Highlights
-
•
COVID-19 patients failing to regain consciousness require neurological assessment.
-
•
Brain imaging and lumbar puncture are required to determine CNS involvement.
-
•
Neurological involvement during COVID-19 infection can be immune mediated.
-
•
Plasmapheresis can be beneficial in COVID-19 patients with autoimmune encephalitis.
Keywords: Autoimmune, Encephalitis, COVID-19, Plasmapheresis
Abstract
Severe SARS-CoV-2 (COVID-19) infection has the potential for a high mortality rate. In this paper, we report the results of plasmapheresis treatment in a series of severely ill patients with COVID-19–related autoimmune meningoencephalitis in the Intensive Care Unit (ICU).
1. Introduction
SARS-CoV-2 (COVID-19) can cause autoimmune disease (Huang et al., 2020) that may be associated with intensive care unit (ICU) admission and high mortality for some patients. Involvement of the nervous system by COVID-19 carries a poor prognosis (Mao et al., 2020). The pathophysiological mechanism of illness and the relationship between COVID-19 and central nervous system (CNS) involvement remain unclear. In this paper, we report the results of plasmapheresis in six patients with COVID-19, confirmed by both chest computed tomography (CT) and reverse real-time transcriptase polymerase chain reaction (PCR); all the patients presented with autoimmune meningoencephalitis during their stay in the ICU.
2. Cases
Between March 11 and April 30, 2020, 332 COVID-19 patients were admitted to our center, 53 of whom required ICU follow-up. Twenty-nine of the ICU patients developed severe acute respiratory distress syndrome (ARDS) and eventually required endotracheal intubation with invasive mechanical ventilation (MV) and deep sedoanalgesia. As per protocol, weaning from the ventilator was planned following recovery of pulmonary symptoms and secondary organ pathologies, with a gradual decrease in sedation. During this process, some patients either failed to regain consciousness or developed agitated delirium, which prompted a neurological examination and investigation with cranial magnetic resonance imaging (MRI) and lumbar puncture (LP). Cerebrospinal fluid (CSF) investigation included protein, glucose, and IgG levels, cell count, viral PCR testing (for both COVID-19 and common seasonal viruses), and oligoclonal band (OGB) testing. CNS involvement was diagnosed in 6 of 29 intubated patients, representing 20.6% of very severely affected patients and 11.3% (6/53) of all ICU patients.
All demographic, clinical and laboratory data, ventilation parameters, and details of medical treatment are shown in Table 1 . Data was retrieved from patients’ files, with consent taken from families during admission. Differences in therapies among patients reflected temporary changes in treatment protocols provided by the Health Ministry for the duration of the pandemic. The time from ICU admission to MRI and LP testing, cerebrospinal fluid results, and MRI results are shown in Table 1. MRI examples are shown in Fig. 1 .
Table 1.
Demographics, Ventilation parameters, Treatment modalities, Laboratory results, MRI and CSF findings and follow-up data of patients.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | ||
|---|---|---|---|---|---|---|---|
| Demographics | Age (yrs) | 49 | 59 | 59 | 51 | 55 | 22 |
| Gender | M | M | M | F | M | M | |
| Co morbidities | None | HT | HT, DM, Obesity | HT, DM | HT | Autism | |
| Ventilation | mode | PCV | PCV | PCV | PCV | PCV | PCV |
