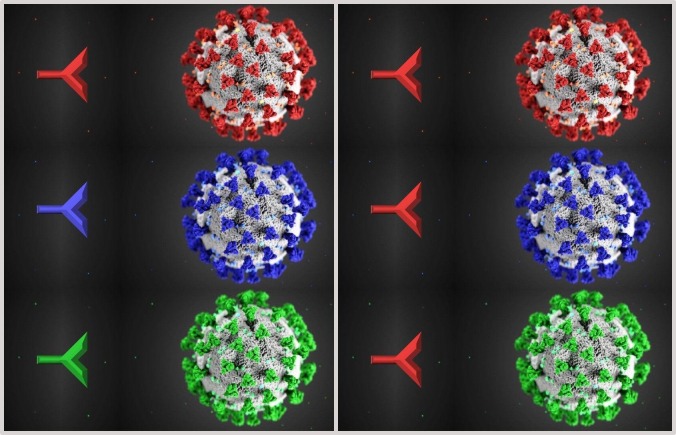
The term «original antigenic sin» (OAS) was coined by T. Francis Jr at the Michigan University in the late 1950s to describe patterns of antibody response to influenza vaccination [1]. The basic concept has been recently summarized by the slang «first flu is forever», which depicts how is important the first imprinting of a dominant viral or bacterial antigen throughout life [2]. In the 70s, T. W. Hoskins of the Christ's Hospital in the West Sussex, working with his collaborators on influenza vaccination, noticed that the development of A/England/42/72 hemagglutinin antibody was less frequent after revaccination of young male students, in whom the A/Hong Kong/68 hemagglutinin antibody had been already induced by previous vaccination [3]. This strange phenomenon went down in history as the «Hoskins paradox», a classical example of OAS. In practice, it refers to the propensity of the human immune system to exploit the immunological memory B and T cells, selected on the basis of a previous contact with a specific epitope, when a new, slightly different, version of the original antigen is encountered, in order to gain time in the attempt to fight the infection. However, in this way, the immune system gets entrapped inside the first response against the antigenic determinant, becoming unable to mount potentially more effective responses during subsequent infections from the mutated pathogen (Fig. 1 ). OAS has been reported not only in relation to influenza virus, but also to dengue virus and human immunodeficiency virus (HIV) [4], [5]. On March 11th, the World Health Organization has declared the ongoing ‘coronavirus-disease-2019′ (COVID-19) an emerging pandemic due to the widespread severe-acute-respiratory-syndrome-coronavirus-2 (SARS-CoV-2), the etiological agent of the disease, first identified in Wuhan [6]. The positive-sense single-stranded RNA of SARS-CoV-2 is almost identical to bat and pangolin coronaviruses; therefore, an animal origin from spillover event is alleged [7]. A recent study on 95 full-length genomic sequences of SARS-CoV-2 strains has highlighted that there may be selective mutations inside the virus [8]; a further study concerning with 86 complete or near-complete genomes of SARS-CoV-2 has provided evidences of genetic diversity and rapid evolution of the virus [9]. The metatranscriptome sequencing of the bronchoalveolar lavage fluid coming from 8 SARS-CoV-2 patients has confirmed that the virus evolves in vivo after infection, a feature which may determine its virulence, infectivity and transmissibility [10]. If we exclude conspiracy theories, SARS-CoV-2 can be hypothetically considered as the natural result of an antigenic shift from SARS-CoV, the etiological agent of the ‘severe acute respiratory syndrome’ (SARS), since they share about 80% of the whole genome and almost all the encoded proteins [11]. During SARS outbreak, it was observed that the onset of ‘acute respiratory distress syndrome’, the most dramatic complication of the disease, overlapped with antiviral immunoglobulin G seroconversion in 80% of patients [12]. Besides, it was found that patients who developed more quickly the anti-spike neutralizing antibody showed a higher risk of dying from the disease [13]. In addition to the formation and tissue deposition of pro-inflammatory immunocomplexes, these alarming data have been explained by means of complement-dependent enhancement and antibody-dependent enhancement (ADE), immunological escape mechanisms also exploited by other viruses, such as dengue virus, Ebola virus and HIV [14], [15], [16], [17], [18]. Briefly, an ineffective immune response against the mutated virus due to OAS can produce a large amount of sub-neutralizing cross-reactive antibodies, which raise inflammation and may paradoxically facilitate the virus entry into host cells, e.g. macrophages, complement mediated or via fragment crystallizable (Fc) receptors. The intracellular presence of the pathogen triggers a pyroptosis process with subsequent release of danger-associated molecular patterns (DAMPs) aimed to invoke in loco further inflammatory cells, which in turn secrete a massive number of cytokines; both ADE and pyroptosis could well explain the «cytokine storm», which has been described in the fatal cases of COVID-19 [19]. J.A. Tetro of the Guelph University has advanced the question if SARS-CoV-2 may receive ADE from other coronaviruses [20]; in this regard, 4 human coronaviruses (HCoV-HKU1, HCoV-NL63, HCoV-OC43, HCoV-229E) are spread all over the world, and they continually circulate among humans causing respiratory infections in adults and children, usually mild as common cold, while the Middle-East-respiratory-syndrome-related-coronavirus (MERS-CoV), responsible for the homonymous, often serious, respiratory illness, has been reported in over 25 countries to date [21]. Faced with this scenario and to the potential adaptive mutation of SARS-CoV-2, the development of an effective subunit vaccine appears quite complicated; therefore, the most viable solution is to focus on an alternative vaccination source able to stimulate the innate immunity rather than the acquired one. The former immunity is more active in children, where the immune system is still immature and prone to receive new antigenic stimuli, while the latter in adults: is maybe here the reason why the child population rarely experiences fatal complications during the ongoing COVID-19 pandemic?…the arduous sentence to near future research lines.
