Abstract
Background and aims
COVID-19 and low levels of vitamin D appear to disproportionately affect black and minority ethnic individuals. We aimed to establish whether blood 25-hydroxyvitamin D (25(OH)D) concentration was associated with COVID-19 risk, and whether it explained the higher incidence of COVID-19 in black and South Asian people.
Methods
UK Biobank recruited 502,624 participants aged 37–73 years between 2006 and 2010. Baseline exposure data, including 25(OH)D concentration and ethnicity, were linked to COVID-19 test results. Univariable and multivariable logistic regression analyses were performed for the association between 25(OH)D and confirmed COVID-19, and the association between ethnicity and both 25(OH)D and COVID-19.
Results
Complete data were available for 348,598 UK Biobank participants. Of these, 449 had confirmed COVID-19 infection. Vitamin D was associated with COVID-19 infection univariably (OR = 0.99; 95% CI 0.99–0.999; p = 0.013), but not after adjustment for confounders (OR = 1.00; 95% CI = 0.998–1.01; p = 0.208). Ethnicity was associated with COVID-19 infection univariably (blacks versus whites OR = 5.32, 95% CI = 3.68–7.70, p-value<0.001; South Asians versus whites OR = 2.65, 95% CI = 1.65–4.25, p-value<0.001). Adjustment for 25(OH)D concentration made little difference to the magnitude of the association.
Conclusions
Our findings do not support a potential link between vitamin D concentrations and risk of COVID-19 infection, nor that vitamin D concentration may explain ethnic differences in COVID-19 infection.
Keywords: COVID-19, Vitamin D, Ethnicity
Highlights
-
•
There is an urgent need to understand risk factors for contracting COVID-19 infection and for poorer prognosis thereafter.
-
•
COVID-19 appears to disproportionately affects black and minority ethnic individuals. The underlying mechanism is unknown.
-
•
One potential mediator could be their higher prevalence of apparent vitamin D deficiency.
-
•
We explored whether blood 25 hydroxyvitamin D (25(OH)D) concentration was associated with COVID-19 risk.
-
•
We found no evidence that (25(OH)D) explains susceptibility to COVID-19 infection, either overall or between ethnic groups.
1. Introduction
A novel coronavirus that manifested in 2019 (SARS-CoV-2) has led to a pandemic of pneumonia-related illness (COVID-19) with an estimated case fatality of around 1%. There is an urgent need to better understand risk factors for contracting the infection and for poorer prognosis thereafter.
There is growing evidence that COVID-19 disproportionately affects black and minority ethnic individuals, with the Intensive Care National Audit and Research Centre reporting that a third of confirmed cases admitted to critical care in England are non-white [1]. This compares with the 2011 Census figures which show that 14% of the general population of England and Wales identify themselves as black and minority ethnic individuals [2]. Similarly, in the United States a pattern of higher risk has been observed in African Americans [3]. Consequently, the relationship between ethnicity and COVID-19 has been identified as an urgent public health research priority [4].
Several factors have been proposed to explain the apparent greater risk of COVID-19 infection in ethnic minority groups. UK Government statistics show that people from black and minority ethnic backgrounds are more likely than white British people to live in the most socioeconomically deprived areas of England [5]. Furthermore, certain minority ethnic groups experience a higher burden of comorbid disease [6], which may put them at higher risk of more severe COVID-19 infection [7,8].
One potential mediator could be the higher prevalence of apparent vitamin D deficiency in black and minority ethnic populations [4]. Vitamin D is variably inversely associated with multiple health outcomes and mortality (although these associations may not be causal) [9]. Most vitamin D results from production in the skin following exposure to ultraviolet (UV) radiation from the sun. Individuals with dark skin have, on average, lower concentrations of blood vitamin D because the melanin in dark skin does not absorb as much UV [10]. Furthermore, deficiency is more common in high latitude countries such as the UK. Whilst most chronic conditions have not been improved by vitamin D supplementation, a recent meta-analysis of randomised trials suggested vitamin D may lessen the risk of acute respiratory infections [11].
In this study, we hypothesised that blood 25 hydroxyvitamin D (25(OH)D) concentration was associated with COVID-19 risk among UK Biobank participants, and explained wholly, or in part, the higher incidence of COVID-19 infection in ethnic minority participants.
