Abstract
Background
Patients with bronchiectasis are at increased risk of developing non-tuberculous mycobacteria lung disease (NTM-LD), and published guidelines recommend regular testing for NTM infection in this patient population.
Objective
This study aimed to survey physicians managing patients with bronchiectasis to understand the perceived risk of NTM to their patients, perceived disease severity and frequency of testing for NTM.
Methods
The study comprised an online survey of hospital-based physicians in the UK, Germany, Italy, France and the Netherlands. The target group were hospital-based physicians who had managed at least 10 adult patients with bronchiectasis over the preceding 12 months.
Results
In total, 280 physicians completed the survey. Most (87%) thought their patients to be at particular risk of NTM, although it was perceived as a moderate risk versus other respiratory pathogens. Most perceived NTM-LD to impact patient morbidity (84%), and 61% indicated that NTM-LD significantly impacted mortality. 68% of all respondents did not test for NTM prior to initiating macrolide monotherapy, despite guidelines recommending testing. The perceived risk of and screening for NTM varied among countries.
Conclusions
The study demonstrates that physicians understand the risk of NTM-LD and associated morbidity in patients with bronchiectasis; however, a minority do not perceive that NTM-LD significantly affects mortality. Greater awareness of the need to test for NTM infection before initiating macrolide monotherapy for bronchiectasis is essential due to potential emergence of drug-resistant NTM.
Keywords: bronchiectasis, atypical mycobacterial infection, respiratory infection
Key messages.
What is the question?
How do physicians managing patients with bronchiectasis perceive the risk and disease severity of non-tuberculous mycobacteria lung disease (NTM-LD) in their patients, and how often and when do they test for NTM?
What is the bottom line?
Physicians managing patients with bronchiectasis understand the association between bronchiectasis and the risk of NTM-LD, although current NTM screening practices prior to introducing macrolide monotherapy treatment are not compliant with existing guidelines.
Why read on?
This study gives an insight into how pulmonologists perceive NTM in relation to their patients with bronchiectasis in terms of disease severity, and highlights a lack of understanding of when patients with bronchiectasis should be tested for NTM infection.
Introduction
Non-tuberculous mycobacteria (NTM) are ubiquitous environmental bacteria, capable of causing opportunistic infection in humans.1 2 NTM lung disease (NTM-LD) is by far the most common clinical manifestation of NTM diseases.1 Two forms of the disease are generally recognised: the slowly progressing nodular-bronchiectatic form, most commonly associated with postmenopausal women and the fibrocavitary form, which has a much more rapid progression and is associated with middle-aged (former) smokers with a history of underlying lung disease.3
In particular, patients with bronchiectasis and chronic obstructive pulmonary disease (COPD), especially those treated with corticosteroids, have been found to have a substantially increased risk of NTM-LD. One study reported an adjusted ORs of 187.5 (95% CI 24.8 to 1417.4) among patients with bronchiectasis and 15.7-fold (95% CI 11.4 to 21.5) among patients with COPD.4 NTM isolation prevalence in patients with bronchiectasis ranges from 2% to 63%.5–8
European guidelines for the management of bronchiectasis recommend regular testing for NTM isolation, particularly where NTM are suspected as a cause of bronchiectasis. Testing should be carried out prior to initiation of long-term macrolide monotherapy to prevent exacerbations, in order to avoid development of macrolide-resistant NTM.9 10 Local guidelines from the UK from 2010 for management of bronchiectasis recommend regular monitoring for NTM if patients prove culture positive for NTM.11 Yet, the physician perception of the risk of NTM-LD in patients with bronchiectasis—and its impact on testing practices—has never been investigated.
In this study, we surveyed hospital-based physicians managing adult patients with bronchiectasis in the UK, Germany, Italy, France and the Netherlands. Our aims were to understand current testing practices in the surveyed countries, and to measure the current perception of risk and severity of NTM-LD in the adult population with bronchiectasis.
Methods
The physician survey was carried out via a secure online platform. Surveys were conducted in the respondent’s native language, and respondents received a financial incentive for participation in the study. The target group were hospital based (spending at least 80% of their time in the clinic) pulmonologists who had managed at least 10 adult patients with bronchiectasis over the preceding 12 months. The survey took place in the UK, Germany, Italy, France and the Netherlands.
