Abstract
Background and Aims
Same day bidirectional endoscopies (esophagogastroduodenoscopies [EGD]s and colonoscopies) are routinely performed. However, the best sequence of procedures is unknown, as is whether the use of carbon dioxide (CO2) affects the preferred sequence of procedures. This study aims to determine the preferred sequence of procedures and choice of insufflation gas (air or CO2) in patients undergoing same day bidirectional endoscopies.
Methods
Two hundred adults with a clinical indication for same day bidirectional endoscopies were randomized equally into four groups: A1 (EGD first, CO2 as insufflator); A2 (EGD first, air as insufflator); B1 (colonoscopy first, CO2 as insufflator); and B2 (colonoscopy first, air as insufflator). All procedures were performed with conscious sedation (Midazolam/Fentanyl). The primary outcome was patients’ overall comfort/satisfaction with the procedures and sedation received, as assessed by questionnaires and validated scoring scales (Nurse-Assessed Patient Comfort Score [NAPCOMS], La Crosse [WI]) collected during the procedures, before discharge, and on day 7 postprocedure.
Results
Two hundred patients were randomized, with data available for 186. Mean Midazolam dose between groups was significantly less in the EGD first groups (P=0.01). During the procedures, no differences were found in patients’ comfort as per the nurse reported NAPCOMS scores (P=0.19) or the Lacrosse (WI) endoscopy scores (P=0.05). On postprocedure days 0 and 7, no differences were found in the patients’ reported Lacrosse (WI) scores, nausea, sore throat, dizziness, satisfaction with sedation or overall level of procedural satisfaction (P>0.05 for each). However, bloating and discomfort were significantly lower in the CO2 arms (P<0.001).
Conclusions
This randomized controlled trial using validated patient comfort scoring assessments for same day bidirectional endoscopies demonstrated that the sequence of procedures affects the sedation used but does not affect overall patient comfort or satisfaction. Lesser sedation is needed in the EGD first group, and less postprocedural abdominal pain/discomfort and bloating is seen with CO2 insufflation.
Keywords: Colonoscopy, Gastroscopy, Quality, Sedation
Same day bidirectional endoscopies (esophagogastroduodenoscopy [EGD] and colonoscopy) are routinely performed in endoscopy units. However, little is known as to whether the order of sequence of the two procedures (i.e., EGD before colonoscopy or vice versa) is of any consequence. Some of those who favour performing EGD before colonoscopy (EGD first approach) argue that the sedation necessary for EGD is then carried over to the colonoscopy and thus allows for a better-tolerated colonoscopy and less overall sedation. Additionally, abdominal bloating caused by insufflation of air during colonoscopy could lead to reduced tolerance of the subsequent EGD (1). Others, however, argue that the gaseous distention of the small intestine and colon with room air caused by performing the EGD first leads to a more difficult and uncomfortable colonoscopy thereafter, likely due to a mechanical effect of air migrating to the proximal colon. Whether carbon dioxide insufflation would obviate this concern has not been assessed.
Studies to date comparing procedural sequences in same day endoscopies have revealed conflicting results. Some show that the approach of using the EGD first allows for better procedural quality (2), decreased overall patient discomfort (3), less sedation (4,5), faster recovery times (5,6), less cardiovascular stress (6) and a much higher chance of determining the diagnosis in the undifferentiated patient (e.g., occult gastrointestinal bleeding) (7). Other studies either show no difference in overall patient discomfort and satisfaction between both procedures (3) or even show preference for colonoscopy before EGD (8). Many of these studies use deep sedation with Propofol (3,4,6,9) while some use no sedation at all (2). Additionally, most of these studies lack validated patient comfort scoring assessments, making generalization of these results difficult.
The use of carbon dioxide (CO2) for insufflation during upper and lower endoscopies has recently become popular over traditionally used room air, especially after studies revealed less postprocedure patient discomfort with the use of CO2 (10). While CO2 would be the preferred agent of choice for insufflation, many North American and European centres continue to use room air possibly due to the expense associated with switching to CO2 and perhaps unawareness of the associated potential benefits (11). Whether the use of CO2 or room air affects the preferred sequence of procedures is also still unknown to date.