2. Subjects
UK Biobank recruited 502,624 participants aged 37–73 years across England, Scotland and Wales between 2006 and 2010. Its aim was to identify the causes of disease and death in middle and old age by following up participants over time. At baseline, biological measurements were recorded and touch-screen questionnaires administered according to a standardised protocol [12,13]. The study received ethical approval from the North West Multi-Centre Research Ethics Committee (REC reference: 16/NW/0274), and was conducted in accord with the principles of the Declaration of Helsinki. All participants gave written informed consent for data collection, analysis, and record linkage.
3. Materials and methods
Baseline data from UK Biobank were linked to COVID-19 test results provided by Public Health England [14], including the specimen date, origin (whether the person was an inpatient or not) and result (positive or negative). Confirmed COVID-19 infection was defined as at least one positive test result. Data were available for the period 16th March 2020 to 14th April 2020.
Exposures were measured at the baseline assessment visits conducted between 2006 and 2010. Blood collection sampling procedures for the study have previously been described and validated [15]. Biochemical assays, including 25(OH)D, a measure of vitamin D status, were performed at a central laboratory on around 480,000 samples. Further details of these measurements can be found in the UK Biobank Data Showcase and Protocol [16]. Vitamin D was imputed with the minimum detectable value (10 nmol/L) if it was below the limit of detection, and the maximum detectable value (375 nmol/L) if too high for detection.
Ethnicity was self-reported, and categorised as white, black, South Asian, or other.
Smoking status was self-reported and categorised as current or non (ex/former) smoker. Blood pressure was measured at the baseline visit using an automated measurement, and the average of available measures used. Height was measured using a Seca 202 height measure. Weight was measured to the nearest 0.1 kg using the Tanita BC-418 MA body composition analyser. Body mass index (BMI) was derived from weight (kg)/height(m) [2]. It was categorised into underweight (BMI<18.5 kg/m2), normal weight (BMI 18.5–24.9 kg/m2), overweight (BMI≥25–29.9 kg/m2) and obese (BMI≥30 kg/m2). Area-level socioeconomic deprivation was assessed by the Townsend score (incorporating measures of unemployment, non-car ownership, non-home ownership and household overcrowding) [17]. Higher scores on the Townsend score represent greater socioeconomic deprivation; scores were categorised into quintiles. Diabetes at baseline was defined as self-reported physician-diagnosed type 1 or type 2 diabetes, a primary care or hospital record of diabetes at or before recruitment (defined as ICD-10 codes E10-E14.9), or diabetes medication. Household income was self-reported and categorised into: <£18,000; £18,000-£30,999; £31,000-£51,999; £52,000-£100,000; or >£100,000. Health was self-rated as excellent, good, fair, or poor. Long-standing illness, disability or infirmity was self-reported as yes or no.
Univariable logistic regression analysis was performed of the association between 25(OH)D concentration (as a continuous variable) and confirmed COVID-19 infection. The model was then adjusted for sex, month of assessment, Townsend deprivation quintile, household income, self-reported health rating, smoking status, BMI quintile, ethnicity, age at assessment, diabetes, systolic blood pressure (SBP), diastolic blood pressure (DBP), and long-standing illness, disability or infirmity. As sensitivity analyses these models were repeated with participants categorised as vitamin D deficient (defined as <25 nmol/L) or not deficient [18] and then categorised as vitamin D insufficient (defined as <50 nmol/L) or sufficient.
Next, univariable logistic regression was performed of the association between ethnicity and confirmed COVID-19 infection. The model was first adjusted for 25(OH)D, then sex, month of assessment, Townsend deprivation quintile, household income, self-reported health rating, smoking status, BMI quintile, age at assessment, diabetes, SBP, DBP, and long-standing illness, disability or infirmity.
Finally, multivariable analysis was performed including an ethnicity∗vitamin D deficiency interaction term. All analyses were undertaken using Stata v14.
4. Results
Results were available for 2724 COVID-19 tests conducted on 1474 individuals. Complete data on (25(OH)D) concentration and covariates were available for 348,598 UK Biobank participants. Of these, 449 had a positive COVID-19 test.