Panel members were recruited via phone, referral schemes and via conferences by M3 Global Research (Abingdon, UK). Registered panellists were required to update their profile on a bimonthly basis, and their credentials were verified against bodies that maintain official registers of pulmonologists in each country. To ensure that the panel was representative of the total population of pulmonologist in each market, the recruitment process was randomised.
M3 Global Research holds European Union (EU) compliance certifications to ensure the protection of panel information (eg, Data Protection Authority (DPA), European Pharmaceutical Market Research Association (EphMRA), British Healthcare Business Intelligence Association (BHBIA), General Data Protection Regulation (GDPR), physician verification). The panel is also ISO 26362 certified.
The following terms were used in the survey: ‘NTM’ when asking questions about testing for the presence of NTM in respiratory secretions; ‘NTM infection’ when asking about risk of contracting NTM leading to NTM-LD, that is, NTM sputum culture positivity; and ‘NTM-LD’ when asking about agreement with statements regarding the clinical impact of NTM-LD.
Spearman’s r (correlation coefficient) was calculated to explore correlations between ordinal responses. Group differences were evaluated by Mann-Whitney U test or X2 test for ordinal or categorical variables, respectively. The physician survey is included in online supplementary appendix 1.
bmjresp-2019-000498supp001.pdf (658.8KB, pdf)
Results
Respondent characteristics
The number of physicians invited, complete and incomplete surveys, and number of respondents screened out are detailed in online supplementary table S1. The survey was closed when the targeted number of surveys were completed: Germany (n=60), the UK, (n=60), Italy (n=60), France (n=60) and the Netherlands (n=40). Relatively few respondents who treated patients with NTM were excluded during the screening process, although more physicians from Germany who were not hospital based were excluded from the survey compared with physicians from other countries (see online supplementary table S2). The sample of respondents included physicians working in academic and general hospitals, and was spread across the regions in the countries (see online supplementary figure S1).
bmjresp-2019-000498supp002.pdf (1.7MB, pdf)
Respondents reported managing an average of 51 patients with bronchiectasis over the preceding 12 months; this ranged from 31 patients in the Netherlands to 66 in the UK, and 74% of respondents had personally managed at least one patient with NTM-LD during this period. On average, respondents managed 10 patients with NTM-LD over the 12-month period, ranging from 4 in the Netherlands to 17 in Germany. Respondents treating more patients with bronchiectasis also managed more patients with NTM-LD (Spearman’s r 0.48, p<0.05).
Perceived risk of NTM infection in patients with bronchiectasis
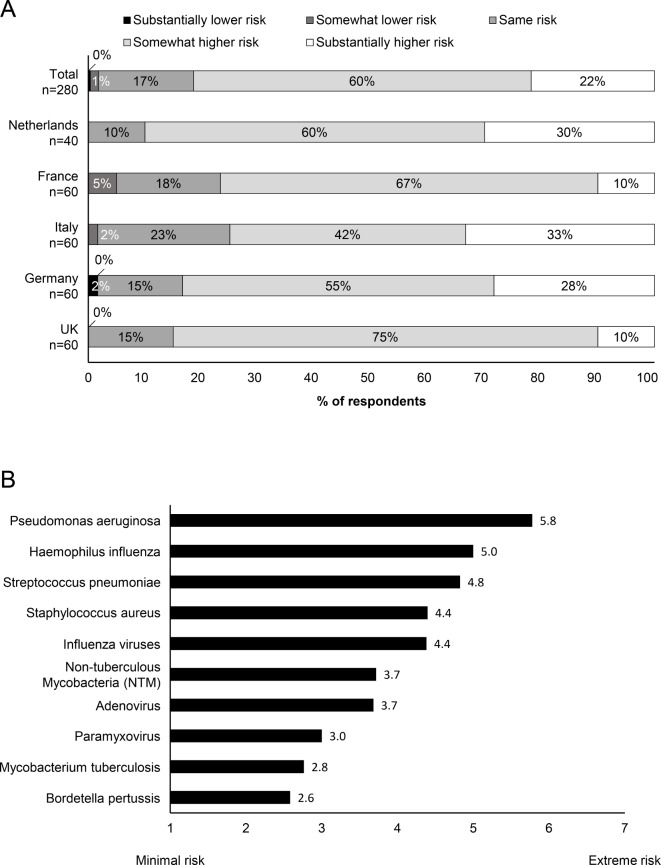
The majority of surveyed physicians (87%; range 77% in Italy to 93% in the Netherlands) considered their patients with bronchiectasis to be at particular risk of NTM infection (table 1A). Overall 82% of respondents considered patients with bronchiectasis to be at somewhat higher or substantially higher risk of NTM infection then patients with moderate-to-severe COPD (figure 1A).
