Highlights
-
•
Influenza viruses accounted for a large proportion of respiratory virus infection even during the epidemic of COVID-19 in Beijing.
-
•
No single symptom or laboratory finding was suggestive of a specific respiratory virus.
-
•
The epidemic history was important for screening of COVID-19.
Keywords: 2019 novel coronavirus, COVID-19, Influenza, Virus, Infection, Epidemic history
Abstract
Background
Coronavirus disease 2019 (COVID-19) is spreading. Here, we summarized the composition of pathogens in fever clinic patients and analyzed the characteristics of different respiratory viral infections.
Methods
Retrospectively collected patients with definite etiological results using nasal and pharyngeal swabs in a fever clinic.
Results
Overall, 1860 patients were screened, and 136 patients were enrolled. 72 (52.94%) of them were diagnosed as influenza (Flu) A virus infection. 32 (23.53%) of them were diagnosed as Flu B virus infection. 18 (13.24%) and 14 (10.29%) of them were diagnosed as COVID-19 and respiratory syncytial virus (RSV) infections, respectively. The COVID-19 group had a higher rate of contact with the epidemic area within 14 days and of clustering onset than other groups. Fever was the most common symptom in these patients. The ratio of fever to the highest temperature was higher in Flu A virus infection patients than in COVID-19 patients. COVID-19 patients had a lower white blood cell count and neutrophil count than Flu A virus and RSV infection groups, but higher lymphocyte count than Flu A and B virus infection groups. The COVID-19 group (83.33%) had a higher rate of pneumonia in chest CT scans than Flu A and B virus infection groups.
Conclusions
Influenza viruses accounted for a large proportion of respiratory virus infection even during the epidemic of COVID-19 in Beijing. No single symptom or laboratory finding was suggestive of a specific respiratory virus; however, epidemic history was significant for the screening of COVID-19.
Viral infection is becoming a disaster threatening human health because of the emergence of new respiratory viruses such as the 2019 novel coronavirus (2019-nCoV), which is spreading in China (Munster et al., 2020), as well as influenza A H1N1, the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV) that emerged in the past decade (DomínguezCherit et al., 2009, Assiri et al., 2013, Lee et al., 2003). 2019-nCoV has been given much attention recently (National Health Commission of the People’s Republic of China, 2020), and the disease caused by 2019-nCoV was named as COVID-19, short for “coronavirus disease 2019”, by World Health Organization (2020). However, other respiratory viruses, even though not that widely spreading, can cause similar symptoms as 2019-nCoV and should not be ignored. Here, we summarized the composition of pathogens in fever clinic patients and analyzed the characteristics of different respiratory viral infections.
Methods
Data collection
Patients with fever (oral temperature > = 37.3 °C) or respiratory symptoms (cough, sputum, pharyngalgia, rhinorrhea, dyspnea, etc.) were advised to go to fever clinics for screening in Beijing. Peking Union Medical College Hospital (PUMCH) has a fever clinic for screening. Also, to strictly control the development of the epidemic situation of COVID-19, all patients with an epidemiologic history of COVID-19 (e.g. there was a history of travel or residence in Wuhan and its surrounding areas and communities with reported cases within two weeks before the onset of the disease; or within 14 days before the onset of the disease, contact with COVID-19 patients (with positive nucleic acid test); or within 14 days before the onset of the disease, contact with patients with fever and respiratory symptoms from Wuhan and its surrounding areas and communities with reported cases; or there was a clustering onset of disease) (National Health Commission of People’s Republic of China, 2020) were required to screen even though without fever or respiratory symptoms at PUMCH. Clustering onset was defined as two or more cases of fever and/or respiratory symptoms that were found in a small area such as family, office, school class, and other places within two weeks. Patient's medical records containing the symptoms, signs, epidemic history, etc., were taken by doctors. The epidemic history included
-
(a)
contact with an epidemic area within two weeks before the onset of the disease;
-
(b)
contact with confirmed or suspected cases within two weeks before the onset of the disease;
-
(c)
contact with poultry, livestock or wild animals (especially dead animals) within two weeks before the onset of the disease;
-
(d)
clustering onset.
Nasal and pharyngeal swabs were collected by clinicians with unified training and sent to the Laboratory of Peking Union Medical College Hospital to test for the presence of influenza A (Flu A) virus, influenza B (Flu B) virus, 2019-nCoV and respiratory syncytial virus (RSV) nucleic acid using real-time Reverse Transcription-Polymerase Chain Reaction (RT-PCR) or Flu A and Flu B virus antigen using a colloidal gold immunochromatography assay. Patients seen on January 19 through February 22, 2020, with positive nucleic acid or antigen test were included in the study. Epidemiologic features, clinical presentation, laboratory findings, and image features of viral infection patients were collected and analyzed. This study was approved by the institutional review board committee of PUMCH.
