Abstract
Tamoxifen is frequently used as adjuvant treatment in premenopausal patients with hormone receptor-positive early breast cancer. According to guidelines, the use of nonhormonal barrier contraception is recommended during tamoxifen treatment and up to 3 months after its interruption prior to attempting conception. Nevertheless, when conception occurs inadvertently during tamoxifen treatment, the effects on the fetus and on the course of pregnancy are still not completely known. Here, we report 3 cases of young women who accidentally became pregnant while taking tamoxifen and perform a systematic review of the literature to provide more elements for better and clear multidisciplinary counselling of women facing this challenging situation.
Keywords: Breast carcinoma, Breast cancer, Pregnancy, Tamoxifen, Teratogenic effects
Introduction
Breast cancer represents the most common tumor diagnosed in women and the most frequent malignancy in patients of reproductive age [1, 2, 3]. In premenopausal patients with hormone receptor-positive early breast cancer, adjuvant endocrine therapy is indicated and may include the administration of tamoxifen for 5–10 years [4, 5, 6, 7, 8, 9]. To avoid the risk of fetal malformation, pregnancy is contraindicated during tamoxifen treatment and up to 3 months after its interruption. Thus, barrier contraception is recommended, even if its uptake may be considered suboptimal [10, 11, 12, 13, 14, 15]. Tamoxifen may also be secreted in breast milk. Hence, women taking tamoxifen should not breastfeed [16, 17].
Here, we report 3 cases of young women who accidentally became pregnant while taking tamoxifen and perform a systematic literature review to provide more elements for better counselling of women facing this challenging situation.
Case 1
A 40-year-old lady underwent quadrantectomy and sentinel lymph node biopsy in May 2011 for a moderately differentiated ductal invasive carcinoma (pT2 pN0(sn), estrogen receptor [ER] 90%, progesterone receptor [PgR] 90%, Ki-67 15%, HER2 negative). Staging showed no evidence of distant metastases. In September 2011, the patient started treatment with tamoxifen 20 mg daily. In June 2012, after 9 months of tamoxifen treatment, a pelvic ultrasound showed a viable 12-week fetus. The patient decided to stop tamoxifen and to continue the pregnancy, which ended with a term vaginal delivery on December 20, 2012. The female newborn weighed 2,785 g and was 49.5 cm in length, appropriate for her gestational age. She subsequently attained all developmental milestones as assessed by her parents. The patient decided not to breastfeed and resumed tamoxifen treatment in February 2013. At present, she is doing well on tamoxifen, and has no evidence of disease 6 years after diagnosis.
Case 2
A 34-year-old lady presented with a left breast lump and underwent radical mastectomy and sentinel lymph node biopsy in May 2011. Histology revealed a moderately differentiated infiltrating ductal carcinoma (pT2 pN0(sn), ER 99%, PgR 90%, Ki-67 36%, HER2 negative). The patient received adjuvant chemotherapy with cyclophosphamide, epirubicin, and 5-fluorouracil for 3 cycles, followed by docetaxel for another 3 cycles. At completion of chemotherapy, tamoxifen 20 mg daily was initiated. A gonadotropin-releasing hormone agonist was given during chemotherapy and continued until June 2014 as part of adjuvant endocrine therapy. In November 2014, still under tamoxifen treatment, the patient became pregnant. According to ultrasound examination and the date of the last menstrual period, gestational age was 8 weeks. The patient decided to stop tamoxifen treatment and to continue her pregnancy. In July 2015, a healthy male infant was delivered at term by cesarean section with Apgar scores of 9/10 at 1 min and 10/10 at 5 min. The baby weighed 3,160 g and no malformation was described. Tamoxifen was resumed after delivery and was stopped in June 2017. At present, both the baby and the mother are well.
Case 3
In November 2007, a 38-year-old lady became pregnant while receiving adjuvant tamoxifen for a previously diagnosed low-grade ductal carcinoma in situ treated with conservative surgery. Tamoxifen was stopped at 7 weeks of gestation and the pregnancy was uneventful until 27 weeks, when a breast lump was discovered. She underwent left breast biopsy, revealing a new low-grade ductal carcinoma in situ. She delivered a healthy male infant by cesarean section at 34 weeks of gestation with normal weight and length according to gestational age (birthweight 2,800 g, length 46 cm). Thereafter, a left radical mastectomy was performed, demonstrating invasive ductal carcinoma (ER 55%, PgR 20%, Ki-67 14%, HER2 negative); adjuvant endocrine therapy with an aromatase inhibitor plus gonadotropin-releasing hormone agonist was prescribed. The boy is now 10 years old and he has attained the developmental milestones for age, except for a problem of dysorthography; the mother is well and free of disease.
Literature Review
To assess the obstetrical and fetal impact of prenatal exposure to tamoxifen, we performed a systematic review of the literature. We searched on PubMed for relevant papers published up to May 5, 2019, with restriction to publications in English, using the following keywords and Boolean operators: breast AND (carcinoma OR cancer OR neoplasm), AND (pregnancy OR pregnant OR gestation), AND (tamoxifen) AND (teratogenic effects). The work was done and reported according to the PRISMA guidelines for reporting of systematic reviews [18]. Guidelines of the American Society of Clinical Oncology (ASCO), European Society for Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN), and International Federation of Gynecology and Obstetrics (FIGO) were also consulted as sources of relevant references. Both series and cases of exposure during pregnancy were considered, dissecting possible confounders such as other concomitant treatment for which a teratogenic effect is described.
