new, and synergistic effect against malaria has been reported [23,24]. Hydroxychloroquine and chloroquine have similar efficacy against malaria. Although hydroxychloroquine has a preferred safety profile, more pharmacodynamic information is available for chloroquine [25], [26], [27].
Several studies point to mechanisms for the potential antiviral action of chloroquine and azithromycin.
Chloroquine was shown to impair early stages of virus replication by interfering with the pH-dependent endosome-mediated viral entry of several different viruses [28]. Due to the alkalization of endosomes, chloroquine was an effective in vitro treatment against Chikungunya virus when added to Vero cells prior to virus exposure. A pH-dependent mechanism of entry of coronavirus into target cells was also reported for SARS-CoV-1 [29]. The activation step that occurs in endosomes at acidic pH results in fusion of the viral and endosomal membranes, leading to the release of the viral SARS-CoV-1 genome into the cytosol. In the absence of antiviral drug, the virus is targeted to the lysosomal compartment where the low pH, along with the action of enzymes, disrupts the viral particle, thus liberating the infectious nucleic acid and, in several cases, enzymes necessary for its replication. Hence, the lysosomal space has been proposed as a major target site to tackle SARS-CoV-2 [30].
Macrolides such as azithromycin are very commonly used antibacterial agents; however, they have also received considerable attention for their anti-inflammatory and immunomodulatory actions. These two properties may ensure some efficacy in a wide spectrum of respiratory viral infections [31,32]. Both in vitro and in vivo studies indicate efficacy of macrolides in respiratory viral infections, including rhinovirus (RV), respiratory syncytial virus (RSV), and influenza virus. Macrolides also reduced the release of proinflammatory cytokines, which were induced by RSV infection, viral titers, RNA of RSV replication, and the susceptibility to RSV infection. Similar effects of macrolides on influenza virus infection and augmentation of IL-12 by macrolides, which is essential in reducing virus yield, were reported [32].
As with the use of any drug or drug combination, the potential benefit has to be weighed against any safety concerns. Prolongation of QT interval and cases of torsades de pointes have been reported for both drugs [18,33]. Hydroxychloroquine prolongs the QT interval and, according to its prescribing information, should not be administered with other drugs that have the potential to induce cardiac arrhythmias [33]. There may be an increased risk of inducing ventricular arrhythmias if the drug is used concomitantly with other arrhythmogenic drugs [4]. Most relevant interaction data with azithromycin is available for chloroquine. QTc interval prolongation was studied in a randomized, placebo-controlled parallel trial in 116 healthy subjects who received either chloroquine (1000 mg) alone or in combination with oral azithromycin (500 mg, 1000 mg, and 1500 mg once daily) [18]. Co-administration of azithromycin increased the QTc interval in a dose- and concentration-dependent manner. Compared with chloroquine alone, the maximum mean (95% upper confidence bound) increases in QTcF were 5 (10) ms, 7 (12) ms and 9 (14) ms with the co-administration of 500 mg, 1000 mg and 1500 mg azithromycin, respectively. Furthermore, studies in pigs revealed that azithromycin/chloroquine combinations did not increase cardiac instability [34]. In a clinical interaction study between azithromycin and chloroquine, no clinically relevant pharmacokinetic changes were observed [23]. However, increases in QTc values were noted. Although mean increases were numerically greater for the azithromycin plus chloroquine group compared with the chloroquine alone group, these differences were not statistically significant. Administered alone and together, chloroquine and azithromycin were well tolerated overall in this study. More investigations with hydroxychloroquine are needed as well as finetuning the optimum dosing regimen to get a clearer picture of the expected risk-benefit assessment in these severe infections. These dose optimization attempts will have to utilize ways to estimate the respective infection site concentration; this is very challenging due to the tremendous differences in subcellular concentrations. Average tissue concentrations based on lung/blood ratios obtained from animal studies have been proposed and applied but may not be appropriate for dose optimization [35].
The unusual pharmacokinetic properties of these two drugs may make them suitable partners for an ion-trapping-mediated mechanism of action where lysosomal drug accumulation delivers local action. These two drugs have very large volumes of distribution. In a widely used long list of pharmacokinetic properties of the most commonly used drugs, chloroquine and azithromycin have the largest volumes of distribution (hydroxychloroquine is not listed) [36].
Short-term use of hydroxychloroquine with azithromycin may have clinical impact early in the progress of COVID [1,2]. An early diagnosis combined with a safe and effective treatment before more serious symptoms occur would be highly desirable. Both of these low-cost drugs have been widely used for decades with acceptable safety that could expedite rapid use if a clinical benefit is established in rigorous safety and efficacy studies. These studies are currently ongoing and will provide solid guidance.
Acknowledgement
The fundamental contributions by and fruitful discussions with Prof. Paul Tulkens (UCLouvain, Belgium) are gratefully acknowledged.