| parameters | PC | 16cmH2O | 20cmH2O | 27cmH2O | 21cmH2O | 28cmH2O | 26cmH2O |
| PEEP | 14cmH2O | 14cmH2O | 16cmH2O | 14cmH2O | 16cmH2O | 14cmH2O | |
| FiO2 | 70% | 50% | 70% | 50% | 60% | 70% | |
| Prone ventilation | 3 times | 2 times | 3 times | No | 3 times | 3 times | |
| Intubation days | 19 | 14 | 14 | 14 | 25 | 15 | |
| Treatment | Antibiotics | LOP/RIT, AZI, CEF, HC, FAV | AZI, HC, FAV | AZI, HC, FAV | AZI, HCFAV | AZI, HCFAV | AZI, HC, FAV |
| WBC 10^3/uL | 26.53 | 20.21 | 17.08 | 11.49 | 42.70 | 17.83 | |
| Platelets 10^3/uL | 202 | 540 | 140 | 660 | 299 | 664 | |
| CRP (<0.5) mg/L | 135 | 82.9 | 32.7 | 142.2 | 732.3 | 431.8 | |
| Serum | D-dimer (0–0.5) mg/L | 6.27 | 6.6 | 0.73 | 0,91 | 6.97 | 7.93 |
| LDH (85–227) IU/L | 560 | 304 | 414 | 271 | 709 | 1110 | |
| IL-6 pg/mL (<7 pg/mL) | 481 | – | – | – | 510 | 9192 | |
| Ferritin (10–291) ng/mL | 1763 | 2918 | 896 | 612 | 5235 | 555 | |
| MRI date (day) | 13 | 15 | 15 | 12 | 15 | 14 | |
| MRI | MRI Result | Encephalitis | Encephalitis | Normal | Normal | Normal | Encephalitis |
| Treatment | Plasmapheresis cycles | 6 | 9 | 1 | 5 | 5 | 3 |
| Outcome | Discharge from ICU | 21 | 19 | Exitus | 21 | Still in ICU | 17 |
| Protein (15–40) mg/dL | 37.6 | 73.2 | 65.7 | 131 | 52.00 | 57.00 | |
| CSF | Glucose (40–70) mg/dL | 130 | 201 | 121 | 120 | 67 | 59 |
| Cell count | 0 | 0 | 0 | 0 | 0 | 0 | |
| CSF IgG mg/L (0 – 3.4 mg/dL | – | 4.27 | 4.68 | 3.23 | 6.66 | 5.71 | |
| IgG Index (<0.60) | – | 0.330 | 0.450 | 0.780 | 0.380 | 0.520 | |
| AlbQ (<9.0) | – | 13.5 | 8.87 | 5.14 | 14.1 | 10.0 | |
| Oligoclonal band | – | None | None | None | None | None | |
HT: Hypertension; DM: Diabetes mellitus; PCV: Pressure controlled ventilation, PC: pressure controlled level. PEEP: positive end-expiratory pressure, FiO2: fraction of inspired oxygen. ICU: Intensive care unit. WBC: White blood cells, CRP: C-reactive protein, LDH: Lactate dehydrogenase, CSF: cerebrospinal fluid, LOP: Lopinavir, RIT. Ritonavir, AZI: Azitromisin, HC: Hydroxychloroquine, CEF: Ceftriaxone, FAV: Faviripavir;. IL-6: interleukin-6. MRI: magnetic resonance imaging, AlbQ: albumin quotient.
Fig. 1.
Case 1: A through C. A: Axial FLAIR showing bilateral segmental frontal cortical hyperintensity together with focal effacement of right frontal sulci. T1WI images showed mild pial-subarachnoid enhancement after I.V. Gad and DWI showed frontal cortical hyperintensity without matching hypointensity on ADC mapping (data not provided). All findings were considered compatible with meningoencephalitis. Following plasmapheresis, there was prominent regression of FLAIR findings at the first (B) and second (C) week’s follow-up imaging. Similarly, DWI hyperintensities disappeared and contrast enhancement regressed following treatment (data not provided). Case 2: D through F. D: Axial FLAIR showing bilateral extensive hyperintense signal changes of the frontal and parietal white matter accompanied by focal frontal cortical hyperintensity. As in Case 1, DWI images showed matching areas of diffusion restrictions (data not provided); the findings were considered compatible with meningoencephalitis. Following plasmapheresis, there was a prominent regression on FLAIR at the first (E) and second (F) week’s follow-up imaging. These also showed linear pial-subarachnoid hyperintensity within the right frontal sulci on the second week’s follow-up FLAIR compatible with subarachnoid hemorrhage (F).
