Highlights
-
•
The severity of COVID-19 in pediatric cases was milder than adults.
-
•
Children younger than 2 years were more susceptible to COVID-19.
-
•
The average length of stay and the time of SARS-CoV-2 clearance were 10.57 and 6.39 days, respectively.
Keywords: Pediatric, Coronavirus disease 2019, Characteristics, Treatment, Outcomes
Abstract
Background
At present, coronavirus disease 2019 (COVID-19) has spread in many countries. We conducted this study to help pediatricians understand the conditions of COVID-19 in children.
Methods
We retrospectively summarized the characteristics, treatment and outcomes of pediatric cases in Wuhan Children's Hospital which was the only designated hospital for children with COVID-19 in Hubei Province. A Cox proportional hazards regression analysis was used to evaluate factors associated with clinical outcomes.
Results
As of February 29, 75 children had been discharged, of which only one was has severe pneumonia and one was critical cases. Children younger than 2 years were more susceptible to COVID-19. All patients have received interferon-α nebulization, and eight cases including the severe and critical cases were co-administrated ribavirin. Five patients with mild pneumonia were given arbidol. Twenty-three patients were given traditional Chinese medicine (TCM). The average length of stay (LOS) and the time of SARS-CoV-2 clearance were 10.57 and 6.39 days, respectively. None of the factors was associated with LOS or time of SARS-CoV-2 clearance.
Conclusions
The severity of COVID-19 in pediatric cases were milder than adults. The efficacy of the antiviral therapy in children with COVID-19 remains to be evaluated.
1. Introduction
In December 2019, a cluster of cases caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in Wuhan, Hubei Province. By April 27, 2020, more than 2.8 million cases have been reported worldwide. For such a threat to global health, the clinical characteristics, treatment strategies and its outcomes are the most concerned issues for clinicians.
People of all ages, including children, are susceptible to coronavirus disease 2019 (COVID-19). However, the sample size in the available reports on the clinical characteristics of children with COVID-19 was usually small [1]. In addition, the outcome of the treatment regimen remains to be verified in children, although a few experts’ consensuses about pediatric COVID-19 have been published [[2], [3], [4], [5]]. Wuhan Children’s Hospital is the only designated hospital for children diagnosed with COVID-19 in Hubei Province. In this study, we retrospectively analyzed the characteristics, treatment and outcome of pediatric cases in this hospital.
2. Methods
The observed cases were pediatric patients who were discharged from the Wuhan Children's Hospital from December 8, 2019 to February 29, 2020 and diagnosed with COVID-19. All cases were documented according to the oropharyngeal or anal swab samples tested positive for SARS-CoV-2 nucleic acid using RT-PCR. Confirmed patients can be discharged if their clinical symptoms improved obviously and respiratory pathogenic nucleic acid is negative for two consecutive times [[3], [4], [5]].
Data, included demographics, clinical, laboratory and radiological information, treatment and outcomes, were collected from patients’ medical records. (1) The severity of illness was defined according to the experts’ consensus [4], that is acute upper respiratory tract infection, mild pneumonia, severe pneumonia and critical cases (Supplementary Table 1). (2) The time of SARS-CoV-2 RNA clearance in respiratory samples was defined as the time from the first time of pathogenic nucleic acid positive to the test was negative without a positive test afterward. (3) Patients were diagnosed with Mycoplasma pneumoniae pneumonia (MPP) if they had clinical manifestations of pneumonia and / or chest radiographs suggestive of pneumonia, and the titer of IgM antibody against MP greater than 1:160.
Descriptive data were presented as number with percentages for categorical variables. Kolmogorov-Smirnov test was used to analyze whether the continuous variables were normally distributed. Medians with interquartile range (IQR) or means with standard deviations (SDs) were used for continuous variables. A Cox proportional hazards regression analysis was used to evaluate factors independently associated with length of hospital stay (LOS) or time of SARS-CoV-2 RNA clearance.