Institutional variation across Canada regarding the sequence of procedures for same day bidirectional endoscopies is currently based on a combination of personal preferences and the few studies available. Given the absence of any formal guidelines in this area, we undertook the current study to determine the preferred sequence of procedures and choice of insufflation gas (air or CO2) that leads to increased patient comfort, satisfaction and decreased sedation needs in patients undergoing same day bidirectional endoscopies. We hypothesize that performing an EGD before colonoscopy with CO2 used as an insufflator is the best-tolerated sequence associated with increased patient satisfaction/comfort and decreased sedation use.
METHODS
This is a randomized, double-blinded (to insufflation gas) controlled trial. Outpatients ≥ 18 years of age with any clinical indication for receiving same day bidirectional endoscopies were considered for inclusion. Exclusion criteria included those with prior bowel or gastrointestinal surgery(s) (exceptions: appendectomy, cholecystectomy or hernia repair); known obstructive or cancerous lesions; active inflammatory bowel disease; hereditary polyposis syndromes; allergies to fentanyl and/or midazolam; difficulties with communication or conditions affecting ability to provide informed consent; neurologic conditions that affected breathing (e.g., GBS, ALS or myasthenia gravis); and those who did not wish to participate in this study. All patients were booked through a centralized booking office and were seen in clinic before endoscopy.
This study was approved by the Queen’s University Health Research Ethics Board and was registered in a national clinical trials database (NCT02635217).
Participants were prospectively randomized into four groups: group A1 (EGD before colonoscopy using CO2 as insufflator), group A2 (EGD before colonoscopy using air as insufflator), group B1 (Colonoscopy before EGD using CO2 as insufflator), and group B2 (colonoscopy before EGD using air as insufflator), with 50 patients randomized to each group. Randomization occurred just before entering the endoscopy room and was accomplished by a clinical research assistant using an online randomizing software (Randomizer, Medsharing, Paris, France).
All bidirectional procedures were conducted in the Endoscopy Suite at Hotel Dieu Hospital, Kingston, Ontario, by one of 10 adult staff gastroenterologists. Both the patients and the gastroenterologists performing the procedures were unblinded to the sequence of the procedures—for obvious reasons—but blinded to the type of insufflation, both of which were assigned via randomization. This was accomplished by covering the main endoscopy processor with a black surface impervious to light and concealing the CO2 insufflator in a covered, painted wooden box. After randomization, which occurred just before commencing the examination, the nurse and physician would leave the room, and a research assistant would activate the appropriate insufflation. All EGDs and colonoscopies were performed using the same model of endoscope and colonoscope respectively (Pentax) using conscious sedation in interval doses (Midazolam and Fentanyl), with dosing determined by the endoscopist. Six sprays of metered nonaerosolized Lidocaine hydrochloride (Lidodan Endotracheal, 12 mg/spray, Odan Laboratories Ltd., Montreal, Quebec) were used for local sedation before the EGD for each participant.
The primary outcome of this study was patients’ overall comfort/satisfaction with the procedures and sedation. The secondary outcome was total sedation use during the procedures.
Various predetermined patient characteristics, key procedural and postprocedural parameters were obtained for each patient group by individuals blinded to the nature of the groups and recorded both during and after the completion of the procedures. The patient’s colonoscopy level of comfort was scored by the endoscopy nurse using the previously NAPCOMS scoring system (12) (see the Appendix). This encompasses the domains of pain, sedation and overall colonoscopy procedure tolerability. Similarly, during the EGD phase, the endoscopy nurse gauged patients’ comfort using the previously validated Lacrosse WI intra-endoscopy sedation comfort scale (13) (see the Appendix). The endoscopy nurses administering the scoring scales were trained on the correct use of the scoring systems via an educational session before the start of this study. Among other procedural data points acquired, the adequacy of bowel preparations for colonoscopies was assessed by the endoscopist performing the procedures using the previously validated Aronchick Bowel Prep scoring system (14).
