Abstract
Basal cell carcinoma (BCC) as a non-melanoma skin cancer type is the most common malignant tumor throughout the world. The incidence is higher in age over 60. The intense of exposure to ultraviolet radiation is one of the known risk factors. Over 50% of BCC of the periocular region initially occur on the lower lid and inner angle. Literature review of treatment options for basal cell carcinoma, which consist of surgery, or combined techniques plus vismodegib, radiotherapy and imiquimod. The first consideration for treatment of periocular BCC is radical surgical excision using Mohs micrographic technique. Functional and esthetic outcome in patients are important after clear excisions and reconstruction should be carefully considered. Radical exenteration is considered in the case of orbital invasion of high-risk aggressive BCC.
Key words: Periocular tumors, basal cell carcinoma, vismodegib, radiotherapy
Introduction
The incidence of all skin malignancies is slowly increasing worldwide, especially the non-melanoma skin cancer types (NMSC). The relative incidence of periocular tumors varies with geographical area and racial group. The most common skin cancer is the basal cell carcinoma (BCC). Over 75% percent of BCC occur in the head and neck region. At about 20% of BBC appear in periocular region.1,2 Orbital invasion is uncommon with a reported incidence about 2%.3 The age of BCC emergence is typically in patients over 60 years. BCC of the eyelids has a high risk of recurrence, especially in infiltrative types. Late diagnosis of BCC can lead to functional and cosmetic defects. Any periocular skin malignancy can invade the orbit region and lead to the probability of radical exenteration. The orbital invasion of BCC is in 2-4% and the risk factors include large size of the primary tumor also multiple recurrences, infiltrative histological subtype, perineural spread, the medial canthus and inner angle localization and patient’s age over seventy. In less than 1% of basal cell carcinomas perineural invasion is present.4,5
Early diagnosis and exact surgery promise better treatment outcome functionality and better cosmetic outcome, but in some cases also other treatment options are necessary to be combined with surgery.6-8
The new last 8th edition of American Joint Committee on Cancer (TNM system) allows more objective and consistent designation of the T category.9
Epidemiology
Nowadays it is impossible to collect the data from cancer registries that would analyze BCC worldwide. Due to data of the American Cancer society in 2012, 5.4 million cases of NMSC were diagnosed in 3.3 million people. In 8 patients from 10 histopathologically BCC was verified. A population-based study estimated that 3.5 million patients with NMSC were treated in the United States in 2006.10 In the Unites States estimated an ageadjusted prevalence and incidence of BCC was 226 to 343 per 100,000 persons per year, respectively.11 Due to other incidencebased mathematical model support approximately 13 million non- Hispanic patients in the United States in 2007 may have had a personal history of NMSC. Today a high prevalence of NMSC not only in the United States is present.12
Approximately 40% of patients who have had one BCC will develop another lesion within five years, although the probability of developing a subsequent BCC following the first BCC is significantly less than after a next BCC (12.8% versus 33.9% percent in first year; 20% versus 51.8% in 2 year interval and 34.6% versus 75% in 5 year interval).13
One of the most important risk factors of BCC is the intermittent intense exposure to ultraviolet (UV) radiation; other risk factors include long-term sun abuse, family history of other skin cancers, immunosuppressive status, previous irradiation or exposure to toxic substances. Short-wavelength UVB radiation (290-320 nm) plays a more important role than long-wavelength UVA radiation (320-400 nm). UVB radiation damages not only DNA and its repair system, but changes the immune system resulting in progressive genetic alterations which are responsible of neoplasm formation. Mutations induced by UV in the TP53 tumor-suppressor gene have been verified in about 50% of BCC lesions. In skin carcinogenesis the mutations play a significant role by activating hedgehog intercellular signaling pathway genes. Patched Ptch-1 mutations promote the development of eyelid BCC.14,15
Risk factors of multiple BCC are important; the high-risk patients have to be followed up for recurrence or development of new lesions and they have to examine their skin regularly.16
There are many factors as risk factors of multiple BCC development mentioned in different previous studies. Important are age,17,18 male sex,19 pale white skin, blue or green iris color, blond or red hair (skin phototype I, II),19,20 frequent sun exposure and sunburn,21,22 severe actinic damage,20 history of previous radiotherapy, 23 increased numbers of identified tumors already present, 24-26 tumor size over 1 cm,17 lesions on trunk,27-29 positive family history of other skin tumors,22 low DNA repair capacity,30 detected tumor necrosis factor (TNF), microsatellite polymorphism, 19,31 PTCH gene polymorphism.32 and glutathione S-transferase and cytochrome P450 polymorphism.19,33
In Europe the high incidence of developed stage of BCC size are associated with a low socioeconomic status, patients who live under socio- economic deprivation are more likely to get BCC. Small BCC (stage T1) are usually easily managed with a good prognosis and cosmetic result.34
