Abstract
Background
Whether certain clinical or laboratory characteristics are able to differentiate cirrhotic patients with upper gastrointestinal bleeds (UGIB) at high-risk inpatient mortality is unknown. The objective of this study is to elucidate patient factors at presentation that are associated with in-hospital mortality.
Methods
A retrospective analysis of cirrhotic patients presenting with UGIB was performed. Baseline characteristics at admission including demographics, clinical and laboratory characteristics were collected. Factors associated with in-hospital mortality were evaluated with logistic regression analyses. The discriminative power of MELD score was evaluated with the use of area under the receiver operating characteristic (ROC) curve.
Results
One hundred and sixteen patients were included in this study. MELD score at presentation was higher in the death cohort (24.0 versus 14.8, P < 0.001) and remained significantly associated with mortality after multivariable adjustment (P < 0.001). ROC analysis of MELD score for death yielded an area under the curve of 0.88. At admission, the death group had lower systolic blood pressure (103 mmHg versus 123 mmHg, P=0.008 and more frequently presented with bright red blood per rectum (46.7% versus 11.9%, P = 0.003). Bilirubin and international normalized ratio were also higher, and albumin was lower in patients who died.
Conclusions
Among cirrhotic patients presenting with UGIB, the severity of symptoms and impairment in hepatic synthetic function is associated with in-hospital mortality. Admission MELD score may be useful in predicting in-hospital mortality.
Keywords: Cirrhosis, ICU, Upper gastrointestinal bleed
Acute upper gastrointestinal bleeding (UGIB) is a common and important complication of portal hypertension. Although the commonest cause of UGIB in cirrhosis is variceal bleeding, nonvariceal bleeding has a higher rate of high-risk endoscopic bleeding stigmata compared to patients without cirrhosis and thus may lead to greater morbidity in this population (1,2). Advancements in pharmacological modalities over the past few decades, such as somatostatin analogues and antibiotic prophylaxis, and introduction of endoscopic interventions such as banding, ligation and transjugular intrahepatic portosystemic shunt procedures have markedly improved survival in cirrhotic patients experiencing UGIB (3). Despite this, acute UGIB represents a considerable health care burden with mortality rates ranging from 15 to 30% (4–7).
There is significant variation in severity of UGIB. The ability to predict clinical outcomes remains a topic of considerable interest as early prognostication may assist in stratifying and treating higher-risk patients more effectively. Additionally, cirrhotic patients admitted to the intensive care unit (ICU) for any cause are a particularly high-risk population with in-hospital mortality ranging from 20 to 100% (8–11). One study retrospectively identified all cirrhotic patients admitted to ICU with acute variceal bleed (AVB) and found a 39% in-hospital mortality rate (11). This study also found that Model for End Stage Liver Disease (MELD) scores (12) was comparable to physiological and organ failure scores such as the Acute Physiology And Chronic Health Evaluation (APHACHE II) score and Sequential Organ Failure Assessment (SOFA) in predicting mortality (11). This finding is consistent with other studies which have shown that higher MELD scores are correlated with short-term mortality in UGIB in AVB (13–15).
While it has been established that there are higher rates of mortality in cirrhotic patients presenting with UGIB, there is currently no reliable method of predicting these outcomes. As such, this study was conducted to elucidate patient factors at presentation that are associated with in-hospital mortality in cirrhotic patients presenting with UGIB.
METHODS
This was a retrospective cohort study conducted at The Ottawa Hospital in Ottawa, Ontario, Canada. The protocol was reviewed and approved by the institutional review board and ethics committee at the Ottawa Hospital Research Institute.
Patient Inclusion and Selection
Details of the study design, inclusion criteria and selection process have been previously reported (16). In summary, all adults (age ≥18 years) discharged from The Ottawa Hospital, from July 2014 to July 2016, with a primary ICD-10 diagnosis code of unspecified cirrhosis of the liver (K70.3, K74.0, K74.6), oesophageal varices (I85), ascites (R18), hepatic failure (K72), peritonitis (K65), hepatoreneal syndrome (K76.7) or postprocedural disorders of the digestive system (K91.8, K91.9) were identified for chart review. Two authors (IC and DY) confirmed a diagnosis of cirrhosis by chart review, the definition of which is described in Tandon et al. (16) The same two authors further identified all cirrhotic patients who were admitted with signs and symptoms of UGIB (hematemesis, bloody nasogastric aspirate, melena, coffee-ground emesis, anemia or hematochezia). Exclusion criteria included severe hemorrhage resulting in death prior to endoscopy, discharge prior to inpatient endoscopy, absence of any endoscopic etiology for UGIB and patients with incomplete medical records. In the setting of recurrent admissions for UGIB, only the first encounter was considered for inclusion.