Table 1.
Percentage of physicians considering patients with bronchiectasis to be at particular risk of NTM infection (A) and estimated percentage of adult patients with bronchiectasis contracting NTM during the course of their disease (B)
| Country (n)* | A: Perceived risk for NTM infection | B: Estimated NTM prevalence* |
| UK | 90% (n=60) | 13% (n=47) |
| Germany | 92% (n=60) | 21% (n=44) |
| Italy | 77% (n=60) | 23% (n=40) |
| France | 87% (n=60) | 17% (n=45) |
| The Netherlands | 93% (n=40) | 12% (n=26) |
| Total | 87% (n=280) | 18% (n=202) |
*Respondents who could not estimate the risk of NTM infection in their patients were not included in this analysis.
NTM, non-tuberculous mycobacteria.
Figure 1.
Perceived risk of NTM and respiratory infections in patients with bronchiectasis (A) Perceived risk of NTM infection in patients with bronchiectasis versus patients with moderate-to-severe COPD. (B) Perceived risk of respiratory infections for patients with bronchiectasis. Risk was rated from 1 (minimal risk) to 7 (extreme risk). COPD, chronic obstructive pulmonary disease.
Survey participants ranked the risk of NTM infection in patients with bronchiectasis (on a scale of 1 (minimal risk) to 7 (extreme risk)) as ‘moderate’, equivalent to adenovirus (rated at 3.7), with Pseudomonas aeruginosa being ranked as the respiratory pathogen with the greatest risk (5.8) and Bordetella pertussis with the lowest risk (2.6) (figure 1B).
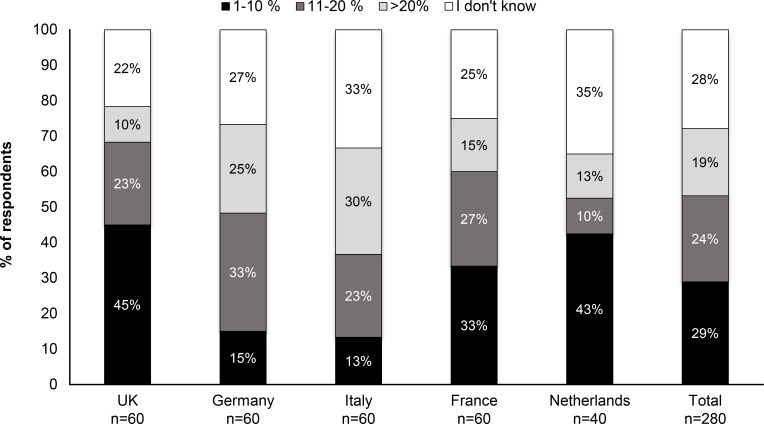
Overall, 53% of respondents estimated the risk of their patients with bronchiectasis contracting NTM to be ≤20%. This rating varied widely between the surveyed countries; for example, in the UK 68% respondents estimated a risk of ≤20%, whereas in Italy only 36% gave this estimate (figure 2). Respondents estimated the number of patients with bronchiectasis who contracted an NTM infection during the course of their disease as 18% (table 1B), which is similar to the overall estimates of the percentage of patients with bronchiectasis that had been tested were tested positive for NTM (17%) (figure 3A).
Figure 2.
Reported proportion of patients with bronchiectasis contracting NTM. NTM, non-tuberculous mycobacteria.
Figure 3.
Testing for NTM infection in patients with bronchiectasis. (A) Proportion of patients with bronchiectasis tested for NTM. (B) Triggers for NTM testing in patients with bronchiectasis. NTM, non-tuberculous mycobacteria.
Perception of NTM severity
Most physicians across the surveyed countries judged NTM-LD as a significant factor for worsening of respiratory function (89%), increasing morbidity and hospitalisation frequency (84%) and mortality risk (61%). A quarter of respondents were unsure about the impact of NTM-LD on mortality risk (table 2, online supplementary table S3).