3. Results
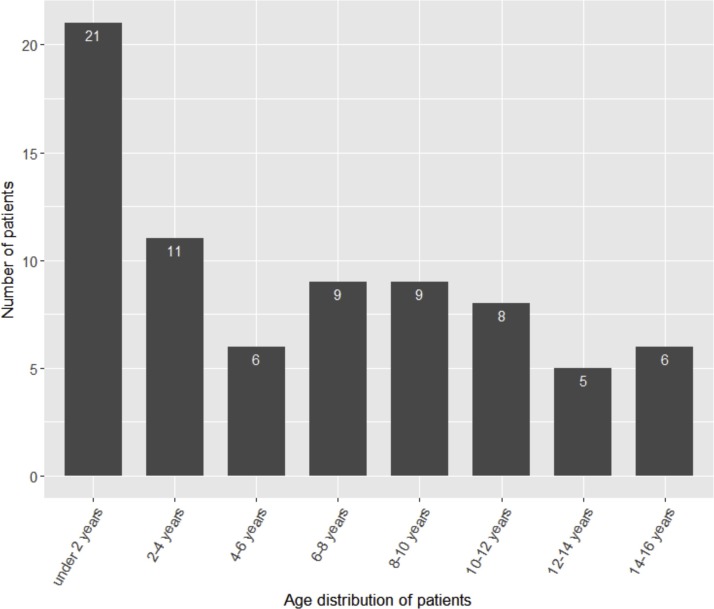
A total of 75 patients have been discharged, and none have died by February 29 (Table 1 ). The mean age was 6.06 years (range 1 month-15 years). As showed in the age distribution chart (Fig. 1 ), children younger than 2 years account for the highest proportion (28.0 %) in pediatric COVID-19. A majority of the children (82.7 %) had family members diagnosed with COVID-19. With the exception of fever (53.3 %) and dry cough (61.3 %), other clinical symptoms were less common. All cases have received chest CT examination. The normal, local patchy shadowing, bilateral patchy shadowing and ground-glass opacity chest CT were observed in 22 (29.3 %), 22 (29.3 %), 26 (34.7 %) and 5 patients (6.7 %), respectively. Most children (58.7 %) did not have complications. 32.0 %, 6.7 %, and 2.7 % of patients had one, two, and three complications, respectively. The most common complication was an increase of creatine kinase-MB [6]. There was only one case with severe pneumonia and one with critical illness. Most of the children (68.0 %) have mild pneumonia, and the remaining children have acute upper respiratory infection (20.0 %) or asymptomatic infection (9.3 %). There were 42 patients co-infected with other pathogens, including mycoplasma pneumoniae (28 cases), bacteria (3 cases, including moraxella catarrhalis, staphylococcus aureus, streptococcus pneumoniae), influenza B virus (3 cases), influenza A virus (1 case), adenoviridae (2 cases), cytomegalovirus (1 case) and respiratory syncytial virus (1 case). But the Mycoplasma pneumoniae was diagosed according to antibody test which can be misleading.
Table 1.
The characteristics of children with COVID-19.
| Patients (n = 75) | |
|---|---|
| Characteristics | |
| Age (years) | 6.06 ± 4.78 |
| Height (cm) | 114.27 ± 37.01 |
| Weight (kg) | 27.85 ± 21.99 |
| BMI | 18.39 ± 4.08 |
| Male sex | 44 (58.67 %) |
| With family member diagnosed with COVID-19 | 62 (82.67 %) |
| Clinical symptoms | |
| Fever | 40 (53.33 %) |
| Dry cough | 46 (61.33 %) |
| Runny nose | 5 (6.67 %) |
| Diarrhea | 4 (5.33 %) |
| Sputum production | 4 (5.33 %) |
| Sore throat | 3 (4.00 %) |
| Fatigue | 3 (4.00 %) |
| Chest Congestion | 3 (4.00 %) |
| Shortness of breath | 2 (2.67 %) |
| Myalgia | 2 (2.67 %) |
| Chest CT | |
| No abnormality | 22 (29.33 %) |
| Local patchy shadowing | 22 (29.33 %) |
| Bilateral patchy shadowing | 26 (34.67 %) |
| Ground-glass opacity | 5 (6.67 %) |
| Severity of illness | |
| Asymptomatic infection | 7 (9.33 %) |
| Acute upper respiratory tract infection | 15 (20.00 %) |
| Mild pneumonia | 51 (68.00 %) |
| Severe pneumonia | 1 (1.33 %) |
| Critical case | 1 (1.33 %) |
| Complications | |
| Increased creatine kinase-MB | 12 (16.00 %) |
| Acute liver injury | 3 (4.00 %) |
| Hypogammaglobulinemia | 2 (2.67 %) |
| Acute kidney injury | 1 (1.33 %) |
| Shock | 1 (1.33 %) |