Clinical Outcomes and Definitions
The primary goal of this study was to investigate clinical, biochemical and endoscopic findings associated with mortality in cirrhotics presenting with UGIB. Other clinical outcomes including packed red blood cell (pRBC) transfusion, hospital length of stay (LOS) and risk of repeat endoscopy or other therapeutic intervention within 72 hours for re-bleeding were also compared.
AVB was defined as the presence of varices on endoscopic assessment according to the Baveno IV-V criteria (17,18). Factors including venous (nonpulsatile) bleeding, active bleeding at the gastroesophageal junction or gastric fundus in the presence of varices, or presence of varices in the absence of other lesions suggested AVB as source of UGIB. Nonvariceal bleeding (NVB) was defined as all other etiologies of UGIB such as, but not limited to, peptic ulcer disease (with active arterial oozing or spurting, visible vessel, adherent clot or red wale sign), portal-hypertensive gastropathy, esophagitis or gastritis, Mallory-Weiss tear and gastric antral vascular ectasia. If there was more than one possible source for gastrointestinal bleeding found on esophagogastroduodenoscopy (EGD), the most responsible lesion was left to the endoscopist’s discretion.
Complications of portal hypertension were also collected. History of prior variceal bleeding was determined via admission documentation on the electronic medical record (EMR). Ascites was considered present if detected on physical examination and/or quantified as more than trace on imaging modalities (ultrasound or CT scan). Hepatic encephalopathy was defined as presence of altered mental status in the absence of other organic pathology. Spontaneous bacterial peritonitis (SBP) was defined as an absolute neutrophil count > 250 cells/mm3 or positive fluid cultures on paracentesis done at the time of admission.
Data Collection
The following data were extracted from the EMR: (a) baseline patient characteristics—gender, age at admission, active alcohol intake, current medication use (nonsteroidal anti-inflammatory drugs [NSAIDs], antiplatelet agents, anticoagulation, proton pump inhibitors (PPIs) and beta-blockers), existing co-morbidities (renal disease, ischemic heart disease, congestive heart failure, metastatic disease) and cirrhosis etiology (alcohol, viral, nonalcoholic steatohepatitis, autoimmune, cryptogenic, other); (b) presenting symptoms—syncope, melena, coffee-ground emesis, hematemesis, hematochezia; (c) vital signs at admission (blood pressure, heart rate, respiratory rate, temperature); (d) hospitalization details—date of admission and discharge, hospital LOS, UGIB interventions (somatostatin analogues, PPI infusion, antibiotic therapy, pRBC transfusion), need for ICU admission and death during current hospitalization; (e) previous cirrhosis complications—esophageal/gastric varices, previous variceal bleed, ascites, hepatic encephalopathy, SBP, hepatorenal syndrome; (f) present cirrhosis complications—ascites, hepatic encephalopathy, SBP, hepatorenal syndrome; (g) hematological/biochemical investigations—white blood cell (×109/L), hemoglobin (g/L), platelet count (×109/L), international normalized ratio (INR), sodium (mmol/L), blood urea nitrogen (mmol/L), creatinine (umol/L), total bilirubin (µmol/L), serum albumin (g/L) and hepatocellular enzymes (AST, ALT, ALP, GGT) (U/L); and (h) endoscopy details—presence of varices, source of UGIB (AVB versus NVB) and rates of repeat endoscopy within 72 hours for re-bleeding.
Statistical Analysis
Categorical values were reported as relative frequencies and proportions, and compared using chi-square and Fisher’s exact test. Continuous variables were reported as mean and standard deviation and compared using Student’s t-test if normally distributed, or with median and interquartile range and compared with Wilcoxon rank sum test if data were skewed. The discriminative power of MELD score was evaluated with the use of area under the receiver operating characteristic (ROC) curve. Multivariable logistic regression was used to evaluate the association between death and MELD score, adjusting for age and systolic blood pressure as continuous variables, gender, hematemesis, bright red blood per rectum and ascites. All analyses were performed using R v3.3 (19).