Periocular localization, clinical examination
More than 50% of the BCCs appear on the lower eyelid, 30% on the medial canthus, 15% on the upper lid and 5% on the lateral canthus.2 Risk factors such as chemical or physical irritation (e.g. of tears) may do more harm to the lower eyelid. BCC with orbital invasion occur more frequently in the medial canthus (average 60%) compared to the lower eyelid (average 30%), upper eyelid (average 6%) or lateral canthus (average 14%).2,35-38
BCC is characterized by pink color but in advanced stages it can have ulceration or bleeding. In a large series of orbital invasion visible or palpable mass was found in all the patients. Mass fixation to orbital bone (35%), limitation of ocular motility (30%) and globe displacement (18%) are serious signs of orbital infiltration. Other important signs are immobile eyelids, tearing secondary to canalicular or sac BCC infiltration and ptosis.2,5
Instead of examination in the daylight and slit lamp examination other imaging methods in patients with orbital invasion are necessary. Computed tomography with bone windows can be used for visualizing bony destruction and magnetic resonance imaging is a better option for visualizing soft tissue changes and rare perineural invasion.2
Diagnostic performance of optical coherence tomography (OCT) does not depend on the lesion’s anatomical location. Diagnostic performance for lesions with mediocre image quality is still better than by clinical and dermoscopic examination. Good OCT image quality is correlated with improved diagnostic performance. 39
High-frequency ultrasound (HFUS) is a new non-invasive technique that allows visualizing skin lesions in vivo to obtain size, shape, and tumor volume. Ex vivo HFUS could increase surgeon confidence on complete tumor excision by visualizing the tumor and surgical margins.40 To avoid unfounded surgical excisions or excisional biopsies a new noninvasive imaging technique, in vivo reflectance confocal microscopy, is available. In a study of Cinotti et al. was found that this method had a high sensitivity (100%) and specificity (70%) for eyelid margin tumors and has a great advance to examine both the skin and the conjunctiva of the eyelid.41
BCC arises from basal cells of the epidermis and clinical symptoms can be variable as mentioned bellow, but the final diagnosis must always be verified by pathological examination. Biopsy (excisional biopsy) is not necessary for all BCC lesions just a simple (even partial) shave biopsy is adequate to establish diagnosis, different from melanoma when depth of invasion is needed for staging. The basic histological types of BCC are superficial, infiltrating and nodular tumors, and those with adnexal differentiation. The most common types of BCC which tend to be less aggressive are nodular and superficial types. The infiltrating subtypes of BCC are more aggressive but rare, morpheaform types do have a higher rate of residual positive margins after excision, as well as a higher risk of recurrence. In cases with orbital invasion most aggressive subtypes are found (80%).2,5,6
Typical clinical manifestation is a single lesion with ulceration, or central scarring and keratosis, with possibility of deep infiltration of BCC, but the presence of inflammation with or without the ulceration increases the possibility to be transformed into more aggressive phenotype.37 Large, recurrent tumors and incompletely excised tumors are factors inducing more aggressive histological types of BCC.14 An aggressive, infiltrative type of BCC showed a high expression of an apoptotic gene Bcl-2 and moderate levels of proliferation associated markers - proliferating cell nuclear antigen. 42 It was found that telomere length was shortened and gene expression correlated with cell proliferation (Bcl-2, Ki-67) was increased in BCC.43
Treatment of periocular basal cell carcinoma - surgery
Surgery is the first line treatment of the BCC, including wide surgical excision and Mohs micrographic surgery with frozen section margin control.44,45 Margin control is necessary to achieve clear resection margins in order to reduce the risk of local recurrence. Treatment needs to be individualized due to general status of patient, tumor stage and histological characteristics (Figure 1). Depending on the stage, localization and pathological variety of the BCC a cure rate of about 95% is achieved after treatment. The recurrence rate after primary surgery is 1%-5% per year.4,6,46,47
After surgery there it is necessary a good interdisciplinary cooperation between ophthalmologist and pathologist. The integrity of the eyelids and its function is important for protecting and preserving the function of the eye globe. The shape of the tumor, the distance from the eyelid margin and its diameter are important to choose the best surgical technique. Reconstruction with a skin flap based on the tumor size in the eyelid is necessary to preserve eyelid function and cosmetic result. Skin flap is only one of so many different factors and techniques that eyelid reconstruction entails, and over simplifies the process of reconstruction. The aim of surgery is to excise the lesion with clear margins 3 mm around the tumor and reestablish eyelid function. The localization of the tumor on the eyelid margin makes surgical reconstruction more difficult. The smaller the size of the tumor (stage T1), the simpler the surgical reconstruction and better functional and cosmetic results can be achieved. The UK National Multidisciplinary Guidelines recommend that non-infiltrative BCC lesions 2 cm should be excised with a margin of 4-5 mm. Margins closer to the tumor (2-3 mm) may be taken in case of limited reconstructive options.46
