Abstract
The outbreak of the novel SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) responsible for coronavirus disease 2019 (COVID-19) has developed into an unprecedented global pandemic. Clinical investigations in patients with COVID-19 has shown a strong upregulation of cytokine and interferon production in SARS-CoV2- induced pneumonia, with an associated cytokine storm syndrome. Thus, the identification of existing approved therapies with proven safety profiles to treat hyperinflammation is a critical unmet need in order to reduce COVI-19 associated mortality. To date, no specific therapeutic drugs or vaccines are available to treat COVID-19 patients. This review evaluates several options that have been proposed to control SARS-CoV2 hyperinflammation and cytokine storm, eincluding antiviral drugs, vaccines, small-molecules, monoclonal antibodies, oligonucleotides, peptides, and interferons (IFNs).
Keywords: Interferons, Recombinant, Coronavirus, COVID-19, SARS-CoV-2
1. Covid-19 pathogenesis
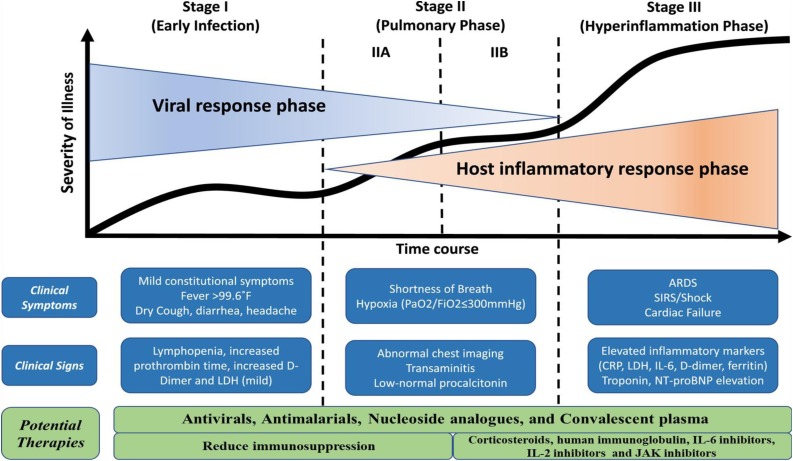
COVID-19, caused by the SARS-CoV2 virus, is a potentially fatal disease that represents a major global public health concern. The SARS-CoV2 virus infects the lower respiratory tract and causes pneumonia in humans, with symptoms that appear milder than SARS or MERS infection, but ultimately becomes a lethal disease of hyperinflammation and respiratory dysfunction [1]. bySARS-CoV2 infection and disease can be approximately divided into three phases: I. an asymptomatic phase with or without detectable virus; II. a non-severe symptomatic phase with upper airway involvement; and III. a severe, potentially lethal disease with hypoxia, 'ground glass' infiltrates in the lung, and progression to acute respiratory distress syndrome (ARDS) with high viral load (Fig. 1 ) [2].
Fig. 1.
COVID-19 pathogenic phases and potential therapeutic targets (modified and adopted from Siddiqi and Mehra, 2020 [38]).
The coronavirus genome encodes four major proteins: spike (S), nucleocapsid (N), membrane (M), and envelope (E). The S protein is responsible for viral entry into target ACEII expressing cells of the body. Approximately 75 percent of the SARS-CoV2 genome is identical to the SARS-CoV genome, and the amino acid residues required for receptor binding are the same between these two viruses; both viruses use the angiotensin converting enzyme 2 (ACE-2) receptor to infect airway epithelial cells and endothelial cells. [3].