Declarations
Funding: No funding
Competing Interests: None
Ethical Approval: Not required
Editor: Jean-Marc Rolain
References
- 1.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med Infect Dis. 2020 Apr 11 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020 Mar 30 doi: 10.1016/j.medmal.2020.03.006. pii: S0399-077X(20)30085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juurlink DN. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ. 2020 Apr 8 doi: 10.1503/cmaj.200528. pii: cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaire S, Tulkens PM, Van Bambeke F. Cellular pharmacokinetics of the novel biaryloxazolidinone radezolid in phagocytic cells: studies with macrophages and polymorphonuclear neutrophils. Antimicrob Agents Chemother. 2010;54(6):2540–2548. doi: 10.1128/AAC.01723-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder RL, Gerber JP. Chloroquine and hydroxychloroquine binding to melanin: Some possible consequences for pathologies. Toxicol Rep. 2014;1:963–968. doi: 10.1016/j.toxrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kazmi F, Hensley T, Pope C, Funk RS, Loewen GJ, Buckley DB. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells) Drug Metab Dispos. 2013;41(4):897–905. doi: 10.1124/dmd.112.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacIntyre AC, Cutler DJ. The potential role of lysosomes in tissue distribution of weak bases. Biopharm Drug Dispos. 1988;9(6):513–526. doi: 10.1002/bod.2510090602. [DOI] [PubMed] [Google Scholar]
- 9.Tett SE, Cutler DJ, Day RO, Brown KF. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988;26(3):303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlier MB, Garcia-Luque I, Montenez JP, Tulkens PM, Piret J. Accumulation, release and subcellular localization of azithromycin in phagocytic and non-phagocytic cells in culture. Int J Tissue React. 1994;16(5-6):211–220. [PubMed] [Google Scholar]
- 11.Tyteca D, Van Der Smissen P, Mettlen M, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP. Azithromycin, a lysosomotropic antibiotic, has distinct effects on fluid-phase and receptor-mediated endocytosis, but does not impair phagocytosis in J774 macrophages. Exp Cell Res. 2002;281(1):86–100. doi: 10.1006/excr.2002.5613. [DOI] [PubMed] [Google Scholar]
- 12.Tyteca D, Van Der Smissen P, Van Bambeke F, Leys K, Tulkens PM, Courtoy PJ. Azithromycin, a lysosomotropic antibiotic, impairs fluid-phase pinocytosis in cultured fibroblasts. Eur J Cell Biol. 2001;80(7):466–478. doi: 10.1078/0171-9335-00180. [DOI] [PubMed] [Google Scholar]
- 13.Van Bambeke F, Montenez JP, Piret J, Tulkens PM, Courtoy PJ, Mingeot-Leclerq MP. Interaction of the macrolide azithromycin with phospholipids. I. Inhibition of lysosomal phospholipase A1 activity. Eur J Pharmacol. 1996;314(1-2):203–214. doi: 10.1016/s0014-2999(96)00552-3. [DOI] [PubMed] [Google Scholar]
- 14.Zheng S, Matzneller P, Zeitlinger M, Schmidt S. Development of a population pharmacokinetic model characterizing the tissue distribution of azithromycin in healthy subjects. Antimicrob Agents Chemother. 2014;58(11):6675–6684. doi: 10.1128/AAC.02904-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aubert-Tulkens G, Van Hoof F, Tulkens P. Gentamicin-induced lysosomal phospholipidosis in cultured rat fibroblasts. Quantitative ultrastructural and biochemical study. Lab Invest. 1979;40(4):481–491. [PubMed] [Google Scholar]
- 16.Cutler DF. Introduction: lysosome-related organelles. Semin Cell Dev Biol. 2002;13(4):261–262. doi: 10.1016/s108495210200054x. [DOI] [PubMed] [Google Scholar]
- 17.Steinman RM, Brodie SE, Cohn ZA. Membrane flow during pinocytosis. A stereologic analysis. J Cell Biol. 1976;68(3):665–687. doi: 10.1083/jcb.68.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfizer. Zithromax product information. 2019; Available from:https://labeling.pfizer.com/ShowLabeling.aspx?id=511.
- 19.Barbour A, Scaglione F, Derendorf H. Class-dependent relevance of tissue distribution in the interpretation of anti-infective pharmacokinetic/pharmacodynamic indices. Int J Antimicrob Agents. 2010;35(5):431–438. doi: 10.1016/j.ijantimicag.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 20.MacIntyre AC, Cutler DJ. role of lysosomes in hepatic accumulation of chloroquine. J Pharm Sci. 1988;77(3):196–199. doi: 10.1002/jps.2600770303. [DOI] [PubMed] [Google Scholar]
- 21.Wibo M, Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole B, Ohkuma S. Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol. 1981;90(3):665–669. doi: 10.1083/jcb.90.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cook JA, Randinitis EJ, Bramson CR, Wesche DL. Lack of a pharmacokinetic interaction between azithromycin and chloroquine. Am J Trop Med Hyg. 2006;74(3):407–412. [PubMed] [Google Scholar]
- 24.Ohrt C, Willingmyre GD, Lee P, Knirsch C, Mihous W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 2002;46(8):2518–2524. doi: 10.1128/AAC.46.8.2518-2524.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4) doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolain JM, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30(4):297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19?Int J Antimicrob Agents2020:105938. [DOI] [PMC free article] [PubMed]
- 29.Yang ZY, Huang Y, Ganesh L, Leung K, Kong WP, Schwartz O. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons G, Bertram S, Glowacka I, Steffen I, Chaipan C, Agudelo J. Different host cell proteases activate the SARS-coronavirus spike-protein for cell-cell and virus-cell fusion. Virology. 2011;413(2):265–274. doi: 10.1016/j.virol.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakeya H, Seki M, Izumikawa K, Kosai K, Morinaga Y, Kurihara S. Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study. PLoS One. 2014;9(3):e91293. doi: 10.1371/journal.pone.0091293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012 doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Concordia PLAQUENIL® hydroxychloroquine sulfate tablets. Product Information. 2015 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf Available from: [Google Scholar]
- 34.Fossa AA, Wisialowski T, Duncan JN, Deng S, Dunne M. Azithromycin/chloroquine combination does not increase cardiac instability despite an increase in monophasic action potential duration in the anesthetized guinea pig. Am J Trop Med Hyg. 2007;77(5):929–938. [PubMed] [Google Scholar]
- 35.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. pii: ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thummel KE, Shen DD, Isoherranen N, Smith HE. Design and optimization of dosage regimens: pharmacokinetic data. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's The Pharmacological Basis of Therapeutics. 11th edition. McGraw-Hill; New York: 2006. [Google Scholar]