Summary
Non-steroidal anti-inflammatory drugs (NSAIDs) have an optional prescription status that has resulted in frequent use, in particular for the symptomatic treatment of fever and non-rheumatic pain. In 2019, a multi-source analysis of complementary pharmacological data showed that using NSAIDs in these indications (potentially indicative of an underlying infection) increases the risk of a severe bacterial complication, in particular in the case of lung infections. First, the clinical observations of the French Pharmacovigilance Network showed that severe bacterial infections can occur even after a short NSAID treatment, and even if the NSAID is associated with an antibiotic. Second, pharmacoepidemiological studies, some of which minimized the protopathic bias, all converged and confirmed the risk. Third, experimental in vitro and in vivo animal studies suggest several biological mechanisms, which strengthens a causal link beyond the well-known risk of delaying the care of the infection (immunomodulatory effects, effects on S. pyogenes infections, and reduced antibiotics efficacy). Therefore, in case of infection, symptomatic treatment with NSAIDs for non-severe symptoms (fever, pain, or myalgia) is not to be recommended, given a range of clinical and scientific arguments supporting an increased risk of severe bacterial complication. Besides, the existence of a safer drug alternative, with paracetamol at recommended doses, makes this recommendation of precaution and common sense even more legitimate. In 2020, such recommendation is more topical than ever with the emergence of COVID-19, especially since it results in fever, headaches, muscular pain, and cough, and is further complicated with pneumopathy, and given experimental data suggesting a link between ibuprofen and the level of expression of angiotensin-converting enzyme 2.
Keywords: Anti-inflammatory agents, non-steroidal; Infections; Respiratory tract infections; Superinfection; COVID-19; Pharmacovigilance; Pharmacoepidemiology
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ANSM
National Agency for the Safety of Medicines and Health Products
- COX-2
inducible cyclooxygenase
- CRPV
Regional Pharmacovigilance Centers
- NSAIDs
non-steroidal anti-inflammatory drugs
- TNF-α
tumor necrosis factor-α
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) form a large heterogeneous pharmacological family, but they all have one thing in common, the inhibition of one of the two cyclooxygenases. These enzymes are involved in the cascade of arachidonic acid, which leads to a decrease in the synthesis of prostaglandins. Their main indication was rheumatic pain of inflammatory origin, but now their use goes far beyond this indication. If for a few decades their prescription has decreased because of their poor safety profile, in particular in the elderly, their status as an optional prescription medication or over the counter has resulted in an increase in their use, in particular for fever and non-rheumatic pain. The mechanism of action explains their most frequent serious adverse effects (digestive disorders, such as gastritis, or digestive ulcer complicated by bleeding, and renal disorders, such as acute renal failure in patients at risk), which are often a cause of hospitalization [1]. These adverse effects have been known for a long time and are most often preventable, if their risk factors are taken into account [2].
More recently, other risks specifically related to their use in fever or non-rheumatic pain (that is symptoms of a potential underlying infection) have been highlighted. Thus, an increased risk of progression towards necrotizing fasciitis, when taking an NSAID in case of skin infection, was mentioned as soon as 1995 with the publication of a series of 14 cases of necrotizing fasciitis that occurred in children with varicella, 35% of whom had been exposed to ibuprofen [3]. The alert drove the Food and Drug Administration to issue a warning against the use of NSAIDs during varicella. Subsequently, several pharmacoepidemiological studies showed that taking NSAIDs (ibuprofen in particular) in case of cutaneous infection in patients with varicella could promote the occurrence of necrotizing fasciitis [4], [5], [6]. In 2004, France thus advised against the use of NSAIDs in case of varicella.
In 2015, the French Regional Pharmacovigilance Centers (CRPVs) collected and assessed several clinical observations of a severe, sometimes fatal, bacterial infection in patients, who took an NSAID for a fever or a pain associated with a bacterial infection. These cases led the National Agency for the Safety of Medicines and Health Products (ANSM) to request a new expert report to the CRPVs, following the previous report in 2002. This new report (presented in 2016) has resulted in the emergence of a signal for severe pulmonary infections, as well as proposals of information and action [7], [8]. In 2018, new observations of serious adverse effects led to a third pharmacovigilance report that focused on severe bacterial complications with ibuprofen and ketoprofen, used for fever or non-rheumatic pain. This last report (presented in 2019) enabled to consolidate the signal, on the basis of the analysis of pharmacovilance cases, and recent pharmacoepidemiological and experimental studies [9], [10], [11]. The level of evidence led the ANSM to share the signal with its European counterparts to initiate a collective analysis (in progress as of April 2020), and to communicate about the aggravating role of NSAIDs in the event of bacterial infection as soon as April 2019 [12]. It was then reminded to favor the use of paracetamol in case of pain or fever, in particular for a common bacterial infection, such as angina, nasopharyngitis, otitis, cough, pulmonary infection, skin lesion or varicella, notably in self-medication. In front of the early clinical manifestations of COVID-19 (fever, headache, muscle pain, and cough) and its further aggravation towards pneumopathy [13], the information regarding the risks of NSAIDs was renewed when the epidemic started, and was widely relayed to the general public and health professionals [14], [15].