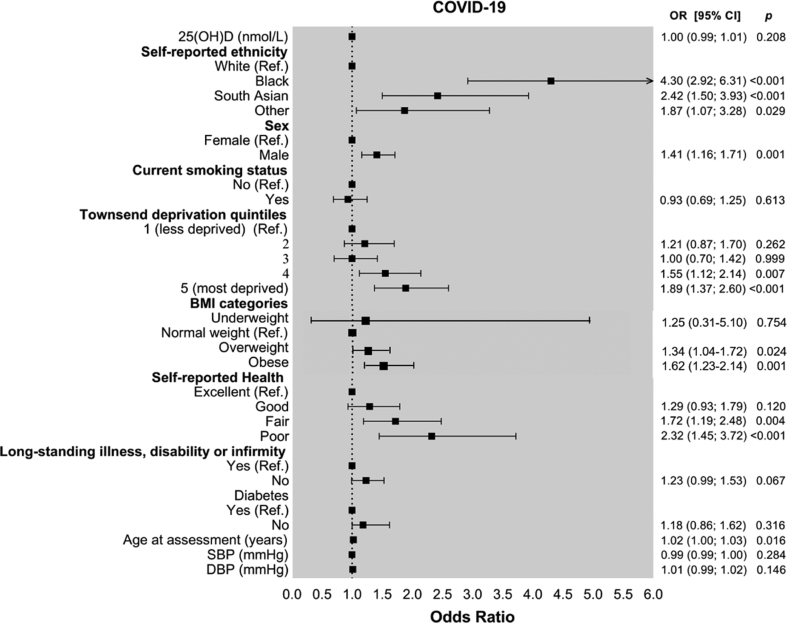
Table 1 presents study participants by presence or absence of positive COVID-19 test result. Median 25(OH)D concentration measured at recruitment was lower in patients who subsequently had confirmed COVID-19 infection (28.7 (IQR 10.0–43.8) nmol/L) than other participants (32.7 (IQR 10.0–47.2) nmol/L). It predicted COVID-19 infection univariably (Table 2; OR = 0.99, 95% CI 0.99–0.999, p = 0.013), but not after adjustment for covariates (OR = 1.00; 95% CI = 0.998–1.01; p = 0.208). Exposures that did predict COVID-19 status in the multivariable logistic regression were male sex (OR = 1.41; 95% CI = 1.16–1.71; p-value = 0.001), higher socioeconomic deprivation (highest vs lowest Townsend quintile OR = 1.89; 95% CI = 1.37–2.60; p-value<0.001), poorer self-reported health status (poor health vs excellent health OR = 2.32; 95% CI = 1.45–3.72; p-value<0.001), age at assessment (OR = 1.02; 95% CI = 1.00–1.03; p-value = 0.016), being overweight (OR = 1.34; 95% CI = 1.04–1.72; p-value = 0.024) or obese (OR = 1.62; 95% CI = 1.23–2.14; p-value = 0.001), and non-white ethnicity (blacks OR = 4.30, 95% CI = 2.92–6.31, p-value<0.001; South Asians OR = 2.42, 95% CI = 1.50–3.93, p-value<0.001) (Fig. 1).
Table 1.
Characteristics of study population by presence or absence of confirmed COVID-19 infection.
| No COVID-19 |
COVID-19 |
P value | ||
|---|---|---|---|---|
| N (%) | N (%) | |||
| Sex | Male | 168,391 (48.37) | 265 (59.02) | <0.001 |
| Female | 179,758 (51.63) | 184 (40.98) | ||
| Self-reported ethnicity | White | 331,464 (95.21) | 385 (85.75) | <0.001 |
| Black | 5022 (1.44) | 32 (7.13) | ||
| South Asian | 5917 (1.70) | 19 (4.23) | ||
| Other | 5746 (1.65) | 13 (2.90) | ||
| Current smoking status | Yes | 312,037 (89.63) | 398 (88.64) | 0.493 |
| No | 36,112 (10.37) | 51 (11.36) | ||
| Townsend deprivation quintile | 1 | 70,669 (20.30) | 61 (13.59) | <0.001 |
| 2 | 70,726 (20.31) | 76 (16.93) | ||
| 3 | 70,644 (20.29) | 64 (14.25) | ||
| 4 | 70,270 (20.18) | 105 (23.39) | ||
| 5 | 65,840 (18.91) | 143 (31.85) | ||
| BMI category | Underweight | 1759 (0.51) | 2 (0.45) | <0.001 |
| Normal weight | 115,410 (33.15) | 95 (21.16) | ||
| Overweight | 148,210 (42.57) | 194 (43.21) | ||
| Obese | 82,770 (23.77) | 158 (35.19) | ||
| Self-reported health rating | Excellent | 60,508 (17.38) | 45 (10.02) | <0.001 |
| Good | 203,640 (58.49) | 227 (50.56) | ||
| Fair | 69,676 (20.01) | 133 (29.62) | ||
| Poor | 14,325 (4.11) | 44 (9.80) | ||
| Long-standing illness, disability or infirmity | Yes | 237,470 (68.21) | 245 (54.57) | <0.001 |
| No | 110,679 (31.79) | 204 (45.43) | ||
| Diabetes | Yes | 329,324 (94.59) | 400 (89.09) | <0.001 |
| No | 18,825 (5.41) | 49 (10.91) | ||
|
| ||||
| Median (IQR) |
Median (IQR) |
|||
| Vitamin D | 32.7 (10.0–47.2) | 28.7 (10.0–43.8) | <0.01 | |
| Age at assessment | 49 (38–57) | 49 (40–58) | <0.05 | |
| SBP | 138 (125–151) | 138 (127–153) | 0.177 | |
| DBP | 82 (75–89) | 83 (76–90) | <0.01 | |
Categorical variables compared by chi [2] test; continuous variables compared by Mann-Whitney U test.
