Abstract
In order to fight the SARS-CoV-2 pandemic infection, there is a growing need and demand for diagnostic tools that are complementary and different from the RT-PCR currently in use. Multiple serological tests are or will be very soon available but need to be evaluated and validated. We have thus tested 4 immunochromatographic tests for the detection of antibodies to SARS-CoV-2. In addition, we assessed the kinetics of antibody appearance using these assays in 22 patients after they were tested positive by RT-PCR. We observed great heterogeneity in antibody detection post-symptom onset. The median antibody detection time was between 8 and 10 days according to the manufacturers. All the tests showed a sensitivity of 60 to 80% on day 10 and 100% on day 15. In addition, a single cross-reaction was observed with other human coronavirus infections. Thus, immunochromatographic tests for the detection of anti-SARS-CoV-2 antibodies may have their place for the diagnostic panel of COVID-19.
Keywords: SARS-CoV-2, COVID-19, Antibody, Lateral flow assay
Background
Since December 2019, the world has been facing a pandemic of COVID-19, an infectious disease caused by SARS-CoV-2, a virus that emerged in China 7. Although RT-PCR testing of SARS-CoV-2 has become the standard method for direct diagnosis, these real-time PCR tests have some limitations, primarily dedicated infrastructure to avoid any biorisk, limited capacity and a long turnaround time 3. There is increasing pressure from the medical community and society to screen the population on a large scale. Serological tests in ELISA format or as immunochromatographic lateral flow assay (LFA) have recently become available from many manufacturers 4 , 2. These serological tests will be complementary to PCR tests both for screening and diagnosis of the population, for the purpose of population exits from containment in different countries and finally for future epidemiological studies. However, it is necessary to evaluate the analytical performance of these assays and also their place in clinical practice. Thus, the objective of our study was to evaluate four immunochromatographic assays for the detection of IgM and IgG antibodies to SARS-CoV-2 and to evaluate the kinetics of their detection by these LFA.
Study design
Study population and specimen
Twenty two patients diagnosed positive in Amiens University hospital for SARS-CoV- 2 on a nasopharyngeal swab using a RT-PCR technique (National Reference Center in Pasteur Institute, Paris, France) were included in our study. The date of reporting of the first symptoms was retrieved from the medical records. The samples were tested regularly during the hospitalization until the tests were positive, with an evaluation at most on day 24 post-symptoms. In order to evaluate a possible cross-reaction with the other human coronaviruses described to date (NL63, HKU1, 229E and OC43), sera following such viral respiratory infection diagnosed in our lab were tested. This project was conducted in accordance with the reference methodology (MR-004 France) in accordance with Article 30 of the GDPR.
Rapid immunochromatographic tests
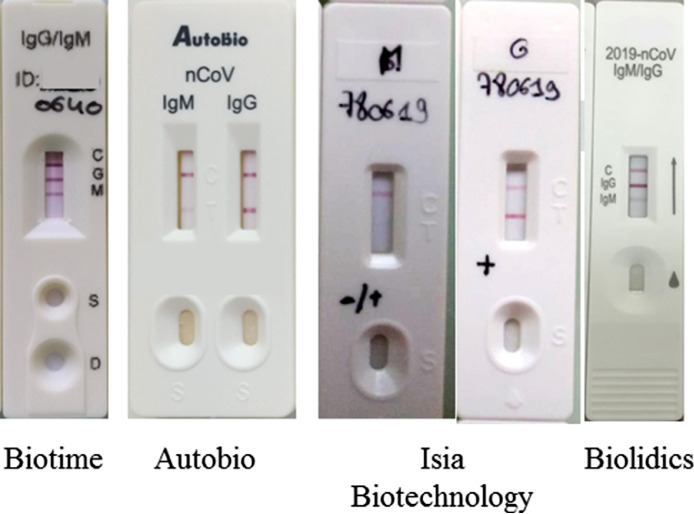
We evaluated 4 immunochromatographic tests for the detection of IgM and IgG directed against SARS-CoV-2 (Fig. 1 ). These tests were kindely provided by Asian manufacturers, namely Biotime Biotechnology Co, Autobio Diagnostics Co, ISIA BIO-Technology Co and Biolidics.