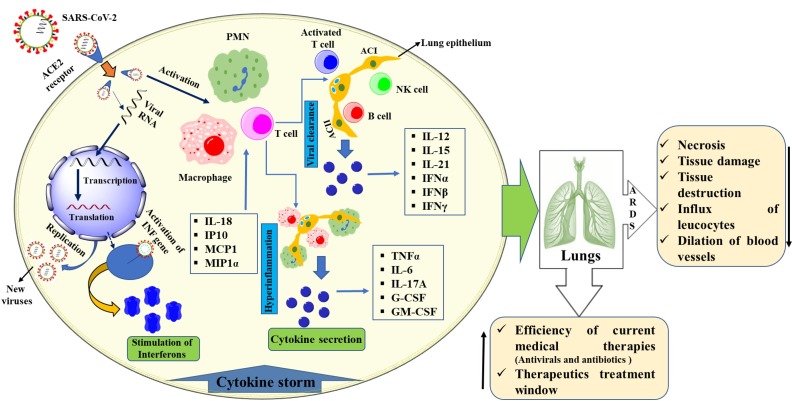
ARDS is the main cause of death in COVID-19 disease, and appears to cause similar immunopathogenic features in SARS-CoV and MERS-CoV infections [4]. One of the main features of ARDS is the cytokine storm - an uncontrolled systemic inflammatory response resulting from the release of pro-inflammatory cytokines and chemokines by immune effector cells [5]. High blood levels of cytokines and chemokines have been detected in patients with COVID-19 infection, including: IL1-β, IL1RA, IL7, IL8, IL9, IL10, basic FGF2, GCSF, GMCSF, IFNγ, IP10, MCP1, MIP1α, MIP1β, PDGFB, TNFα, and VEGFA [6]. The ensuing cytokine storm triggers a violent inflammatory immune response that contributes to ARDS, multiple organ failure, and finally death in severe cases of SARS-CoV-2 infection, similar to SARS-CoV and MERS-CoV infections [5]. Patients infected with COVID-19 showed higher leukocyte numbers, abnormal respiratory findings, and increased levels of plasma pro-inflammatory cytokines [4] (Fig. 2 ) [7]. The direct cause of death from acute COVID-19 involves cytokine storm damage to lungs and multiple organs of the body: heart, kidney and liver, leading to multiple organexhaustion [8,9,11,12].
Fig. 2.
Schematic representation of COVID-19 pathogenesis and cytokine storm with possible effects. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ACE2: angiotensin-converting enzyme 2; PMN: polymorphonuclear granulocyte; AC: alveolar cell; NK: natural killer).
2. Interferons as a potential therapy for COVID-19
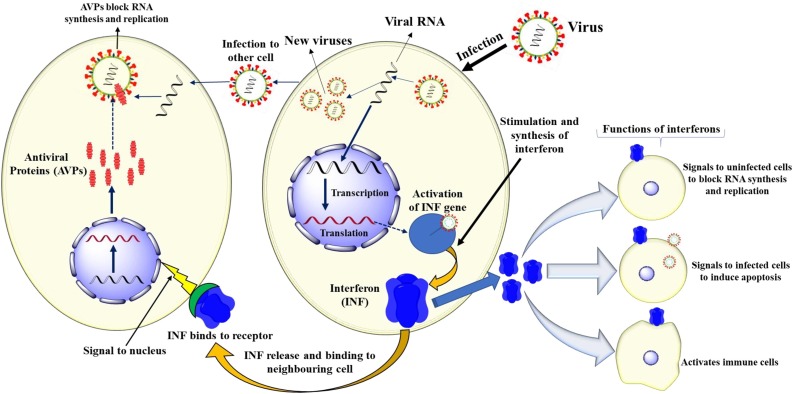
New therapeutic interventions will likely require a long lead time for the development of approved drugs. Thus, in light of the dire need and urgency to identify the treatment and control of COVID-2019, a repurposing of IFNs and other approved drugs is a potential option in drug development for the control of coronavirus infection. The potential drug options for SARS-CoV-2 infection include the use of enzyme inhibitors, nucleosides, host-targeted agents, convalescent plasma and IFNs [13,14]. Interferons (IFN) enhance the immune system in several ways, by exhibiting various biological functions including antiviral, antiproliferative, immunomodulatory and developmental activities [15] (Fig. 3 ). IFNs employed therapeutically are manufactured using recombinant DNA technology and multiple clinically approved IFNs are available: IFN α-2a (Roferon), IFN α-2b (Intron A), IFN α-n1 (Wellferon), IFN α-n3 (Alferon), IFN α -con 1 (Infergen), IFN β-1a (Rebif), IFN β-1b (Betaferon), IFN β -1a (Avonex), IFN β -1b (Betaseron), IFN α -2a (Pegasys), IFN α -2b (PegIntron), IFN α P-2b (Sylatron), and IFN γ-1b (Acimmune) [18,19].