In a randomized study compared Mohs micrographic surgery to radiotherapy local failure rate was 1% in group of patients treated with surgery and 7.5% in group treated with radiotherapy. Patients after surgery considered their cosmetic outcome as good or better (surgery 87%, radiotherapy 69%).48-50
Mohs micrographic surgery has been regarded as the best method of removing BCC with minimal recurrence rate, it is expensive and time consuming and not recommended usually for orbital infiltration due to difficulties by obtaining orientation of specimens from orbital soft tissue. The risk of false-negative results with standard frozen section techniques is higher. Therefore, paraffin section histology remains the choice of margin control for BCCs with orbital invasion.2,4 In prospective studies in patients with periocular BCC managed by Mohs micrograft surgery using fresh-frozen tissue sections was a recurrence rate of 0% in 5 year interval for primary tumors and 7.8% for recurrent tumors. This is the treatment of choice for periocular BCC, Mohs for periocular BCC followed by reconstruction has definitely not lost popularity and is the standard of care in many places.44,45 But due to second intention healing, it has lost popularity because of the long healing process and potential functional alteration of important anatomical structures if certain wound healing factors are not carefully taken into consideration.51
Mohs surgery is often undertaken in specialized centers and hence may not be accessible (e.g.. often long travel is required), is not suitable for patients who require sedation/general anesthesia as it needs several passes often in one day but that in conjunction of an ophthalmologist with an oculoplastic surgeon a reconstruction can take place. In some studies the rate of tissue sparing of 46% can be reached by using it for primary infiltrative growth pattern BCC.52
Ho et al. in their one of the largest series of BCC excisions with the non-Mohs rapid paraffin technique performed the reconstruction few days after the histology results were available. Only when the margins were clear, plastic reconstruction was performed. In condition the margins were involved, further excision was performed using frozen section or paraffin examination until a negative margin was obtained.36
Monitoring of surgical margins with frozen sections during the operation or later paraffin sections is necessary. The surgeon should repair the surgical defect after obtaining tumor negative margins. Conway et al. evaluated the recurrence of primary BCC infiltrating the eyelid margins after resection with and without intraoperative frozen section control. There was no tumor recurrence in group with control.53,54
When unmonitored perioperative excision is performed aggressive histological subtypes of BCC are more likely to be incompletely excised. Exenteration of the orbit is considered only in cases of extensive orbital invasion. It may be combined with adjunctive radiotherapy when margins are not clear or in high-risk aggressive infiltrative types of tumors.8 Madge et al. in their study of patients with orbital invasion by inner angle canthal BCC treated with non-exenterating surgery found final margins were clear in 18 of 20 patients. After 3 years interval, in only one patient the BCC was relapsed. Due to their results conservative (non-exenterating) surgery with margin control in this highly selected group of patients can have good results in BCC recurrence.3
A multidisciplinary team must collaborate in planning management of BCC with orbital invasion (Figure 1). Shields et al. in their study in patients indicated for exenteration reported in 4 of the 9 skin tumors allowed some eyelid sparing.6 Ben et al. in group of patients with exenterations reported complications in 23.5%.7 Complications can include fistula formations into sinuses, to the nasolacrimal duct, tissue necrosis, chronically exposed bone, cerebrospinal fluid leak, pain or secondary infections. The time interval after exenteration for healing with granulation can occasionally far exceed the usual 3-4 months. Cosmetic defects after exenteration are covered by individual epithesis (Figure 2). [The photographic documentation is published with the consent of the patients.]
Figure 1.
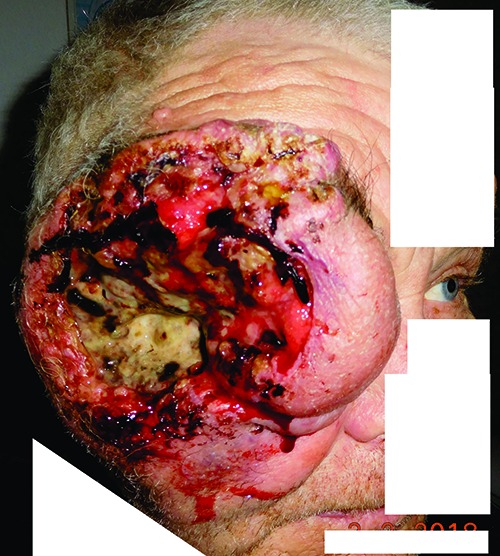
Patient with large periocular basal cell carcinoma on his right side. According to patients history tumor started to develop 3 years before from a small lesion in the lower eyelid. Later without any treatment. The first visit of this patient at Dept. of Ophthalmology, Faculty of Medicine, Comenius University.
Figure 2.
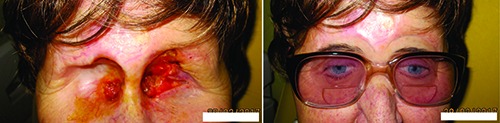
Patient after biorbital exenteration of the orbit due to basal cell carcinoma - left, individualized epithesis of the same patient - right (Dept. of Ophthalmology, Faculty of Medicine, Comenius University).
Other treatment modalities - vismodegib, imiquimod
Primary treatment of BCC is surgery but there is no effective therapy for locally advanced or infiltrative BCC.