Statistics
Statistical analysis was finished by SPSS Statistics 17.0. The normality of the distribution was assessed using the Kolmogorov-Smirnov test. Group t-test was applied to the normal distribution data, and the Mann–Whitney U test was applied to the non-normal distribution data. Data are shown as means ± SD or median(25%–75%). A Chi-square test was used for comparison of two or multiple rates or components. Analyses were presented as two-sided comparisons. A P-value of less than 0.05 was considered to be significant.
Results
The etiology composition and demographic characteristics of the patients
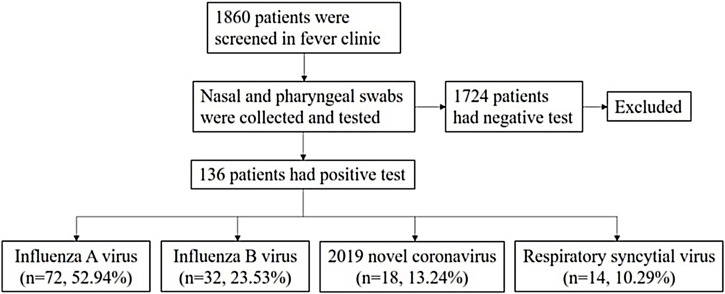
Overall, 1860 patients were screened in a fever clinic. 136 (7.31%) patients with positive nasal and pharyngeal swab tests for Flu A virus, Flu B virus, 2019-nCoV, or RSV were enrolled in the analysis. The selection process and etiological composition are shown in Figure 1 ; the baseline features of the patients are presented in Table 1 . The median age of the patients was 37.00(27.00–58.75). The ratio of males and females was 1:1.31. Seventy-two (52.94%) patients were diagnosed with Flu A virus infection. Thirty-two (23.53%) of the patients were diagnosed with Flu B virus infection. Eighteen (13.24%) and 14 (10.29%) of the patients were diagnosed with COVID-19 and RSV infection, respectively. There was no statistical difference in the sex ratio among the four groups (P > 0.05). No statistical differences were found in the age of the patients among Flu A virus infection, COVID-19, and RSV infection groups (P > 0.05). However, the age of Flu B virus infection group [32.00(26.00–37.00)] was younger than other groups (P < 0.05). The COVID-19 group had a higher rate of contact with an epidemic area within 14 days and clustering onset than other groups (P < 0.05). No other epidemic history was statistically different among the four groups (P > 0.05). 36 (26.47%) patients had comorbidities, the most common of which was hypertension (8.96%). The less common comorbidities were malignant tumor (7.46%), heart disease (5.97%), diabetes mellitus (5.22%), chronic lung disease (4.48%), autoimmune disease (2.99%), chronic liver disease (1.49%) and chronic kidney disease (0.75%). RSV infection patients had a higher risk of having comorbidities than the COVID-19 group (P = 0.010); however, no differences were found in the rate of comorbidities among other groups (P > 0.05).
Figure 1.
The screening procedure of the patients.
Table 1.
The baseline features of respiratory virus-infected patients.