N number; BMI body mass index; IQR inter-quartile range; SBP systolic blood pressure; DBP diastolic blood pressure.
Table 2.
Association between Vitamin D and confirmed COVID-19 infection.
| Univariable |
Multivariablea |
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Vitamin D (nmol/L) | 0.99 (0.99–0.999) | 0.013 | 1.00 (0.998–1.01) | 0.208 |
| Vitamin D deficient (<25 nmol/L) | 1.37 (1.07–1.76) | 0.011 | 0.92 (0.71–1.21) | 0.564 |
| Vitamin D insufficient (<50 nmol/L) | 1.19 (0.99–1.44) | 0.068 | 0.88 (0.72–1.08) | 0.232 |
OR odds ratio; CI confidence interval.
Adjusted for ethnicity, sex, month of assessment, Townsend deprivation quintile, household income, self-reported health rating, smoking status, BMI category, age at assessment, diabetes, SBP, DBP, and long-standing illness, disability or infirmity.
Fig. 1.
Forest plot of factors associated with COVID-19 infection.
When participants were categorised into vitamin D deficient (<25 nmol/L) and not deficient the pattern of results was similar to those observed with vitamin D concentration entered as a continuous variable (univariable OR = 1.37, 95% CI = 1.07–1.76, p-value = 0.011; adjusted OR = 0.92, 95% CI = 0.71–1.21, p-value = 0.564) (Table 2). When participants were categorised into vitamin D insufficient (<50 nmol/L) and sufficient there was no association with COVID-19 infection either univariably (OR = 1.19, 95% CI = 0.99–1.44, p-value = 0.068), nor multivariably (OR = 0.88, 95% CI = 0.72–1.08, p-value = 0.232) (Table 2).
In the study, 331,849 (95.20%) participants were white, 5054 (1.45%) black, 5936 (1.70%) South Asian, and 5759 (1.65%) other. Of the 449 participants with confirmed COVID-19 infection, 385 (85.75%) were white, 32 (7.13%) black, 19 (4.23%) South Asian, and 13 (2.90%) other. Median 25(OH)D concentration was 33.8 (IQR 10.0–48.1) nmol/L in white participants, 21.0 (IQR 10.0–29.9) in black participants, 14.5 (IQR 15.5–22.1) in South Asian participants, and 23.3 (IQR 10.0–33.7) nmol/L in others. In this study 38,778 (11.69%) white, 1834 (36.29%) black, 3403 (57.33%) South Asian, and 1671 (29.02%) of other participants were vitamin D deficient at baseline.
In logistic regression, black ethnicity and South Asian ethnicity were both associated with confirmed COVID-19 infection univariably (OR = 5.49, 95% CI = 3.82–7.88, p-value<0.001; OR = 2.76, 95% CI = 1.74–4.39, p-value<0.001 respectively) compared with whites. Adjustment for 25(OH)D concentration made little difference to the magnitude of the associations (Table 3). Results were similar when, instead of adjusting for 25(OH)D concentration, adjustment was made separately for vitamin D deficiency and vitamin D insufficiency.
Table 3.
