Abstract
Background
Polyacrylamide hydrogel (PAAG) was used as an injectable implant for augmentation mammoplasty for over 30 years, but its use was ceased due to various related complications. The only way to treat these complications is PAAG removal, but this causes breast ptosis, nipple retraction, breast asymmetry, and skin laxity.
Objectives
This article reports a new technique for breast reshaping after PAAG removal without prosthesis implantation.
Method
From January 2015 to June 2018, twenty-three patients underwent periareolar mammoplasty with the tissue folding technique (PMTFT) for breast reshaping after PAAG removal. Postoperative breast shape and the degree of satisfaction of the patients were evaluated during follow-up.
Results
All patients recovered well without severe complications. All patients were satisfied with their postoperative breast shape and their symptoms were relieved after surgery.
Conclusions
PMTFT provides satisfactory postoperative breast shape results. Economical, practical, and technical advantages were found over traditional prosthesis-mediated breast reconstruction. PMTFT can be an ideal surgical choice in appropriate cases.
Keywords: Periareolar mammoplasty, Tissue folding technique, Polyacrylamide hydrogel, Augmentation mammoplasty
Introduction
Polyacrylamide hydrogel (PAAG) originated in Ukraine and was used for augmentation mammoplasty implants from 1987. To date, PAAG has been applied in more than 30 countries from the late 1980s. More than 200,000 women in China have undergone PAAG augmentation mammoplasty [1]. As a jelly-like substance, PAAG can integrate with fatty tissue after being injected into the breast. Satisfactory appearance results and convenient manipulation made PAAG, advertised as “artificial fatty tissue,” a star in the field of augmentation mammoplasty. Although biological safety has only been reported in several animal experiments, PAAG as an injectable augmentation mammoplasty implant has been reported with varying degrees of side effects, such as pain, infection, migration (e.g., to the axilla, the forearms, the thoracic-abdominal wall, the abdominal wall, and the legs), mass formation, breast cancer, and diffuse stiffness [2]. Chen et al. [3] reported that the complication rate of PAAG for injection augmentation mammoplasty was over 20%.
Surgical removal of PAAG is the only method for treating gel-related complications. Simple PAAG removal surgery is associated with postoperative breast ptosis, nipple retraction, breast asymmetry, and skin laxity (Fig. 1). To solve this aesthetic problem, immediate or delayed augmentation mammoplasty after PAAG removal was implemented, including prosthesis implantation and autologous tissue breast reconstruction. Use of these methods was restricted due to prosthesis-related complications and technique-related issues. In this study, we present a novel surgery technique for breast reconstruction after PAAG removal, which uses periareolar mammoplasty with the tissue folding technique (PMTFT) to obtain a satisfactory cosmetic appearance without prosthesis implants or complex autologous tissue reconstruction. Its value will be assayed in further clinical practice.
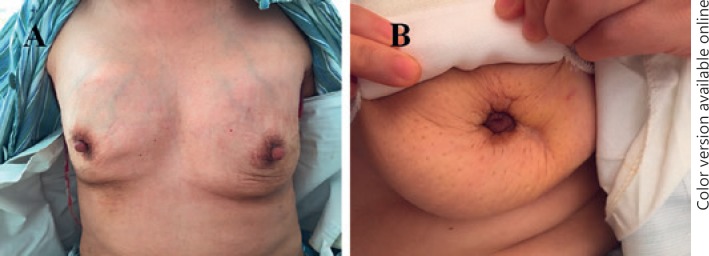
Fig. 1.
Unsatisfactory appearance of patients who underwent PAAG removal surgery. A The patient had breast asymmetry and skin laxity after PAAG removal. B The patient had nipple retraction after PAAG removal.
Materials and Methods
Patients
The results of 23 patients who underwent PMTFT after PAAG removal in our department from January 2015 to June 2018 were retrospectively collected. Patients who suffered from acute inflammation were not included in this study. Both ultrasound and magnetic resonance imaging (MRI) were performed for estimation of the volume of PAAG and breast tissue and the distribution and migration of PAAG before the surgery. Patients diagnosed with synchronous breast cancers by intraoperative frozen pathology were excluded from this study.