| Total | Flu A virus | Flu B virus | COVID-19 | RSV | |
|---|---|---|---|---|---|
| n | 136 | 72 | 32 | 18 | 14 |
| Sex (M/F) | 59/77 | 28/44 | 14/18 | 11/7 | 6/8 |
| Age | 37.00(27.00-58.75) | 38.50(27.00-62.75) | 32.00(26.00-37.00) | 39.00(34.00-60.25) | 47.64 ± 17.92 |
| Epidemiologic features (within two weeks) | |||||
| Contact with epidemic area | 16 (11.76%) | 3 (4.17%) | 2 (6.25%) | 11 (61.11%) | 0 |
| Contact with confirmed or suspected cases | 33 (24.26%) | 17 (23.61%) | 5 (15.63%) | 7 (38.89%) | 4 (28.57%) |
| Contact with poultry, livestock or wild animals | 0 | 0 | 0 | 0 | 0 |
| Clustering onset | 18 (13.24%) | 4 (5.56%) | 3 (9.78%) | 11 (61.11%) | 0 |
| Comorbidities | |||||
| yes | 36 (26 .47%) | 21 (29.17%) | 7 (21.88%) | 1 (5.56%) | 7 (50.00%) |
| no | 100 (73.53%) | 51 (70.83%) | 25 (78.13%) | 17 (94.44%) | 7 (50.00%) |
| Symptoms (days from onset to visit) | |||||
| Fever | 130 (95.59%) | 72 (100%) | 31 (96.88%) | 15 (83.33%) | 12 (85.71%) |
| Tmax, ℃ | 38.50(38.00-39.00) | 38.53 ± 0.56 | 38.38 ± 0.56 | 38.00(37.60-38.50) | 38.00(37.70-38.90) |
| Cough | 89 (65.44%) | 51 (70.83%) | 21 (65.63%) | 9 (50.00%) | 8 (57.14%) |
| Sputum | 60 (41.18%) | 32 (44.44%) | 13 (40.63%) | 9 (50.00%) | 6 (42.86%) |
| Pharyngalgia | 63 (46.32%) | 39 (54.17%) | 14 (43.75%) | 4 (22.22%) | 6 (42.86%) |
| Rhinorrhea | 61 (44.85%) | 35 (48.61%) | 16 (50.00%) | 6 (33.33%) | 4 (28.57%) |
| Myalgia or fatigue | 75 (55.15%) | 41 (56.94%) | 23 (71.88%) | 6 (33.33%) | 5 (35.71%) |
| Headache | 69 (50.74%) | 40 (55.56%) | 21 (65.63%) | 2 (11.11%) | 6 (42.86%) |
| Dyspnea | 12 (8.82%) | 7 (9.72%) | 2 (6.25%) | 2 (11.11%) | 1 (7.14%) |
| Chest pain | 8 (5.88%) | 4 (5.56%) | 3 (9.38%) | 0 | 1 (7.14%) |
| Diarrhea | 4 (2.94%) | 1 (1.39%) | 1 (3.13%) | 1 (5.56%) | 1 (7.14%) |
| Days from onset to visit | 2.00(1.00-3.00) | 2.00(1.00-3.00) | 2.00(1.00-3.00) | 3.00(2.00-4.00) | 3.00(2.00-7.00) |
| SpO2, % (at room air) | 98.00(97.00-100.00) | 99.00(97.00-100.00) | 98.00(98.00-100.00) | 98.06 ± 1.63 | 98.5(96.00-99.25) |
Notes: Flu A: influenza A; Flu B: influenza B; COVID-19: coronavirus disease 2019; RSV: respiratory syncytial virus; M: male; F: female; Tmax: the highest temperature; SpO2: pulse oxygen saturation.
Symptoms of different respiratory virus-infected patients
Fever was the most common symptom in respiratory viral infection patients. All patients with Flu A virus infection had a fever; the ratio was higher than that in COVID-19 and RSV infection patients (P < 0.05). The highest temperature was higher in the Flu A infection group (38.53 ± 0.56) than in the COVID-19 group [38.00(37.60–38.50)] (P = 0.004). The percentage of pharyngalgia in the COVID-19 group was lower than in the Flu A group (P = 0.018). The Flu B virus infection group had a higher rate of myalgia or fatigue than the COVID-19 and RSV infection groups (P = 0.016 and 0.047, respectively). The rate of headache in Flu A and B viral infection patients was higher than that in the COVID-19 group (P = 0.001 and <0.001 respectively). No other symptoms such as cough, sputum, rhinorrhea, etc. were found statistically different among the groups (P > 0.05). The days from onset to visit and the pulse oxygen saturation (SpO2) at room air were not different among the groups (P > 0.05).
The laboratory findings and image features of different respiratory viral infections
There was no statistical difference in complete blood count parameters between Flu A and B infection groups (P > 0.05). COVID-19 patients had a lower white blood cell (WBC) count and neutrophil count than the Flu A virus infection group (P = 0.022 and 0.002, respectively) and RSV infection group (P < 0.001 and <0.001, respectively). Also, Flu A (P = 0.019 and 0.027 respectively) and Flu B (P = 0.001 and 0.005 respectively) virus infection groups had lower WBC and neutrophil count than the RSV infection group. The lymphocyte count was lower in Flu A and B virus infection groups than in the COVID-19 group (P = 0.001 and 0.005 respectively) and RSV infection group (P = 0.015 and 0.038 respectively); however, there was no lymphocyte count difference between the COVID-19 group and RSV infection group (P = 0.600). The RSV infection group had lower hemoglobin levels than other groups (P < 0.05), and higher C-reactive protein (CRP) than the COVID-19 (P = 0.025) and Flu B infection groups (P = 0.049). CRP was not statistically different among Flu A and B infection and COVID-19 groups (P > 0.05). No other differences in laboratory parameters were found between the groups (P > 0.05).