Results
The 3 patients reported on here had spontaneous pregnancies while taking tamoxifen during the 1st trimester, they all stopped the medication within the 12th week, and all had uneventful pregnancies, with normal newborns.
To contextualize our reports, we searched the literature for data on teratogenicity of tamoxifen in animals and conducted a systematic review on the effects of tamoxifen during pregnancy in humans, focusing on fetal wellbeing and the malformation rate.
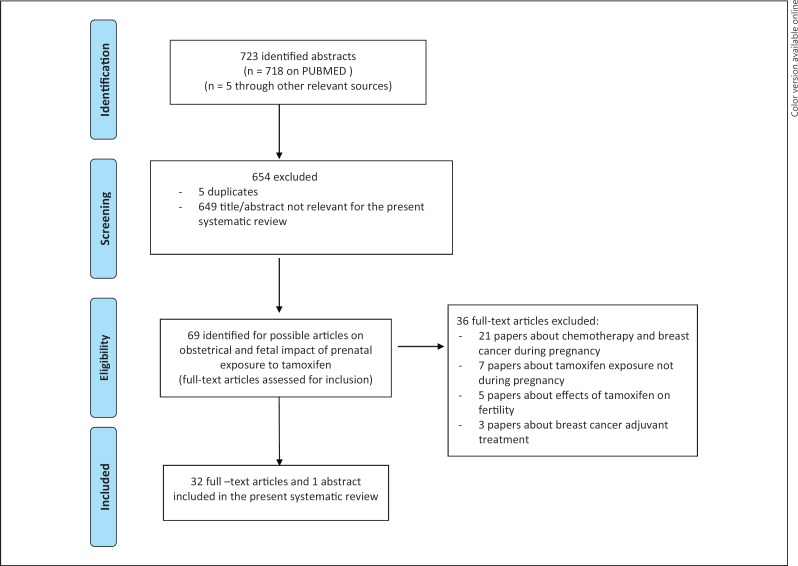
The systematic research revealed 723 papers; of these, 718 papers were found on PubMed and 5 from other relevant sources. A total of 32 full-text articles and 1 abstract were deemed eligible and were included in this review; 12 papers reported data on teratogenic effects of tamoxifen in animals [8, 11, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28], and 21 were case reports, case series, and reviews about tamoxifen exposure during human pregnancies [8, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48] (Fig. 1).
Fig. 1.
PRISMA flowchart summarizing the process for the identification of eligible articles.
The animal studies showed an increase in the incidence of abnormalities in the reproductive tracts of the offspring [11, 19, 20, 21] and irregularly ossified ribs in rat pups (“kinky ribs” and “wavy rib syndrome”) [11, 22]. Tamoxifen produced epithelial changes similar to those caused by diethylstilbestrol (DES) or clomiphene citrate [8], known teratogenic agents. Tamoxifen exposure resulted in metaplastic changes of the uterine epithelium of fetal rats and vaginal adenosis in newborn mice. Cunha et al. [23] examined the effect of tamoxifen in 54 genital tracts isolated from 4- to 19-week-old human female fetuses and grown for 1–2 months in untreated athymic nude mice or host mice. They showed that tamoxifen in human fetal genital tracts resulted in delayed proliferation and maturation of the vaginal epithelium. Prenatal treatment of guinea pigs with tamoxifen showed delayed vaginal opening in female offspring, which was similar to DES-exposed female guinea pigs [24]. The long-term effects of tamoxifen use and whether it may increase gynecological cancers in daughters (as DES) are still unknown; in pregnant rats, tamoxifen exposure during gestation has been associated with breast cancer in the female offspring [25, 26]. Yamasaki et al. [27] performed an in utero and lactational exposure assay using tamoxifen at doses of 0.12, 0.6, or 3 μg/kg/day in rats. A delay in preputial separation and hypospadia were detected at the doses of 0.6 and 3 μg/kg/day. No negative consequences for male and female fertility were found. Nobakht et al. [28] showed that tamoxifen treatment of pregnant rats could affect hippocampus formation in prenatal and postnatal rats.
The effects of tamoxifen during human pregnancy have been described in 17 case reports [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45], in a trial on breast cancer prevention [46], in the Lareb and INCIP databases [47], in the records provided by AstraZeneca [8], and in a prospective analysis of pregnancies having occurred during and after treatment with trastuzumab and/or lapatinib in patients with early breast cancer [48]. Our systematic review of the literature identified a total of 249 fetuses exposed to tamoxifen during the 1st trimester of pregnancy or thereafter, including our 3 cases (Table 1). Among the 249 cases cited, there were 68 live births. Among the live births, 1 case showed genital tract abnormalities (ambiguous genitalia with clitoromegaly and labial fusion) in a female fetus exposed to tamoxifen for 20 weeks [29]. In another case, Goldenhar syndrome was reported, including right-sided microtia (underdevelopment of the exterior ear) and hemifacial microsomia (underdevelopment of the lower half of one side of the face). This infant, who also had preauricular skin tags, had been exposed to tamoxifen during the 1st and 2nd trimesters. In addition, the baby had been exposed to diagnostic X-rays and marijuana or cocaine at least once during the first 6 weeks of gestation [30]. Finally, another case report described a Pierre Robin sequence after 6 weeks of tamoxifen exposure. Noncraniofacial anomalies (acetabular and sacral dysplasia) were also reported [31]. The AstraZeneca Safety Database divided the cases according to the duration of the exposure [8]. In the group of fetuses exposed during the 1st trimester only, 2 live births with congenital anomalies were included: 1 with a triple X karyotype and anomalies of the external genitalia (clitoris and labia) and 1 with idiopathic chylothorax. In the group of fetuses exposed after the 1st trimester, there was just 1 case of congenital malformation of one hand. This database also included 74 patients with unknown duration of tamoxifen exposure; of these, 6 women gave birth to 6 babies with congenital anomalies (Table 1).