Fig. 3.
Mechanism of interferon biosynthesis and their functions.
In a recent study with MERS-CoV infected patients, the combination of Remdesivir and IFNbeta revealed superior antiviral activity, compared to the effect of lopinavir and ritonavir [20]. Treatment of these patients with oral ribavirin and subcutaneous pegylated IFN alpha-2a demonstrated significant improvement in survival, provided that adequate monitoring and assessment was available [21,22]. Remdesivir and IFN beta may likewise prove useful in the treatment of COVID-19 [[14], [15], [16]], particularly since recent clinical trials have demonstrated that Remdesivir shortened the length of time in hospital intensive care for Covid-19 patients.
Earlier studies showed that coronaviruses including MERS, SARS, human coronavirus 229E, and avian infectious bronchitis virus (IBV) were susceptible to IFN treatment [17,23]. In patients with MERS infection, a combination of ritonavir plus IFN α2a or IFN α-2b resulted in significantly improved survival after 14 days of treatment. The combination of ritonavir and IFNβhad no significant effect on clinical outcome in patients infected with MERS, but the combination of ribavirin (1), ritonavir (2) and IFN α-2a inhibited viremia within 48h of treatment [13]. The use of recombinant IFNs (IFN -α, IFN -β and exogenous IFNs) in the treatment of SARS-CoV2, SARS-CoV and MERS-CoV demonstrated that IFN response inhibited protein synthesis and replication of the virus [[24], [25], [26]]. IFN α and γ, alone or in combination, showed partial efficacy against the animal coronaviruses, as well as inhibiting SARS-CoV replication in vitro. IFN β had the highest potency, demonstrating prophylactic protection and antiviral potential post infection [27]. Therefore, it may be worthwhile to test the safety and efficacy of human and recombinant IFNs in SARS-CoV-2-infected patients, alone or in combination with other antiviral drugs.
3. Potential combination approaches for COVID-19
At present, there are no Food and Drug Administration (FDA)-approved drugs specifically indicated for the treatment of patients with COVID-19, with the exception of the recently studied Remdesivir. It was shown that Remdesivir reduced the patients' time in ICU from fifteen days to eleven days. Originally developed as a small molecule compound against Ebola virus, Remdesivir acts by inhibiting the viral RNA dependent RNA polymerase. However, it is difficult to imagine how the direct antiviral properties of Remdesivir could be potently active during the immunopathogenic ARDS phase of COVID-19 disease, suggesting that other off-target effects may be attributed to the drug. Further studies, particularly amongst patient populations at earlier stages of the disease, are warranted to resolve these issues.
A number of drugs and combinational therapies have been identified using previously approved drugs that targett clathrin-mediated endocytosis, viral protease, regulate immunity, inhibit the inflammatory cytokine surge, improve pulmonary function and reduce lung viral loads (Table 1 ). At present, treatment of COVID-19 cytokine storm focuses primarily on support and symptomatic treatment of inflammation, cytokine storm and compromised respiratory function [28]. Recently, a number of specific anti-cytokine approaches have proven effective in the treatment of a variety of cytokine storm syndromes, and include drugs targeting interleukin-1 (IL-1), IL-6, IL-18, and interferon-gamma [29]. While randomized trials will be needed to confirm which, if any, of these therapeutics are effective in Covid-19-infected patients with cytokine storm syndrome, IL-6 blockade using anti-IL6 antibody has recently been reported, with successful outcomes in some individuals [10]. While working to prevent future outbreaks of coronavirus infections with vaccine development and new or re-purposed anti-viral medicines, it remains of utmost importance to use the knowledge at our disposal to treat those patients most at risk of dying from Covid-19-induced cytokine storms.