Cases 1, 2, and 6 had pathological MRI findings showing cortical or white matter hyperintensities, contrast enhancement, and sulcal hemorrhagic features, all of which are considered compatible with meningoencephalitis (MRI positive). Cases 3–5 had scans within normal limits (MRI negative). CSF was examined in all six patients, and OGB was tested in all but one case. Lumber puncture revealed high CSF protein levels without pleocytosis in all cases. No evidence of an active CNS infection could be determined, as there was no pleocytosis in the CSF, and PCR was negative for viruses. The albumin quotient was elevated in three of five patients, and OGB was negative in all five. Therefore, plasmapheresis with albumin was initiated and performed on alternate days on the hypothesis of an autoimmune CNS involvement for both MRI positive and negative cases. Patients regained consciousness after the 3rd cycle (Case 1), 2nd cycle (Case 2), and 1st cycle (Cases 4 and 6) of plasmapheresis and could be extubated. After the 1st cycle of plasmapheresis, the clinical picture in Case 3 worsened, with refractory fever around 40.5 °C and ferritin levels that peaked at 15,669 ng/mL. Cardiac arrest developed and CPR was unsuccessful. Therapy in Case 5 continued in the ICU after 5 cycles of plasmapheresis for reactivation of cytomegalovirus infection.
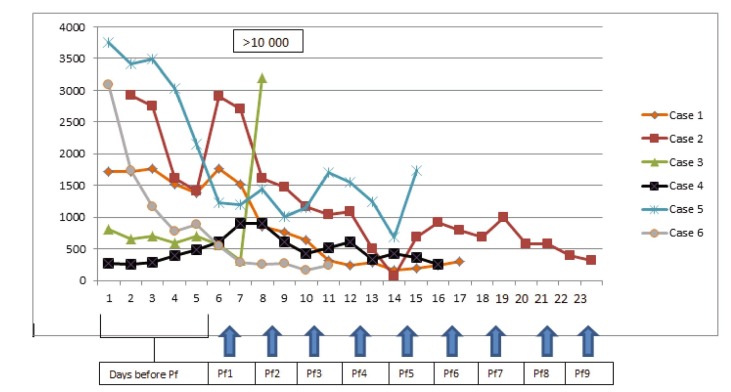
The most striking laboratory improvement following plasmapheresis was observed in serum ferritin levels in clinically improving patients (Fig. 2 ). Furthermore, MRI findings were reversible in all three patients starting from the first week’s MRI control (Fig. 1).
Fig. 2.
Ferritin levels before and after plasmapheresis. (Pf: plasmapheresis).
3. Discussion
To the best of our knowledge, this is the first relatively large series on the effect of plasmapharesis in COVID-19–related autoimmune encephalitis. The patients presented in this report were all critically ill with severe ARDS followed by a failure to recover consciousness or severe agitation during the weaning period. Another common feature was their persistently high inflammatory markers despite recovery from pulmonary or other organ pathologies. We observed increased levels of inflammatory acute-phase reactants such as ferritin, fibrinogen, CRP, IL-6 in sera, and bilateral cerebral inflammation compatible with meningoencephalitis on MRI. There was no evidence of an active CNS infection, including COVID-19. Apart from Case 3, patients started to recover soon after the initiation of a sequential plasmapheresis and Cases 1, 2–4, and 6 were soon discharged from the ICU to a normal ward.
COVID-19 is an ongoing viral pandemic; different aspects of neurological involvement are increasingly reported, ranging from headache, stroke, and central and peripheral nervous system involvement (Duong et al., 2020, Helms et al., 2020, Moriguchi et al., 2020, Poyiadji et al., 2020). However, the mechanism by which the CNS is affected is as yet unclear, and treatment approaches are not well defined. High amounts of cytokines induction by SARS-CoV (He et al., 2006), MERS-CoV (Faure et al., 2014), and the current COVID-19 (Huang et al., 2020) infections have been reported. In addition, previous studies have shown that increased amounts of proinflammatory cytokines in serum were associated with disease severity (Baig et al., 2020). In a recently published retrospective multicenter study of 150 confirmed COVID-19 cases from Wuhan, China, the authors suggested that COVID-19 mortality might be due to virus-activated cytokine storm syndrome (Ruan et al., 2020).