Table 1.
Summary of the reported cases with exposure to tamoxifen during pregnancy
| Study [Ref.], year | Cases, n | Tamoxifen indication | Tamoxifen exposure | Complications of pregnancy | GA at delivery, weeks | Way of delivery | Fetal outcome | Weight at delivery, g | Long-term neonatal outcome | Confounders (other exposures during pregnancy) |
|---|---|---|---|---|---|---|---|---|---|---|
| Koizumi and Aono [34], 1986 | 2 | Hyperprolactinemia and infertility due to pituitary adenoma | 1st trimester | NR | Case 1: 41; Case 2: 37 | Vaginal | Healthy at birth; no malformations | Case 1: 3,240; Case 2: 2,600 | NR | Bromocriptine |
| Clark [46], 1993 | 85 | Prevention of breast cancer | Unknown | No fetal anomalies | No information about duration of tamoxifen exposure and pregnancy outcome | |||||
| Cullins et al. [30], 1994 | 1 | Adjuvant hormone therapy for node-negative breast cancer | 1st and 2nd trimesters | Preterm labor and chorioamnionitis | 26 | CS | Normal karyotype; Goldenhar syndrome | 896 | NR | X-ray (bone scan using 99mTc) and drugs (cocaine and marijuana) |
| DiPaola et al. [44], 1997 | 1 | Multiple chemotherapy regimens for metastatic melanoma | 2nd and 3rd trimesters | CS due to progression of the tumor in the mother | 30 | CS | Healthy at birth; no malformations | 1,520 | Normal milestones up to 15 months | Carmustine, dacarbazine, cisplatin |
| Tewari et al. [29], 1997 | 1 | Hormone therapy for metastatic breast cancer | 1st and 2nd trimesters | Induction of labor due to deteriorating condition of the patient | 29 | Vaginal | Normal karyotype; ambiguous genitalia | 1,360 | NR | NR |
| Isaacs et al. [32], 2001 | 1 | Primary endocrine therapy for meta-static breast cancer | During whole pregnancy | CS due to deteriorating condition of the patient | 31 | CS | Normal karyotype; healthy at birth | 1,940 | Normal up to 2 years of age | Radiation and radiotherapy |
| Öksüzoglu and Güler [35], 2002 | 1 | Adjuvant hormone therapy for node-negative breast cancer | 1st trimester | NR | NR | NR | Healthy at birth; no malformations | NR | Normal milestones at 27 months | None |
| Andreadis et al. [41], 2004 | 1 | Hormone therapy for metastatic breast cancer | During whole pregnancy | Preterm labor | 35 | CS | Healthy at birth; no malformations | 2,070 | Normal milestones at 12 months | Biphosphonate, chemotherapy (FEC) and radiotherapy exposure |
| Li et al. [33], 2007 | 1 | Multiple chemotherapy regimens for metastatic melanoma | 1st and 2nd trimesters | NO | 34 | CS | Healthy at birth; no malformations | 2,750 | NR | Multiple chemotherapy regimens (carmustine, dacarbazine, cisplatin) during 1st and 2nd trimesters |
| Berger and Clericuzio [31], 2008 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | 3rd trimester gestational diabetes; preeclampsia | 32 | Vaginal | Normal karyotype; microretrognathia, cleft of the secondary palate, glossoptosis: Pierre Robin sequence + left acetabular and sacral dysplasia | 1,983 | Unknown | 3rd trimester gestational diabetes; preeclampsia; use of ramelteon |
| Beale et al. [42], 2009 | 1 | Adjuvant hormone therapy for breast cancer | 1st and 2nd trimesters | Oligohydramnios; preterm premature rupture of membranes at 31+2 weeks | 31+6 | CS | Twin A: no malformations; renal failure at 12 weeks of age Twin B: no malformations | Twin A: 1,590 Twin B: 1,705 | Twin A: death at 13 weeks of age after respiratory arrest Twin B: unknown | Methadone; trastuzumab during 1st and 2nd trimesters; cigarette smoking; nifedipine tocolysis; preterm delivery |
| Warraich and Smith [43], 2009 | 1 | Adjuvant hormone therapy for breast cancer | Until 28 weeks | Anhydramnios | 37 | CS | No malformations; severe pulmonary hypoplasia and atelectasis | 2,690 | Death a few days after birth | Herceptin and goserelin assumption; exposure to radioactive scans |
| Grandvuillemin et al. [45], 2009 | 1 | NR | Until 16 weeks | NR | Medical abortion during 2nd trimester for maternal reason | NR | Female fetus with an enlarged clitoris | NR | NR | NR |
| Koca et al. [36], 2010 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | Polyhydramniosis | 39 | CS | Healthy at birth; no malformations | 3,150 | Healthy in her 66th month | Radiotherapy and chemotherapy (FEC) exposure |
| Koca et al. [37], 2013 | 3 | Case 1: adjuvant endocrine therapy for metastatic breast cancer Case 2: adjuvant hormone therapy for breast cancer + LHRH Case 3: adjuvant hormone therapy | 1st trimester | NR | NR | NR | Healthy at birth in cases 1 and 2 Case 3: voluntary medical abortion at 6 weeks | NR | Case 1: normal up to 2 years of age Case 2: NR | Case 1: chemotherapy (paclitaxel, carboplatin, doxorubicin) |