RESULTS
Patients
A total of 116 patients were included in this study; 30 (26%) were admitted to the ICU during the index admission; the remainder were admitted to other medical services. A total of 15 of the 116 patients died during the admission, 13 of which were admitted initially to the ICU. Two patients that died during the hospitalization did not require ICU admission at presentation. Of the 30 patients admitted to the ICU, 17 were intubated prior to EGD. Of the 15 patients who died, 9 died of complications from gastrointestinal bleeding, 2 died of subsequent sepsis and 1 died of each of hepatorenal syndrome, liver failure, myocardial infarction and surgical emergencies.
Baseline patient characteristics are summarized in Table 1. There was no significant difference in age, gender, active alcohol use or etiology of cirrhosis between the two groups. The prevalence of congestive heart failure was not different in the two groups, however, a larger proportion of patients who died had a history of CAD (26.7 versus 5.0%, P = 0.02) and acute kidney injury (73.3 versus 31.0%, P = 0.003). There was also no difference in home medications at admission including PPI, NSAIDs, beta-blockers, antiplatelets or anticoagulants between the two groups. Similarly, there was no difference in the prevalence of known esophageal varices, previous variceal bleed, previous hepatic encephalopathy, previous spontaneous bacterial peritonitis, previous hepatorenal syndrome or ascites at admission.
Table 1.
Baseline characteristics of study population
| No death (n = 101) | Death (n = 15) | P value | |
|---|---|---|---|
| Patient Characteristics* | |||
| Age, mean ± SD, years | 56.7 (10.2) | 59.4 (11.6) | 0.34 |
| Female, number, (%) | 30 (29.7) | 4 (26.7) | 1.00 |
| ICU admission at presentation | 17 (16.8) | 13 (86.7) | <0.001 |
| Active EtOH use, number (%) | 57 (57.0) | 6 (42.9) | 0.39 |
| History of CHF, number (%) | 4 (4.0) | 2 (13.3) | 0.17 |
| History of CAD, number (%) | 5 (5.0) | 4 (26.7) | 0.02 |
| AKI on admission, number (%) | 31 (31.0) | 11 (73.3) | 0.003 |
| Current Medications, number (%) | |||
| Proton-pump inhibitor | 50 (49.5) | 7 (46.7) | 1.00 |
| NSAIDs† | 23 (22.8) | 1 (6.7) | 0.19 |
| B-Blockers‡ | 30 (29.7) | 3 (20.0) | 0.55 |
| Antiplatelet agents (any)§ | 14 (13.9) | 2 (13.3) | 1.00 |
| Aspirin | 11 (10.9) | 1 (6.7) | 1.00 |
| Dual Therapy | 2 (2.0) | 1 (6.7) | 0.34 |
| Anticoagulation¶ | 3 (3.0) | 2 (13.3) | 0.12 |
| Cirrhosis Etiology, number (%)‖ | 0.32 | ||
| Alcohol | 57 (56.4) | 10 (66.7) | |
| NASH | 12 (11.9) | 1 (6.7) | |
| Viral | 29 (28.7) | 3 (20.0) | |
| Autoimmune/PBC/PSC | 2 (2.0) | 0 (0.0) | |
| Cryptogenic | 0 (0.0) | 1 (6.7) | |
| Other | 1 (1.0) | 0 (0.0) | |
| Cirrhosis Complications, number (%)** | |||
| Known esophageal varices | 53 (52.5) | 10 (66.7) | 0.41 |
| Previous variceal bleed | 20 (19.8) | 4 (26.7) | 0.51 |
| History of HE | 14 (13.9) | 5 (33.3) | 0.07 |
| History of SBP | 4 (4.0) | 1 (6.7) | 0.51 |
| History of HRS | 1 (1.0) | 0 (0.0) | 1.00 |
| Ascites at admission | 73 (72.3) | 14 (93.3) | 0.11 |
Bold values indicate a P value <0.05 and have reached statistical significance.
*Patient characteristics: SD, standard deviation; EtOH, alcohol; CHF, congestive heart failure; CAD, coronary artery disease; AKI, acute kidney injury; CKD, chronic kidney disease.
†NSAIDS, nonsteroidal anti-inflammatory drugs including ibuprofen, naproxen, celecoxib, indomethacin.
‡B-blockers, metoprolol, propanolol, bisoprolol, carvedilol.