Upon completion of the procedures, participants who were alert and deemed ready to be discharged from the endoscopy recovery unit according to the unit’s discharge protocol (Modified Aldrete Score of nine or greater) (15) were asked to complete the following questionnaires: (a) the validated patients’ self-reported version of the Lacrosse WI scale assessing the patients’ overall level of comfort during each procedure (13) (see the Appendix) and (b) a Likert scale asking participants to rate their overall level of comfort and satisfaction (with procedures and sedation) and their levels of bloating, nausea, sore throat and dizziness at the end of both procedures (see the Appendix). Participants were also then asked to repeat both these surveys again 7 days postprocedure to ensure consistency of responses outside the periprocedural time frame. These were recall surveys completed via a telephone interview with the blinded research assistant.
Statistics
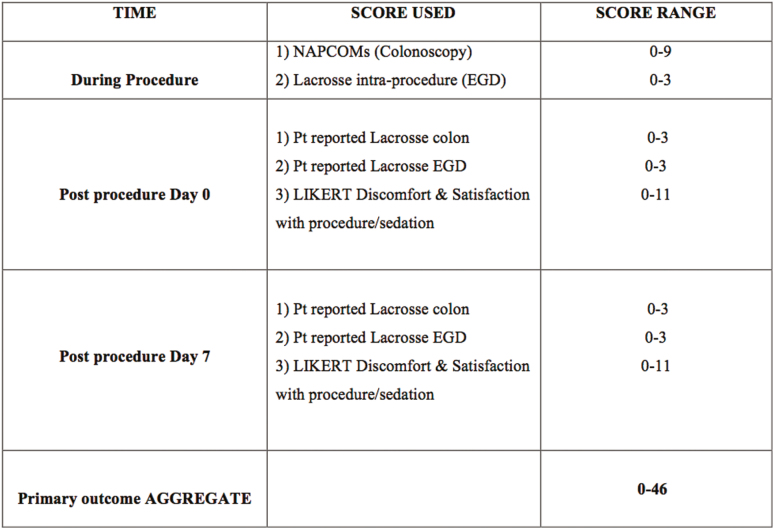
The primary outcome was the difference between the four groups on the aggregate comfort/satisfaction scores including nurse reported intraprocedural scores (NAPCOMS, Lacrosse score [EGD]) and patient reported postprocedural scores (immediate postprocedure scores and day 7 scores of colonoscopy discomfort, EGD discomfort, global discomfort, satisfaction with level of sedation and satisfaction with overall procedures) (Figure 1). A three-point difference was considered to be clinically reasonable between the worst tolerated and the best tolerated. Given the range of scores (0 to 46, with the mid point of 23), we estimated the sample size using scores of 22 and 25. The sample size was calculated with a power of 80% and an alpha of 0.05, and a two-sided test suggested that a sample of 44 per group is a reasonable estimate. As a precaution, 50 patients per group were recruited to account for incomplete procedures and withdrawals.
Figure 1.
Aggregate of various scores related to patient comfort and satisfaction with sedation/procedures used for the primary outcome (both during procedures and on postprocedural days 0 and 7). Individual scoring scales are displayed in the Appendix.
Continuous data were analyzed as means using the ANOVA test, with Tukey’s test used for pairwise comparisons. Nonparametric data were examined using the Kruskal-Wallis test. Categorical data were analyzed using Chi square tests. P value<0.05 was considered significant.
RESULTS
Between January 2016 and November 2017, 200 patients were randomized, and 187 underwent attempts at both colonoscopy and upper endoscopy (see CONSORT diagram, Figure 2). Six patients had their procedure(s) cancelled due to the following factors: withdrawal of consent for procedure (n=2), unprepared colons (n=3) and severe hypertension (n=1). In addition, the diagnosis of colon cancer via colonoscopy precluded the need for EGD in five patients; and a clear source of anemia was found on EGD, and therefore, colonoscopies were not performed in two patients.
Figure 2.
CONSORT flow diagram.
Mean age of the participants was 61.4 years (range 19 to 84 years), and 106 were female. The most common indication for bidirectional endoscopy was anemia. There were no significant differences in BMI, chronic opioid use, prior diagnoses of depression or resident involvement in the procedures between groups (Table 1).
Table 1.