| Ishizuka and Satou [38], 2016 | 1 | Adjuvant hormone therapy for breast cancer | 1st and 2nd trimesters | NR | At term | CS | Healthy at birth; no malformations | 2,544 | Normal up to 5 years of age | Goserelin while pregnancy unknown |
| Jyoti et al. [39], 2016 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | NO | 39 | CS | Healthy at birth; no malformations | Normal but not specified | NR | NR |
| Mohamed and Mirghani [40], 2017 | 1 | Adjuvant hormone therapy for node-negative breast cancer | 1st and 2nd trimesters | NO | At term | NR | Healthy at birth; no malformations | NR | NR | NR |
| AstraZeneca safety database | 37 | NR | 1st trimester | NR | NR | NR | 2 live births with congenital anomalies: 1 girl delivered at 29 weeks with XXX chromosomes and also a phallus-like clitoris and huge labia, and 1 with idiopathic chylothorax; 2 elective terminations with fetal defects; 6 spontaneous abortions; 6 live births without congenital anomalies; 4 elective terminations (no fetal defects or unknown); 17 unknown | NR | NR | NR |
| AstraZeneca safety database | 15 | After 1st trimester | 1 live birth with congenital anomaly: congenital hand malformation; 1 elective termination with fetal defects; 9 live births without congenital anomaly; 1 elective termination (no fetal defects or unknown); 3 unknown | |||||||
| AstraZeneca safety database | 10 | During whole pregnancy | 1 live birth with congenital anomaly: Goldenhar syndrome (Cullins' report); 8 live births without congenital anomalies; 1 elective termination (no fetal defects or unknown) | |||||||
| AstraZeneca safety database | 74 | Unknown | 6 live births with congenital anomaly: 1 with cleft palate, 1 with ear malformation, 1 with trisomy 21, 1 with a small degree of labial fusion, 1 with craniofacial defects, and 1 with slight clitoral hypertrophy; 1 stillbirth with fetal defects; 3 elective terminations with fetal defects; 1 stillbirth without fetal defects; 5 spontaneous abortions; 1 ectopic pregnancy; 11 live births without congenital anomaly; 10 elective terminations (no fetal defects or unknown); 36 unknown | |||||||
| Lambertini et al. [48], 2019 | 1 | Hormone therapy for HER2-positive early breast cancer | 1st trimester | NR | 40 | CS | Healthy at birth; no malformations | 3,145 | NR | Lapatinib + trastuzumab exposure |
| Lareb database | 2 | NR | 1st trimester | NR | Case 1: at term Case 2: induced abortion | NR | Case 1: no congenital anomalies Case 2: induced abortion | NR | NR | NR |
| INCIP database | 2 | NR | 1st trimester | NR | Case 1: spontaneous abortion at 10 weeks Case 2: at term | NR | Case 1: spontaneous abortion Case 2: no congenital anomalies | NR | NR | NR |
| Current clinical case 1 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | NO | 40 | Vaginal | Healthy at birth; no malformations | NR | Normal up to 6 years of age | NR |
| Current clinical case 2 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | NO | At term | CS | Healthy at birth; no malformations | 3,160 | Regular | NR |
| Current clinical case 3 | 1 | Adjuvant hormone therapy for breast cancer | 1st trimester | NO | 34 | CS | Healthy at birth; no malformations | 2,800 | Regular until the last follow-up visit | NR |
GA, gestational age; NR, not reported; CS, cesarean section; LHRH, luteinizing hormone-releasing hormone.
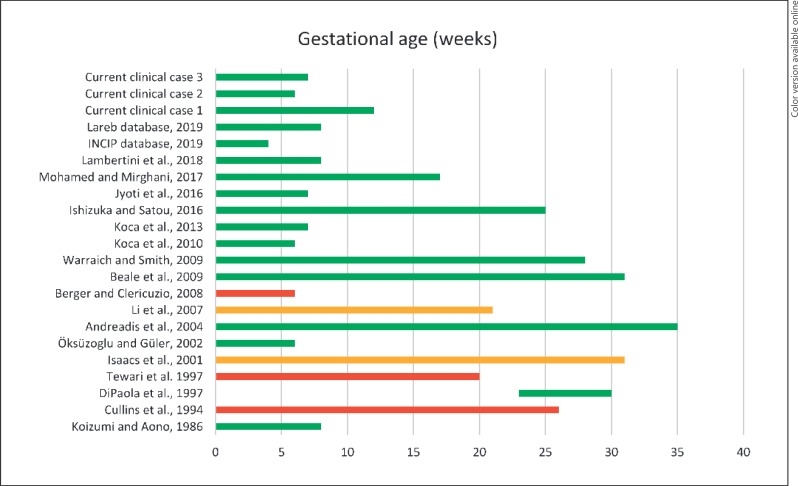
Hence, among the 68 live births reported, there were 12 babies with congenital malformations (18%) [8, 29, 30, 31] and 56 without major anomalies (81%) [8, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 47, 48]; of these, 10 fetuses had been exposed to tamoxifen only from the 2nd trimester [8, 44], while 33 had been exposed from the 1st trimester. In 19 pregnancies, tamoxifen was stopped before the end of the 1st trimester [8, 34, 35, 36, 37, 39, 47, 48], while in the other 16, administration continued till delivery [8, 32, 33, 38, 40, 42, 43]. Two of these 56 live births without major anomalies showed minor malformations; preauricular skin tags were reported in an otherwise normal infant following exposure to tamoxifen during the entire pregnancy [32]. Microphthalmos and severe hypermetropia were diagnosed in an infant at 1 year of age. This pregnancy had been exposed to tamoxifen both in the 1st and in the 2nd trimester, together with carmustine, dacarbazine, and cisplatin [33] (Fig. 2).