In last decades a hedgehog pathway inhibitor vismodegib was invented as an adjuvant therapy or in certain cases as a prior medical treatment for periocular and orbital BCC.55-57
In pathogenesis of BCC but also basal cell nevus syndrome alterations in hedgehog signaling are involved. The hedgehog signal transduction pathway plays an important role in cell proliferation. Alterations in hedgehog signaling may transform an intraepithelial neoplasia into the invasive cell carcinoma.14 Indication is for advanced stage of BCC where no effective surgery is possible or failed or radiotherapy is not sufficient. It is used in patients when radical surgery leads to visual acuity loss, double vision or loss of the whole eye globe or orbital structures (exenteration). Vismodegib can be used to treat basal cell nevus syndrome (Gorlin syndrome) in patients not amenable to surgery with multiple cutaneous lesions of the periocular region and head.58-61 Vismodegib reduces the size of tumors while it represses the hedgehog pathway by directly inhibiting the G protein-coupled receptor protein.62,63
Kahana et al. published the first histopathological description of the effects of vismodegib treatment. After 5 months interval of treatment, the surgical specimen failed to show nuclear immunoreactivity for the proliferation marker Ki-67and residual squamous cells exhibited degenerative cytologic features.64
Many studies confirmed that vismodegib is effective in BCC patients, but the long-term results after finishing treatment are still unknown. Demirci et al. in four patients with orbital BCC present vismodegib as the only treatment. In their results it was described a partial response after 7 months - a mean tumor reduction (over 80%). In 2 patients taking vismodegib as adjuvant therapy complete remission in 7 months interval was present and no evidence of clinical recurrence in 15 months was present. In 2 two patients with extensive infiltration after 14 months of oral treatment a complete clinical remission was present.65
In study of Gill et al. in 7 patients with extensive periocular or orbital BCC treated with vismodegib in 11 weeks interval in 7 patients were demonstrated recurrent tumors previously excised with controlled margins by frozen sections or Mohs microsurgery. The mean follow-up duration of this study was 7 months. One (14%) patient showed disease progression.60 In a case report of advanced recurrent periocular BCC the periorbital tumor regressed after vismodegib therapy in 3 months, but recurred after 9 months and patient was finally treated with orbital exenteration. The reason was the resistance to vismodegib.61
Vismodegib in cream form or per os therapy is generally well tolerated. Side effects like alopecia, muscle spasms, fatigue, nausea, decreased appetite, weight loss, dysosmia, or diarrhea are sometimes the reasons to give up the therapy with vismodegib. Necessity of additional long term studies is required to estimate the risks factors to establish the treatment criteria for periocular BCC.66
In elderly skin cancer patient should be ideally implemented also a geriatric assessment in clinical therapy decision. Current clinical practice guidelines for BCC and non melanoma skin cancer only partially address geriatric aspects of cancer care, such as limited life-expectancy, geriatric comorbidities and but also treatment compliance.67
Topical immunotherapy - imiquimod - may be an alternative treatment for patients with periocular BCC when the surgery is not possible to be performed. Imiquimod is an immune modulator which induces apoptosis in tumor cells and stimulates innate and adaptive immunity. Large number of studies reported the use of imiquimod in a 5% cream form for nodular skin BCC. Usually the cream is applied once per day, five times per week at least 8 to 16 weeks depending on the tumor stage. In a study with 19 patients they evaluated the efficacy of topical immunotherapy for the treatment of BCC and the histological clearance rate was about 90% after 3 months and 84% at 40 months. The 3-year interval of histological clearance rate was 100% for lesions up to 10 mm.61 In another study of 15 patients remission was observed in all patients after 3 months of imiquimod treatment and clinical remission at 24 months.68 In a study of ten patients with BCC lesions, 80% showed clinical and histological progress and remained asymptomatic for 12 months.69 Garcia-Martin et al. compared the efficacy, cosmetic results and tolerance of imiquimod and radiotherapy. In the imiquimod cream group all patients had complete clinical clearance at 24 months of follow-up. In the radiotherapy group patients received treatment 2 or 3 times per week for 5 weeks with a dose of 300 cGy per session (total administered dose was 4000-7000 cGy). Esthetic and functional results were superior in the imiquimod group, but radiotherapy was better tolerated.62 In the study of 24 patients the recurrence rate of periocular nodular BCC following treatment with imiquimod was low. The 3-year histological clearance rate was 100% (8/8) for lesions less than 1 cm and 81.8% (9/11) for lesions more than 1 cm, but significant neoadjuvant effects in larger lesion were observed.70 Vismodegib frequently needs to be undertaken in a specialist center so access for patient is not always easy, because patients with advanced disease are often elderly and this can limit their option.
Adjunctive radiotherapy possibilities
As an adjunctive therapy to high-risk aggressive BCC with perineural invasion radiotherapy is used or, it can be applied to residual inoperable tumors. It can be indicated to patients with comorbidities with high risk of surgery. National organization guidelines have established that standard therapy of BCC is complete surgical removal and radiation therapy is an option only for inoperable tumors. In certain patients it can be the only treatment modality when the post-operative defect would be cosmetically disfiguring and comorbidity is present. Irradiation therapy after histological sample should be considered as the multidisciplinary management. Today there are several types of radiotherapy for BCC: external radiotherapy and also interstitial brachytherapy. Very few studies have been conducted to define the optimum treatment in terms of recurrence rate, cosmetic outcome and sideeffects. In most of studies, the overall local control rate was between 80-100% and over 90% of patients reported good or excellent cosmetic outcome. About 25% of patients undergo radiation therapy alone for invasive orbital BCC.71 In case report of a 73-year-old man with recurrent BCC in the inner canthus extending to the orbit treated with CyberKnife stereotactic body radiation therapy the patient enjoyed rapid tumor regression, with complete remission after 6 months without toxicity.72 Brachytherapy HDR Ir192 can be used also in patients with BCC.73
The side effects after external radiotherapy but also brachytherapy can be dry eye, secondary cataract, ectropion, cicatrization of the lacrimal duct, secondary neovascular glaucoma, radiation retinopathy and maculopathy, radiation optic neuropathy which can lead to blindness.