Fig. 1.
Design of these 4 immunochromatographic tests for the detection of antibodies against SARS-CoV-2.
For the Biotime, Autobio, and Biolidics tests the detection of IgM and IgG is performed on the same diagnostic cassette. For ISIA 2 different cassettes are available. Each test requires between 10 and 20 µL of serum, plasma or whole blood and is read 10 to 15 minutes after the sample and diluent have been deposited.
For the Biotime and Biolidics assays, respectively 15 and 17 of the 22 patients could be tested for lack of immunochromatographic tests.
Results
Delay between symptoms onset and first SARS-CoV-2 antibodies detection
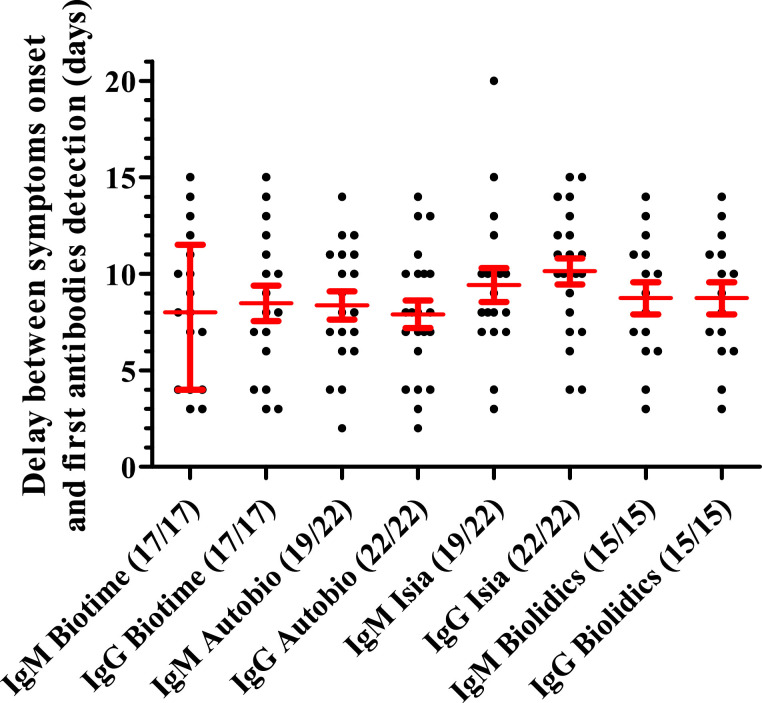
Longitudinal immunochromatographic testing in all patients shows heterogeneity in the time to detection of antibodies after symptom reporting (Fig. 2 ). The median antibody detection time was 8 days since onset of symptoms for Autobio and Biotime (IgM or IgG), 9 days for Biolidics (IgM or IgG) and 9 and 10 days for ISIA IgM and IgG respectively (Fig. 2 and supplementary data). IgG was detected in all patients on day 15 since onset of symptoms, while IgM was not detected in 3 patients with Autobio and ISIA. IgM was detected before IgG in 1, 1, 7 and 0 patients with the Biotime, Autobio, ISIA and Biolidics assays respectively. In the other cases, IgM was detected at the same time as IgG. Thus, the diagnostic interest of detecting IgM directed against CoV-2-SARS appears limited.
Fig. 2.
Delay between symptoms onset and first SARS-CoV-2 antibodies (IgM or IgG) detection by the four assays. Dots represent each positive assay performed. Red bars represent median with interquartile range for delay (days) between symptoms onset and antibodies detection. The number of positive patients over the total number of tested patients is indicated in brackets.