Fig. 2.
Duration of tamoxifen exposure during pregnancy. Pregnancies without malformations are depicted in green; pregnancies complicated by major malformations are depicted in red; pregnancies with minor malformations are depicted in orange. The letter by Clark [46] and the AstraZeneca Safety Database analysis [8] are not presented in this graph, as the exact duration of tamoxifen exposure was not specified.
In summary, if we consider all the 248 pregnancies (249 fetuses) with in utero exposure to tamoxifen, 68 live births were reported, while there were 25 elective terminations, 12 spontaneous abortions, 1 ectopic pregnancy, 2 stillbirths, and 55 pregnancies whose outcome was unknown. As the letter of Clark [46] reporting on 85 healthy women who became pregnant while receiving chemoprophylactic tamoxifen did not describe the obstetric outcome of pregnancies or the exposure duration, we decided not to include these data in our systematic review.
Discussion and Conclusions
According to current guidelines [49, 50], use of tamoxifen is contraindicated during pregnancy and should be postponed after delivery. Nevertheless, as shown in a recent survey investigating physicians' knowledge, practice, and attitudes regarding fertility and pregnancy issues in young breast cancer patients, 25 and 36% of the respondents disagreed or were neutral, respectively, on the statement that endocrine therapy should be avoided in pregnant patients [51]. In the present paper, we reported 3 more cases of patients accidentally exposed to tamoxifen during the 1st trimester of pregnancy and performed a systematic review of the literature to gather preclinical and clinical data about tamoxifen exposure during pregnancy and its effect on pregnancy and newborns.
Our 3 patients were exposed to tamoxifen during the 1st trimester only, without subsequent fetal malformations (Fig. 1). In our review, we documented 2 newborns with minor malformations [32, 33], whereas major malformations were observed in 12 infants of the 68 live births (17.6%) [8, 29, 30, 31]. As a point of reference, the prevalence of major malformations in the general population of the USA is 3% [52, 53].
The malformations in the reproductive tract were consistent with those reported in developmental toxicity studies in rats. Craniofacial malformations were observed in another 3 infants. Similar craniofacial malformations have been observed in cases of retinoic acid embryopathy, leading some researchers to hypothesize that tamoxifen may act on early organogenesis in a way similar to that of retinoic acid drugs [31, 53]. Thus, the apparent rate of malformations among all tamoxifen-exposed offspring, regardless of the severity of the malformations (both minor and major) or the gestational stage at exposure, was 20.5% (14/68). Both spontaneous abortions and stillbirths were documented in the literature, but no effective causal link with tamoxifen use can be established because of the paucity of cases. Notably, some patients received tamoxifen together with chemotherapy, illicit drugs, and X-rays, which may represent major confounding factors (Table 1).
Because of animal studies and case reports showing congenital abnormalities after tamoxifen exposure during pregnancy and because of the lack of long-term data on pediatric outcomes, no definitive conclusions on the teratogenic risk following tamoxifen use in pregnant women can be drawn. Although international guidelines contraindicate tamoxifen use during pregnancy, each case of accidental exposure must be approached individually. Our case series and systematic review of the literature supports the indication to stop tamoxifen during pregnancy and reinforces the need to provide adequate information on contraception to patients undergoing adjuvant endocrine therapy. The choice of contraceptive method among breast cancer survivors should consider the benefits of the method versus the risk of cancer recurrence. Thus, the ideal contraception method would be nonhormonal. In fact, the World Health Organization (WHO) contraindicates any hormonal contraception during breast cancer treatment. The first choice should be a copper intrauterine device [54]. A Cochrane study showed that in women with breast cancer treated with tamoxifen, the levonorgestrel-releasing intrauterine system led to a reduction in the incidence of endometrial polyps and hyperplasia [55]. Barrier methods (condoms and diaphragms) may also be chosen. Their limited efficacy is a major concern if pregnancy must be stringently controlled during breast cancer treatment. Female or male sterilization may also be discussed with couples who do not wish to have another pregnancy. Emergency contraception may be used if needed [56].
Patients receiving tamoxifen and interested in having a pregnancy should be informed about the need for a washout period of 3 months before conceiving. If an inadvertent pregnancy occurs, the potential risks for the fetus and newborn should be clearly discussed with patients, along with possible options, allowing women to make an informed decision. This also highlights the importance of framing an appropriate and patient-centered reproductive counselling in a dedicated setting.
Statement of Ethics
The authors have no ethical conflicts to disclose. The subjects gave informed consent to published their clinical history anonymously.