Overall, 77 (56.62%) patients underwent a chest CT scan, and 41 (53.25%) of them were diagnosed with viral pneumonia by chest CT scan. The distribution of patients who underwent a chest CT scan, and the image features are shown in Table 2 . The COVID-19 group (83.33%) had a higher rate of pneumonia in the chest CT scan than Flu A (48.39%, P = 0.018) and Flu B (14.29%, P < 0.001) viral infection groups, but had no difference with the RSV infection group (64.29%, P = 0.252). Patients with Flu B infection were more likely to have a normal chest CT scan compared with other virus infection groups (P < 0.05). Most of the pneumonia patients had multiple ground-glass opacity in the chest CT scan, there were no differences in opacity among the four groups (P > 0.05).
Table 2.
The laboratory findings and imaging features of respiratory virus-infected patients.
| Flu A virus (n = 72) | Flu B virus (n = 32) | COVID-19 (n = 18) | RSV (n = 14) |
|
|---|---|---|---|---|
| White blood cell count, ×10⁹/L | 6.44 ± 2.23 | 5.44 ± 2.36 | 5.02 ± 1.62 | 11.49 ± 5.93 |
| Neutrophil count, ×10⁹/L | 4.91 ± 2.25 | 3.87 ± 2.19 | 2.70(1.95-3.25) | 8.03(4.33-11.73) |
| Lymphocyte count, ×10⁹/L | 0.81(0.50-1.41) | 1.01 ± 0.44 | 1.61 ± 0.65 | 1.80 ± 1.06 |
| Hemoglobin, g/L | 136.59 ± 21.04 | 145.20 ± 24.19 | 143.56 ± 21.69 | 117.00 ± 31.54 |
| Platelet count, ×10⁹/L | 196.00 ± 57.59 | 193.00 ± 49.29 | 214.67 ± 60.40 | 222.64 ± 122.11 |
| C-reactive protein, mg/L | 15.50(3.57-58.21) | 6.02(2.58-20.75) | 10.30(0.92-21.52) | 36.73(11.61-99.55) |
| Pneumonia in chest CT | 15/31 (48.39%) | 2/14 (14.29%) | 15/18 (83.33%) | 9/14 (64.29%) |
| Solitary lesion | 2 (13.33%) | 0 | 4 (26.67%) | 0 |
| Multiple lesion | 13 (86.67%) | 2 (100%) | 11 (73.33%) | 9 (100%) |
| Consolidation | 1 (6.67%) | 0 | 1 (6.67%) | 1 (11.11%) |
| Ground-glass opacity | 14 (93.33%) | 2 (100%) | 14 (93.33%) | 8 (88.89%) |
Notes: Flu A: influenza A; Flu B: influenza B; COVID-19: coronavirus disease, 2019; RSV: respiratory syncytial virus.
Discussion
In this study, even though conducted during the spreading of 2019-nCoV, more than half of the patients with positive etiology had the Flu A virus infection in Beijing. Less common was the Flu B virus infection. This is similar to that previously reported (Al-Baadani et al., 2019). Only 11.94% and 10.45% of the patients had COVID-19 and RSV infections, respectively. Although COVID-19 should be taken seriously, other respiratory viral infections, especially influenza, should not be ignored. To distinguish COVID-19 from other respiratory virus infections, the epidemic history was an essential criterion because, compared to the other three groups, the COVID-19 group had a higher clustering onset, and a higher rate of contact with an epidemic area within 14 days.
Similar to what has been reported for COVID-19 (Huang et al., 2020), fever was the most common symptom in respiratory virus infection patients. In this study, the ratio of fever to the highest temperature was higher in Flu A virus infection patients than in COVID-19 patients. Given the absence of fever and low-grade fever in COVID-19 patients and its high risk (Guan et al., 2020, Li et al., 2020), it was practical to ask fever-free patients with respiratory symptoms to screen as previously recommended (Li et al., 2020). Even though the rate of pharyngalgia, headache, myalgia, and fatigue were relatively higher in influenza infection groups, it could not be said that the presence of these symptoms was meaningful for excluding COVID-19 because they were not specific symptoms in different respiratory virus infection. In fact, no specific symptoms were helpful in distinguishing COVID-19 from other respiratory virus infections (Li et al., 2020).