Table 2.
Agreement with statements regarding impact of NTM-LD on morbidity and mortality
| Countries | Strongly agree | Agree | Unsure | Disagree | Strongly disagree |
| ‘NTM-LD significantly increases morbidity and leads to more frequent hospitalisations’* | |||||
| UK | 12 | 73 | 12 | 3 | 0 |
| Germany | 25 | 58 | 15 | 2 | 0 |
| Italy | 13 | 67 | 10 | 10 | 0 |
| France | 22 | 70 | 8 | 0 | 0 |
| The Netherlands | 13 | 65 | 20 | 3 | 0 |
| Mean percentage | 17 | 67 | 13 | 4 | 0 |
| ‘NTM-LD has no significant impact on mortality risk, as mortality is determined by the underlying condition’ | |||||
| UK | 3 | 10 | 23 | 55 | 8 |
| Germany | 0 | 7 | 18 | 53 | 22 |
| Italy | 0 | 13 | 32 | 43 | 12 |
| France | 2 | 20 | 28 | 32 | 18 |
| The Netherlands | 3 | 10 | 25 | 53 | 10 |
| Mean percentage | 1 | 12 | 25 | 47 | 14 |
The percentage of physicians choosing an answer is shown. n=280.
*Agreement with this statement weakly correlated with perceived risk of NTM in patients with bronchiectasis (Spearman’s r 0.185, p<0.01) and number of patients with NTM managed (Spearman’s r 0.174, p<0.01).
NTM-LD, non-tuberculous mycobacterial lung disease.
Testing for NTM infection in patients with bronchiectasis
The majority of respondents (85%) tested at least some of their patients with bronchiectasis for NTM culture positivity, and this proportion was similar in all countries (see online supplementary figure S2A). Of those not testing their patients for NTM infection, 78% indicated they had not suspected NTM in any patient, 17% preferred to send the patient to an expert for diagnosis and 5% were unaware how to test and diagnose NTM (see online supplementary figure S2B).
Those physicians who did not test for NTM sputum culture positivity perceived NTM as a lower risk (2.8 vs 3.9, p<0.01). Additionally, these physicians managed significantly fewer patients with NTM-LD (2.9 vs 11.7, p<0.01) and fewer patients with bronchiectasis (30 vs 55, p<0.01) than those who did test. Respondents who tested their patients with bronchiectasis for NTM tested 51% (range: 40% (France) to 70% (the Netherlands)) of their patients (figure 3A). Overall, an average of 17% patients who were tested were estimated to be positive for NTM (figure 3A).
Overall, 49% of respondents tested all patients with bronchiectasis for NTM culture positivity at diagnosis or initial presentation. Changes in radiological features of the lung that lead to suspicion of NTM-LD were the main prompt for surveyed physicians to test for NTM. (figure 3B).
In terms of frequency of testing for sputum culture positivity, 64% of respondents test at least once per year (see online supplementary figure S3). There was a trend for managing a greater number of patients with NTM among those respondents testing every 6 months in comparison to those physicians testing less frequently for NTM (14.4 vs 8.6 patients, p<0.05). Referral to or management by other physicians was the most important reason for less frequently testing for NTM, reported by 49% of respondents (see online supplementary table S4).
Management of patients with bronchiectasis
Physicians reported that 42% of their patients with bronchiectasis received long-term (≥3 months) macrolide monotherapy (range: 35% in France to 60% in the Netherlands, p=0.06) (figure 4A). Only 38% of physicians testing for NTM carried out the test prior to initiating macrolide monotherapy to manage patients with bronchiectasis (range: 24% (Germany) to 66% (UK)) (figure 4B). Considering all physicians surveyed, 68% do not test for NTM prior to initiating macrolide monotherapy. In terms of guidelines followed for management of respiratory diseases, 79% respondents’ employed European Respiratory Society (ERS) guidelines, 40% American Thoracic Society (ATS) guidelines (which are not specific for patients with bronchiectasis) and 34% local or national guidelines overall across the surveyed countries (data not shown). Testing for NTM before initiating macrolide monotherapy was not related to following/using guidelines for treatment decisions. Namely, 94% of physicians reported to follow or use the guidelines in either case, that is, testing or not testing for NTM before initiating macrolide monotherapy.