Table 1.
Combinational remedies and drugs as potential targets for COVID-19.
| Name of the agent/therapy | Targeted virions infection | Target virion mechanism | References |
|---|---|---|---|
| Thalidomide and Glucocorticoids | SARS-CoV-2 | Regulate immunity, inhibit the inflammatory cytokine surg |
[30] |
| Remdesivir and IFNa2 | SARS-CoV-2 | Improves pulmonary function and reduces lung viral loads | [13] |
| Chloroquine and Hydroxychloroquine | SARS-CoV-2 | attenuation of cytokine production and inhibition of autophagy - shown recently to be ineffective in clinical studies | [31] |
| Lopinavir and Ritonavir | HIV, MERS-CoV and SARS-CoV-2 | Protease inhibitor, inhibits 3CLpro | [13] |
| Lopinavir, oseltamivir and ritonavir | SARS-CoV-2 | Targetiviral protease | [32] |
| Lopinavir, ritonavir, and interferon beta | MERS-CoV and SARS-CoV-2 | Slightly reduced viral load and improved pulmonary function | [20] |
| Convalescent plasma | SARS-CoV-2, SARS-CoV and MERS-CoV | Inhibited virus entry to the target cells, suppressed viraemia by anti-SARS-CoV2 antibody | [13] |
| Hydroxychloroquine and Azithromycin | SARS-CoV-2 | Viral load reduction through inhibition of replication | [33] |
| Camostat mesilate Hydroxychloroquine | SARS-CoV-2 | Inhibitor of the host cell serine protease and angiotensin receptor blockers | [34] |
| Darunavir and Umifenovir | SARS-CoV-2 | Viral load reduction through inhibition of replication | [35] |
| Ribavirin and Interferon-α | SARS-CoV-2 | Lowered the risk of acute respiratory distress syndrome (ARDS) and death | [36] |
| Hydroxychloroquine and Nitazoxanide | SARS-CoV-2 | Adjuvant therapy in Covid-19 | [37] |
Declaration of Competing Interest
There are no financial or other interests related to this review that represent a conflict of interest.
Acknowledgements
This work was supported by Zhejiang University special scientific research fund for COVID-19 prevention and control, National Natural Science Fund of China (81522049, 31870135, 31571735), Zhejiang Provincial Wanren Program for Leading Talents of Science and Technology Innovation (2018R52050), Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (2018-62-3), the “Dawn” Program of Shanghai Education Commission (16SG38), Shanghai Science and Technology Committee Project (17JC1404300), Opening Project of Zhejiang Provincial Preponderant and Characteristic Subject of Key University (Traditional Chinese Pharmacology, Zhejiang Chinese Medical University (ZYAOXZD2019001)).
Biographies

Dr. Shivraj Nile obtained M.Sc. in Biotechnology (2004) and PhD in Life Science, SRTM, University, India (2010). He worked as KU-Brain Pool post-doctoral fellow (2012-2014), Konkuk University, He currently working as Associate Professor, Zhejiang Chinese Medical University, China. Previously worked as Assistant Professor, Konkuk University, Korea (2014-2018) and SRTM, University, Nanded (2010-2014). Received RGNF Research Fellowship (UGC, India) (2006-2010). He had 9 years research experience in food science, phytochemistry, biotechnology and nanotechnology. Dr. Nile had more than 85 research publications including Crit. Rev. Food Sci, Nutr, Trends in Food Sci Technol, Nano-Micro Lett, J. Clean Prod, Food Chem, Food Chem Toxicol, Ind Crops Products, Nutrition, Food Res Int, Food Function, Phytomedicine, Frontiers in Pharmacology, and Food Review Int. Also having 3 patents and 6 research projects on his credit and He received 12 national and international research awards. Associate editor for e-Food journal, Combinatorial Chemistry & High Throughput Screening, Journal Recent Patents on Food Nutrition & Agriculture, Current Pharmaceutical Analysis, Journal of Nutrition & Health, Journal of Analytical & Molecular Techniques, International Journal of Recent Trends in Science & Technology, and Journal of Environmental Studies. His expertise is food science, biochemistry, pharmacology, natural products, nanotechnology, and drug discovery. His research mainly focused on functional food, natural colorants, drug development, food nanotechnology and phytomedicine.