Disclosure Statement
A. Brunello served as a consultant for Roche and Eli Lilly, and she is part of the advisory board for Eisai and for Eli Lilly; L. Del Mastro received honoraria from Takeda and personal fees from Ipsen and Takeda outside the submitted work; M. Lambertini served as a consultant for Teva, and he received honoraria from Theramex outside the submitted work; F.A. Peccatori received honoraria from Roche, AstraZeneca, Clovis, Takeda, and Ipsen outside the submitted work; the other authors do not have any financial disclosure to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
A. Brunello was the medical oncologist that provided care to the patient in case 1 and contributed to writing of the manuscript; L. Del Mastro and L. Miglietta were the medical oncologists that provided care to the patient in case 2 and contributed to write the manuscript; B. Buonomo and M. Lambertini developed the research strategy, searched the literature according to the PRISMA methodology, and contributed to writing of the manuscript; B. Buonomo and S. Noli performed the data analysis, worked on the images and editorial adaptations, and contributed to writing of the manuscript; M. Lambertini and F.A. Peccatori coordinated the project, contributed to writing of the manuscript, and edited the last version of the paper; all authors contributed, revised parts of the manuscript, and agreed on the final version of the manuscript.
Acknowledgements
For the conduction of this study, M. Lambertini acknowledges the support from the ESMO for a Translational Research Fellowship at the Institut Jules Bordet in Brussels (Belgium).
References
- 1.Peccatori FA, Lambertini M, Scarfone G, Del Pup L, Codacci-Pisanelli G. Biology, staging, and treatment of breast cancer during pregnancy: reassessing the evidences. Cancer Biol Med. 2018 Feb;15((1)):6–13. doi: 10.20892/j.issn.2095-3941.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SM, Newman LA, Partridge AH. Breast cancer in young women: rare disease or public health problem? JAMA Oncol. 2015 Oct;1((7)):877–8. doi: 10.1001/jamaoncol.2015.2112. [DOI] [PubMed] [Google Scholar]
- 3.Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: a population-based study. Lancet Oncol. 2017 Dec;18((12)):1579–89. doi: 10.1016/S1470-2045(17)30677-0. [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KA, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2018 doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 5.Curigliano G, Burstein HJ, P Winer E, Gnant M, Dubsky P, Loibl S, et al. St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2017 De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017 Aug;28((8)):1700–12. doi: 10.1093/annonc/mdx308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3) Breast. 2017 Oct;35:203–17. doi: 10.1016/j.breast.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Lacchetti C, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor-Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression. J Clin Oncol. 2016 May;34((14)):1689–701. doi: 10.1200/JCO.2015.65.9573. [DOI] [PubMed] [Google Scholar]
- 8.Braems G, Denys H, De Wever O, Cocquyt V, Van den Broecke R. Use of tamoxifen before and during pregnancy. Oncologist. 2011;16((11)):1547–51. doi: 10.1634/theoncologist.2011-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006 May;11((5)):422–34. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 10.Castro-Sanchez A, Martinez-Cannon BA, Platas A, Mohar A, Fonseca A, Vega Y, et al. Suboptimal Use of Effective Contraceptive Methods in Young Mexican Women With Breast Cancer. J Glob Oncol. 2018 Oct;4((4)):1–7. doi: 10.1200/JGO.18.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barthelmes L, Gateley CA. Tamoxifen and pregnancy. Breast. 2004 Dec;13((6)):446–51. doi: 10.1016/j.breast.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Nolvadex . Physicians' Desk Reference Health. 57th ed. Thomsen Healthcare; 2003. [Google Scholar]
- 13.Mehta DK, editor, editor. Pregnancy. British National Formulary No. 45. London: British Medical Association and Royal Pharmaceutical Society; 2004. p. p. 726. [Appendix 4] [Google Scholar]
- 14.Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation. 6th ed. Philadelphia: Williams & Wilkins; 2002. pp. pp.1307–13. [Google Scholar]
- 15.Pagani O, Ruggeri M, Manunta S, Saunders C, Peccatori F, Cardoso F, et al. Pregnancy after breast cancer: are young patients willing to participate in clinical studies? Breast. 2015 Jun;24((3)):201–7. doi: 10.1016/j.breast.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Zubor P, Kubatka P, Kapustova I, Miloseva L, Dankova Z, Gondova A, Bielik T, Krivus S, Bujnak J, Laucekova Z, Kehrer C, Kudela E, Danko J. Current approaches in the clinical management of pregnancy-associated breast cancer-pros and cons. EPMA J. 2018 Jun;9((3)):257–270. doi: 10.1007/s13167-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royal College of Obstetricians and Gynaecologists . Pregnancy and Breast Cancer (Green- Top Guideline No. 12) [Internet] London: Royal College of Obstetricians and Gynaecologists; 2011. Available from: https://www. rcog.org.uk/en/guidelines-research-services/ guidelines/gtg12/ [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Jul;339(jul21 1):b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamness GC, Bannayan GA, Landry LA, Jr, Sheridan PJ, McGuire WL. Abnormal reproductive development in rats after neonatally administered antiestrogen (tamoxifen) Biol Reprod. 1979 Dec;21((5)):1087–90. doi: 10.1095/biolreprod21.5.1087. [DOI] [PubMed] [Google Scholar]