Figure 4.
Use of macrolides in patients with bronchiectasis. (A) Prescription of long-term macrolide (eg, clarithromycin, azithromycin) monotherapy (≥3 months) to patients with bronchiectasis. (B) Testing for NTM before initiating a macrolide monotherapy for the treatment of bronchiectasis (respondents who did not test for NTM did not answer this question). NTM, non-tuberculous mycobacteria.
Discussion
A low index of suspicion among physicians regarding the risk of NTM-LD and its severity may mean that physicians are less likely to test their patients with chronic lung disease for NTM culture positivity. This may lead to NTM-LD going undetected, development of macrolide resistance and worse longer-term outcomes for patients.12 13 Our study aimed to identify how physicians perceive risk of NTM infection in patients with bronchiectasis, how they monitor their patients for NTM infection and whether they adhere to published guidelines for management of respiratory diseases.
Bronchiectasis is an even stronger risk factor for the development of NTM-LD in comparison with COPD,4 and most surveyed physicians appeared to have an understanding of this. This risk is perceived as moderate by the participating physicians in comparison with the risk of contracting P. aeruginosa and Haemophilus influenzae, probably since these are regularly identified as causative agents of exacerbations in patients with COPD and bronchiectasis.9 14 15
Despite this, there was a general agreement among the surveyed physicians that NTM-LD significantly increases morbidity, leading to increased hospitalisations, and that it has a large clinical impact, in line with recently published observations.16 In contrast, the proportion of respondents who perceived a significant impact of NTM-LD on mortality was somewhat lower overall. Studies exploring the impact of NTM-LD on mortality have recently been published, showing that NTM-LD is associated with an increased risk of death irrespective of other comorbidities.16–18 Previous studies showed that patients with specific NTM-LD manifestations like cavitary disease19 or specific NTM species like Mycobacterium xenopi20 have a higher mortality risk. Specific NTM-LD registries may provide further evidence on the respective risk factors in the future.21 22
The perception of risk of NTM among respondents was associated with the number of patients with NTM managed, for example, physicians who manage more patients with NTM-LD have a better awareness of NTM-LD. This reinforces the idea that NTM-LD should be managed in collaboration with physicians with expertise in the treatment of these patients as recommended in national guidelines in the UK and USA.3 23 Another possible explanation for the perceived lack of impact of NTM on mortality is insufficient data regarding the long-term follow-up of patients with NTM, as registries for patients with NTM-LD have only recently been started in Europe.21 22
We identified that most surveyed physicians tested their patients for NTM infection and approximately half tested all of their patients with bronchiectasis at initial presentation or diagnosis. Testing behaviour was significantly associated with the number of NTM-LD and patients with bronchiectasis managed, with those physicians who did not test their patients for NTM tending to manage fewer patients with NTM or bronchiectasis than those who did test.
These findings differ from a recent Europe-wide longitudinal cohort study forming part of the European bronchiectasis registry (European Multicentre Bronchiectasis Audit and Research Collaboration, EMBARC) which shows approximately 30% of patients were tested for NTM infection, regardless of the number of relevant comorbidities present.24 As the EMBARC registry is a much larger study covering a wide range of European countries and centres, it may be more reflective of trends in the whole of Europe rather than the five countries surveyed in our study. Other triggers for testing included radiology results, structural lung defects and worsening symptoms. This is an encouraging finding as it shows many physicians are aware of the risk of NTM infection in patients with bronchiectasis. Our finding of common triggers of NTM testing including increased symptoms or complications is in line with results from EMBARC showing that severe exacerbations are a predictor of NTM testing in patients with bronchiectasis.24 In this study, the main predictor for NTM testing by far was a history of NTM infection. It is unclear how this finding applies to the patient population surveyed in our study as information regarding whether respondent’s patients had previously suffered from NTM-LD was not available.