Risk factors of recurrence and prevention
The risk of recurrence of BCC after surgery is estimated at 5%- 15%. The rate of recurrence depends on the localization, size, infiltration, histological type and previous treatment options. The most recurrences appear on the lower eye lid and in the medial canthus and in infiltrative types. Aggressive histological forms of BCC are associated with a higher risk of recurrence. In a 5-year review of periocular BCC, none of the patients with primary nodular BCC suffered recurrences, but the recurrence rate of infiltrative BCC reached up to 4%. Lesions with perineural invasion are usually regarded as more aggressive and are associated with higher rates of recurrences.35,36,74
Recurrence rate has been reported as 1%, but 3% for more aggressive forms of BCC. The recurrence rate of periorbital BCC after exenteration may be lower than after local excision or radiotherapy alone.2,4 Patients with mixed, infiltrative, morpheaform, micronodular, or any recurrent BCC should be followed up for at least 5 years interval after therapy. Patients with primary nodular BCC may be discharged after one 6-month follow-up once complete excision with clear margins was confirmed. But all patients are advised to be monitored for new lesions not only in the periocular, head and neck region, but elsewhere. The monitoring schedule does vary in many centers - whilst there is some logic to histological severity and the intensity and frequency of monitoring should include specific factors (not least their preference) that would come into play with the schedule and personalize the follow- up.75 One of important prognostic factors can be Ki-67, assay of Ki-67 expression is simple and repeatable and is recommended for evaluation of proliferative activity of malignant tumors. In most cases of BCC the values of the Ki-67 index are higher in recurrence tumors than in primary BCC.76,77 The mechanism of BCC should be further studied in order to discover additional treatment options for patients unable to undergo surgery. Avoiding extensive sun exposure can reduce the incidence of head and neck and also periocular skin cancers. Long term randomized studies with longer follow-up are required to better understand treatment outcome in patients with BCC treated with different modalities. The results of adjunctive radiotherapy, vismodegib and imiquimod also require further studies to evaluate and compare their longterm effects. Additional long term studies are required to estimate the risks factors to establish the treatment criteria for periocular BCC, because infiltrative types leading to orbital invasion may be associated even though rarely, but death. Orbital invasion may often be clinically silent, clinicians need to be alert to the possibility in high-risk tumors and consider appropriate imaging, because exenteration of the orbit is mutilating procedure, leading to cosmetic defects and wide social and psychological problems. In the large defects only relatively satisfying cosmetic result can be achieved with an individualized facial prosthesis.7
Literature search
A web-based literature search using databases such as PubMed, Scopus, Embase, Google Scholar and Directory of Open Access Journals (DOAJ) was carried out. The keywords like basal cell carcinoma, eyelid, periocular were used to search the databases. The search was limited to articles published in English language. A total of 9 490 articles were screened, and 55 articles were included in the review.
References
- 1.Saleh GM, Desai P, Collin JRO, et al. Incidence of eyelid basal cell carcinoma in England: 2000-2010. Br J Ophthalmol 2017;101:209-12. [DOI] [PubMed] [Google Scholar]
- 2.Sun MT, Wu A, Figueira E, et al. Management of periorbital basal cell carcinoma with orbital invasion. Future Oncol Lond Engl 2015;11:3003-10. [DOI] [PubMed] [Google Scholar]
- 3.Madge SN, Khine AA, Thaller VT, et al. Globe-sparing surgery for medial canthal Basal cell carcinoma with anterior orbital invasion. Ophthalmology 2010;117:2222-8. [DOI] [PubMed] [Google Scholar]
- 4.Sun MT, Wu A, Huilgol SC, Selva D. Accuracy of Biopsy in Subtyping Periocular Basal Cell Carcinoma. Ophthal Plast Reconstr Surg 2015;31:449-51. [DOI] [PubMed] [Google Scholar]
- 5.Leibovitch I, McNab A, Sullivan T, et al. Orbital invasion by periocular basal cell carcinoma. Ophthalmology 2005;112: 717-23. [DOI] [PubMed] [Google Scholar]
- 6.Shields JA, Shields CL, Demirci H, et al. Experience with eyelid- sparing orbital exenteration: the 2000 Tullos O. Coston Lecture. Ophthal Plast Reconstr Surg 2001;17:355-61. [DOI] [PubMed] [Google Scholar]
- 7.Ben Simon GJ, Schwarcz RM, Douglas R, et al. Orbital exenteration: one size does not fit all. Am J Ophthalmol 2005;139:11-7. [DOI] [PubMed] [Google Scholar]