§Antiplatelet agents, monotherapy or dual therapy of aspirin, clopidogrel, ticagrelor and/or prasugrel.
¶Anticoagulation, warfarin, low-molecular weight heparin, unfractionated heparin, Dabigatran, Rivaroxaban or Apixaban.
‖Cirrhosis etiology: NASH, nonalcoholic steatohepatitis; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis.
**Cirrhosis complications: HE, hepatic encephalopathy; SBP, spontaneous bacterial peritonitis; HRS, hepatorenal syndrome.
Clinical Presentation
Presenting vitals are summarized in Table 2. Patients who died had lower mean systolic blood pressure at presentation (103.9 ± 19.9 mmHg versus 123.1 ± 26.5 mmHg, P = 0.008). Patients who died during the admission more frequently presented with bright red blood per rectum (46.7 versus 11.9%, P = 0.003; Table 3). There was no difference in presentation with syncope (13.3 versus 5.9%, P = 0.28), melena (40.0 versus 55.3%, P = 0.28), hematemesis (53.3 versus 57.4%, P = 0.79) or coffee-ground emesis (33.3 versus 19.8%, P = 0.31).
Table 2.
Admission vitals for patients with cirrhosis and upper gastrointestinal bleeding
| No death (n = 101) | Death (n = 15) | P value | |
|---|---|---|---|
| Vital signs, mean (SD) | |||
| Heart rate | 99.4 (20.0) | 101.9 (19.0) | 0.65 |
| Systolic blood pressure | 123.1 (26.5) | 103.9 (19.9) | 0.008 |
| Respiratory rate | 18.1 (3.1) | 19.8 (5.0) | 0.08 |
Bold values indicate a P value <0.05 and have reached statistical significance.
Table 3.
Presenting symptoms of patients with cirrhosis and upper gastrointestinal bleeding
| No death (n = 101) | Death (n = 15) | P value | |
|---|---|---|---|
| Symptoms, number (%) | |||
| Syncope | 6 (5.9) | 2 (13.3) | 0.28 |
| Melena | 56 (55.4) | 6 (40.0) | 0.28 |
| Hematochezia | 12 (11.9) | 7 (46.7) | 0.003 |
| Hematemesis | 58 (57.4) | 8 (53.3) | 0.79 |
| Coffee-ground emesis | 20 (19.8) | 5 (33.3) | 0.31 |
Bold values indicate a P value <0.05 and have reached statistical significance.
Laboratory Investigations
There were significant differences in several hematological and biochemical investigations between patients that died during admission compared to those who did not (Table 4). These included: higher white blood cell count (13.6 ± 4.8 versus 9.5 ± 5.3, P = 0.006), higher INR (1.8: interquartile range [IQR] [1.7, 2.5] versus 1.4: IQR [1.3, 1.6], P < 0.001), higher total bilirubin (86.0: IQR [56.5, 129.0] versus 29.0 IQR [16.0, 54.5], P < 0.001) and lower albumin levels (21.4 ± 5.6 versus 26.2 ± 6.0, P = 0.005).
Table 4.
Admission laboratory investigations for patients with cirrhosis and upper gastrointestinal bleeding
| No death (n = 101) | Death (n = 15) | P value | |
|---|---|---|---|
| Laboratory investigations* | |||
| Hemoglobin (g/L), mean (SD) | 93.0 (23.8) | 92.1 (21.3) | 0.89 |
| Platelet count (×109/L), mean (SD) | 127.3 (71.9) | 124.7 (60.7) | 0.90 |
| White blood cell count (×109/L), mean (SD) | 9.5 (5.3) | 13.6 (4.8) | 0.006 |
| INR, median (IQR) | 1.4 (1.3, 1.6) | 1.8 (1.7, 2.5) | <0.001 |
| Sodium (mM), median (IQR) | 138.5 (135.0, 142.0) | 135.0 (133.0, 139.0) | 0.06 |
| Total Bilirubin (µM), median (IQR) | 29.0 (16.0, 54.5) | 86.0 (56.5, 129.0) | <0.001 |
| Albumin (g/L), mean (SD) | 26.2 (6.0) | 21.4 (5.6) | 0.005 |
Bold values indicate a P value <0.05 and have reached statistical significance.
SD, standard deviation; IQR, interquartile range.