- 20.Iguchi T, Hirokawa M, Takasugi N. Occurrence of genital tract abnormalities and bladder hernia in female mice exposed neonatally to tamoxifen. Toxicology. 1986 Dec;42((1)):1–11. doi: 10.1016/0300-483x(86)90087-9. [DOI] [PubMed] [Google Scholar]
- 21.Diwan BA, Anderson LM, Ward JM. Proliferative lesions of oviduct and uterus in CD-1 mice exposed prenatally to tamoxifen. Carcinogenesis. 1997 Oct;18((10)):2009–14. doi: 10.1093/carcin/18.10.2009. [DOI] [PubMed] [Google Scholar]
- 22.Tucker MJ, Adam HK, Patterson JS. Tamoxifen. In: Laurence DR, McLean AE, Wetherall M, editors, editors. Safety testing of new drugs: laboratory predictions and clinical performance. London: Academic Press; 1984. pp. pp.125–61. [chapter 6] [Google Scholar]
- 23.Cunha GR, Taguchi O, Namikawa R, Nishizuka Y, Robboy SJ. Teratogenic effects of tamoxifen on the developing human female genital tract. Hum Pathol. 1987;18:1132–43. doi: 10.1016/s0046-8177(87)80381-7. [DOI] [PubMed] [Google Scholar]
- 24.Hines M, Alsum P, Roy M, Gorski RA, Goy RW. Estrogenic contributions to sexual differentiation in the female guinea pig: influences of diethylstilbestrol and tamoxifen on neural, behavioral, and ovarian development. Horm Behav. 1987 Sep;21((3)):402–17. doi: 10.1016/0018-506x(87)90024-9. [DOI] [PubMed] [Google Scholar]
- 25.Durrani S, Akbar S, Heena H. Breast Cancer During Pregnancy. Cureus. 2018 Jul;10((7)):e2941. doi: 10.7759/cureus.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halakivi-Clarke L, Cho E, Onojafe I, Liao DJ, Clarke R. Maternal exposure to tamoxifen during pregnancy increases carcinogen-induced mammary tumorigenesis among female rat offspring. Clin Cancer Res. 2000 Jan;6((1)):305–8. [PubMed] [Google Scholar]
- 27.Yamasaki K, Noda S, Muroi T, Mitoma H, Takakura S, Sakamoto S. Effects of in utero and lactational exposure to tamoxifen in SD rats. Toxicol Lett. 2005 Apr;156((2)):289–96. doi: 10.1016/j.toxlet.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Nobakht M, Najafzadeh N, Kordestani Shargh B. Effects of tamoxifen on morphological and ultrastructural aspects of developing hippocampus of rat. Iran Biomed J. 2009 Oct;13((4)):237–43. [PubMed] [Google Scholar]
- 29.Tewari K, Bonebrake RG, Asrat T, Shanberg AM. Ambiguous genitalia in infant exposed to tamoxifen in utero [letter] Lancet. 1997 Jul;350((9072)):183. doi: 10.1016/S0140-6736(97)24029-8. [DOI] [PubMed] [Google Scholar]
- 30.Cullins SL, Pridjian G, Sutherland CM. Goldenhar's syndrome associated with tamoxifen given to the mother during gestation. JAMA. 1994 Jun;271((24)):1905–6. [PubMed] [Google Scholar]
- 31.Berger JC, Clericuzio CL. Pierre Robin sequence associated with first trimester fetal tamoxifen exposure. Am J Med Genet A. 2008 Aug;146A((16)):2141–4. doi: 10.1002/ajmg.a.32432. [DOI] [PubMed] [Google Scholar]
- 32.Isaacs RJ, Hunter W, Clark K. Tamoxifen as systemic treatment of advanced breast cancer during pregnancy—case report and literature review. Gynecol Oncol. 2001 Mar;80((3)):405–8. doi: 10.1006/gyno.2000.6080. [DOI] [PubMed] [Google Scholar]
- 33.Li RH, Tam WH, Ng PC, Mok TS, Tam B, Lau TK. Microphthalmos associated with Dartmouth combination chemotherapy in pregnancy: a case report. J Reprod Med. 2007 Jun;52((6)):575–6. [PubMed] [Google Scholar]
- 34.Koizumi K, Aono T. Pregnancy after combined treatment with bromocriptine and tamoxifen in two patients with pituitary prolactinomas. Fertil Steril. 1986 Aug;46((2)):312–4. doi: 10.1016/s0015-0282(16)49531-2. [DOI] [PubMed] [Google Scholar]
- 35.Oksüzoglu B, Güler N. An infertile patient with breast cancer who delivered a healthy child under adjuvant tamoxifen therapy. Eur J Obstet Gynecol Reprod Biol. 2002 Aug;104((1)):79. doi: 10.1016/s0301-2115(01)00552-8. [DOI] [PubMed] [Google Scholar]
- 36.Koca T, Akgun Z, Yucel SB, Dag NZ, Teomete M. Pregnancy a short time after multimodal therapy for bilateral breast cancer: a case report and review of literature. J Oncol Pharm Pract. 2011 Dec;17((4)):440–3. doi: 10.1177/1078155210384755. [DOI] [PubMed] [Google Scholar]
- 37.Koca E, Kuzan TY, Babacan T, Turkbeyler IH, Furkan S, Altundag K. Safety of tamoxifen during pregnancy: 3 case reports and review of the literature. Breast Care (Basel) 2013 Dec;8((6)):453–4. doi: 10.1159/000357321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishizuka S, Satou S. A case of delivery of healthy infant in breast cancer patient incidentally treated with goserelin acetate and tamoxifen during pregnancy. Breast Cancer. 2016 Jan;23((1)):164–6. doi: 10.1007/s12282-013-0469-z. [DOI] [PubMed] [Google Scholar]