Both the ERS bronchiectasis and European/US Cystic Fiboris (CF) guidelines recommend testing for NTM culture positivity prior to initiation of macrolide monotherapy.9 10 Despite most respondents stating that they follow ERS guidelines, all aspects of these recommendations do not appear to be put into practice. This is also reflected by other studies that show that adherence to guideline recommendations is often poor.25–27
Long-term macrolide monotherapy is often used to prevent exacerbations inpatients with bronchiectasis,9 28 reflected in the high proportion of the patients with bronchiectasis reported in our survey receiving the treatment. However, macrolide monotherapy is not recommended for treatment of NTM-LD, as there is an increased risk of macrolide resistance emergence, which decreases the chance of an NTM-LD cure.12 13 Since 68% of survey respondents do not test their patients for NTM prior to starting macrolide treatment, education of physicians, alongside promotion and enforcement of current guidelines9 is necessary. While studies still have to show whether the risk of macrolide-resistant NTM disease is increased in patients receiving macrolide maintenance treatment, case reports have recorded this phenomenon.29 Conversely, macrolide monotherapy may be beneficial and even reduce the incidence of NTM culture positivity in patients with bronchiectasis who are not yet infected with NTM, as suggested by a study of patients with CF.30
Our survey has several limitations: The sample size of this survey (n=280) is limited relative to the European population; however, there was good regional coverage within countries among the respondents. Moreover, as the information collected on the rate of NTM testing and risk was based on estimates by the responding physicians rather than on data from medical records, this may have resulted in some degree of over and/or under-representation of the real rates of testing and level of risk. Similarly the fact that the physicians received a small incentive for their participation may have resulted in overreporting of treated patients with bronchiectasis. Our study also did not explore how patients with bronchiectasis or NTM-LD are managed in individual countries, or the severity of bronchiectasis disease in the patients managed by the respondents. This information could help to understand the noted differences between countries, however, further research is needed here.
The results indicate that physicians treating adult patients with bronchiectasis are aware of the association between bronchiectasis and the risk of NTM-LD. The majority of physicians perceive that NTM-LD can lead to severe health consequences, although a minority do not perceive that it can lead to a significant increase in mortality. Testing practices when diagnosing bronchiectasis as well as prior to starting macrolide maintenance therapy must be improved to be compliant with existing guidelines and improve the care of patients with bronchiectasis.
Acknowledgments
IDR Medical (Cheltenham, UK) performed the survey on behalf of the authors. Medical writing support was provided by Christopher Lamb of Physicians World Europe GmbH (Mannheim, Germany).
Footnotes
Contributors: All authors contributed to the study design and data analysis, and reviewed and approved the manuscript.
Funding: Funding for this study was provided by Insmed.
Competing interests: JvI: Advisory board member for Insmed. Supported by a personal grant from the Netherlands organisation for scientific research NOW/ ZonMW (Veni 016.176.024). RvdL: Employee of Insmed Netherlands BV. MO: Employee of Insmed Germany GmbH.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 2014;6:210–20. 10.3978/j.issn.2072-1439.2013.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu U-I, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis 2015;15:968–80. 10.1016/S1473-3099(15)00089-4 [DOI] [PubMed] [Google Scholar]
- 3.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 4.Andréjak C, Nielsen R, Thomsen Vibeke Ø, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax 2013;68:256–62. 10.1136/thoraxjnl-2012-201772 [DOI] [PubMed] [Google Scholar]
- 5.Máiz L, Girón R, Olveira C, et al. Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect Dis 2016;16:437 10.1186/s12879-016-1774-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirsaeidi M, Hadid W, Ericsoussi B, et al. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis 2013;17:e1000–4. 10.1016/j.ijid.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksamit TR, O'Donnell AE, Barker A, et al. Adult patients with bronchiectasis: a first look at the US bronchiectasis research registry. Chest 2017;151:982–92. 10.1016/j.chest.2016.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shteinberg M, Stein N, Adir Y, et al. Prevalence, risk factors and prognosis of nontuberculous mycobacterial infection among people with bronchiectasis: a population survey. Eur Respir J 2018;51. 10.1183/13993003.02469-2017. [Epub ahead of print: 10 May 2018]. [DOI] [PubMed] [Google Scholar]