*Laboratory investigations: INR, international normalized ratio; BUN, blood urea nitrogen.
Endoscopic Findings
Of the 116 patients included in this cohort, 112 had endoscopy performed within 24 hours. On endoscopy, 73 had AVB and 43 had NVB. Of those with NVB, 17 were diagnosed with peptic ulcer disease (PUD), 11 with esophagitis/gastritis, 4 with Mallory-Weiss tear, 5 with angiodysplasia and 5 with gastric antral vascular ectasia. There was no significant difference in rates of AVB between patients who died in-hospital and those who did not (80.0 versus 62.4%, P = 0.297). Furthermore, there was no difference in type of AVB (P = 0.45): of the 12 patients who died with AVB, 11 had esophageal varices and 1 had esophagogastric varices compared with the 63 patients who survived following AVB; 48 of which had esophageal varices, 6 with esophagogastric varices and 9 with isolated gastric varices. Of those who died, two required transjugular intrahepatic portosystemic shunt during hospitalization for bleeding compared with only one among the patients who survived.
Clinical Outcomes
Clinical outcomes are summarized in Table 5. The death group received a higher median number of pRBCs (5.0 units: IQR [2.0, 8.0] versus 2.0 units: IQR [2.0, 4.0], P = 0.008). Repeat EGD within 72 hours was also required more frequently for re-bleeding in the death group (40.0 versus 13.9%, P = 0.03). In those who rebled in the death group, 33.3% were from a bleeding varix, 33.3% from oozing portal-hypertensive gastropathy (PHG) and 33.3% were lesions that were unable to be identified. In patients who did not die, 42.9% rebled from a varix, 28.6% were lesions that were unable to be identified, 7.1% from oozing PHG, 14.3% from a bleeding polyp and 7.1% from gastric antral vascular ectasia. There was no significant difference in-hospital LOS (median 6.4 days: IQR [4.5, 15.3] versus 4.8 days [3.0, 7.0], P = 0.09).
Table 5.
Clinical outcomes of patients with cirrhosis and upper gastrointestinal bleeding
| No death (n = 101) | Death (n = 15) | P value | |
|---|---|---|---|
| Units of pRBC required, median (IQR)† | 2.0 (2.0, 4.0) | 5.0 (2.0, 8.0) | 0.008 |
| Length of Stay, median (IQR)† | 4.8 (3.0, 7.0) | 6.4 (4.5, 15.3) | 0.09 |
| Pre-endoscopic HGB, mean (SD) | 93.0 (23.8) | 92.1 (21.3) | 0.89 |
| Repeat EGD for re-bleed within 72 h, N (%)† | 14 (13.9) | 6 (40.0) | 0.03 |
Bold values indicate a P value <0.05 and have reached statistical significance.
*IQR, interquartile range.
†EGD, esophagogastroduodenoscopy for re-bleeding within 72 h of initial bleeding episode.
MELD Score and Multivariate Analysis
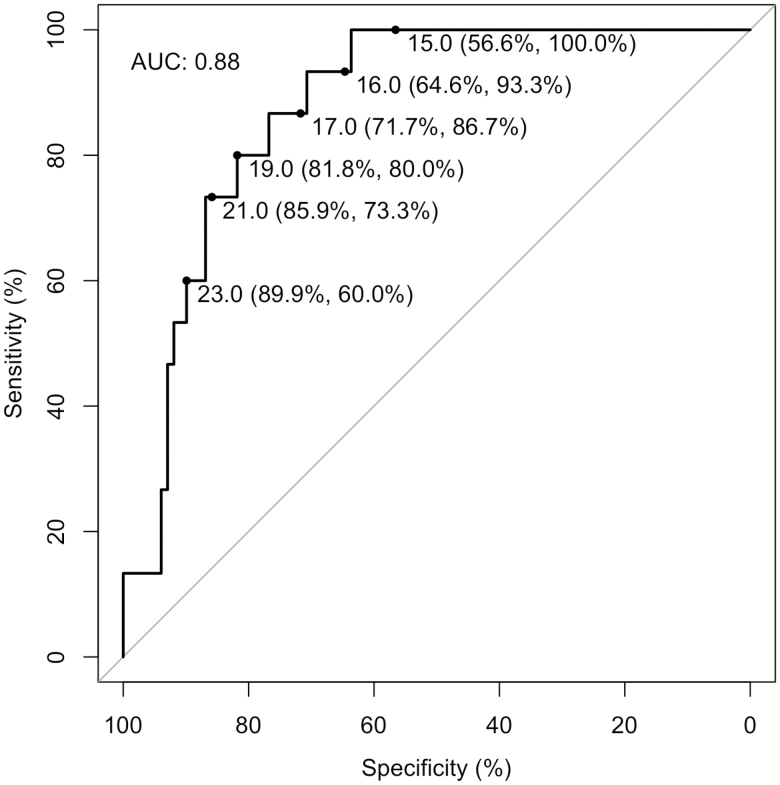
Calculated MELD scores at admission were higher in the death group (24.0 ± 6.1 versus 14.8 ± 5.6, P < 0.001). Table 6 presents the results from the adjusted logistic regression analysis. A one unit increase in MELD score was associated with 1.31 (95% confidence interval [CI]: 1.13, 1.51) times the odds of death. Bright red blood per rectum was associated with over 12 times the odds of death (odds ratio: 12.48; 95% CI: 1.99, 78.33). ROC analysis of MELD score for death yielded an area under the curve of 0.88. Figure 1 demonstrates sensitivities and specificities at various MELD cut-offs for in-hospital mortality.