- 8.Furdova A, Lukacko P. Periocular Basal Cell Carcinoma Predictors for Recurrence and Infiltration of the Orbit. J Craniofac Surg 2017;28:e84-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding S, Sagiv O, Guo Y, et al. Change in Eyelid Carcinoma T Category With Use of the 8th Versus 7th Edition of the American Joint Committee on Cancer: Cancer Staging Manual. Ophthal Plast Reconstr Surg 2019;35:38-41. [DOI] [PubMed] [Google Scholar]
- 10.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol 2010;146:283-7. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: A retrospective cohort study. J Am Acad Dermatol 2016;75:957-966.e2. [DOI] [PubMed] [Google Scholar]
- 12.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol 2010;146:279-82. [DOI] [PubMed] [Google Scholar]
- 13.Wehner MR, Linos E, Parvataneni R, et al. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatol 2015;151:382-8. [DOI] [PubMed] [Google Scholar]
- 14.Celebi ARC, Kiratli H, Soylemezoglu F. Evaluation of the ‘Hedgehog’ signaling pathways in squamous and basal cell carcinomas of the eyelids and conjunctiva. Oncol Lett 2016;12:467-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin VT, Merritt H, Esmaeli B. Targeting EGFR and sonic hedgehog pathways for locally advanced eyelid and periocular carcinomas. World J Clin Cases 2014;2:432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallaji Z, Rahimi H, Mirshams-Shahshahani M. Comparison of risk factors of single Basal cell carcinoma with multiple basal cell carcinomas. Indian J Dermatol 2011;56:398-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Iersel CA, van de Velden HVN, Kusters CDJ, et al. Prognostic factors for a subsequent basal cell carcinoma: implications for follow-up. Br J Dermatol 2005;153:1078-80. [DOI] [PubMed] [Google Scholar]
- 18.Levi F, Randimbison L, Maspoli M, et al. High incidence of second basal cell skin cancers. Int J Cancer 2006;119:1505-7. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran S, Fryer AA, Lovatt TJ, et al. Combined effects of gender, skin type and polymorphic genes on clinical phenotype: use of rate of increase in numbers of basal cell carcinomas as a model system. Cancer Lett 2003;189:175-81. [DOI] [PubMed] [Google Scholar]
- 20.Karagas MR, Stukel TA, Greenberg ER, et al. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA 1992;267:3305-10. [PubMed] [Google Scholar]
- 21.Lear JT, Tan BB, Smith AG, et al. Risk factors for basal cell carcinoma in the UK: case-control study in 806 patients. J R Soc Med 1997;90:371-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallberg P, Kaaman T, Lindberg M. Multiple basal cell carcinoma. A clinical evaluation of risk factors. Acta Derm Venereol 1998;78:127-9. [DOI] [PubMed] [Google Scholar]
- 23.Badri T, Zeglaoui F, Kochbati L, et al. [Multiple basal cell carcinomas following radiation therapy for nasopharyngeal cancer]. Presse Medicale Paris Fr 1983 2006;35:55-7. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Ruczinski I, Jorgensen TJ, et al. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst 2008;100:1215-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol 2000;136:1524-30. [DOI] [PubMed] [Google Scholar]
- 26.Revenga F, Paricio JF, Vázquez MM, Del Villar V. Risk of subsequent non-melanoma skin cancer in a cohort of patients with primary basal cell carcinoma. J Eur Acad Dermatol Venereol JEADV 2004;18:514-5. [DOI] [PubMed] [Google Scholar]
- 27.Lear JT, Smith AG, Strange RC, Fryer AA. Patients with truncal basal cell carcinoma represent a high-risk group. Arch Dermatol 1998;134:373. [DOI] [PubMed] [Google Scholar]
- 28.Lovatt TJ, Lear JT, Bastrilles J, et al. Associations between ultraviolet radiation, basal cell carcinoma site and histology, host characteristics, and rate of development of further tumors. J Am Acad Dermatol 2005;52:468-73. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran S, Fryer AA, Smith A, et al. Cutaneous basal cell carcinomas: distinct host factors are associated with the development of tumors on the trunk and on the head and neck. Cancer 2001;92:354-8. [DOI] [PubMed] [Google Scholar]
- 30.Wei Q, Matanoski GM, Farmer ER, et al. DNA repair related to multiple skin cancers and drug use. Cancer Res 1994;54:437-40. [PubMed] [Google Scholar]
- 31.Hajeer AH, Lear JT, Ollier WE, et al. Preliminary evidence of an association of tumour necrosis factor microsatellites with increased risk of multiple basal cell carcinomas. Br J Dermatol 2000;142:441-5. [DOI] [PubMed] [Google Scholar]
- 32.Strange RC, El-Genidy N, Ramachandran S, et al. PTCH polymorphism is associated with the rate of increase in basal cell carcinoma numbers during follow-up: preliminary data on the influence of an exon 12-exon 23 haplotype. Environ Mol Mutagen 2004;44:469-76. [DOI] [PubMed] [Google Scholar]