Ms. Arti Nile finished her master’s degree in Biotechnology (2012), SRTM, University, India. She is having 3 years industrial experience as CRA and CDM. Currently she is perusing her PhD in Food Science, Konkuk University, Korea. Her major research focused on utilization of fruit and crop waste towards bioactive compound extraction and determination of biological activities. She got research fellowship from NRF-Korea for her PhD work and she published more than 10 research papers in various international journals. She is also having good experience with clinical research and data management.

Dr. Jiayin Qiu obtained her PhD in Pharmacology, Southern Medical University, China (2014). She worked as a visiting student in Weizman Institute of Science in Isreal (2012-2014). She currently working as postdoctoral in Zhejiang Chinese Medical University, China. Host Youth Program of National Natural Science Foundation of China (2016-2018) and Natural Science Foundation of Guangdong Province (2015-2017). Dr. Qiu had 19 research publications including J Biol Chem, Frontiers in Pharmacology and Journal of Antimicrobial Chemotherapy etc. Her research mainly focused on Anti-virus Pharmacolo gy.

Prof. Lin Li is a professor of pharmacology of School of Pharmaceutical Sciences, Southern Medical University. She was awarded with “The Outstanding Teacher of Southern Medical University”. Prof. Li obtained her PhD in Pharmacology, Southern Medical University, China (2010). In 2012, Prof. Li was trained by David Geffen School of Medicine in University of California for teaching skills. During 2008 to 2010, she worked as a visiting scientist in New York Blood Center. Prof. Li is the director of World Federation of Chinese Medicine Societies, the secretary general of Division of Anti-inflammatory and Immunology of Chinese Pharmacological Society (CPS). Prof. Li mainly engaged in anti-viral drug development and HIV latency research. She was awarded about seven research grants. Prof. Li has already published more than 30 SCI papers in academic journals like J Antimicrob Chemother, J. Acquir. Immune Defic. Syndr., Retrovirology, Antimicrob. Agents Chemother, with a total impact factor over 100.

Prof. Xu Jia obtained his PhD in Biochemistry and Molecular Biology, Fu Dan University, China (2012). He worked as a Professor in Chengdu Medical College since 2012. Received National Natural Science Foundation of China (2013, 2014, 2018) and Sichuan science and technology fund for outstanding youth (2014). He became the head of the research and innovation team of universities in Sichuan province for research on bacterial resistance and anti-infection (2015). Honors he received: outstanding contribution of Sichuan provincial commission of health and family planning (2017), Sichuan thousand talents program (2018), Sichuan academic and technological leader (2018), Sichuan new youth (2019). Prof. Dr. Jia had more than 20 research publications including Cell, Nucleic Acids Research and Frontiers in Microbiology etc. The related results on the mechanism of bacterial resistance were published in the journal Cell. His expertise is on antibiotic resistance, the structure and function of non-coding RNAs, especially riboswitch, SARS-CoV-2, COVID-19, antivirus drugs, bacterial epidemiology, antibacterial drugs and aging.