Table 6.
Multivariable logistic regression of select variables associated with inpatient mortality
| Adjusted* odds ratio (95% CI) | P value | |
|---|---|---|
| Age (per year) | 1.07 (0.99, 1.16) | 0.09 |
| Male | 0.22 (0.03, 1.74) | 0.15 |
| MELD Score (per 1 unit) | 1.31 (1.13, 1.51) | <0.001 |
| Hematemesis | 0.97 (0.20, 4.78) | 0.97 |
| Bright red blood per rectum | 12.5 (1.99, 78.3) | 0.007 |
| Systolic blood pressure (per 1 unit) | 0.97 (0.93, 1.01) | 0.10 |
| Ascites | 8.03 (0.44, 146) | 0.16 |
Bold values indicate a P value <0.05 and have reached statistical significance.
*Adjusted for the variables in the table.
CI, confidence interval; MELD, Model for End Stage Liver Disease.
Figure 1.
Receiver operating characteristic curve for in-hospital mortality with sensitivity and specificity at different Model for End Stage Liver Disease (MELD) scores.
DISCUSSION
UGIB in cirrhosis is associated with poor outcomes and higher mortality rates compared to noncirrhotic patients (11,20,21). Early identification of patients at risk for in-hospital mortality may assist with timely initiation of appropriate management and improvement in clinical outcomes. As such, understanding clinical factors associated with inpatient mortality is critical in improving our understanding of the natural history of UGIB in cirrhosis.
In the current study, patient demographic factors including age and gender were not associated with inpatient mortality. Furthermore, there was no difference in etiology of UGIB in patients who died compared with those who did not. However, factors associated with severe UGIB, including hypotension and hematochezia, and underlying severity of the liver disease (characterized by baseline MELD score) appeared to be associated with in-hospital death.
The results of our study suggest that more advanced liver disease is associated with worse clinical outcomes when presenting with UGIB. The MELD score has previously been assessed as a prognostic tool in AVB (11,13–15). Bambha et al. performed a multicenter retrospective trial on 16 centers in North America assessing predictors of 6-week mortality in AVB and determined that MELD scores were significantly associated with mortality (hazard ratio [HR]1.08, 95% CI 1.03 to 1.12, P = 0.003), while the Child-Pugh-Turcotte score was not shown to be useful in predicting mortality (13). Similarly, Amitrano et al. assessed 172 patients admitted for first episode of AVB and found that the baseline MELD score was highly predictive of 6-week mortality (14). They subsequently performed an ROC curve analysis and found a MELD score cuff-off of 15 to have a sensitivity of 78% (95% CI 62 to 90), specificity of 72% (95% CI 64 to 79) and a C-index of 0.80 (95% CI: 0.74 to 0.86). MELD score for in-hospital mortality has also been assessed in other studies, similarly finding that MELD scores were significantly higher in cirrhotic patients with AVB who died during hospitalization (11,22). The relatively higher MELD cut-offs for death in our study may be a reflection of continued improvement of care and clinical outcomes in cirrhotic patients presenting with UGIB (3,4,23).The results of our study suggest that regardless of the etiology of the bleed, MELD score is associated with increased in-hospital mortality and may be a simple and useful tool to prognosticate cirrhotic patients presenting with UGIB. This is further supported by the finding that individual components of the MELD score are also significantly worse in patients who died. Specifically, in the present cohort, a MELD score cut-off of 19 resulted in a sensitivity of 82.5% and a specificity of 80.0% in predicting in-hospital mortality.