Association between ethnicity and confirmed COVID-19 infection.
| Univariable |
Adjusted for Vitamin D concentration |
Multivariablea |
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| White (referent) | 1 | 1 | 1 | |||
| Black | 5.49 (3.82–7.88) | <0.001 | 5.32 (3.68–7.70) | <0.001 | 4.30 (2.92–6.31) | <0.001 |
| South Asian | 2.76 (1.74–4.39) | <0.001 | 2.65 (1.65–4.25) | <0.001 | 2.42 (1.50–3.93) | <0.001 |
| Other | 1.95 (1.12–3.39) | 0.018 | 1.90 (1.09–3.32) | 0.024 | 1.87 (1.07–3.28) | 0.029 |
OR odds ratio; CI confidence interval.
Also adjusted for sex, month of assessment, Townsend deprivation quintile, household income, self-reported health rating, smoking status, BMI category, age at assessment, diabetes, SBP, DBP, and long-standing illness, disability or infirmity.
There was no significant interaction between ethnicity and vitamin D deficiency (OR = 0.90; 95% CI = 0.66–1.23; p-value = 0.515).
5. Discussion
Our findings are consistent with previous studies [7,19] in demonstrating a higher risk of confirmed COVID-19 infection in ethnic minority groups. Vitamin D has been suggested as possibly protective of COVID-19 infection [[20], [21], [22]] and, if so, could plausibly play a role in ethnic variations in COVID-19 infection. However, we found no association between 25(OH)D and COVID-19 infection after adjusting for potential confounders. Therefore, despite 25(OH)D concentration being lower in black and minority ethnic participants, there was no evidence that it might play a role in their higher risk of COVID-19 infection.
It has been suggested in some media outlets that language barriers may contribute to ethnic differences in COVID-19 risk. This is unlikely to contribute to the risk we observed in UK Biobank because all participants spoke English (albeit with the possibility of fluency variation). The association with ethnicity was only slightly attenuated after adjustment for socioeconomic and lifestyle differences in white and black and minority ethnic participants; the risk of COVID-19 remained around 4-fold in black participants and more than 2-fold in South Asians. Further studies are required to determine the mechanisms underlying ethnic variations in risk of COVID-19 infection and its severity. It may be that pathways related to cardiometabolic conditions or differences in cardiorespiratory reserve, or potentially other social factors, are more relevant, as we have recently discussed [23].
Our study replicated findings showing increased risk of COVID-19 in black and minority ethnic individuals, in men, and in people who are overweight or obese. It is surprising that we did not observe an association between diabetes or blood pressure and COVID-19 risk. Other studies have shown increased risk of hospitalisation and severe illness requiring ventilation in patients with diabetes and hypertension [7,24]. However, ours is a relatively healthy general population cohort. Furthermore, we do not have information on the severity of COVID-19 and have included all positive tests rather than only severe cases.
We did not show an independent association between smoking and COVID-19. Evidence from the literature is mixed. Initial studies suggested that current smoking increases the risk of severe infection [25]. However, this has since been disputed with some evidence that smoking may even protect against initial infection [26].
The strengths of UK Biobank include its extensive phenotyping which enables the adjustment for demographic and lifestyle risk factors, disease and ill health, its large sample size, and its central processing laboratory for biochemical assays. However, it is not representative of the general population, in that participants live in less socioeconomically deprived areas, are predominantly Caucasian, and have fewer self-reported health conditions [27]. We have demonstrated that ethnic differences in COVID-19 infection exist in this relatively healthy population. Baseline measurements, including 25(OH)D concentration and health status, were obtained a decade ago. It would be preferable to have measurements immediately preceding development of COVID-19. However, 25(OH)D concentrations vary more by season than year, and generally track over time [28].
Our study is the first to assess whether there is an association between blood 25(OH)D concentration and COVID-19 risk. We found no such link, suggesting that measurement of 25(OH)D would not be useful to assess risk in clinical practice. Furthermore, our results suggest that vitamin D is unlikely to be the underlying mechanism for the higher risk observed in black and minority ethnic individuals and vitamin D supplements are unlikely to provide an effective intervention.
6. Conclusion
Our analyses of UK Biobank data provided no evidence to support a potential role for (25 (OH)D) concentration to explain susceptibility to COVID-19 infection either overall or in explaining differences between ethnic groups.