Baseline demographics and indications for procedures
| EGD first with CO2 (Group A1) (n=47) |
EGD first with air (Group A2) (n=48) |
Colon first with CO2 (Group B1) (n=44) |
Colon first with air (Group B2) (n=48) |
P value | ||
|---|---|---|---|---|---|---|
| Mean Age (years) | 61.7 | 61.2 | 62.3 | 59.5 | 0.72 | |
| Female (% of group) | 49 | 63 | 55 | 61 | 0.54 | |
| Mean BMI | 29.1 | 29.3 | 30.9 | 30.1 | 0.57 | |
| Indication for colonoscopy (n) |
Anemia | 27 | 35 | 28 | 28 | 0.14 |
| Screening/Surveillance | 14 | 10 | 14 | 16 | ||
| Change in bowel habit | 6 | 0 | 2 | 3 | ||
| Rectal bleeding | 0 | 0 | 0 | 1 | ||
| Abdominal pain | 0 | 2 | 0 | 0 | ||
| Inflammatory Bowel Disease | 0 | 1 | 0 | 0 | ||
| Indication for Esophagogastroscopy (n) | Anemia/GI bleeding | 27 | 32 | 30 | 28 | 0.59 |
| Dysphagia | 4 | 3 | 0 | 1 | ||
| Dyspepsia | 5 | 4 | 3 | 8 | ||
| GERD/Barrett’s | 5 | 4 | 3 | 5 | ||
| Portal hypertension | 3 | 3 | 2 | 3 | ||
| Other | 3 | 1 | 6 | 3 | ||
| Prior bidirectional endoscopy?- Yes (n) | 5 | 12 | 8 | 12 | 0.24 | |
| Regular opioid use (n) | 4 | 3 | 3 | 8 | 0.26 | |
| Prior diagnosis of depression | 15 | 11 | 8 | 11 | 0.33 | |
With respect to the primary outcome (mean aggregate comfort/satisfaction scores), a significant difference was seen (P=0.03, Table 2); however, in individual analyses, the difference was driven by a significant difference in mean scores between the EGD first with CO2 group and EGD first with air group (P=0.047). No differences were found in patients’ comfort as per the nurse-reported NAPCOMS scores (P=0.19) or the Lacrosse (WI) endoscopy scores (P=0.05) during the procedures. On postprocedure days 0 and 7, again, no differences were found in the patients’ reported Lacrosse (WI) scores, nausea, sore throat, dizziness, satisfaction with sedation and overall level of procedural satisfaction (P>0.05 for each). Bloating and pain/discomfort were significantly lower in both of the CO2 groups (P<0.001) but did not differ according to the sequence of procedures. There was a significant difference in the mean midazolam dose between groups (P=0.01), with the EGD first groups requiring less overall (Table 3). No significant differences were seen in the doses of Fentanyl between groups.
Table 2.
Tukey’s post hoc testing between all four groups shows that the significant difference in the primary outcome is between EGD first with CO2 (group A1) and EGD first with air group (group A2)
| EGD first with CO2 (Group A1) |
EGD first with Air (Group A2) |
Colonoscopy first with CO2 (Group B1) |
Colonoscopy first with Air (Group B2) |
P value | |
|---|---|---|---|---|---|
| Aggregate patient comfort score with possible Range 0–46 (Mean ± SD) |
8.9 ± 7.1 | 12.8 ± 9.1 | 8.9 ± 5.5 | 10.8 ± 5.43 | 0.03 |
P =0.047
P>0.05 between all other groups
Table 3.
Comparisons between all four groups with regards to the secondary outcome (total sedation use)
| EGD first with CO2 (Group A1) |
EGD first with air (Group A2) |
Colon first with CO2 (Group B1) |
Colon first with air (Group B2) |
P value | |
|---|---|---|---|---|---|
| Mean duration of gastroscopy (mean, minutes) |
11.9 | 13.2 | 15.6 | 13.5 | 0.32 |
| Mean duration of colonoscopy (mean, minutes) |
32.5 | 30.6 | 32.8 | 31.4 | 0.88 |
| Total Fentanyl-mcg (mean ± D) | 126 ± 42 | 124 ± 39 | 133 ± 47 | 130 ± 43 | 0.7 |
| Total Midazolam, mg (mean ± SD) | 2.4 ± 0.8 | 2.4 ± 0.9 | 2.7 ± 1.0 | 2.9 ± 0.9 | 0.013 |
| EGD Fentanyl-mcg, (mean ± SD) | 83 ± 31 | 82 ± 25 | 53 ± 20 | 31 ± 30 | <0.0001 |
| EGD Midazolam, mg (mean ± SD) | 1.9 ± 0.7 | 1.9 ± 0.8 | 1.4 ± 0.7 | 1.5 ± 0.8 | 0.001 |
| Colonoscopy Fentanyl, mcg (mean ± SD) | 45 ± 32 | 45 ± 33 | 85 ± 37 | 81 ± 32 | <0.0001 |
| Colonoscopy Midazolam, mg (mean ± SD) | 0.5 ± 0.6 | 0.7 ± 0.7 | 1.4 ± 0.6 | 1.4 ± 0.6 | <0.0001 |
EGD, Esophagogastroduodenoscopy
The quality of the procedures appeared similar, with no differences seen in bowel preparation or duration of procedures between all four groups.