Fig. 1.
In an ideal immune system (on the left) to SARS-CoV-2 and its antigenic variants corresponds always a specific adaptive immunity, as shown by the color matching (red-red, blue-blue, green-green) between a symbolic antibody and the spike proteins, which surround the outer surface of the virion conferring to it the characteristic corona aspect on electron microscopy; in an OAS model (on the right), the specific adaptive immune response (red antibody) is only mounted against the original virus (red colored) and it is also used to fight the mutated versions (blue colored and green colored) of the virus, resulting in a maladaptive response less specific and less effective [the 3D illustration of SARS-CoV-2 with the spike proteins in red has been created by Alissa Eckert, MS, and Dan Higgins, MAM, at the Centers for Disease Control and Prevention (CDC) of Atlanta, Georgia, USA, placed in the public domain and thus free of any copyright restrictions].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Francis T. On the doctrine of original antigenic sin. Proc Am Philos Soc. 1960;104(6):572–578. [Google Scholar]
- 2.Viboud C., Epstein S.L. First flu is forever. Science. 2016;354(6313):706–707. doi: 10.1126/science.aak9816. [DOI] [PubMed] [Google Scholar]
- 3.Hoskins T.W., Davies J.R., Allchin A., Miller C.L. Controlled trial of inactivated influenza vaccine containing the a-Hong Kong strain during an outbreak of influenza due to the a-England-42-72 strain. Lancet. 1973;2(7821):116–120. doi: 10.1016/s0140-6736(73)93062-6. [DOI] [PubMed] [Google Scholar]
- 4.Mongkolsapaya J., Dejnirattisai W., Xu X.N., Tangthawornchaikul N. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9(7):921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 5.Klenerman P., Zinkernagel R.M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature. 1998;394(6692):482–485. doi: 10.1038/28860. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [accessed 11 March 2020].
- 7.Li X., Zai J., Zhao Q., Nie Q. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J Med Virol. 2020 doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang C., Liu Z., Chen Z., Huang X. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020 doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81 doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen Z, Xiao Y, Kang L, Ma W, et al. Genomic diversity of SARS-CoV-2 in coronavirus disease 2019 patients. Clin Infect Dis 2020; doi:10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed]
- 11.Xu J., Zhao S., Teng T., Abdalla A.E. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Zhang F., Yu W., He T. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J Med Virol. 2006;78(1):1–8. doi: 10.1002/jmv.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S.F., Tseng S.P., Yen C.H., Yang J.Y. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451(2):208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yip M.S., Leung N.H., Cheung C.Y., Li P.H. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J. 2014;11:82. doi: 10.1186/1743-422X-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katzelnick L.C., Gresh L., Halloran M.E., Mercado J.C. Antibody-dependent enhancement of severe dengue disease in humans. Science. 2017;358(6365):929–932. doi: 10.1126/science.aan6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takada A., Feldmann H., Ksiazek T.G., Kawaoka Y. Antibody-dependent enhancement of Ebola virus infection. J Virol. 2003;77(13):7539–7544. doi: 10.1128/JVI.77.13.7539-7544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willey S., Aasa-Chapman M.M., O'Farrell S., Pellegrino P. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology. 2011;8:16. doi: 10.1186/1742-4690-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta P., McAuley D.F., Brown M., Sanchez E. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zumla A., Chan J.F., Azhar E.I., Hui D.S. Coronaviruses - drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]