| Acute respiratory distress syndrome | 1 (1.33 %) |
| Number of complications | |
| 0 | 44 (58.67 %) |
| 1 | 24 (32.00 %) |
| 2 | 5 (6.67 %) |
| ≥3 | 2 (2.67 %) |
| Comorbidities | |
| Sinus tachycardia | 1(1.33 %) |
| Epilepsy as a sequel of previous viral encephalitis | 1(1.33 %) |
| History of atrial septal defect surgery | 2 (2.67 %) |
| Growth retardation | 1(1.33 %) |
| Fatty liver disease | 1(1.33 %) |
| Anaphylactoid purpura | 1(1.33 %) |
| Other pathogens | |
| Mycoplasma pneumonia | 28 (37.33 %) |
| Bacteria | 3 (4.00 %) |
| Influenza B virus | 3 (4.00 %) |
| Influenza A virus | 1 (1.33 %) |
| Adenoviridae | 2 (2.67 %) |
| Cytomegalovirus | 1 (1.33 %) |
| Respiratory syncytial virus | 1 (1.33 %) |
Fig. 1.
Age distribution of the pediatric patients with COVID-19 (n = 75).
The treatment and clinical outcomes were showed in Table 2 . All patients received recombinant human interferon α1b nebulization (Shenzhen Kexing Biological Engineering Co., Ltd. and Beijing Sanyuan Gene Pharmaceutical Co., Ltd., China, 1−4 μg/kg/d bid). Eight cases (10.7 %) including the severe and critical patients were co-administrated with ribavirin (10 mg/kg/d bid, I.V.). Twenty-three patients (30.7 %) were given traditional Chinese medicine (TCM) for antiviral therapy, of whom 14 were mild pneumonia, and the rest were acute upper respiratory infections or asymptomatic infections. In addition, 5 cases (6.7 %) with mild pneumonia received arbidol orally (10 mg/kg/d tid for children <50 kg; 0.6 g/d tid for children ≥50 kg) which is indicated for infection caused by influenza virus in a few countries. The mean duration from onset of symptoms to initiation of antiviral therapy was 4.91 days.
Table 2.
The treatment and outcomes of children with COVID-19.
| Patients (n = 75) | |
|---|---|
| Treatment | |
| Interferon α1b | 75 (100 %) |
| Ribavirin | 8 (10.67 %) |
| Arbidol | 5 (6.67 %) |
| Traditional Chinese medicine | 23 (30.67 %) |
| Oseltamivir | 20 (26.67 %) |
| Antibacterial agents | 37 (49.33 %) |
| Azithromycin | 30 (40.00 %) |
| Days from onset of symptoms to initiation of antiviral therapy | 4.91 ± 3.18 |
| Outcomes | |
| Days of temperature return to normal | 5.87 ± 4.85 |
| Days of improvement of cough | 7.60 ± 4.04 |
| Days of hospital stay | 10.57 ± 3.22 |
| Days of SARS-CoV-2 clearance | 6.39 ± 2.29 |
| Days of pulmonary CT improvement | 9.45 ± 2.52 |
| Adverse drug reaction | |
| Rash | 3 |
| Diarrhea | 5 |
| Hematologic toxicity | 1 |
Twenty patients (26.7 %) were administrated oseltamivir, of which 4 were positive for influenza virus nucleic acid and the rest were empirical treatment. Half of the patients have been treated with antibacterial agents (first- or second-generation cephalosporins), but most cases discontinued it after COVID-19 was confirmed. It is worth noting that 37.3 % of patients were co-infected with Mycoplasma pneumoniae, and all of them were given azithromycin orally.