Therefore, the objective of this paper is to summarize the current state of knowledge that justifies to avoid NSAIDs in case of symptoms suggestive of a COVID-19 infection.
Role of NSAIDs in worsening lung infections
Clinical data from French Pharmacovigilance Network
The 2019 CRPVs report integrates the medical and pharmacological analysis of all the clinical observations collected and assessed by the French Pharmacovigilance Network. The analysis was conducted by two experts (APJB and JM), and focused in particular on elements of anamnesis, chronology, and clinical descriptions [9], [10], [11]. There were 124 cases of acute complicated community-acquired pneumonia (103 cases with purulent pleurisy, 5 cases with pulmonary abscess, 4 cases with sepsis, 2 cases with purulent pericarditis, 2 cases with focus of necrosis), where the role of an NSAID was suspected (113 cases with ibuprofen and 11 cases with ketoprofen). The clinical characteristics of these cases are particularly illustrative of the severity of the complications. Patients were children in half of the cases (63/124 cases), often young (30% of infants), or young adults (median age 37 years for ibuprofen and 45 years for ketoprofen), and had no risk factor or co-morbidities (exclusion criterion). The median duration of NSAID therapy before hospitalization was short (4 days for ibuprofen and 2 days for ketoprofen). The most frequent reasons for taking or prescribing ibuprofen were febrile pulmonary symptoms such as cough and dyspnea (33% of the cases), fever (21% of the cases), influenza-like illness (13% of the cases), and pneumonia (6% of cases). In about 1/3 of the cases (39/113 cases for ibuprofen and 0/11 cases ketoprofen), an antibiotic therapy was associated with the NSAID, either at the same time or shortly after, but before hospitalization. In most cases, it was community-acquired pneumococcal pneumonia (73% of cases) while streptococcal infections came second (S. pyogenes or other). Deaths (4/124 cases) always concerned adults.
Pharmacoepidemiological data
French as well as foreign teams (United States, United Kingdom, and Poland) conducted studies in children or adults with bacterial or viral pulmonary infections, which allowed to assess the risk in various real-life settings [16]. All the studies converge and confirm that NSAID exposure in case of pulmonary infections increases the risk of severe pulmonary complications (Table 1 ) [17], [18], [19], [20], [21], [22], [23], [24], with odds ratio ranging from 1.94 to 8.1, as detailed in the 2019 CRPVs report [9] and the reviews of Voiriot et al. [25], [26].
Table 1.
Pharmacoepidemiological studies assessing the role of non-steroidal anti-inflammatory drugs (NSAIDs) in the aggravation of pulmonary infections.
| Authors Date Country |
Design | Participants Age |
Exposure | Outcome | Odds ratio (95% confidence interval) |
|---|---|---|---|---|---|
| Byington et al. [17] 2002 United States |
Case-control | 540 children with bacterial community-acquired pneumonia < 19 years |
Ibuprofen Paracetamol |
Empyema | Ibuprofen: 4.0 (2.5–6.5) Paracetamol: non-significant |
| François et al. [18] 2010 France |
Case-control | 767 children with community-acquired pneumonia 28 days–15 years |
Ibuprofen Aspirin Glucocorticoids Other NSAIDs |
Pleural effusion, lung abscess, or cavitation on presentation or during hospital stay | Ibuprofen: 2.57 (1.51–4.35) Aspirin: 1.72 (0.69–4.99) Glucocorticoids: 1.41 (0.58–3.41) Other NSAIDs: 2.41 (0.68–8.56) |
| Voiriot et al. [19] 2011 France |
Nested case-control | 90 adults with community-acquired pneumonia in intensive care unit 52 ± 15 years |
NSAIDs | Pleural empyema, or lung cavitation | 8.1 (2.3–28.0) |
| Messika et al. [20] 2014 France |
Case-control | 106 adults with pneumococcal community-acquired pneumonia in intensive care unit 57.5 (44.5–74.1) years |
NSAIDs | Pleural effusion, or pulmonary abscess | 4.04 (1.06–15.44) |
| Elemraid et al. [21] 2015 United Kingdom |
Nested case-control | 160 children with pneumonia ≤ 16 years |
Ibuprofen | Pleural empyema | 1.94 (97.5% credible interval: 0.80–3.18) |
| Le Bourgeois et al. [22] 2016 France |
Case-control | 166 children with acute viral infection 3 months–15 years |
NSAIDs Paracetamol |
Pleural empyema within 15 days after diagnosis of acute viral infection | NSAIDs: 2.79 (1.40–5.58) Paracetamol: 1.53 (0.83–2.82) |
| Basille et al. [23] 2017 France |
Nested case-control | 221 adults with community-acquired pneumonia in intensive care unit 64.8 ± 17.3 years |