- 9.Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017;50:1700629 10.1183/13993003.00629-2017 [DOI] [PubMed] [Google Scholar]
- 10.Floto RA, Olivier KN, Saiman L, et al. Us cystic fibrosis Foundation and European cystic fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: Executive summary. Thorax 2016;71:88–90. 10.1136/thoraxjnl-2015-207983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasteur MC, Bilton D, Hill AT, et al. British thoracic Society guideline for non-CFbronchiectasis. Thorax 2010;65:i1–58. 10.1136/thx.2010.136119 [DOI] [PubMed] [Google Scholar]
- 12.Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in complex lung disease. Am J Respir Crit Care Med 2006;174:928–34. 10.1164/rccm.200603-450OC [DOI] [PubMed] [Google Scholar]
- 13.Aksamit TR, Philley JV, Griffith DE. Nontuberculous mycobacterial (NTM) lung disease: the top ten essentials. Respir Med 2014;108:417–25. 10.1016/j.rmed.2013.09.014 [DOI] [PubMed] [Google Scholar]
- 14.Loebinger MR, Wells AU, Hansell DM, et al. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009;34:843–9. 10.1183/09031936.00003709 [DOI] [PubMed] [Google Scholar]
- 15.McDonnell MJ, Aliberti S, Goeminne PC, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016;71:1110–8. 10.1136/thoraxjnl-2016-208481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diel R, Jacob J, Lampenius N, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J 2017;49:1602109 10.1183/13993003.02109-2016 [DOI] [PubMed] [Google Scholar]
- 17.Diel R, Lipman M, Hoefsloot W. High mortality in patients with Mycobacterium avium complex lung disease: a systematic review. BMC Infect Dis 2018;18:206 10.1186/s12879-018-3113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marras TK, Campitelli MA, Lu H, et al. Pulmonary nontuberculous Mycobacteria–Associated deaths, Ontario, Canada, 2001–2013. Emerg Infect Dis 2017;23:468–76. 10.3201/eid2303.161927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh W-J, Moon SM, Kim S-Y, et al. Outcomes of Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J 2017;50:1602503 10.1183/13993003.02503-2016 [DOI] [PubMed] [Google Scholar]
- 20.Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med 2010;181:514–21. [DOI] [PubMed] [Google Scholar]
- 21.Aliberti S, Codecasa LR, Gori A, et al. The Italian registry of pulmonary non-tuberculous mycobacteria - IRENE: the study protocol. Multidiscip Respir Med 2018;13:33. 10.1186/s40248-018-0141-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aliberti S, Polverino E, Chalmers JD, et al. The European multicentre bronchiectasis audit and research collaboration (EMBARC) ERS clinical research collaboration. Eur Respir J 2018;52:1802074 10.1183/13993003.02074-2018 [DOI] [PubMed] [Google Scholar]
- 23.Haworth CS, Banks J, Capstick T, et al. British thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax 2017;72:ii1–64. 10.1136/thoraxjnl-2017-210927 [DOI] [PubMed] [Google Scholar]
- 24.Loebinger M, Ringshausen F, Haworth C, et al. Characteristics of patients with pulmonary non-tuberculous mycobacterial infection in bronchiectasis: data from the EMBARC registry. Eur Respir J 2018;52. [Google Scholar]
- 25.Adjemian J, Prevots DR, Gallagher J, et al. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc 2014;11:9–16. 10.1513/AnnalsATS.201304-085OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marras TK, Prevots DR, Jamieson FB, et al. Opinions differ by expertise in Mycobacterium avium complex Disease. Ann Am Thorac Soc 2014;11:17–22. 10.1513/AnnalsATS.201305-136OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Ingen J, Wagner D, Gallagher J, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J 2017;49:1601855 10.1183/13993003.01855-2016 [DOI] [PubMed] [Google Scholar]
- 28.McShane PJ, Naureckas ET, Tino G, et al. Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013;188:647–56. 10.1164/rccm.201303-0411CI [DOI] [PubMed] [Google Scholar]
- 29.Kipourou M, Manika K, Papavasileiou A, et al. Immunomodulatory effect of macrolides: at what cost? Respir Med Case Rep 2016;17:44–6. 10.1016/j.rmcr.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coolen N, Morand P, Martin C, et al. Reduced risk of nontuberculous mycobacteria in cystic fibrosis adults receiving long-term azithromycin. J Cyst Fibros 2015;14:594–9. 10.1016/j.jcf.2015.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2019-000498supp001.pdf (658.8KB, pdf)
bmjresp-2019-000498supp002.pdf (1.7MB, pdf)