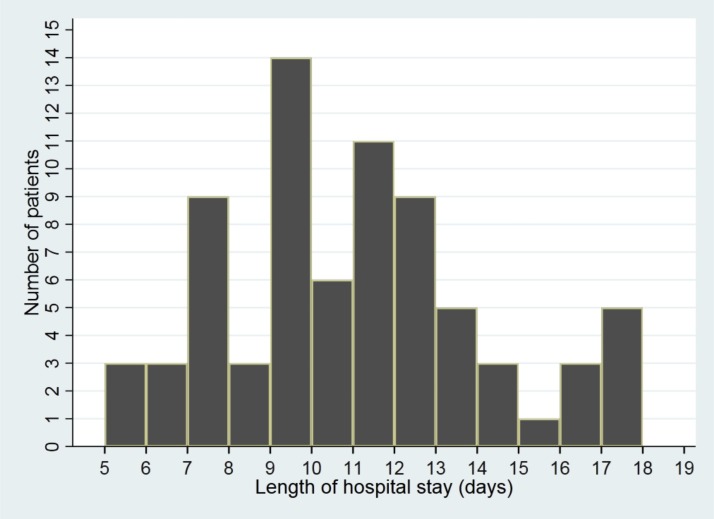
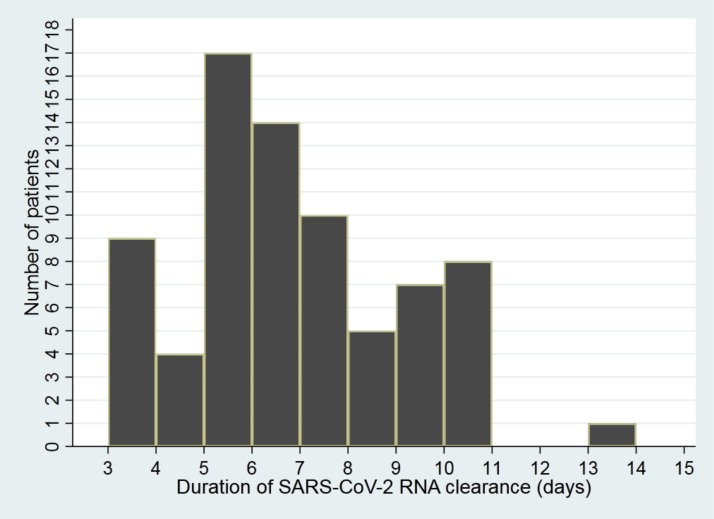
The average LOS was 10.6 days (Fig. 2 ). In a multivariate Cox model analysis, we failed to observe any factor associated with LOS (Table 3 ). The mean time of SARS-CoV-2 RNA clearance was 6.4 days (Fig. 3 ). In the multivariate Cox model analysis, none of the factors was related to the time of SARS-CoV-2 RNA clearance.
Fig. 2.
The length of hospital stay of the pediatric patients with COVID-19 (n = 75).
Table 3.
Multivariate analysis of length of hospital stay and SARS-CoV-2 RNA clearance.
| Variable | Length of hospital stay |
Time of SARS-CoV-2 RNA clearance |
||
|---|---|---|---|---|
| HR (95 %CI) | P | HR (95 %CI) | P | |
| Age | 1.02 (0.95−1.10) | 0.62 | 1.03 (0.95−1.10) | 0.48 |
| BMI | 1 (0.93−1.07) | 0.90 | 1 (0.92−1.07) | 0.91 |
| Sex | 0.7 (0.41−1.21) | 0.21 | 0.77 (0.46−1.28) | 0.32 |
| Severity of illness | 0.76 (0.49−1.16) | 0.20 | 0.96 (0.61−1.51) | 0.87 |
| NO. of complications | 0.94 (0.72−1.24) | 0.68 | 0.92 (0.76−1.12) | 0.43 |
| Mycoplasma pneumonia | 2.69 (0.97−7.45) | 0.06 | 0.44 (0.12−1.61) | 0.21 |
| Bacteria | 0.77 (0.22−2.69) | 0.69 | 0.76 (0.22−2.61) | 0.67 |
| Virus | 0.43 (0.16−1.15) | 0.09 | 1.29 (0.50−3.29) | 0.60 |
| Traditional Chinese medicine | 0.69 (0.35−1.38) | 0.29 | 0.77 (0.39−1.53) | 0.46 |
| Arbidol | 1.62 (0.37−7.01) | 0.52 | 0.51 (0.11−2.30) | 0.38 |
| Ribavirin | 0.54 (0.18−1.61) | 0.27 | 1.86 (0.53−6.53) | 0.33 |
| Days from symptoms to antiviral therapy | 0.99 (0.91−1.07) | 0.73 | 0.98 (0.90−1.07) | 0.62 |
| Oseltamivir | 1.1 (0.53−2.31) | 0.80 | 1 (0.53−1.89) | 0.99 |
| Antibacterial agents | 0.86 (0.48−1.55) | 0.62 | 1.05 (0.58−1.88) | 0.88 |
| Azithromycin | 0.55 (0.22−1.34) | 0.19 | 2.26 (0.62−8.21) | 0.21 |
Fig. 3.