Ibuprofen | Parapneumonic pleural effusion, pleural empyema, or lung abscess | 2.57 (1.02–6.64) |
| Krenke et al. [24] 2018 Poland |
Nested case-control | 203 children with community-acquired pneumonia 2 months–17 years |
Ibuprofen Paracetamol |
Parapneumonic effusion, pleural empyema, necrotizing pneumonia, or lung abscess | Ibuprofen: 5.06 (1.47–17.35) Paracetamol: 2.75 (1.22–6.16) |
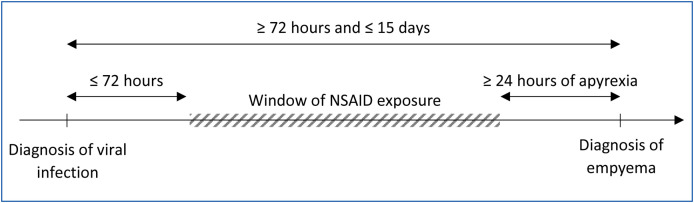
It should be noted that in the issue of NSAIDs and infections, an important limitation of pharmacoepidemiological studies is due to protopathic bias, since exposure to the drug of interest (NSAID) may be linked to the first symptoms of the outcome of interest (fever or pain due to the onset of a pulmonary complication). Thus, the risk is to wrongly suspect the NSAID whereas it is just an early marker of the onset of the complication. Interestingly, the French study carried out by Le Bourgeois et al. was specifically designed to minimize the protopathic bias as well as certain confounding factors [22]. More precisely, this matched case-control study, conducted in 15 French pediatric departments, included children with acute respiratory viral infections, in order to study the risk of developing empyema in the following 15 days. To minimize the protopathic bias, children were excluded if they did not have a minimum of 24 hours of apyrexia between the end of the symptoms of the viral infection and the diagnosis of empyema, or if there were less than 72 hours between the onset of the viral infection and the diagnosis of empyema. NSAID exposure was defined as the use of an NSAID by prescription or self-medication starting within 72 hours of the onset of the viral infection and before apyrexia, or during the corresponding time window for the matched control (Fig. 1 ). To minimize the confounding factors related to physician practices or geographic location, the cases and matched controls came from the same source population, and were recruited by the same physician. Finally, 83 cases of empyema, among which 32 had been exposed to ibuprofen, were compared to 83 matched controls, among which 21 had been exposed to ibuprofen, and 1 to ketoprofen. The study found an increased risk of empyema as of the first day of NSAID exposure (adjusted odds ratio: 2.79, 95% confidence interval: 1.40–5.58), but not with paracetamol (conditional odds ratio: 1.53, 95% confidence interval: 0.83–2.82), and a decreased risk with 6 days of antibiotic exposure.
Figure 1.
Definition of the window of non-steroidal anti-inflammatory drug (NSAID) exposure for cases to minimize protopathic bias in the study according Le Bourgeois et al. [22].
The significance of these results was underlined by Professor Paul Little from the University of Southampton [27], the principal investigator of the Pragmatic trial of Ibuprofen, Paracetamol and Steal, and the Internet Doctor Trial. The Pragmatic trial of Ibuprofen, Paracetamol and Steal was conducted in primary care on 889 patients with respiratory tract infection, to assess different symptom management strategies (paracetamol, ibuprofen, or both, and inhalations). It showed that new consultations for a progression of the symptoms and for complications of the initial pathology were more frequent in the ibuprofen group [28]. The Internet Doctor Trial was conducted on 3044 patients, to assess personalized advice on the management of respiratory infections delivered via an interactive website. It also highlighted a poor control of severe symptoms in the ibuprofen group [29].
Experimental data and pharmacological plausibility
Experimental in vitro and in vivo animal studies suggest several biological mechanisms involved in the deleterious effect of NSAIDs in case of an infection (in particular immunomodulatory effects, effects on S. pyogenes infections, and reduced antibiotics efficacy), which supports the existence of a causal link between NSAIDs and complications. The causal link thus goes beyond the well-known effect of eliminating symptoms of inflammation (fever, pain, and edema) by NSAIDs, which can lead to a delay in the appropriate therapeutic management of infections (in particular the initiation of antibiotic therapy as soon as possible), and allow complications to develop. This effect is probably marginal as both clinical and experimental data show an aggravation of bacterial infections, even in case of antibiotic therapy associated with NSAIDs.