Autoimmune disorders of the nervous system, such as Bickerstaff’s brainstem encephalitis (BBE) and Guillain-Barre syndrome (GBS), have been described following previous coronavirus infections (Kim et al., 2017, Tsai et al., 2004). Likewise, some recent reports have hypothesized the association of SARS-CoV-2 infection with GBS (Toscano et al., 2020, Zhao et al., 2020 May), while others have reported systemic inflammatory response syndrome (SIRS) precipitated by severe viral infections as well as SARS-CoV-2 (Wu et al., 2020). In this setting, Mehta et al. (2020) suggested immunosuppression to prevent permanent organ damage, while Ritchie and Singanayagam (2020) pointed out the delicate balance between helpful antiviral immunity and the potential harm done by an interleukin storm. While a case report from China presented three severely ill COVID-19 patients who benefitted from plasma exchange (Ma et al., 2020), Keith et al. (2020) extrapolated their results from severe sepsis, and based on the hypothesis of an extreme host response with cytokine storm, advocated for a plasmapheresis trial in fulminant COVID cases. In this regard, our cases may highlight the treatment effect of plasmapheresis in COVID-19 patients with autoimmune encephalitis. Furthermore, a few key points in our series can support a cytokine storm occurring in the severely ill ICU-treated COVID-19 patients: 1) increased level of inflammatory markers, with ferritin, as a useful marker for disease severity; 2) high protein levels and albumin quotient in CSF without pleocytosis; 3) reversible MRI findings; and 4) dramatic improvement following plasmapheresis in both clinical and laboratory findings. At this point, we can only speculate that the reasons there was no pathology on some MRI scans despite pathological CSF have to do with the timing of MRI testing or individual responses to the disease.
In conclusion, the presented cases emphasize the importance of suspecting neurological involvement in severely affected COVID-19 patients and the potential benefit of plasmapheresis in such patients. Furthermore, the CSF results observed here point toward an autoimmune/antibody-mediated involvement hypothesis for both the meninges and the cerebral parenchyma during severe COVID-19 infection. Further studies are necessary to support or refute this hypothesis and thus augment the therapeutic approach in these patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ritchie Andrew I, Singanayagam Aran. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? The Lancet. 2020;395(10230):1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. Epub 2020 Mar 13. [DOI] [PubMed] [Google Scholar]
- Duong L., Xu P., Liu A. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 Infection in Downtown Los Angeles, Early April 2020. Brain, Behavior. Immun. 2020 doi: 10.1016/j.bbi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure E., Poissy J., Goffard A. Distinct immune response in two MERS-CoV-infected patients: can we go from bench to bedside? PLoS One. 2014;9 doi: 10.1371/journal.pone.0088716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C. Neurologic features in severe SARS-CoV-2 infection. N Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, et all. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497-506. [DOI] [PMC free article] [PubMed]
- Keith P., Day M., Perkins L., Moyer L., Hewitt K., Wells A. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID-19. Crit Care. 2020;24:128. doi: 10.1186/s13054-020-2836-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.E., Heo J.H., Kim H.O., Song S.H., Park S.S., Park T.H., Ahn J.Y., Kim M.K., Choi J.P. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13:227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Xia P., Zhou Y., Liu Z., Zhou X., Wang J., Li T. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;1(214) doi: 10.1016/j.clim.2020.108408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J. Neurological manifestations of hospitalized patients with COVID-19 inWuhan, China: a retrospective case series study. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID 19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033-1034. [DOI] [PMC free article] [PubMed]
- Moriguchi T., Harii N., Goto J., Harada D., Sugawara H., Takamino J., Ueno M. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020;3(94):55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyiadji N., Shahin G., Noujaim D., Stone M., Patel S., Griffith B. COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology. 2020;31 doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, Franciotta D, et al. Guillain-Barré Syndrome Associated With SARS-CoV-2. N Engl J Med . 2020 Apr 17;NEJMc2009191. doi: 10.1056/ NEJMc2009191. [DOI] [PMC free article] [PubMed]
- Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, Chang YC. 2004. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004; 61, 1669–1673. https://doi.org/10.1001 /archneur.61.11. 1669. [DOI] [PubMed]
- Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré Syndrome Associated With SARS-CoV-2 Infection: Causality or Coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. Epub 2020 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behavior. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]