The duration of SARS-CoV-2 RNA clearance in the pediatric patients with COVID-19 (n = 75).
4. Discussion
Under the current global epidemic situation, clinicians' knowledge of the COVID-19 in children is generally lacking. We summarize the characteristics, treatment and outcomes of pediatric patients discharged from our hospital, hoping to provide a reference for diagnosis and treatment of children in other countries.
Our data suggested that infected family members were the main cause of COVID-19 in children, so healthy adults were recommended as the caregivers of children. Young children are more dependent on their family members and infants are not suitable for wearing masks, which may explain why children younger than 2 years are the most vulnerable group to COVID-19. The age distribution in our center was consistent with previous studies [7,8]. Overall, the severity of COVID-19 in pediatric cases was milder than adults. Chinese Center for Disease Control and Prevention has analyzed the illness severity of 44,415 adult and pediatric patients, and found that severe and critical cases accounted for nearly 20 % [9]. A epidemiological study in Chinese children with COVID-19 (n = 2143) showed that severe and critical illness accounted for 5.8 % [10,11]. In this study only 2.7 % of children were severe or critical cases. However, the severity of the disease may be underestimated because our data were calculated based on discharged patients rather than all confirmed cases, and mild cases were more likely to be discharged. The major clinical symptoms of children were similar to those of adults, such as fever and cough. But the typical symptoms (e.g. fatigue and sputum) of children were less frequent than that of adults [6]. In addition, the features of chest CT for pediatric patients were also different from adults, which has been specifically discussed by our colleagues [11].
The antiviral drugs for pediatric COVID-19 recommended in the current consensuses were summarized in Supplementary Table 2. Interferon-α nebulization and TCM were recommended for all cases, especially children with mild pneumonia. Since most of the children were relatively mild, this study was not powered to test the efficacy of these medications on outcomes.
As early as 2003, the in vitro study has found that interferon-α could effectively inhibit SARS-CoV replication [12], but its efficacy in patients remains inconclusive [13]. In our center and another three hospitals in Zhejiang Province, China, all children were given interferon-α by aerosolisation [9]. Consequently, the efficacy of interferon remains to be evaluated. In fact, interferon was commonly used in combination with ribavirin rather than alone in the treatment of SARS or Middle East respiratory syndrome (MERS) [14]. However, the efficacy of this combination in patients was still controversial [14,15]. In our center, we have combined interferon with ribavirin for severe and critically ill patients. In the study of Qiu et al., none of the patients received ribavirin, whereas, 39 % of the patients used lopinavir-ritonavir (LPV/r) [9]. Regardless of the illness severity and other baseline characteristics, the days of hospital stay (14 ± 3 vs. 10.57 ± 3.22 days) and SARS-CoV-2 clearance (10 ± 2 vs. 6.39 ± 2.29 days) in their centers were not shorter than ours. Yet the efficacy needs to be confirmed by multi-center and large-sample research.
Several TCM treatment schedules have been proposed against COVID-19. TCM has played an important role in the treatment of adult COVID-19, especially the mild cases [13]. Xia et al. compared the clinical outcomes of combined Chinese and western medicine (34 cases) and that of western medicine (18 cases) in adult cases of COVID-19. They found that the patients who received combined Chinese and western medicine had better clinical outcomes, such as the improvement time of clinical symptom, length of hospital stay, and cure rate [10]. However, its efficiency and safety in children remain to be verified.