Adverse events were similar between groups as well, with most captured in the patient questionnaires. There were two serious adverse events: one patient presented to the emergency room the day following the endoscopy with abdominal bloating and was diagnosed with ascites, and the second patient had postpolypectomy bleeding requiring a short admission to hospital. Neither appeared related to the study interventions.
DISCUSSION
Bidirectional endoscopies are routinely performed in most endoscopy suites for a variety of different indications. While individual and institutional practices have so far guided the order of sequence of both procedures—EGD before colonoscopy or vice versa—there lacked a definitive study of procedures using conscious sedation evaluating if one sequence of procedures was in any way better than the other, with the focus primarily being patient satisfaction and comfort. In the recent past, some studies have attempted to answer this question, but the focus has either been nonpatient-related outcomes, or the procedures have varied based on the choice of depth of sedation or agents used (deep versus moderate sedation, Propofol versus combinations of benzodiazepines and narcotics) or anesthesia involvement. Additionally, most studies have used CO2 as the insufflating agent of choice, and while many centres are attempting to switch to CO2 today, traditional room air still remains the gas predominantly used for insufflation during endoscopy (11), making generalization of results to these patients difficult. Finally, most studies assessing patients’ comfort have either focused on comfort during or immediately after procedures using either Likert scales or scales that have not been fully validated.
Given the continued uncertainty regarding this practical and daily issue, we conducted this large randomized controlled trial incorporating previously validated scoring tools to assess patients’ comfort during and after procedures. The ultimate goal of this study was to determine the procedural sequence that works best for patient comfort and satisfaction during bidirectional endoscopies. In our trial, light (conscious) sedation was used, as is common practice in most endoscopy units (16). Both insufflating agents (i.e., traditional room air and CO) were studied in order to assess the effects these gases may have. Lastly, patients were contacted again at day 7 postprocedure in an effort to eliminate the possibility of delayed sedative effects biasing patients’ responses immediately after their procedures. To our knowledge, this is the first study to date that addresses all these factors.
In concordance with the few previously published studies in this area (4,5), our results showed that the overall sedation requirements (specifically Midazolam) in the EGD first group were less than the colonoscopy first group; however, contrary to most others, we showed no differences in overall patient satisfaction/discomfort between the two sequences of procedures both on days 0 and 7 postprocedure. Research from the ICU literature looking at patients’ recollections of ICU procedural pain reveals that patients, when interviewed 3 to 16 months after hospitalization and asked about their recall of procedural pain intensity and pain distress during their admission, did so with much higher scores than those reported in the ICU (17). As humans, it is often challenging for anyone to re-experience a moment in time that was particularly ‘unpleasant’ for them; and we may tend to overestimate the challenges we faced during that time. The fact that no differences were reported by patients between the two sequences of procedures a week after the procedures in many ways strengthens the findings observed on day 0 in our study.
