To the Editor,
After the SARS pandemic in 2002–2003 and MERS pandemic in 2012–2013, coronavirus disease 2019 (COVID-19), an emerging infectious disease caused by a single-stranded enveloped RNA Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), is a current global pandemic impacting >3,800,000 patients and 265,902 deaths worldwide. The association between the disease severity of COVID-19 and preexisting cardiovascular disease (CVD) risk remains unknown. The aim of this meta-analysis is to determine the association of preexisting CVD risk and the disease severity of COVID-19.
We systematically searched MEDLINE, Embase, Cochrane Database of Systematic Reviews, Scopus, and Web of Science as well as preprint servers from December 2019 through April 10, 2020, for observational studies that reported the association between preexisting CVD risk and COVID-19. There were no limitations on language or date of publication. Data were independently reviewed by two investigators. Conflicts were resolved through consensus. The strategies included MeSH and Embase terms as well as keywords including coronavirus, COVID-19, 2019nCoV, SARS-CoV-2, severe acute respiratory syndrome coronavirus 2, CVD, CVD events, diabetes mellitus (DM), hypertension (HTN), smoking, myocardial injury, cardiac injury, coronary artery disease (CAD), acute myocardial infarct, acute coronary syndrome, or heart failure (HF). Inclusion criteria are as follows: (1) patients were confirmed to have been infected by SARS-CoV-2; and (2) numbers of the preexisting CVD risk, numbers of severe cases and non-severe cases were reported. Reviews, editorials, non-human studies, duplicate publications, only children cases, letters without sufficient data, studies without preexisting CVD risk were excluded. We classified severe disease as patients with confirmed COVID-19 who admitted to the intensive care unit, developed acute respiratory distress syndrome, elevated troponin, the progression of the disease, required mechanical ventilation, or death. Quality assessment was performed by two independent reviewers using the Newcastle-Ottawa quality assessment scale, a validated scale for non-randomized studies in meta-analyses. We assigned scores of 0–3, 3.5–6, and 6.5–9 for low, moderate, and high quality of studies, respectively. Random-effects meta-analyses were used. Subgroup analyses were performed to explore the potential sources of heterogeneity. We defined substantial heterogeneity between the studies if I 2 > 50%. All statistical analyses were performed with Stata version 11 (Stata Corp), and all tests were two-sided with a significance level of 0.05.
We identified 13 observational studies. A total of 49,076 confirmed COVID-19 cases (10,009 were severe cases and 7773 were non-severe cases), 50 smoking, 3568 DM, 3490 HTN, 69 HF, 113 CAD, and 4761 CVD events were included. The proportion of DM, HTN, CAD, HF and CVD events were all statistically significant higher in patients with severe COVID-19 disease compared to non-severe COVID-19 disease [DM: OR = 3.02, 95% CI (2.07–4.42), I 2 = 39.7%, P < 0.001; HTN: OR = 4.56, 95% CI (1.96–10.59), I 2 = 91.7%, P < 0.001; CAD: OR = 6.85, 95% CI (3.81–12.3), I 2 = 48.1%, P < 0.001; HF: OR = 9.77, 95% CI (5.36–17.79), I 2 = 0%, P < 0.001; CVD events: OR = 8.89, 95% CI (4.04–19.5), I 2 = 66.9%, P < 0.001]. However, the proportion smoking was not statistically significant higher in patients with severe COVID-19 disease compared to non-severe COVID-19 disease [smoking: OR = 2.75, 95% CI (0.46–16.3), I 2 = 92.3%, P < 0.001] (Fig. 1 ). Only one study reported angiotensin-converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB) use accounted for 21.1% of severe COVID-19 disease, but 5.8% of non-severe COVID-19 disease. There were no significant interactions among the subgroups using the quality assessment. There was no study markedly affecting the summary estimate or P-values for heterogeneity of all groups.
Fig. 1.