Immunomodulatory effects of NSAIDs
Basic research studies suggest that NSAIDs disrupt the resolution of the inflammatory process [25], [26], [30]. Schematically, the immunomodulatory effects of NSAIDs are biphasic:
-
•
in the initial phase, the synthesis of eicosanoids (prostaglandins E2, prostacyclin, or leukotriene B4) is inhibited by NSAIDs, which limits locoregional recruitment of neutrophils at the site of infection, and disturbs their intrinsic properties (adhesion, degranulation, oxidative stress, and phagocytosis). Consequently, NSAIDs could alter the capacity of antibacterial immune defenses, and promote the regional spread of the infection despite the administration of appropriate antibiotic therapy. Interestingly, earlier studies had also highlighted the important role of prostacyclin as a protective prostaglandin regulating capillary permeability, thus minimizing the inflammatory response to tumor necrosis factor-α (TNF-α) [31], [32];
-
•
in the late phase, inhibition by NSAIDs of inducible cyclooxygenase (COX-2) blocks the class switch of lipid mediators, preventing the local release of the mediators specialized in the resolution of inflammation (lipoxins, resolvins, and protectins). The recruitment of macrophages, necessary for the clearance of apoptotic neutrophils, is impacted, which could promote the sustainability of the locoregional inflammatory reaction.
The hypothesis of the 2-phase effect of NSAIDs has already been shown on a mouse model of chemically induced acute lung injury [33]. In this study, prior drug inhibition of COX-2 was associated with less pulmonary recruitment of neutrophils in the initial phase, and increased inflammation from the 48th hour and prolonged beyond, contributing to delayed healing of animals. These late effects of COX-2 have been found in other animal models [34], [35].
Other studies suggest that NSAIDs could also affect the severity of bacterial infections through several mechanisms, such as an increase in the production of inflammatory cytokines (TNF-α, interleukin-1, and interleukin-6) [30], [34], [35], [36], [37], [38]. It can also be noted that profens, in particular ibuprofen, inhibit the fatty acid amide hydrolase (the enzyme that degrades anandamide, one of the main mediators of the endocannabinoid system), while endocannabinoids are mentioned in the aggravation of infections, and especially sepsis [39], [40], [41], [42], [43].
Effects of NSAIDs on S. pyogenes infections
Several studies have also shown an effect of NSAIDs, in particular ibuprofen, on the spread of S. pyogenes infection [44], [45], [46]. Even if the role of NSAIDs was assessed in another type of infection (severe soft tissue infections), the results are particularly enlightening, and can probably be extrapolated to pulmonary infections.
The study by Weng et al. [44] focused on mice inoculated with S. pyogenes by the intramuscular route. The groups compared consisted of 14 mice treated with 50 mg/kg/day of oral ibuprofen in 3 doses for 7 days, and 12 mice treated with physiological serum. The main results were higher mortality at 10 days (72% versus 0%, P < 0.001), more infiltration of macrophages and extensive tissue damage, as well as higher tissue concentrations of interleukin-6 (P = 0.0001) and TNF-α (P = 0.001) in mice treated with ibuprofen.
In the study of Hamilton et al. [45], an NSAID (ketorolac tromethamine, ibuprofen, or indomethacin) was given 1 hour after inoculating the mice with S. pyogenes, and 7 hours before administrating antibiotics. Both NSAIDs and antibiotics (penicillin or clindamycin) were continued for 72 hours. The control group did not receive NSAIDs. In the study, NSAID exposure increased the severity of the infection and accelerated the death of mice. When combined with antibiotic therapy, the NSAID reduced or delayed its efficacy. Again, this study does not support the hypothesis that the use of an NSAID in the event of a bacterial infection would delay the diagnosis and the antibiotic treatment, but is in favor of a specific effect of NSAIDs, which would increase the severity of S. pyogenes infections.
Finally, studies on models of muscle injury have shown that ibuprofen impairs muscle regeneration, which increases the expression of vimentin, a protein responsible for the adhesion of S. pyogenes, and thus facilitates its proliferation [47], [48], [49].
NSAIDs in case of COVID-19 infection
Although accumulating evidence support the existence of a deleterious effect of NSAIDs in case of an infection in several settings, there are currently no clinical studies demonstrating that such risk applies in case of COVID-19. However, mechanistic arguments suggest that taking an NSAID for symptoms suggestive of COVID-19 may be more harmful than beneficial.