Surgical Technique
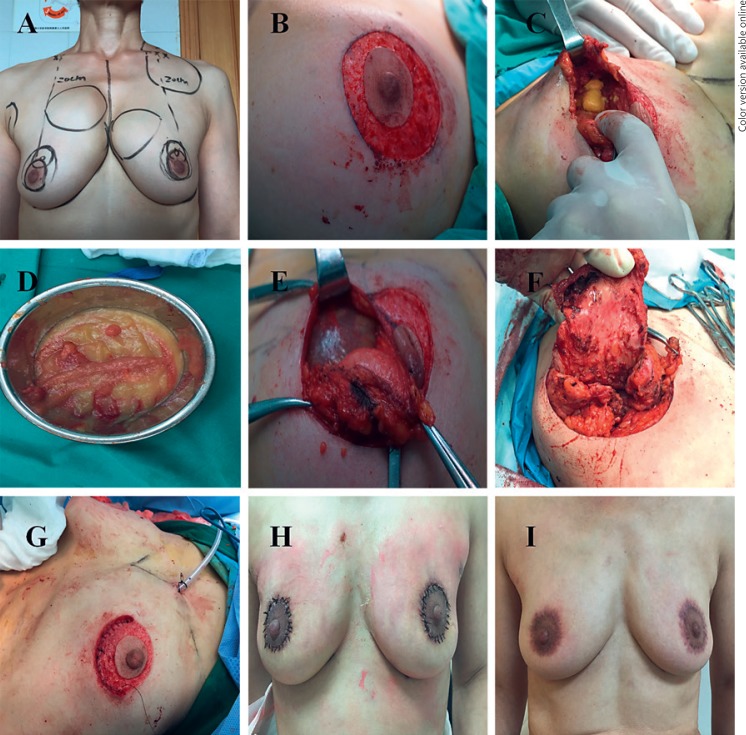
Prior to the surgery, the new nipple position and areola size were designed and marked on the skin in a standing position (Fig. 2A). Under general anesthesia, the patient was placed in a supine position with both arms stretched before sterilization. According to the new nipple and areola position, 2 concentric circle-shaped incisions were made (i.e., inner ring and outer ring incisions). The depth of the 2 incisions was restricted, retaining the dermis layer to preserve the blood supply to the nipple areola complex (NAC). The skin between the 2 rings was deepithelialized (Fig. 2B). The separation of the subcutaneous level extended to the border of external upper quadrant and the inner upper quadrant of the breast. Then a pseudocapsule was opened to expose the PAAG (Fig. 2C). All visible PAAG with involved glandular and muscular tissues were aspirated or resected (Fig. 2D). The excised PAAG, pseudocapsule, and mass were sent for frozen pathological analysis to exclude malignant tumor. The glandular flap originating from the surrounding mammary tissue in the external upper quadrant and the inner quadrant was folded as tissue flap and was mounded into the posterior space of the breast (Fig. 2E, F). The flap was adjusted to ensure a satisfactory shape and positioning were achieved before sutured, correcting nipple inversion after PAAG removal and reshaping the submammary folds. Drainage was placed after hemostasis (Fig. 2G). The outer ring was tightened to match the inner ring by performing a string suture. Finally, the skin of the inner and outer rings was sutured (Fig. 2H).
Fig. 2.
The patient underwent PMTFT for breast reshaping following PAAG removed. A The patient had PAAG migration to her chest wall and clavicle area before the surgery. B The skin between the 2 rings was deepithelialized after performance of the double ring incision. C PAAG was removed from patient. D Appearance after the PAAG was taken out. E PAAG was totally cleaned from the breast. F The dissected acroscopic flap was folded and mounded into the posterior space of the breast. G A drainage was placed and the incision sutured. H One week after the patient accepted PMTFT. I Shape of the breast 6 months after the surgery.
Clinical Assessment
Patient demographics and comorbidities were recorded. Pathological reports revealed chronic inflammation and foreign-body reaction without any evidence of malignancy. The appearance and shape of the breast were assessed and recorded via images. Postoperative feedback of satisfaction was obtained 6 months after the surgery by telephone. A questionnaire containing 4 categories (i.e., very good, good, moderate, and poor) was used to assess the shape, size, position, and overall impression of the new breast.
Results
PMTFT after PAAG removal was performed in 23 patients. The characteristics of patients are summarized in Table 1. The mean age of the patients was 43.7 years (range 30–62) and the BMI ranged from 19.3 to 22.6 (mean 21.3). Preoperative symptoms included: lump (14 out of 23), pain (9 out of 23), PAAG migration (3 out of 23), swelling (1 out of 23), and anxiety (1out of 23). The average drainage duration was 6.3 days and the mean postoperative hospitalization was 7.3 days. All patients recovered well without severe complications. Only 1 patient suffered infection of the areola incision but recovered after a dressing change.