- 39.Jyoti B, Bharat C, Ankita N, Munita B, Sudeep G. Pregnancy on tamoxifen: case-report and review of literature. South Asian J Cancer. 2016 Oct-Dec;5((4)):209–10. doi: 10.4103/2278-330X.195347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohamed KEH, Mirghani S. Breast Cancer Case using Tamoxifen during Pregnancy: A Case Report and Literature Review. Sudan Journal of Medical Sciences. 2017;12((2)) [Google Scholar]
- 41.Andreadis C, Charalampidou M, Diamantopoulos N, Chouchos N, Mouratidou D. Combined chemotherapy and radiotherapy during conception and first two trimesters of gestation in a woman with metastatic breast cancer. Gynecol Oncol. 2004 Oct;95((1)):252–5. doi: 10.1016/j.ygyno.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Beale JM, Tuohy J, McDowell SJ. Herceptin (trastuzumab) therapy in a twin pregnancy with associated oligohydramnios. Am J Obstet Gynecol. 2009 Jul;201((1)):e13–4. doi: 10.1016/j.ajog.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 43.Warraich Q, Smith N. Herceptin therapy in pregnancy: continuation of pregnancy in the presence of anhydramnios. J Obstet Gynaecol. 2009 Feb;29((2)):147–8. doi: 10.1080/01443610802643774. [DOI] [PubMed] [Google Scholar]
- 44.Dipaola RS, Goodin S, Ratzell M, Florczyk M, Karp G, Ravikumar TS. Chemotherapy for metastatic melanoma during pregnancy. Gynecol Oncol. 1997 Sep;66((3)):526–30. doi: 10.1006/gyno.1997.4805. [DOI] [PubMed] [Google Scholar]
- 45.Grandvuillemin A, Rousseau T, Laurent N, Meyer F, Lebouvier M, Disson-Dautriche A. A case of sexual ambiguity under tamoxifen during pregnancy. Fundam Clin Pharmacol. 2009;23(Suppl 1):37. [Google Scholar]
- 46.Phillips DH, Venitt S. Safety of prophylactic tamoxifen. Lancet. 1993 Jun;341((8858)):1485–6. doi: 10.1016/0140-6736(93)90934-9. [DOI] [PubMed] [Google Scholar]
- 47.Schuurman TN, Witteveen PO, van der Wall E, Passier JL, Huitema AD, Amant F, et al. Tamoxifen and pregnancy: an absolute contraindication? Breast Cancer Res Treat. 2019 May;175((1)):17–25. doi: 10.1007/s10549-019-05154-7. [DOI] [PubMed] [Google Scholar]
- 48.Lambertini M, Martel S, Campbell C, Guillame S, Hilbers FS, Schuenly U, et al. Pregnancies during and after trastuzumab and/or lapatinib in patients with human epidermal growth factor receptor 2-positive early breast cancer: analysis from the NeoALTTO (BIG 1-06) and ALTTO (BIG 2-06) trials. Cancer. 2019 Jan 15;125((2)):307–16. doi: 10.1002/cncr.31784. [DOI] [PubMed] [Google Scholar]
- 49.Peccatori FA, Azim HA, Jr, Orecchia R, Hoekstra HJ, Pavlidis N, Kesic V, et al. ESMO Guidelines Working Group Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013 Oct;24(Suppl 6):vi160–70. doi: 10.1093/annonc/mdt199. [DOI] [PubMed] [Google Scholar]
- 50.Loibl S, Schmidt A, Gentilini O, Kaufman B, Kuhl C, Denkert C, et al. Breast cancer diagnosed during pregnancy: adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol. 2015 Nov;1((8)):1145–53. doi: 10.1001/jamaoncol.2015.2413. [DOI] [PubMed] [Google Scholar]
- 51.Lambertini M, Di Maio M, Pagani O, Curigliano G, Poggio F, Del Mastro L, et al. The BCY3/BCC 2017 survey on physicians' knowledge, attitudes and practice towards fertility and pregnancy-related issues in young breast cancer patients. Breast. 2018 Dec;42:41–9. doi: 10.1016/j.breast.2018.08.099. [DOI] [PubMed] [Google Scholar]
- 52.Correa A, Cragan JD, Kucik JE, Alverson CJ, Gilboa SM, Balakrishnan R, et al. Reporting birth defects surveillance data 1968-2003. Birth Defects Res A Clin Mol Teratol. 2007 Feb;79((2)):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 53.National Toxicology Program NTP Monograph: Developmental Effects and Pregnancy Outcomes Associated With Cancer Chemotherapy Use During Pregnancy. NTP Monogr. 2013 May;((2)):i-214. [PubMed] [Google Scholar]
- 54.Gompel A, Ramirez I, Bitzer J, European Society of Contraception Expert Group on Hormonal Contraception Contraception in cancer survivors - an expert review Part I. Breast and gynaecological cancers. Eur J Contracept Reprod Health Care. 2019 Jun;24((3)):167–74. doi: 10.1080/13625187.2019.1602721. [DOI] [PubMed] [Google Scholar]
- 55.Dominick S, Hickey M, Chin J, Su HI. Levonorgestrel intrauterine system for endometrial protection in women with breast cancer on adjuvant tamoxifen. Cochrane Database Syst Rev. 2015 Dec;((12)):CD007245. doi: 10.1002/14651858.CD007245.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Medica AC, Stark SS, Hadnott TN, Dietz AC, Romero SA, Natarajan L, et al. Use of emergency contraception among female young adult cancer survivors. Fertil Steril. 2018 Jun;109((6)):1114–1120.e1. doi: 10.1016/j.fertnstert.2018.02.136. [DOI] [PMC free article] [PubMed] [Google Scholar]