On the one hand, a study assessed the effect of ibuprofen on cardiac fibrosis in a rat model of diabetes, and showed that ibuprofen increases the level of expression of angiotensin-converting enzyme 2 (ACE2) [50]. However, in COVID-19, the level of expression of ACE2 could play a major role. ACE2 seems to be the gateway for SARS-CoV-2 to enter the human body, by acting as a receptor for SARS-CoV-2 [51], [52], [53]. Some in vitro studies have found a positive correlation between the level of expression of ACE2 and the risk of coronavirus infection [54]. Although the exact role of NSAIDs remains to be clarified, this suggests a potentially deleterious action during the viral contamination phase of COVID-19 [55].
On the other hand, current evidence suggests that the immune responses induced by SARS-CoV-2 infection are two-phased. During the incubation and non-severe stages, an immune response is required to eliminate the virus and prevent progression to severe stages [56], a defense mechanism that NSAIDs may block. During the severe phase, lung damage seems to be related to an acute immune reaction and a cytokine storm, which may raise the question of using anti-inflammatory drugs at this stage [56], [57], [58]. It should be noted that a French clinical trial was announced, whose objective is to assess the efficacy of naproxen, in addition to the standard care currently used in intensive care units, on all-cause mortality at 30 days [59], [60]. This clinical trial is based on basic research studies that suggest a dual effect of naproxen, with an antiviral effect shown on the influenza virus [61], [62], in addition to its well-known anti-inflammatory effect. Designed and powered primarily on an efficacy objective (with 584 patients randomized in 2 parallel groups), this trial will not make it possible to conclude on the risk of naproxen in this situation.
Therefore, pending further research, a pragmatic and cautionary approach would be to avoid the use of NSAIDs for symptomatic treatment of non-severe symptoms of COVID-19. This cautious attitude is notably recommended by Paul Little [63], and the National Institute for Health and Care Excellence [64].
Conclusion
In 2019, the multi-source analysis conducted by the CRPVs, which integrated complementary pharmacological data (clinical, pharmacoepidemiological, and experimental), confirmed and amplified the conclusions of the previous reports on the role of NSAIDs (in particular ibuprofen, ketoprofen) in the aggravation of bacterial infections (in particular pulmonary infections). Therefore, in case of infection, symptomatic treatment with NSAIDs for non-severe symptoms (fever, pain, or myalgia) is not to be recommended, given a range of clinical and scientific arguments supporting an increased risk of severe bacterial complication. Besides, the existence of a safer drug alternative, with paracetamol at recommended doses, makes this recommendation of precaution and common sense even more legitimate. In 2020, such recommendation is more topical than ever with the emergence of COVID-19, especially since it results in fever, headaches, muscular pain, and cough, and is further complicated with pneumopathy, and given experimental data suggesting a link between ibuprofen and the level of expression of ACE2.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
The authors would like to thank Véronique Channaut Soeiro for her valuable help in translating the manuscript, and for her careful proofreading.
References
- 1.Pirmohamed M., James S., Meakin S., Green C., Scott A.K., Walley T.J. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore N., Duong M., Gulmez S.E., Blin P., Droz C. Pharmacoepidemiology of non-steroidal anti-inflammatory drugs. Therapies. 2019;74(2):271–277. doi: 10.1016/j.therap.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Brogan T.V., Nizet V., Waldhausen J.H.T., Rubens C.E., Clarke W.R. Group A streptococcal necrotizing fasciitis complicating primary varicella: a series of fourteen patients. Pediatr Infect Dis J. 1995;14(7):588–593. doi: 10.1097/00006454-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Choo P.W., Donahue J.G., Platt R. Ibuprofen and skin and soft tissue superinfections in children with varicella. Ann Epidemiol. 1997;7(7):440–445. doi: 10.1016/s1047-2797(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 5.Zerr D.M., Alexander E.R., Duchin J.S., Koutsky L.A., Rubens C.E. A case-control study of necrotizing fasciitis during primary varicella. Pediatrics. 1999;103(4):783–790. doi: 10.1542/peds.103.4.783. [DOI] [PubMed] [Google Scholar]