Table 1.
Patient demographics
| Characteristics | Patients, n (%) |
|---|---|
| Age (years) | |
| <40 | 9 (39) |
| 40–49 | 8 (35) |
| 50–59 | 5 (22) |
| >60 | 1 (4) |
| Major symptom1 | |
| Lump | 14 |
| Pain | 9 |
| PAAG migration | 3 |
| Swelling | 1 |
| Anxiety | 1 |
| PAAG injection duration (years) | |
| <10 | 1 (4) |
| 10–15 | 17 (74) |
| >15 | 5 (22) |
Some patients presented with more than 1 symptom.
The average time of postoperative follow-up was 14.8 months (6–24 months). Images comparing the appearance and shape of the breasts before and after surgery are shown in Figure 3. The questionnaire administered to the 23 patients after surgery revealed 19 “very good” and 4 “good” results. Of the 4 patients who answered “very good,” 3 complained about mild pain and 1 had hypoesthesia of the nipples. After reviewing the medical records, the patients with postoperative pain were all found to have preoperative complaints of pain. Actually, their symptoms of pain were partially relieved after surgery according to results at follow-up.
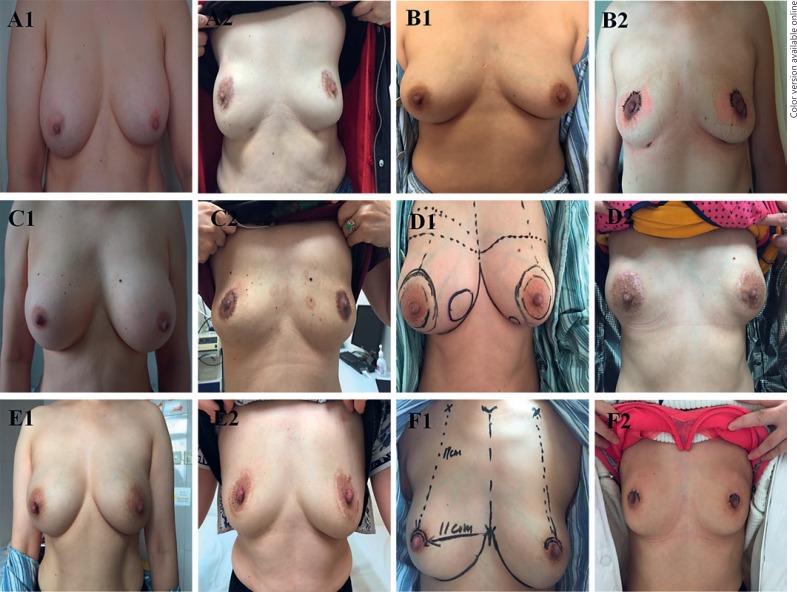
Fig. 3.
Images comparing the appearance and shape of the breasts before and after surgery. A–F Photos from different patients. The numbers 1 and 2 indicate the appearance of same patient before and after the surgery.
Discussion
The complication rate of PAAG injection was higher than that of other material-mediated breast augmentation mammoplasty. The complications included pain, infection, PAAG migration, mass formation, breast cancer, and diffuse stiffness [4]. The estimated population of women receiving PAAG injection for breast augmentation from 1997 to 2006 is 200,000 in China [5]. Complications and potential toxicity have led to increasing demands for PAAG removal. Debridement surgery may be the best and only method for treating PAAG-related complications [6].
The simplest way to remove PAAG is via blunt aspiration and drainage. This method only requires a small incision to insert a suction device, but only free hydrogel can be removed. Blunt aspiration is an easy and minimally invasive process, but its disadvantage is also obvious. Gel in a liquid state can be aspirated, leaving residual gel unremoved. Pathologically, a pseudocapsule is the margin between breast tissue and PAAG, formed by collagen, foreign material, and infiltrated macrophages. A pseudocapsule may be regarded as another state of PAAG that should be removed during surgery. Debridement surgery resecting both PAAG and the capsule is more recommended than simple PAAG aspiration [7]. However, debridement surgery without mammoplasia usually results in an unsatisfactory postoperative appearance of the breast. Mastoptosis, breast deformity, and retraction of the nipple are usually found, while the breasts appears “deflated.”