This retrospective observational cohort study demonstrates that a number of simple clinical parameters at time of presentation can be used to assess subsequent clinical outcomes including in-hospital mortality in cirrhotic patients presenting with UGIB. Specifically, patients with higher MELD scores are at an increased risk of mortality when presenting with UGIB. A strength of this study is the inclusion of consecutive cirrhotic patients who were admitted with AVB or NVB. Given that there is no reliable way to delineate the etiology of UGIB pre-endoscopically, the results of this study are more applicable to the clinical setting as the majority of prior studies retrospectively assessed AVB only. Despite this, one of the major limitations of this study is that it is a single center retrospective study performed in an academic hospital. While our center has a large catchment area representative of a variety of clinical settings and cirrhotic populations, there may be referral bias in those patients admitted to the ICU directly from a peripheral institution where earlier intervention could have been initiated but not captured in our EMR. As such, there may be hemodynamic instability that would not have been apparent in our chart review. Furthermore, given the retrospective nature of this study, there may be confounding variables associated with in-hospital mortality in this patient cohort that are not captured here such as other co-morbidities and larger prospective studies on this topic are warranted to explore these variables. Finally, the main limitation of our study is the relatively small sample size of patients who died due to their UGIB. Our study may be underpowered to detect differences in factors associated with in-hospital mortality. While the results of this small study are hypothesis-generating, larger multicenter studies are necessary to further elaborate clinical factors that can be used to predict death in cirrhotic patients presenting with gastrointestinal bleeding.
In summary, UGIB in cirrhotic patients is a common clinical scenario and is associated with significant morbidity and mortality. Our study suggests that severity of the clinical symptoms (hematochezia), severity of the underlying liver disease (synthetic dysfunction and MELD score) and presentation (hemodynamic instability/hypotension) are associated with increased risk of in-hospital mortality. MELD score in particular may be a powerful tool in predicting inpatient mortality in this patient population and larger dedicated studies are warranted to further validate its clinical utility. These readily available clinical factors may be useful in prognosticating cirrhotic patients presenting with UGIB with subsequent improvement in triage and clinical decision making in this relatively common clinical scenario.
Conflict of interest disclosure: None reported by all authors.
Funding sources: None reported by all authors.
Author contributions: K.B.: Data acquisition, data analysis, drafting of manuscript, critical revision of manuscript. P.T.: Data acquisition, data analysis, drafting of manuscript, critical revision of manuscript. S.F.: Data analysis, drafting of manuscript, critical revision of manuscript. D.Y.: Data acquisition, data analysis, critical revision of manuscript. I.C.: Data acquisition, critical revision of manuscript. K.W.: Study concept design, data analysis, drafting of manuscript, critical revision of manuscript, study supervision. E.K.: Study concept design, data analysis, drafting of manuscript, critical revision of manuscript, study supervision.