- 6.Lesko S.M., O’Brien K.L., Schwartz B., Vezina R., Mitchell A.A. Invasive group A streptococcal infection and nonsteroidal anti-inflammatory drug use among children with primary varicella. Pediatrics. 2001;107(5):1108–1115. doi: 10.1542/peds.107.5.1108. [DOI] [PubMed] [Google Scholar]
- 7.CRPV de Tours, CRPV d’Angers . 2016. Point AINS et risque infectieux – Comité technique de pharmacovigilance – CT012016043. https://ansm.sante.fr/content/download/97701/1241193/version/1/file/CR_CT_Pharmacovigilance_CT012016043_17-05-2016.pdf [Accessed May 4, 2020 (18 pp.)] [Google Scholar]
- 8.Jonville-Béra A., Sassier M., Vigier C., Laine P. Assessing the relationship between the use of NSAIDs for pain or fever and serious bacterial infection. Fundam Clin Pharmacol. 2017;31:5–20. [Google Scholar]
- 9.CRPV de Tours, CRPV de Marseille . 2019. Rapport d’expertise – Infections bactériennes graves (de la peau et des tissus mous, pleuro-pulmonaires, neurologiques et ORL) rapportées avec l’ibuprofène ou le kétoprofène dans le traitement symptomatique de la fièvre ou de douleur non rhumatologique. https://www.ansm.sante.fr/content/download/159487/2090277/version/1/file/Rapport+_PV_AINS-Tours_Marseille_+2019.pdf [Accessed May 4, 2020 (81 pp.)] [Google Scholar]
- 10.Soeiro T., Bourneau-Martin D., Micallef J., Jonville-Béra A.P. Multi-source comprehensive review on whether ibuprofen exacerbates bacterial infections. Br J Clin Pharmacol. 2020:30. https://bpspubs.onlinelibrary.wiley.com/doi/epdf/10.1111/bcp.14266. Abstract OC045. [Accessed May 4, 2020 (49 pp.)] [Google Scholar]
- 11.Soeiro T., Jonville-Béra A.P., Micallef J. Re: Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. doi: 10.1136/bmj.m1185. https://www.bmj.com/content/368/bmj.m1185/rr-1. [Accessed May 4, 2020] [DOI] [PubMed] [Google Scholar]
- 12.ANSM . 2019. Anti-inflammatoires non stéroïdiens (AINS) et complications infectieuses graves – Point d’information. https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Anti-inflammatoires-non-steroidiens-AINS-et-complications-infectieuses-graves-Point-d-Information [Accessed May 4, 2020] [Google Scholar]
- 13.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministère des Solidarités et de la Santé . 2020. J’ai des symptômes, je suis malade COVID-19. https://solidarites-sante.gouv.fr/soins-et-maladies/maladies/maladies-infectieuses/coronavirus/tout-savoir-sur-le-covid-19/article/j-ai-des-symptomes-je-suis-malade-covid-19 [Accessed May 4, 2020] [Google Scholar]
- 15.Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;17:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 16.Montastruc J.L., Benevent J., Montastruc F., Bagheri H., Despas F., Lapeyre-Mestre M. What is pharmacoepidemiology? Definition, methods, interest and clinical applications. Therapies. 2019;74(2):169–174. doi: 10.1016/j.therap.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Byington C.L., Spencer L.Y., Johnson T.A., Pavia A.T., Allen D., Mason E.O. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34(4):434–440. doi: 10.1086/338460. [DOI] [PubMed] [Google Scholar]
- 18.François P., Desrumaux A., Cans C., Pin I., Pavese P., Labarère J. Prevalence and risk factors of suppurative complications in children with pneumonia: suppurative complications of pneumonia. Acta Paediatr. 2010;99(6):861–866. doi: 10.1111/j.1651-2227.2010.01734.x. [DOI] [PubMed] [Google Scholar]
- 19.Voiriot G., Dury S., Parrot A., Mayaud C., Fartoukh M. Nonsteroidal anti-inflammatory drugs may affect the presentation and course of community-acquired pneumonia. Chest. 2011;139(2):387–394. doi: 10.1378/chest.09-3102. [DOI] [PubMed] [Google Scholar]
- 20.Messika J., Sztrymf B., Bertrand F., Billard-Pomares T., Barnaud G., Branger C. Risks of nonsteroidal anti-inflammatory drugs in undiagnosed intensive care unit pneumococcal pneumonia: younger and more severely affected patients. J Crit Care. 2014;29(5):733–738. doi: 10.1016/j.jcrc.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Elemraid M.A., Thomas M.F., Blain A.P., Rushton S.P., Spencer D.A., Gennery A.R. Risk factors for the development of pleural empyema in children: risk factors of pediatric empyema. Pediatr Pulmonol. 2015;50(7):721–726. doi: 10.1002/ppul.23041. [DOI] [PubMed] [Google Scholar]
- 22.Le Bourgeois M., Ferroni A., Leruez-Ville M., Varon E., Thumerelle C., Brémont F. Nonsteroidal anti-inflammatory drug without antibiotics for acute viral infection increases the empyema risk in children: a matched case-control study. J Pediatr. 2016;175 doi: 10.1016/j.jpeds.2016.05.025. [47–53.e3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basille D., Plouvier N., Trouve C., Duhaut P., Andrejak C., Jounieaux V. Non-steroidal anti-inflammatory drugs may worsen the course of community-acquired pneumonia: a cohort study. Lung. 2017;195(2):201–208. doi: 10.1007/s00408-016-9973-1. [DOI] [PubMed] [Google Scholar]