Clinical sensitivity of different assays for SARS-CoV-2 antibodies
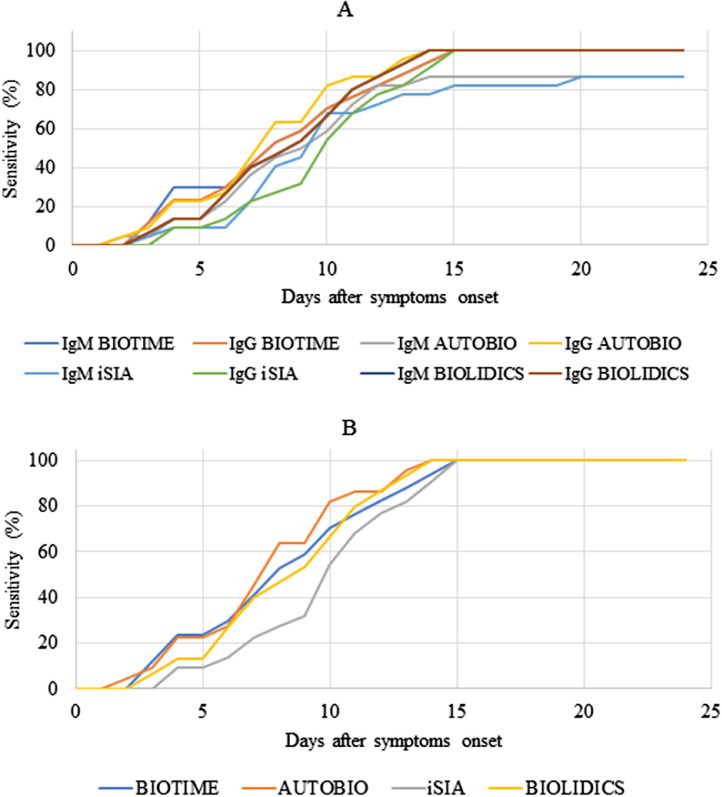
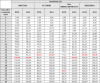
The clinical sensitivity of the different tests could be assessed longitudinally during follow-up and we observed an increasing sensitivity in the post-symptom period (Fig. 3 and Table 1 ). As described above, the clinical sensitivity of IgM does not appear to be superior to IgG for these immunochromatographic tests. With either IgM or IgG detection for a patient on days 5, 10 and 15 since onset of symptom, we calculated a clinical sensitivity between 9 and 24%, 67 and 82% and 100% respectively (Fig. 3B and Table 1). The Autobio test appears to have better sensitivity at day 10 (81.82%) versus 70.59%, 68.18% and 66.67% for Biotime, ISIA and Biolidics respectively (not significant).
Fig. 3.
Sensitivity of different assays for SARS-CoV-2 antibodies in relation to time between onset of symptoms and assay performing. (A) represents results for IgM and IgG testing separately. (B) represents results admitting positivity of the assay if IgM and/or IgG are detected.
Table 1.
Sensitivity of the four assays in relation to the time between onset of symptoms and assay performing.
 |
Specificity of different assays for SARS-CoV-2 antibodies
In order to evaluate the specificity of these different immunochromatographic tests, particularly regarding previous infections to other viruses of the human coronavirus family, we evaluated 12 sera from patients who had a RT-PCR diagnosis of respiratory infection by different coronaviruses in 2019 (Table 2 ). Of the 41 tests performed, only one (Autobio) was positive from the serum of a patient with a respiratory diagnosis 39 days previously of HCoV-229E. The same test for the other three samples with HCoV-229E was negative each time. Cross-reactions with these different coronaviruses therefore appear to be limited but may require further investigation.
Table 2.
Specificity of the four assays relative to other viruses of the Coronaviridae.
| Detected coronavirus | Serum (days after diagnosis by PCR) | BIOTIME |
AUTOBIO |
ISIA BIOTECHNOLOGY |
BIOLIDICS |
||||
|---|---|---|---|---|---|---|---|---|---|
| IGM | IGG | IGM | IGG | IGM | IGG | IGM | IGG | ||
| HCoV-OC43 | 86 | N | N | ||||||
| HCoV-OC43 | 170 | N | N | ||||||
| HCoV-OC43 | 174 | N | N | ||||||
| HCoV-OC43 | 176 | N | N | ||||||
| HCoV-OC43 | 301 | N | N | ||||||
| HCoV-229E | 39 | N | N | N | P | N | N | N | N |
| HCoV-229E | 14 | N | N | N | |||||
| HCoV-229E | 761 | N | N | ||||||
| HCoV-229E | 56 | N | N | ||||||
| HCoV-NL63 | 16 | N | N | N | N | N | N | N | N |
| HCoV-NL63 | 561 | N | N | ||||||
| HCoV-HKU1 | 24 | N | N | N | N | N | N | ||
N: negative P: positive.