Prof. Guoyin Kai obtained his PhD in Biochemistry and Molecular Biology, Shanghai Jiaotong University, China (2005). He worked as a visiting scholar in Brookhaven National Laboratory in USA (2012-2013). He currently working as Professor, Zhejiang Chinese Medical University, China. Previously worked as associate Professor (2005-2012) and Professor (2012-2017) in Shanghai Normal University, China (2005-2017). Received Excellent Youth Talent Project from National Science Fund (2016-2018) and Meiji Life Science Award (China) (2014). Prof. Dr. Kai had more than 100 research publications including Metab Eng, Chem Eng J, New Phytol, Nano-Micro Lett, Crit. Rev. Food Sci, Nutr, J Exp Bot, Food Chem, Nanomedicine, Phytomedicine, J. Agr Food Chem, Food Chem Toxicol, and PNAS etc. Also having 17 Chinese patents and 28 research projects as PI and received 14 research awards. His expertise is on biotechnology and bioactive compounds and their biological evaluation. His research mainly focused on natural product, phytomedicine and biotechnology
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cytogfr.2020.05.002.
Contributor Information
Xu Jia, Email: jiaxu@cmc.edu.cn.
Guoyin Kai, Email: guoyinkai1@126.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi Y., Wang Y., Shao C. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H., Liu S.M., Yu X.H., Tang C.L., Tang C.K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int. J. Antimicrobial Agents. 2020:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Analysis. 2020 doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothan H.A., Siddappa N., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X., Wang T., Cai D., Hu Z., Chen J., Liao H., Zhi L., Wei H., Zhang Z., Qiu Y., Wang J., Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020 doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tisoncik J.R., Korth M.J., Simmons C.P., Farrar J., Martin T.R., Katze M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020 doi: 10.1016/j.jaut.2020.102452. 102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 12.Wang G., Cao K., Liu K., Xue Y., Roberts A.I., Li F. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2018;25:1209–1223. doi: 10.1038/s41418-017-0006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020 doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang B.X., Fish E.N. Global virus outbreaks: interferons as 1st responders. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.101300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kindler E., Thiel V., Weber F. Interaction of SARS and MERS Coronaviruses with the antiviral interferon response. Adv. Virus Res. 2016;96:219–243. doi: 10.1016/bs.aivir.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan E.L., Ooi E.E., Lin C.Y., Tan H.C., Ling A.E.B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10(4):581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppangala N. Recombinant Interferon as Drugs. https://www.biotecharticles.com/Healthcare-Article/Recombinant-Interferon-as-Drugs-196.html
- 20.Sheahan T.P., Sims A.C., Leist S.R., Schafer A., Won J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omrani A.S., Saad M.M., Bahloul K.Baig A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouadma L., Lescure F.X., Lucet J.C., Yazdanpanah Y., Timsit J.F. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hensley L.E., Fritz L.E., Jahrling P.B., Karp C.L., Huggins J.W., Geisbert T.W. Interferon-beta 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10(2):317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falzarano D., Wit E., Rasmussen A.L., Feldmann F., Feldmann H. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat. Med. 2013;19(10):1313–1317. doi: 10.1038/nm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C.C., Wang X.J., Wang H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov. Today. 2019;24:726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zumla A., Chan, Azhar E.I., Hui D.S.C., Yuen K.Y. Coronaviruses -drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.https://www.cusabio.com/COVID-19-Cytokine-Storm. Accessed on 01/05/2020.
- 29.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C., Qi F., Shi K., Li Y., Li J., Chen Y., Pan J., Zhou T., Lin X., Zhang J., Luo Y., Li X., Xia J. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 Pneumonia. Preprints. 2020 doi: 10.1002/ctm2.35. 2020020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 32.Muralidharan M., Sakthivel R., Velmurugan D., Gromiha M.M. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- 33.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-converting enzyme 2 and anti-hypertensives (angiotensin receptor blockers and angiotensin converting enzyme inhibitors) in coronavirus disease 2019 (COVID-19) Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costanzo M., De-Giglio M.A.R., Roviello G.N. SARS-CoV-2: Recent Reports on Antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr. Med. Chem. 2020;27 doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 36.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 37.Okasha K.M. 2020. Hydroxychloroquine and Nitazoxanide Combination Therapy for COVID-19.https://clinicaltrials.gov/ct2/show/NCT04361318 [Google Scholar]
- 38.Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J. Heart Lung Transplant. 2020 doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.