Author contributions
JPP and NS had the original concept. CEH undertook the statistical analyses. All authors interpreted the results. CEH, JPP and NS drafted the manuscript. All authors revised the manuscript and approved the final version.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
CEH is funded by Health Data Research-UK (Ref. Edin-1).
SVK acknowledges funding from a NRS Senior Clinical Fellowship (SCAF/15/02), the Medical Research Council (MC_UU_12017/13) and the Scottish Government Chief Scientist Office (SPHSU13).
CLN is supported by the Medical Research Council (MR/R024774/1).
NS receives funding from the British Heart Foundation Research Excellence Award (RE/18/6/34217).
Contributor Information
Naveed Sattar, Email: naveed.sattar@glasgow.ac.uk.
Jill P. Pell, Email: jill.pell@glasgow.ac.uk.
References
- 1.Intensive Care National Audit and Research Centre . 2020. Covid-19 study case mix programme. [Google Scholar]
- 2.Office for National Statistics 2011 Census. 2011. https://www.ons.gov.uk/census/2011census cited; Available from.
- 3.John Hopkins Coronavirus Resource Centre 2020. https://coronavirus.jhu.edu/data/racial-data-transparency cited; Available from.
- 4.Khunti K., Singh A.K., Pareek M., Hanif W. Is ethnicity linked to incidence or outcomes of covid-19? BMJ. 2020;369:m1548. doi: 10.1136/bmj.m1548. [DOI] [PubMed] [Google Scholar]
- 5.UK Government Ethnicity facts and figures. 2018. https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/people-living-in-deprived-neighbourhoods/latest cited; Available from.
- 6.Forouhi N.G., Sattar N. CVD risk factors and ethnicity–a homogeneous relationship? Atherosclerosis Suppl. 2006;7:11–19. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. medRxiv. 2020 [Google Scholar]
- 8.Tu H., Tu S., Gao S., Shao A., Sheng J. The epidemiological and clinical features of COVID-19 and lessons from this global infectious public health event. J Infect. 2020 doi: 10.1016/j.jinf.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair R., Maseeh A., Vitamin D. The "sunshine" vitamin. J Pharmacol Pharmacother. 2012;3:118–126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. 2019;23:1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins R. What makes UK Biobank special? Lancet. 2012;379:1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 13.Sudlow C., Gallacher J., Allen N., Beral V., Burton P., Danesh J. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armstrong J., Rudkin J., Allen N., Crook D., Wilson D., Wyllie D. 2020. Dynamic linkage of COVID-19 test results between Public Health England’s Second Generation Surveillance System and UK Biobank. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott P., Peakman T.C. Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 16.UK biobank. https://www.ukbiobank.ac.uk/ cited; Available from.
- 17.Townsend P., Phillimore P. In: Health and deprivation: inequality and the North. Beattie A., editor. Crrom Helm Ltd; London: 1987. [Google Scholar]
- 18.Bolland M.J., Avenell A., Grey A. Should adults take vitamin D supplements to prevent disease? BMJ. 2016;355:i6201. doi: 10.1136/bmj.i6201. [DOI] [PubMed] [Google Scholar]
- 19.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020 doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakovac H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589. doi: 10.1152/ajpendo.00138.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020:12. doi: 10.3390/nu12061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes J.M., Subramanian S., Laird E., Anne Kenny R. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North - supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020 doi: 10.1111/apt.15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sattar N., McInnes I.B., McMurray J.J.V. Obesity a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 24.Richardson S., Hirsch J.S., Narasimhan M. Presenting Characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Tao Z.W., Lei W., Ming-Li Y., Kui L., Ling Z. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyara M., Tubach F., Pourcher V., Morelot-Panzini C., Pernet J., Haroche J. Low incidence of daily active tobacco smoking in patients with symptomatic COVID-19. Qeios. 2020 doi: 10.32388/WPP19W.3. [DOI] [Google Scholar]
- 27.Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T. Comparison of sociodemographic and health-related Characteristics of UK biobank participants with those of the general population. Am J Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng J.E., Hovey K.M., Wactawski-Wende J., Andrews C.A., Lamonte M.J., Horst R.L. Intraindividual variation in plasma 25-hydroxyvitamin D measures 5 years apart among postmenopausal women. Canc Epidemiol Biomarkers Prev. 2012;21:916–924. doi: 10.1158/1055-9965.EPI-12-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]