To solve the problem of breast deformity, several methods for reshaping have been developed. Silicone prosthesis implantation (immediate or delayed) is most commonly practiced after PAAG removal [8]. The ideal position for prostheses placement is in the submuscular layer, which is recognized as a theoretical “no gel plane.” Excision of all of the gel is almost impossible. There remains more or less material in or beyond the surgery area [9]. Therefore, synchronous silicone prosthesis implantation is not commonly recommended by most authors to avoid gel-related capsular contracture and inflammation [10]. Although there is still no significant evidence pointing to an increased complication rate, supporters agree that the indications for immediate prosthesis implantation should be restrictive [11]. Delayed silicone prosthesis implantation yields a satisfactory breast appearance with a relatively lower morbidity rate, but its disadvantages are a higher expense due to delayed surgery and anesthesia [12]. A comparison of PMTFT versus immediate/delayed prosthesis implantation is shown in Table 2.
Table 2.
Comparison of PMTFT vs. immediate/delayed prosthesis implantation
| Immediate prosthesis implantation | Delayed prosthesis implantation | PMTFT | |
|---|---|---|---|
| Prosthesis implantation | Yes | Yes | No |
| Risk of inflammation | High | Low | Low |
| Prosthesis-related complications | High | Low | No |
| Second operation | No | Yes | No |
| Economic cost | Moderate | High | Low |
Autologous reconstruction is another choice after PAAG removal. As part of a mature technique practiced for decades, the pedicled flap is an appropriate donor tissue for breast reconstruction [13]. But applying autologous flap transplantation in PAAG removal patients entails cosmetic problems concerning surgical trauma and the naked appearance. One-stage autologous fat transplantation is not recommended by most studies because of a high inflammation risk, while delayed operation is acceptable [14, 15].
In this study we presented a novel breast reconstruction method after PAAG removal, abbreviated as PMTFT. This technique yields a satisfactory mammary appearance without second-stage prosthesis implantation and has value for further clinical practice. By performing a double ring incision, the dermal blood supply to the NAC tissue is protected. This access strategy can provide better surgical exposure than traditional areola or submammary incision and reduce the intraoperative difficulty in manipulation. Better intraoperative vision leads to a shorter surgical time as well as less traction of NAC tissue, thus decreasing the risk of NAC ischemia. Some aesthetic problems after PAAG removal may be mastoptosis and skin laxity after loss of breast tissue. Appropriate design of the double ring may help to correct the skin laxity and mastoptosis through string suture tightening of the 2 rings and adjustment of the position of the inner ring. Synchronous areola reduction can be performed in patients with large areolas who are willing to undergo this operation. The shape of the submammary folds plays a key role in assessing the postoperative shape of the breast. Via the folding technique, the acroscopic glandular flap is mounded into the posterior mammary space. The lower part of the breast and submammary folds is enhanced, resulting in a relatively satisfactory shape of the breast.
This study has some limitations. A longer follow-up and larger cohort of patients are required to confirm the surgical results.
Conclusion
PMTFT is a new surgical technology for breast reconstruction following PAAG removal. With a satisfied breast shape after surgery, this procedure is economically, practically, and technically advantageous. It can be an ideal choice for breast reshaping in appropriate cases after PAAG removal.
Statement of Ethics
Informed consent was obtained from all of the patients for use of their data in accordance with the ethical standards of our institutional ethical committee.
Disclosure Statement
The authors have no conflicts of interests to declare.
Funding Sources
This work was supported by the Shanghai Municipal Commission of Science and Technology (No. 16401933200) and the National Natural Science Foundation of China (NNSFC; No. 81503579).
Author Contributions
Yantao Cai, Bin Liu, Mingjuan Liao, Liu He, and Chenfang Zhu performed the research. Liu He and Chenfang Zhu designed this study. Yantao Cai and Mingjuan Liao analyzed the data. Yantao Cai and Bin Liu followed up the patients and wrote this paper. Chenfang Zhu provided advice and critical discussions on the project. All of the authors read and approved the final version of this paper.