- 33.Ramachandran S, Fryer AA, Smith AG, et al. Basal cell carcinomas: association of allelic variants with a high-risk subgroup of patients with the multiple presentation phenotype. Pharmacogenetics 2001;11:247-54. [DOI] [PubMed] [Google Scholar]
- 34.Lim LT, Agarwal PK, Young D, et al. The Effect of Socio- Economic Status on Severity of Periocular Basal Cell Carcinoma at Presentation. Ophthal Plast Reconstr Surg 2015;31:456-8. [DOI] [PubMed] [Google Scholar]
- 35.Furdová A, Horkovičová K, Babál P, et al. [Non-melanotic Tumors of the Eyelids Skin and Inner Corner - Basocellular Carcinoma]. Ceska Slov Oftalmol Cas Ceske Oftalmol Spolecnosti Slov Oftalmol Spolecnosti 2015;71:293-301. [PubMed] [Google Scholar]
- 36.Ho SF, Brown L, Bamford M, et al. 5 Years review of periocular basal cell carcinoma and proposed follow-up protocol. Eye 2013;27:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzoutzos K, Batistatou A, Kitsos G, et al. Retrospective clinicopathological study of 129 cancerous and 18 precancerous lesions of the eyelids in North-Western Greece. Int Ophthalmol 2017;37:203-8. [DOI] [PubMed] [Google Scholar]
- 38.Collins GL, Nickoonahand N, Morgan MB. Changing demographics and pathology of nonmelanoma skin cancer in the last 30 years. Semin Cutan Med Surg 2004;23:80-3. [DOI] [PubMed] [Google Scholar]
- 39.Holmes J, von Braunmühl T, Berking C, et al. Optical coherence tomography of basal cell carcinoma: influence of location, subtype, observer variability and image quality on diagnostic performance. Br J Dermatol 2018;178:1102-10. [DOI] [PubMed] [Google Scholar]
- 40.Pasquali P, Freites-Martinez A, Fortuño-Mar A. Ex vivo highfrequency ultrasound: A novel proposal for management of surgical margins in patients with non-melanoma skin cancer. J Am Acad Dermatol 2016;74:1278-80. [DOI] [PubMed] [Google Scholar]
- 41.Cinotti E, Perrot JL, Campolmi N, et al. The role of in vivo confocal microscopy in the diagnosis of eyelid margin tumors: 47 cases. J Am Acad Dermatol 2014;71:912-918.e2. [DOI] [PubMed] [Google Scholar]
- 42.Bălăşoiu AT, Mănescu MR, Bălăşoiu M, et al. Histological and immunohistochemical study of the eyelid basal cell carcinomas. Romanian J Morphol Embryol Rev Roum Morphol Embryol 2015;56:803-10. [PubMed] [Google Scholar]
- 43.Zhang L, Huang X, Zhu X, et al. Differential senescence capacities in meibomian gland carcinoma and basal cell carcinoma. Int J Cancer 2016;138:1442-52. [DOI] [PubMed] [Google Scholar]
- 44.Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part I: periocular basal cell carcinoma experience over 7 years. Ophthalmology 2004;111:624-30. [DOI] [PubMed] [Google Scholar]
- 45.Malhotra R, Huilgol SC, Huynh NT, Selva D. The Australian Mohs database, part II: periocular basal cell carcinoma outcome at 5-year follow-up. Ophthalmology 2004;111:631-6. [DOI] [PubMed] [Google Scholar]
- 46.Newlands C, Currie R, Memon A, et al. Non-melanoma skin cancer: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016;130:S125-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: a brief literature review of clinical diagnosis and treatment. OncoTargets Ther 2017;10:2483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koyfman SA, Cooper JS, Beitler JJ, et al. ACR Appropriateness Criteria(®) Aggressive Nonmelanomatous Skin Cancer of the Head and Neck. Head Neck 2016;38:175-82. [DOI] [PubMed] [Google Scholar]
- 49.Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? Results of a randomized study. Br J Cancer 1997;76:100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S-S, Zhao Y, Zhao H, et al. A retrospective study of 2228 cases with eyelid tumors. Int J Ophthalmol 2018;11:1835-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno-Arias GA, Izento-Menezes CM, Carrasco MA, Camps-Fresneda A. Second intention healing after Mohs micrographic surgery. J Eur Acad Dermatol Venereol JEADV 2000;14:159-65. [DOI] [PubMed] [Google Scholar]
- 52.van Kester MS, Goeman JJ, Genders RE. Tissue-sparing properties of Mohs micrographic surgery for infiltrative basal cell carcinoma. J Am Acad Dermatol 2019;80:1700-3. [DOI] [PubMed] [Google Scholar]
- 53.Conway RM, Themel S, Holbach LM. Surgery for primary basal cell carcinoma including the eyelid margins with intraoperative frozen section control: comparative interventional study with a minimum clinical follow up of 5 years. Br J Ophthalmol 2004;88:236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menesi W, Buchel EW, Hayakawa TJ. A reliable frozen section technique for basal cell carcinomas of the head and neck. Plast Surg Oakv Ont 2014;22:179-82. [PMC free article] [PubMed] [Google Scholar]