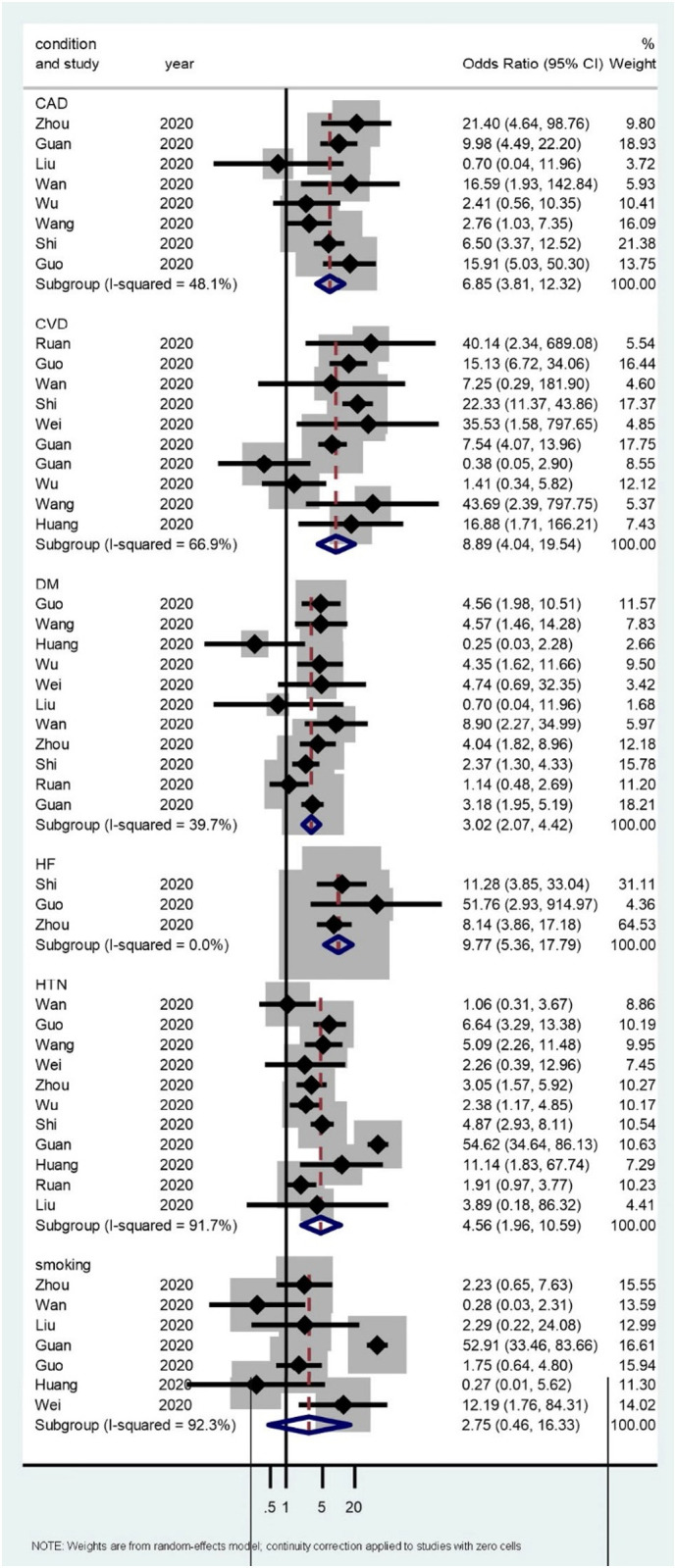
The association of preexisting cardiovascular risk and the disease severity of COVID-19. The forest plot of point estimates and confidence intervals also includes results for variance, used in the inverse variance correction.
There are two main findings from the present study. First, although smoking has been demonstrated to be a CVD risk factor and has known adverse effects on cardiopulmonary function,1 surprisingly, we could not find the association of the COVID-19 severity and smoking. This may be confounded by the frequency of smoking, duration of smoking, or lung function. Second, we found that DM, HTN, CAD, HF, overall CVD events were associated with severe COVID-19 cases, compared to non-severe COVID-19 cases. Previous studies showed that age, high body mass index, and low CD4 T cell count were independently associated with severe COVID-19 disease.2 , 3
There are certain limitations to this study. First, as discussed earlier, the frequency and duration of smoking are not captured in the included studies. Second, we could not differentiate between medication non-compliance or severity of CVD risk. For instance, poorly controlled DM or HTN is probably associated with more severe COVID-19 disease. Last, our results may be confounded by poor sleep hygiene, nutritional status, physical inactivity, or depression.4 , 5
In conclusion, patients with preexisting CVD risk, such as DM and HTN risk, as well as patients with established CVD, are associated with a higher risk of developing severe COVID-19 disease. Smoking was not associated with the severity of the COVID-19 case. Only one study reported ACEI/ARB use and a higher proportion in severe COVID-19 disease, compared to non-severe COVID-19 disease.
References
- 1.Messner B., Bernhard D. Smoking and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. [DOI] [PMC free article] [PubMed]
- 3.Peng Y.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua xin xue guan bing za zhi. 2020;48 doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 4.Ibarra-Coronado E.G., Pantaleón-Martínez A.M., Velazquéz-Moctezuma J. The bidirectional relationship between sleep and immunity against infections. J Immunol Res. 2015;2015:678164. doi: 10.1155/2015/678164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Zhang L., Lei Y. Meta-analysis of infectious agents and depression. Sci Rep. 2014;4:4530. doi: 10.1038/srep04530. [DOI] [PMC free article] [PubMed] [Google Scholar]