References
- 1.Christensen LH, Breiting VB, Aasted A, Jørgensen A, Kebuladze I. Long-term effects of polyacrylamide hydrogel on human breast tissue. Plast Reconstr Surg. 2003 May;111((6)):1883–90. doi: 10.1097/01.PRS.0000056873.87165.5A. [DOI] [PubMed] [Google Scholar]
- 2.Bello G, Jackson IT, Keskin M, Kelly C, Dajani K, Studinger R, et al. The use of polyacrylamide gel in soft-tissue augmentation: an experimental assessment. Plast Reconstr Surg. 2007 Apr;119((4)):1326–36. doi: 10.1097/01.prs.0000254824.13065.3b. [DOI] [PubMed] [Google Scholar]
- 3.Cheng NX, Wang YL, Wang JH, Zhang XM, Zhong H. Complications of breast augmentation with injected hydrophilic polyacrylamide gel. Aesthetic Plast Surg. 2002 Sep-Oct;26((5)):375–82. doi: 10.1007/s00266-002-2052-4. [DOI] [PubMed] [Google Scholar]
- 4.Ono S, Ogawa R, Hyakusoku H. Complications after polyacrylamide hydrogel injection for soft-tissue augmentation. Plast Reconstr Surg. 2010 Oct;126((4)):1349–57. doi: 10.1097/PRS.0b013e3181ead122. [DOI] [PubMed] [Google Scholar]
- 5.Jin R, Luo X, Wang X, Ma J, Liu F, Yang Q, et al. Complications and treatment strategy after breast augmentation by polyacrylamide hydrogel injection: summary of 10-year clinical experience. Aesthetic Plast Surg. 2018 Apr;42((2)):402–9. doi: 10.1007/s00266-017-1006-9. [DOI] [PubMed] [Google Scholar]
- 6.Ohtake N, Koganei Y, Itoh M, Shioya N. Postoperative sequelae of augmentation mammaplasty by injection method in Japan. Aesthetic Plast Surg. 1989;13((2)):67–74. doi: 10.1007/BF01571471. [DOI] [PubMed] [Google Scholar]
- 7.Yu L, Wang J, Zhang B, Zheng DN, Zhu C. Treatment of breast injection with polyacrylamide hydrogel with infiltrated fascia capsule removal: report on 104 cases. Aesthetic Plast Surg. 2012 Oct;36((5)):1120–7. doi: 10.1007/s00266-012-9928-8. [DOI] [PubMed] [Google Scholar]
- 8.Unukovych D, Khrapach V, Wickman M, Liljegren A, Mishalov V, Patlazhan G, et al. Polyacrylamide gel injections for breast augmentation: management of complications in 106 patients, a multicenter study. World J Surg. 2012 Apr;36((4)):695–701. doi: 10.1007/s00268-011-1273-6. [DOI] [PubMed] [Google Scholar]
- 9.Khedher NB, David J, Trop I, Drouin S, Peloquin L, Lalonde L. Imaging findings of breast augmentation with injected hydrophilic polyacrylamide gel: patient reports and literature review. Eur J Radiol. 2011 Apr;78((1)):104–11. doi: 10.1016/j.ejrad.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Miglioretti DL, Rutter CM, Geller BM, Cutter G, Barlow WE, Rosenberg R, et al. Effect of breast augmentation on the accuracy of mammography and cancer characteristics. JAMA. 2004 Jan;291((4)):442–50. doi: 10.1001/jama.291.4.442. [DOI] [PubMed] [Google Scholar]
- 11.Luo SK, Chen GP, Sun ZS, Cheng NX. Our strategy in complication management of augmentation mammaplasty with polyacrylamide hydrogel injection in 235 patients. J Plast Reconstr Aesthet Surg. 2011 Jun;64((6)):731–7. doi: 10.1016/j.bjps.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Patlazhan G, Unukovych D, Pshenisnov K. Breast reconstruction and treatment algorithm for patients with complications after polyacrylamide gel injections: a 10-year experience. Aesthetic Plast Surg. 2013 Apr;37((2)):312–20. doi: 10.1007/s00266-012-0045-5. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khyatt W, Goyal A, Mansel RE. Nipple-sparing skin-sparing mastectomy and vertical latissimus dorsi flap reconstruction for bilateral fibromatosis of the breast. Clin Breast Cancer. 2010 Feb;10((1)):E1–2. doi: 10.3816/CBC.2010.n.012. [DOI] [PubMed] [Google Scholar]
- 14.Fox BS. Autologous fat injection and breast augmentation. Med J Aust. 1988;149((5)):149–284. doi: 10.5694/j.1326-5377.1988.tb120617.x. [DOI] [PubMed] [Google Scholar]
- 15.Longo B, Laporta R, Sorotos M, Pagnoni M, Gentilucci M, Santanelli di Pompeo F. Total breast reconstruction using autologous fat grafting following nipple-sparing mastectomy in irradiated and non-irradiated patients. Aesthetic Plast Surg. 2014 Dec;38((6)):1101–8. doi: 10.1007/s00266-014-0406-3. [DOI] [PubMed] [Google Scholar]