- 55.Dirix L, Rutten A. Vismodegib: a promising drug in the treatment of basal cell carcinomas. Future Oncol Lond Engl 2012;8:915-28. [DOI] [PubMed] [Google Scholar]
- 56.Sekulic A, Migden MR, Basset-Seguin N, et al. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer 2017;17:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macha MA, Batra SK, Ganti AK. Profile of vismodegib and its potential in the treatment of advanced basal cell carcinoma. Cancer Manag Res 2013;5:197-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yin VT, Pfeiffer ML, Esmaeli B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthal Plast Reconstr Surg 2013;29:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Erdem GU, Sendur MAN, Ozdemir NY, et al. A comprehensive review of the role of the hedgehog pathway and vismodegib in the management of basal cell carcinoma. Curr Med Res Opin 2015;31:743-56. [DOI] [PubMed] [Google Scholar]
- 60.Gill HS, Moscato EE, Chang ALS, et al. Vismodegib for periocular and orbital basal cell carcinoma. JAMA Ophthalmol 2013;131:1591-4. [DOI] [PubMed] [Google Scholar]
- 61.Papastefanou VP, René C. Secondary Resistance to Vismodegib After Initial Successful Treatment of Extensive Recurrent Periocular Basal Cell Carcinoma with Orbital Invasion. Ophthal Plast Reconstr Surg 2017;33:S68-70. [DOI] [PubMed] [Google Scholar]
- 62.Garcia-Martin E, Gil-Arribas LM, Idoipe M, et al. Comparison of imiquimod 5% cream versus radiotherapy as treatment for eyelid basal cell carcinoma. Br J Ophthalmol 2011;95:1393-6. [DOI] [PubMed] [Google Scholar]
- 63.Karabulut GO, Kaynak P, Ozturker C, et al. Imiquimod 5% cream for the treatment of large nodular basal cell carcinoma at the medial canthal area. Indian J Ophthalmol 2017;65:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kahana A, Worden FP, Elner VM. Vismodegib for Eye- Threatening Orbital Basal Cell Carcinoma: A Clinicopathologic Report. JAMA Ophthalmol 2013;131:1364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demirci H, Worden F, Nelson CC, et at. Efficacy of Vismodegib (Erivedge) for Basal Cell Carcinoma Involving the Orbit and Periocular Area. Ophthal Plast Reconstr Surg 2015;31:463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahradyan A, Howell AC, Wolfswinkel EM, et al. Updates on the Management of Non-Melanoma Skin Cancer (NMSC). Healthc Basel Switz 2017;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcovich S, Colloca G, Sollena P, et al. Skin Cancer Epidemics in the Elderly as An Emerging Issue in Geriatric Oncology. Aging Dis 2017;8:643-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Martin E, Idoipe M, Gil LM, et al. Efficacy and tolerability of imiquimod 5% cream to treat periocular basal cell carcinomas. J Ocul Pharmacol Ther Off J Assoc Ocul Pharmacol Ther 2010;26:373-9. [DOI] [PubMed] [Google Scholar]
- 69.Carneiro RC, de Macedo EMS, Matayoshi S. Imiquimod 5% cream for the treatment of periocular Basal cell carcinoma. Ophthal Plast Reconstr Surg 2010;26:100-2. [DOI] [PubMed] [Google Scholar]
- 70.de Macedo EMS, Carneiro RC, de Lima PP, et al. Imiquimod cream efficacy in the treatment of periocular nodular basal cell carcinoma: a non-randomized trial. BMC Ophthalmol 2015;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Velter C. Place de la radiothérapie dans le traitement du carcinome basocellulaire. Ann Dermatol Vénéréologie 2018; 145:VS30-5. [DOI] [PubMed] [Google Scholar]
- 72.Pontoriero A, Iatì G, Conti A, et al. Treatment of periocular basal cell carcinoma using an advanced stereotactic device. Anticancer Res 2014;34:873-5. [PubMed] [Google Scholar]
- 73.Furdová A, Lukačko P, Lederleitner D. [HDR 192Ir brachytherapy in treatment of basal cell carcinoma of the lower eyelid and inner angle - our experience]. Ceska Slov Oftalmol Cas Ceske Oftalmol Spolecnosti Slov Oftalmol Spolecnosti 2013;69:75-9. [PubMed] [Google Scholar]
- 74.Honavar SG, Manjandavida FP. Tumors of the ocular surface: A review. Indian J Ophthalmol 2015;63:187-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bader RS. What are the NCCN guidelines for follow-up and surveillance of patients with basal cell carcinoma (BCC)? [Internet]. 2019. [cited 2019 Nov 9]. Available from: https://www.medscape.com/answers/276624-100209/whatare-the-nccn-guidelines-for-follow-up-and-surveillance-ofpatients-with-basal-cell-carcinoma-bcc [Google Scholar]
- 76.Süngü N, Kiran MM, Tatli Doğan H, et al. Evaluation of p53 and Ki67 Expression Profiles in Basal Cell Carcinomas in a Usual and an Unusual Location. Turk Patoloji Derg 2018;34:165-70. [DOI] [PubMed] [Google Scholar]
- 77.Kramer E, Herman O, Frand J, et al. Ki67 as a biologic marker of basal cell carcinoma: a retrospective study. Isr Med Assoc J IMAJ 2014;16:229-32. [PubMed] [Google Scholar]