References
- 1. Christensen E, Fauerholdt L, Schlichting P, Juhl E, Poulsen H, Tygstrup N. Aspects of the natural history of gastrointestinal bleeding in cirrhosis and the effect of prednisone. Gastroenterology 1981;81(5):944–52. [PubMed] [Google Scholar]
- 2. González-González JA, García-Compean D, Vázquez-Elizondo G, Garza-Galindo A, Jáquez-Quintana JO, Maldonado-Garza H. Nonvariceal upper gastrointestinal bleeding in patients with liver cirrhosis. Clinical features, outcomes and predictors of in-hospital mortality. A prospective study. Ann Hepatol 2011;10(3):287–95. [PubMed] [Google Scholar]
- 3. Chalasani N, Kahi C, Francois F, et al. . Improved patient survival after acute variceal bleeding: A multicenter, cohort study. Am J Gastroenterol 2003;98(3):653–9. [DOI] [PubMed] [Google Scholar]
- 4. Carbonell N, Pauwels A, Serfaty L, et al. . Improved survival after variceal bleeding in patients with cirrhosis over the past two decades. Hepatology 2004;40(3):652–9. [DOI] [PubMed] [Google Scholar]
- 5. Vergara M, Clèries M, Vela E, et al. . Hospital mortality over time in patients with specific complications of cirrhosis. Liver Int 2013;33(6):828–33. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt ML, Barritt AS, Orman ES, Hayashi PH. Decreasing mortality among patients hospitalized with cirrhosis in the United States from 2002 through 2010. Gastroenterology 2015;148(5):967–977.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. D’Amico G, De Franchis R; Cooperative Study Group Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology 2003;38(3):599–612. [DOI] [PubMed] [Google Scholar]
- 8. Zauner C, Schneeweiss B, Schneider B, et al. . Short-term prognosis in critically ill patients with liver cirrhosis: An evaluation of a new scoring system. Eur J Gastroenterol Hepatol 2000;12(5):517–22. [DOI] [PubMed] [Google Scholar]
- 9. Das V, Boelle PY, Galbois A, et al. . Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med 2010;38(11):2108–16. [DOI] [PubMed] [Google Scholar]
- 10. Cholongitas E, Senzolo M, Patch D, et al. . Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther 2006;23(7):883–93. [DOI] [PubMed] [Google Scholar]
- 11. Al-Freah MA, Gera A, Martini S, et al. . Comparison of scoring systems and outcome of patients admitted to a liver intensive care unit of a tertiary referral centre with severe variceal bleeding. Aliment Pharmacol Ther 2014;39(11):1286–300. [DOI] [PubMed] [Google Scholar]
- 12. Kamath PS, Kim WR; Advanced Liver Disease Study Group The model for end-stage liver disease (MELD). Hepatology 2007;45(3):797–805. [DOI] [PubMed] [Google Scholar]
- 13. Bambha K, Kim WR, Pedersen R, et al. . Predictors of early re-bleeding and mortality after acute variceal haemorrhage in patients with cirrhosis. Gut 2008;57(6):814–20. [DOI] [PubMed] [Google Scholar]
- 14. Amitrano L, Guardascione MA, Bennato R, et al. . MELD score and hepatocellular carcinoma identify patients at different risk of short-term mortality among cirrhotics bleeding from esophageal varices. J Hepatol 2005;42(6):820–5. [DOI] [PubMed] [Google Scholar]
- 15. Chalasani N, Kahi C, Francois F, et al. . Model for end-stage liver disease (MELD) for predicting mortality in patients with acute variceal bleeding. Hepatology 2002;35(5):1282–4. [DOI] [PubMed] [Google Scholar]
- 16. Tandon P, Bishay K, Fisher S, et al. . Comparison of clinical outcomes between variceal and non-variceal gastrointestinal bleeding in patients with cirrhosis. J Gastroenterol Hepatol 2018;33(10):1773–9. [DOI] [PubMed] [Google Scholar]
- 17. Thabut D, Rudler M, Dib N, et al. ; French Club for the Study of Portal Hypertension (CFEHTP) Multicenter prospective validation of the Baveno IV and Baveno II/III criteria in cirrhosis patients with variceal bleeding. Hepatology 2015;61(3):1024–32. [DOI] [PubMed] [Google Scholar]
- 18. Ahn SY, Park SY, Tak WY, et al. . Prospective validation of Baveno V definitions and criteria for failure to control bleeding in portal hypertension. Hepatology 2015;61(3):1033–40. [DOI] [PubMed] [Google Scholar]
- 19. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 20. Barkun A, Sabbah S, Enns R, et al. ; RUGBE Investigators The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol 2004;99(7):1238–46. [DOI] [PubMed] [Google Scholar]
- 21. Marmo R, Koch M, Cipolletta L, et al. . Predictive factors of mortality from nonvariceal upper gastrointestinal hemorrhage: a multicenter study. Am J Gastroenterol 2008;103(7):1639–47; quiz 1648. [DOI] [PubMed] [Google Scholar]
- 22. Lyles T, Elliott A, Rockey DC. A risk scoring system to predict in-hospital mortality in patients with cirrhosis presenting with upper gastrointestinal bleeding. J Clin Gastroenterol 2014;48(8):712–20. [DOI] [PubMed] [Google Scholar]
- 23. McCormick PA, O’Keefe C. Improving prognosis following a first variceal haemorrhage over four decades. Gut 2001;49(5):682–5. [DOI] [PMC free article] [PubMed] [Google Scholar]