When performing a bidirectional endoscopy, in most cases the endoscopist starts with a reasonable yet safe dose of sedation until the patient is comfortably sedated. The first procedure (EGD or colonoscopy) then begins until completion; the stretcher’s position is reversed, and the second procedure then begins. Additional sedation requirements are usually then gauged by patient discomfort and intolerability at the beginning of the second procedure. The general tendency, based on anecdotal evidence, seems to be that as the second procedure is nearing its end, patients are generally partially or fully awake, which may equate to a faster recovery; and therefore, the threshold to use additional sedation in the final moments of the procedure can be moderately high. State dependency refers to the phenomenon in which the amount of information recalled by patients depends on congruity between the physical or psychological state during the learning phase and the state during the recall phase (18). Therefore, it makes intuitive sense to believe that as the patient is waking up, their recollection of the final few minutes of the second procedure would be much clearer than the rest. Providing an adequate yet safe regimen of sedation influences not only the quality of the examination but also the physician’s and patient’s satisfaction with the sedation (19). Hence, rushing through the final moments of a procedure with an inadequately sedated patient would most likely translate into an improper examination with perhaps a lower level of patient satisfaction with that procedure.
As with most endoscopists, in our unit every effort is made to ensure that patients are comfortable for the duration of both procedures, and this was achieved using objective assessments via validated intraprocedural scores. Our average procedure times for performing the gastroscopy alone in the group with colonoscopy first were 15.6 minutes (CO2 as insufflator) and 13.5 minutes (air as insufflator). This is almost twice the amount of time spent for a gastroscopy in some of the other bidirectional studies (5). As per performance measures highlighted by the European Society of Gastrointestinal Endoscopy recently, completeness of an upper endoscopy cannot solely be defined by reaching the duodenum; a longer inspection time translates into a more complete examination and is related to higher diagnostic yields (20). In fact, in a retrospective study by Teh et al. studying 837 patients, it was shown that an endoscopist who took on average at least 7 minutes to perform an EGD was twice as likely to detect high-risk gastric lesions (defined as biopsy proven intestinal metaplasia, gastric dysplasia, gastric atrophy, or cancer) and three times as likely to detect a case of dysplasia or cancer than those who took less than 7 minutes (21). Based on the pharmacodynamic profile of Midazolam given via IV, the onset of action is about 2 minutes after the injection, with maximum effect obtained in about 5 to 10 minutes (22). Perhaps our patients in the colonoscopy first group were optimally sedated for the gastroscopy part, and this may have been the basis of patients being equally satisfied/comfortable regardless of whether the EGD came first or last.
There is no doubt that CO2 is a much better insufflator than air. Although it has clear safety advantages in advanced polypectomy and ERCP cases, the advantages of CO2 for routine endoscopy seem to be limited to much less postprocedural abdominal discomfort reported by patients along with speedier recovery times (10). In our study, patients in the CO2 groups reported less postprocedural abdominal discomfort/pain and bloating, consistent with previous results. And as expected, this remained the case regardless of whether the EGD or the colonoscopy was performed first. These results add yet another reason for CO2 to be used as an insufflator of choice, especially in several North American outpatient endoscopy centres and nontertiary care hospitals where traditional room air continues to be used for colonoscopic insufflation.
CONCLUSION
In conclusion, this is the first randomized controlled trial using validated patient comfort scoring assessments both during and after the procedures, showing that for same day bidirectional endoscopies, the sequence of procedures affects the sedation used but does not affect overall patient comfort or satisfaction. Lesser sedation is needed in the EGD first group, and less postprocedural abdominal pain/discomfort and bloating is seen with CO2 insufflation. For same day bidirectional endoscopies, guidelines should therefore strongly recommend performing the EGDs first with CO2 insufflation.