Discussion
In this study we demonstrated the kinetics of detection of antibodies to SARS-CoV-2 using immunochromatographic LFA. These simple rapid unit tests are also easy to read (Fig. 1). Profiling early humoral response were already observed with ELISA assay 1 , 8.
We calculated increasing clinical sensitivities over time from the onset of symptoms in patients. Moreover, we did not observe any real added value in IgM staining from these immunochromatographic tests. With this kind of test, it is very difficult to distinguish the very recent infection from the older one because some patients present early with IgG without IgM and for some a detectable IgM threshold appears later. Serological ELISA tests from research laboratories or portfolios of in vitro diagnostic manufacturers may allow a clearer distinction between IgM and IgG kinetics and their respective interest. Nevertheless, the value of these point of care tests seems obvious in countries with limited resources but perhaps also as the epidemic progresses in individuals in the form of self-homemade assay from a drop of blood on the fingertip. This type of test could be easily delivered to individuals and thus limit contact. In addition, in countries with strong health systems, these rapid detection tests may also find their way as doctor tests in emergency departments. During this COVID-19 epidemic there is a rebound in symptoms on days 7 to 10 leading to a visit to a doctor or an emergency department. In Fig. 3 and Table 1, we have calculated a sensitivity of these tests between 22% and 81% during this post-symptom reporting period. Even if symptom reporting remains very subjective and time-varying but if we add an average incubation period of 5 days 5, we can say that at 14-15 days post-infection these tests seem reliable.
Finally, regarding specificity that we evaluated with respect to sera of other common Coronavirus infections, we observed a single cross-reaction. However, we were unable to test serum from people formerly infected with SARS-CoV 6. We would probably have many more cross reactions between these very close viruses as already report. Also, due to the lack of available tests, we could not test for specificity with samples containing antibodies to other viruses (HIV, HCV, HBV and others pathogens).
In conclusion, we described the kinetics of detection of post-symptom antibodies in 22 patients using immunochromatographic rapid tests and demonstrated the good performance of these tests for the detection of antibodies after SARS-CoV-2 infection. Our results suggest that these rapid and simple tests should be seriously considered in this time of health and political crisis to monitor both symptomatic and non-symptomatic patients.
Declaration of Competing Interest
All authors have no conflict of interest to declare
References
- 1.Guo L., Ren L., Yang S., Xiao M., Chang D., Yang F., Dela Cruz C.S., Wang Y., Wu C., Xiao Y., Zhang L., Han L., Dang S., Xu Yan, Yang Q., Xu S., Zhu H., Xu Yingchun, Jin Q., Sharma L., Wang L., Wang J. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19) Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haveri A., Smura T., Kuivanen S., Österlund P., Hepojoki J., Ikonen N., Pitkäpaasi M., Blomqvist S., Rönkkö E., Kantele A., Strandin T., Kallio-Kokko H., Mannonen L., Lappalainen M., Broas M., Jiang M., Siira L., Salminen M., Puumalainen T., Sane J., Melin M., Vapalahti O., Savolainen-Kopra C. Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Euro Surveill. Bull. Eur. Sur Mal. Transm. Eur. Commun. Dis. Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.11.2000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y., Yao L., Li J., Chen L., Song Y., Cai Z., Yang C. Stability Issues of RT-PCR Testing of SARS-CoV-2 for Hospitalized Patients Clinically Diagnosed with COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z., Yi Y., Luo X., Xiong N., Liu Y., Li S., Sun R., Wang Y., Hu B., Chen W., Zhang Y., Wang J., Huang B., Lin Y., Yang J., Cai W., Wang X., Cheng J., Chen Z., Sun K., Pan W., Zhan Z., Chen L., Ye F. Development and Clinical Application of A Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020 doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linton N.M., Kobayashi T., Yang Y., Hayashi K., Akhmetzhanov A.R., Jung S.-M., Yuan B., Kinoshita R., Nishiura H. Incubation Period and Other Epidemiological Characteristics of 2019 Novel Coronavirus Infections with Right Truncation: A Statistical Analysis of Publicly Available Case Data. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z., McGoogan J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]