- 24.Krenke K., Krawiec M., Kraj G., Peradzynska J., Krauze A., Kulus M. Risk factors for local complications in children with community-acquired pneumonia. Clin Respir J. 2018;12(1):253–261. doi: 10.1111/crj.12524. [DOI] [PubMed] [Google Scholar]
- 25.Voiriot G., Chalumeau M., Messika J., Basille D., Philippe B., Ricard J.D. Risques associés à la prise d’anti-inflammatoires non stéroïdiens au cours de la pneumonie. Rev Mal Respir. 2018;35(4):430–440. doi: 10.1016/j.rmr.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Voiriot G., Philippot Q., Elabbadi A., Elbim C., Chalumeau M., Fartoukh M. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J Clin Med. 2019;8(6) doi: 10.3390/jcm8060786. [pii: E786] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Little P. Ibuprofen use in viral infection is associated with subsequent empyema. J Pediatr. 2017;180:291–294. doi: 10.1016/j.jpeds.2016.10.058. [DOI] [PubMed] [Google Scholar]
- 28.Little P., Moore M., Kelly J., Williamson I., Leydon G., McDermott L. Ibuprofen, paracetamol, and steam for patients with respiratory tract infections in primary care: pragmatic randomised factorial trial. BMJ. 2013;347(2):f6041. doi: 10.1136/bmj.f6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Little P., Stuart B., Andreou P., McDermott L., Joseph J., Mullee M. Primary care randomised controlled trial of a tailored interactive website for the self-management of respiratory infections (Internet Doctor) BMJ Open. 2016;6(4):e009769. doi: 10.1136/bmjopen-2015-009769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jahr J., Grände P.O. Prostacyclin counteracts the increase in capillary permeability induced by tumour necrosis factor-α. Intensive Care Med. 1996;22(12):1453–1460. doi: 10.1007/BF01709568. [DOI] [PubMed] [Google Scholar]
- 32.Dorris S.L., Peebles R.S., Jr. PGI 2 as a regulator of inflammatory diseases. Mediators Inflamm. 2012;2012:926968. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukunaga K., Kohli P., Bonnans C., Fredenburgh L.E., Levy B.D. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174(8):5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 34.Gilroy D.W., Colville-Nash P.R., Willis D., Chivers J., Paul-Clark M.J., Willoughby D.A. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 35.Levy B.D., Clish C.B., Schmidt B., Gronert K., Serhan C.N. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2(7):612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 36.Perianin A., Roch-Arveiller M., Giroud J.P., Hakim J. In vivo interaction of nonsteroidal anti-inflammatory drugs on the locomotion of neutrophils elicited by acute non-specific inflammations in the rat—Effect of indomethacin, ibuprofen and flurbiprofen. Biochem Pharmacol. 1984;33(14):2239–2243. doi: 10.1016/0006-2952(84)90661-0. [DOI] [PubMed] [Google Scholar]
- 37.Pettipher E.R., Wimberly D.J. Cyclooxygenase inhibitors enhance tumour necrosis factor production and mortality in murine endotoxic shock. Cytokine. 1994;6(5):500–503. doi: 10.1016/1043-4666(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 38.Stevens D.L. Could nonsteroidal anti-inflammatory drugs (NSAIDs) enhance the progression of bacterial infections to toxic shock syndrome? Clin Infect Dis. 1995;21(4):977–980. doi: 10.1093/clinids/21.4.977. [DOI] [PubMed] [Google Scholar]
- 39.Fowler C.J., Tiger G., Stenström A. Ibuprofen inhibits rat brain deamidation of anandamide at pharmacologically relevant concentrations. Mode of inhibition and structure-activity relationship. J Pharmacol Exp Ther. 1997;283(2):729–734. [PubMed] [Google Scholar]
- 40.Fowler C., Naidu P., Lichtman A., Onnis V. The case for the development of novel analgesic agents targeting both fatty acid amide hydrolase and either cyclooxygenase or TRPV1. Br J Pharmacol. 2009;156(3):412–419. doi: 10.1111/j.1476-5381.2008.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duggan K.C., Hermanson D.J., Musee J., Prusakiewicz J.J., Scheib J.L., Carter B.D. (R)-Profens are substrate-selective inhibitors of endocannabinoid oxygenation by COX-2. Nat Chem Biol. 2011;7(11):803–809. doi: 10.1038/nchembio.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sardinha J., Kelly M.E.M., Zhou J., Lehmann C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediators Inflamm. 