Supplementary Material
Acknowledgements
Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Queen’s University (23). REDCap is a secure, web-based application designed to support data capture for research studies, providing 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
References
- 1. Kurien M, Din S, Dear KL, Elphick DA. Same day bidirectional endoscopy- does the order of the procedures matter?Gut 2011;60(Suppl 1). [Google Scholar]
- 2. Cho JH, Kim JH, Lee YC, et al. Comparison of procedural sequences in same-day bidirectional endoscopy without benzodiazepine and propofol sedation: Starting at the bottom or the top. J Gastroenterol Hepatol 2010;25(5):899–904. [DOI] [PubMed] [Google Scholar]
- 3. Choi JS, Youn YH, Lee SK, et al. Which should go first during same-day upper and lower gastrointestinal endoscopy? A randomized prospective study focusing on colonoscopy performance. Surg Endosc 2013;27(6):2209–15. [DOI] [PubMed] [Google Scholar]
- 4. Hsieh YH, Lin HJ, Tseng KC. Which should go first during same-day bidirectional endosocopy with propofol sedation?J Gastroenterol Hepatol 2011;26(10):1559–64. [DOI] [PubMed] [Google Scholar]
- 5. Chen SW, Cheng CL, et al. Optimal procedural sequence for same-day bidirectional endoscopy with moderate sedation: A prospective randomized study. J Gastroenterol Hepatol 2018;33(3):689–95. [DOI] [PubMed] [Google Scholar]
- 6. Cao Y, Yang J, Li J, et al. Comparison of procedural sequences in same-day painless bidirectional endoscopy: Single-center, prospective, randomized study. Dig Endosc 2017;29(3):330–7. [DOI] [PubMed] [Google Scholar]
- 7. Zuckerman G, Benitez J. A prospective study of bidirectional endoscopy (colonoscopy and upper endoscopy) in the evaluation of patients with occult gastrointestinal bleeding. Am J Gastroenterol. 1992;87(1):62–6. [PubMed] [Google Scholar]
- 8. Kavitha K, Bharathi R, Blum S, Remy P. Same day dual endoscopy: Does the sequence matter?Gastrointestinal Endoscopy 2006;63(5):AB145. [Google Scholar]
- 9. Carter D, Lahat A, Papageorgiou NP, Goldstein S, Eliakim R, Bardan E. Comparison of procedural sequence in same-day consecutive bidirectional endoscopy using moderate sedation. J Clin Gastroenterol 2014;48(3):236–40. [DOI] [PubMed] [Google Scholar]
- 10. Sajid MS, Caswell J, Bhatti MI, et al. Carbon dioxide insufflation vs conventional air insufflation for colonoscopy: A systematic review and meta-analysis of published randomized controlled trials. Colorectal Dis 2015;17(2):111–23. [DOI] [PubMed] [Google Scholar]
- 11. Lo SK, Fujii-Lau L, Enestvedt BK, et al. ; ASGE Technology Committee. The use of carbon dioxide in gastrointestinal endoscopy. Gastrointest Endosc 2016;83(5):857–65. [DOI] [PubMed] [Google Scholar]
- 12. Rostom A, Ross ED, Dubé C, et al. Development and validation of a nurse-assessed patient comfort score for colonoscopy. Gastrointest Endosc 2013;77(2):255–61. [DOI] [PubMed] [Google Scholar]
- 13. Munson GW, et al. Intraprocedural evaluation of comfort for sedated outpatient upper endoscopy and colonoscopy. Gastroenterol Nurs 2011;34(4):296–301. [DOI] [PubMed] [Google Scholar]
- 14. Aronchick CA, Lipshutz WH, Wright SH, et al. A novel tableted purgative for colonoscopic preparation: Efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc 2000;52(3):346–52. [DOI] [PubMed] [Google Scholar]
- 15. Aldrete JA. The postanesthesia score revisited. J Clin Anesth 1995;7(1):89–91. [DOI] [PubMed] [Google Scholar]
- 16. Froehlich F, Harris JK, Wietlisbach V, et al. ; JJ EPAGE Study Group. Current sedation and monitoring practice for colonoscopy: An International Observational Study (EPAGE). Endoscopy 2006;38(5):46l–9. [DOI] [PubMed] [Google Scholar]
- 17. Puntillo K, Max A, Chaize M, Chanques G, Azoulay E. Patient recollection of ICU procedural pain and post ICU burden: The memory study. Crit Care Med 2016;44(11):1988–95. [DOI] [PubMed] [Google Scholar]
- 18. Kessels RPC. Patients’ memory for medical information. J R Soc Med 2003;96(5):219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell GD. Preparation, premedication, and surveillance. Endoscopy 2004;36:23–31. [DOI] [PubMed] [Google Scholar]
- 20. Bisschops R, Areia M. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterol J 2016;4(5):629–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teh JL, Tan JR, Lau LJF, et al. Longer examination time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol 2015;13:480–7. e2. [DOI] [PubMed] [Google Scholar]
- 22. Pharmacodynamic profile of Midazolam <https://www.medicines.org.uk/emc/medicine/25804> (Accessed February 18, 2018).
- 23. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc 2004;59:482–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.