Arbidol is an antiviral agent indicated for upper respiratory tract infection caused by influenza virus in Russia and China. In the treatment of COVID-19, arbidol was often combined with LPV/r, and some researches showed that this combination was associated with better clinical outcomes than LPV/r alone [15,16]. Zhu et al. compared the efficacy of arbidol monotherapy with LPV/r, and found that patients in the arbidol group had a shorter duration of positive RNA test (P < 0.01) [17]. On February 19, arbidol was included in National recommendations for diagnosis and treatment of respiratory infections caused by 2019-nCoV (the 6th edition). Then we started using arbidol. So far, only 5 discharged patients have been treated with arbidol, and all of them were mild cases, so the efficacy of this drug remained to be evaluated with a larger sample.
Although limited medications were suitable for pediatric COVID-19, most children recovered within two weeks, which may be attributed to the less severity of children than adults. The outcomes of the antiviral therapy on COVID-19 need to be evaluated in a larger sample of pediatric patients.
Funding statement
None.
CRediT authorship contribution statement
Hui Peng: Investigation, Validation. Ping Gao: Conceptualization. Qiong Xu: Investigation. Maochang Liu: Supervision. Jing Peng: Validation. Yang Wang: Formal analysis. Hua Xu: Conceptualization, Validation.
Declaration of Competing Interest
All authors certify no potential conflicts of interest.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jcv.2020.104425.
Contributor Information
Yang Wang, Email: cattop3211@qq.com.
Hua Xu, Email: etcp_hust@163.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Cai J., Xu J., Lin D. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa198. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pediatric Branch of Hubei Medical Association, Pediatric Branch of Wuhan Medical Association, Pediatric Medical Quality Control Center of Hubei [Recommendation for the diagnosis and treatment of novel coronavirus infection in children in Hubei (Trial version 1)] Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(2):96–99. doi: 10.7499/j.issn.1008-8830.2020.02.003. (in Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese Medical Association Society of Pediatrics, Chinese Journal of Pediatrics Editorial Board [Recommendations for the diagnosis, prevention and control of the 2019 novel coronavirus infection in children (first interim edition)] Zhonghua Er Ke Za Zhi. 2020;58(0):E004. doi: 10.3760/cma.j.issn.0578-1310.2020.0004. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 4.Shen K., Yang Y., Wang T. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts’ consensus statement. World J. Pediatr. 2020;7:1–9. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z.-M., Fu J.-F., Shu Q. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020;5:1–7. doi: 10.1007/s12519-020-00345-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Rhee J.W., Cheng P. Cardiovascular complications in patients with COVID-19: consequences of viral toxicities and host immune response. Curr. Cardiol. Rep. 2020;22(5):32. doi: 10.1007/s11886-020-01292-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng F., Liao C., Fan Q.H. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr. Issues Pharm. Med. Sci. Pract. 2020;40(2):275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J. Med. Virol. 2020 doi: 10.1002/jmv.25807. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu H., Wu J., Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30198-5. S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y., Mo X., Hu Y. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;16 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 11.Cruz A.T., Zeichner S.L. COVID-19 in children: initial characterization of the pediatric disease. Pediatrics. 2020;16 doi: 10.1542/peds.2020-0834. [DOI] [PubMed] [Google Scholar]
- 12.Cinatl J., Morgenstern B., Bauer G. Treatment of SARS with human interferons. Lancet. 2003;362(9380):293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo E., Zhang D., Luo H. Treatment efficacy analysis of traditional Chinese medicine for novel coronavirus pneumonia (COVID-19): an empirical study from Wuhan, Hubei Province, China. Chin. Med. 2020;15:34. doi: 10.1186/s13020-020-00317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omrani A.S., Saad M.M., Baig K. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14(11):1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Yang B., Li Q. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;16 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Lisi, Li Chunna, Zeng Qi. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019:a retrospective cohort study. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.002. 11;S0163-4453(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z., Lu Z., Xu T. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.060. 10;S0163-4453(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.