2014;2014:978678. doi: 10.1155/2014/978678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lafreniere J., Lehmann C. Parameters of the endocannabinoid system as novel biomarkers in sepsis and septic shock. Metabolites. 2017;7(4):55. doi: 10.3390/metabo7040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng T.C., Chen C.C., Toh H.S., Tang H.J. Ibuprofen worsens Streptococcus pyogenes soft tissue infections in mice. J Microbiol Immunol Infect. 2011;44(6):418–423. doi: 10.1016/j.jmii.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Hamilton S.M., Bayer C.R., Stevens D.L., Bryant A.E. Effects of selective and nonselective nonsteroidal anti-inflammatory drugs on antibiotic efficacy of experimental group A streptococcal myonecrosis. J Infect Dis. 2014;209(9):1429–1435. doi: 10.1093/infdis/jit594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ture Z., Demiraslan H., Kontas O., Alp E., Doganay M. The role of nonsteroidal anti-inflammatory drugs intramuscular injection in the development and severity of deep soft tissue infection in mice. Fundam Clin Pharmacol. 2018;32(2):147–154. doi: 10.1111/fcp.12336. [DOI] [PubMed] [Google Scholar]
- 47.Bryant A.E., Bayer C.R., Huntington J.D., Stevens D.L. Group A streptococcal myonecrosis: increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685–1692. doi: 10.1086/504261. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton S.M., Bayer C.R., Stevens D.L., Lieber R.L., Bryant A.E. Muscle injury, vimentin expression, and nonsteroidal anti-inflammatory drugs predispose to cryptic group A streptococcal necrotizing infection. J Infect Dis. 2008;198(11):1692–1698. doi: 10.1086/593016. [DOI] [PubMed] [Google Scholar]
- 49.Bryant A.E., Bayer C.R., Aldape M.J., Stevens D.L. The roles of injury and nonsteroidal anti-inflammatory drugs in the development and outcomes of severe group A streptococcal soft tissue infections. Curr Opin Infect Dis. 2015;28(3):231–239. doi: 10.1097/QCO.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiao W., Wang C., Chen B., Zhang F., Liu Y., Lu Q. Ibuprofen attenuates cardiac fibrosis in streptozotocin-induced diabetic rats. Cardiology. 2015;131(2):97–106. doi: 10.1159/000375362. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.5Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. [271–280.e8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hofmann H., Geier M., Marzi A., Krumbiegel M., Peipp M., Fey G.H. Susceptibility to SARS coronavirus S protein-driven infection correlates with expression of angiotensin-converting enzyme 2 and infection can be blocked by soluble receptor. Biochem Biophys Res Commun. 2004;319(4):1216–1221. doi: 10.1016/j.bbrc.2004.05.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.SFPT . 2020. ACE2, IEC/ARAII et infections à COVID-19. https://www.em-consulte.com/em/covid-19/IEC-ARA2-et-COVID19-22-mars-2020.pdf [Accessed May 4, 2020 (14 pp.)] [Google Scholar]
- 56.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa248. [(Epub ahead of print) PubMed PMID: 32161940; PubMed Central PMCID: PMC7108125, pii:ciaa248] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ministère des Solidarités et de la Santé . 2020. Ministère des Solidarités et de la Santé – Financement en urgence de 11 projets de recherche appliquée en santé pour près de 9 millions d’euros. https://solidarites-sante.gouv.fr/actualites/presse/communiques-de-presse/article/ministere-des-solidarites-et-de-la-sante-financement-en-urgence-de-11-projets [Accessed May 4, 2020] [Google Scholar]
- 60.ClinicalTrials.gov . 2020. Efficacy of addition of naproxen in the treatment of critically ill patients hospitalized for COVID-19 infection (ENACOVID)https://www.clinicaltrials.gov/ct2/show/NCT04325633 [Accessed May 4, 2020] [Google Scholar]
- 61.Lejal N., Tarus B., Bouguyon E., Chenavas S., Bertho N., Delmas B. Structure-based discovery of the novel antiviral properties of naproxen against the nucleoprotein of influenza A virus. Antimicrob Agents Chemother. 2013;57(5):2231–2242. doi: 10.1128/AAC.02335-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tarus B., Bertrand H., Zedda G., Di Primo C., Quideau S., Slama-Schwok A. Structure-based design of novel naproxen derivatives targeting monomeric nucleoprotein of Influenza A virus. J Biomol Struct Dyn. 2015;33(9):1899–1912. doi: 10.1080/07391102.2014.979230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;368:m1185. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 64.NICE . 2020. COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community. https://www.nice.org.uk/guidance/NG163 [Accessed May 4, 2020] [PubMed] [Google Scholar]