As previously reported, COVID-19 patients had low or normal WBC and neutrophil count (Huang et al., 2020, Guan et al., 2020). And in this study, it was found that COVID-19 patients even had lower WBC counts and neutrophil counts than Flu A virus and RSV infection groups. Although it was reported that COVID-19 patients had low lymphocyte counts (Huang et al., 2020, Guan et al., 2020), this study found that the lymphocyte count was higher in COVID-19 than in Flu A and B virus infection patients. These results showed that low WBC, neutrophil, and lymphocyte counts were suggestive of viral infection, but could not distinguish different between the respiratory-virus groups. Also, even though the CRP level was higher in the RSV infection group than in COVID-19 and Flu B infection groups, it was not statistically different in Flu A infection, Flu B infection, and COVID-19 groups. Lower WBC and lymphocyte levels and higher CRP levels might be more valuable in monitoring disease severity rather than in screening for COVID-19 (Guan et al., 2020). The value of CBC parameters and CRP deserve more study in respiratory viral infections.
The percentage of pneumonia in the Chest CT scan was higher in the COVID-19 group than in Flu A and B virus infection group. This finding was similar to other studies that showed COVID-19 patients were likely to have pneumonia (Huang et al., 2020, Guan et al., 2020). And the possibility of COVID-19 should be considered if pneumonia was found in a chest CT scan during the spreading of 2019-nCoV, as was recommended in a screening procedure in a fever clinic (Li et al., 2020).
There are some limitations to this study. First, only one center was enrolled in the study. It might not be that representative of the whole city of Beijing. Second, most patients, especially the COVID-19 patients, were mild cases on the first visit. They were not representative of respiratory viral infections of varying severity. Third, no follow-up data were available in this study, therefore it was impossible to analyze the whole clinical course of different virus infections.
Conclusions
Influenza viruses accounted for a large proportion of cases respiratory virus infection, even during the epidemic of 2019-nCoV, in Beijing. No single symptom nor laboratory finding was indicative of a specific respiratory virus; however, the epidemic history was valuable for screening out COVID-19 patients. A combination of epidemic history, symptoms, laboratory findings, chest CT scans, and finally, a pathogenic examination was essential for an accurate diagnosis of respiratory virus infection.
Funding
This study was funded by the Chinese Academy of Medical Sciences’ Innovation Fund for Medical Sciences (2020-I2M-CoV19-002).
Declarations of interest
None.
Ethical approval
The work was approved by the institutional review board committee of Peking Union Medical College Hospital.
Authors’ contribution
Huadong Zhu, Jihai Liu, Yan Li, Jiangshan Wang, Xuezhong Yu, Jun Xu and Yi Li designed the study. Yan Li, Qiwen Yang, Yingchun Xu provided the study materials; Yan Li, Jiangshan Wang and Chunting Wang collected and assembled the data; Yan Li, Jiangshan Wang and Chunting Wang, Huadong Zhu and Jihai Liu analyzed and interpreted the data; Yan Li wrote the manuscript and all the authors revised it.
Acknowledgment
None.
References
- Al-Baadani A.M., Elzein F.E., Alhemyadi S.A., Khan O.A., Albenmousa A.H., Idrees M.M. Characteristics and outcome of viral pneumonia caused by influenza and Middle East respiratory syndrome-coronavirus infections: a 4-year experience from a tertiary care center. Ann Thorac Med. 2019;14:179–185. doi: 10.4103/atm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DomínguezCherit G., Lapinsky S.E., Macias A.E., Pinto R., Espinosa-Perez L., de la Torre A. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Hui D., Wu A., Chan P., Cameron P., Joynt G.M. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–1994. doi: 10.1056/NEJMoa030685. [DOI] [PubMed] [Google Scholar]
- Li Yan, Xu Shengyong, Du Tiekuan, Xu Jun, Li Yi, Yu Xuezhong. Clinical features of 2019 novel coronavirus infection patients and a feasible screening procedure. Chin J Emerg Med. 2020;29:320–324. http://www.cem.org.cn/default/content/index/id/12353 [Google Scholar]
- Munster V.J., Koopmans M., van Doremalen N., van Riel D., de Wit E. A novel coronavirus emerging in China – Key Questions for Impact Assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 2020 National Health Commission of the People’s Republic of China. Epidemic update and risk assessment of 2019 Novel Coronavirus. January 28, 2020. http://www.chinacdc.cn/yyrdgz/202001/P020200128523354919292.pdf.
- National Health Commission of People’s Republic of China. Diagnosis and treatment of 2019 novel coronavirus pneumonia (6th trial version). February 18, 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf.
- World Health Organization . 2020. Novel Coronavirus (2019-nCoV): situation report, 22.https://apps.who.int/iris/handle/10665/330991 [Google Scholar]