Abstract
Background
Oral corticosteroid (OCS) treatment for severe asthma is associated with substantial disease burden. Thus, OCS dosage reduction is desirable. Relative efficacy of biologics in reducing OCS treatment for severe, uncontrolled asthma is not fully characterized.
Objective
We performed a matching‐adjusted indirect comparison (MAIC) to assess the relative effects on OCS treatment reduction of three biologic asthma treatments.
Methods
In MAIC of benralizumab vs. mepolizumab and vs. dupilumab, patient‐level data from the Phase III benralizumab OCS‐sparing trial, ZONDA, were weighted to match treatment effect–modifying patient characteristics in comparator trials.
Results
After matching adjustment, mean difference between benralizumab and mepolizumab for OCS reduction was 6.08% (95% CI −22.22‐34.38; P = .67) by week 24, and odds ratio of OCS elimination was 2.32 (95% CI 0.48‐11.15; P = .29). A trend in annual asthma exacerbation rate reduction favouring benralizumab over mepolizumab was observed, although it was not statistically significant (rate ratio [RR] = 0.56 [95% CI 0.28‐1.13; P = .11]). Mean difference between benralizumab and dupilumab for OCS reduction was −0.71% (95% CI −20.56‐19.15; P = .94), and odds ratio of OCS elimination was 2.26 (95% CI 0.52‐9.84; P = .28). A non‐significant trend in annual asthma exacerbation rate reduction favouring benralizumab over dupilumab was observed (RR = 0.50 [95% CI 0.20‐1.28; P = .15]). Effective sample size was 49% (72 vs. 148) and 25% (36 vs. 142) of original sample size for MAIC of benralizumab vs. mepolizumab and benralizumab vs. dupilumab, respectively.
Conclusions and Clinical Relevance
Following patient baseline characteristics matching across clinical trials, benralizumab demonstrated efficacy comparable to mepolizumab and dupilumab for OCS dosage reduction, OCS elimination, and annual exacerbation rate reduction. Comparatively low effective sample sizes indicated substantial differences for patient populations between ZONDA and mepolizumab and dupilumab trials.
Keywords: benralizumab, dupilumab, interleukin‐5, interleukin‐5 receptor, matching‐adjusted indirect comparison, mepolizumab
1. INTRODUCTION
Patients with severe asthma have substantial disease burden and are susceptible to frequent exacerbations.1, 2 This burden is even greater for patients receiving maintenance treatment with oral corticosteroids (OCS) who may experience increased risk of chronic comorbidities including type 2 diabetes mellitus, osteoporosis, and cataracts; neuropsychiatric effects including insomnia and depression; and infections and cardiovascular, metabolic, and gastrointestinal complications.3, 4, 5 For patients who initiated short‐term or maintenance treatment with systemic corticosteroids (SCS) followed over a median of more than 7 years, increasing cumulative SCS exposure resulted in greater risk of potentially life‐changing adverse outcomes, even for some patients with cumulative exposure of only 0.5‐<1 g.3 Reduction of OCS exposure is therefore an important treatment goal for patients with severe OCS‐dependent asthma.
OCS‐sparing potential has been demonstrated by three biologics approved for treatment of severe asthma: benralizumab, an interleukin (IL)‐5 receptor alpha–directed cytolytic monoclonal antibody; mepolizumab, an anti–IL‐5 monoclonal antibody; and dupilumab, a monoclonal antibody that inhibits IL‐4 and IL‐13.6, 7, 8, 9, 10, 11 In the ZONDA clinical trial (NCT02075255),7 benralizumab treatment enabled patients with severe, uncontrolled OCS‐dependent asthma and baseline blood eosinophil counts ≥ 150 cells/µL to achieve and maintain asthma control while reducing OCS dosage. Following an 8‐week OCS optimization phase, benralizumab 30 mg was administered subcutaneously every 4 weeks (Q4W) or every 8 weeks (Q8W; first three doses Q4W). Median OCS dosage reduction from baseline was 75% with benralizumab vs. 25% with placebo (P < .001). Moreover, benralizumab Q8W treatment resulted in a 70% reduction in annual exacerbation rate vs. placebo (P < .001).7 The SIRIUS clinical trial (NCT01691508)6 compared mepolizumab 100 mg Q4W with placebo for patients with severe asthma and eosinophil counts ≥ 300 cells/µL in the year before screening or ≥ 150 cells/µL during the 3‐ to 8‐week OCS dosage optimization phase. Median OCS reduction from baseline was 50% with mepolizumab vs. 0% with placebo (P = .007). Mepolizumab treatment was also associated with a 32% reduction in mean annual exacerbation rate vs. placebo (P = .04).6 In the LIBERTY ASTHMA VENTURE trial (NCT02528214),8 the optimization phase was 16 weeks, with dosage reduction possible every 4 weeks. Dupilumab treatment (300 mg administered subcutaneously every 2 weeks) was associated with a least squares mean reduction in OCS dosage of 70.1% with dupilumab vs. 41.9% with placebo (P < .001) for patients with severe asthma. There was also a 59% reduction in annual exacerbation rate with dupilumab vs. placebo (95% confidence interval [CI] 37‐74).8
Although these data are useful to clinicians, data comparing the OCS‐sparing potential of benralizumab, mepolizumab, and dupilumab would be even more helpful in interpreting comparative efficacy for patients with severe asthma receiving OCS maintenance treatment. However, there have been no head‐to‐head trials with these treatments.
Indirect treatment comparison (ITC) via matching‐adjusted indirect comparison (MAIC) allows for comparison of treatments across clinical trials.12, 13 MAIC is an accepted methodology that has been used to compare biologics for multiple sclerosis,14 psoriasis,12 haemophilia,15 multiple myeloma,16 and asthma.17 In our recent MAIC analysis of studies identified by a systematic literature review, benralizumab and mepolizumab were comparable for reduction of annual asthma exacerbation rates after weighted adjustment of baseline population characteristics. Benralizumab had a slightly greater effect on forced expiratory volume in 1 second (FEV1), but this was not statistically significant at all time‐points.17
MAIC is a population‐adjusted ITC designed to reduce bias by matching patient‐level data from clinical trials of one treatment with aggregate data reported for comparator trials.8 Treatment effect–modifying variables that differ across studies are used to weight patient‐level data for one treatment to approximate the characteristics of patients from the comparator trial. For example, patients with exacerbation rates similar to the aggregate rate of the comparator population are weighted more heavily when modelling study outcomes, similar to a propensity score. Patients who are substantially different from the comparator population have less weight on the outcome. Therefore, this matching adjustment can simulate results as they would be if the same patient population had been used to assess the compared treatments.8
This second publication in a series of MAIC analyses of data for patients with severe, uncontrolled asthma compares benralizumab with mepolizumab and with dupilumab for effects on mean percentage of OCS dosage reduction, patients who achieved elimination of OCS treatment, and annualized exacerbation rate.
2. METHODS
2.1. Overview
This MAIC analysis followed guidance for well‐designed, population‐adjusted ITCs.10 The systematic literature review (conducted August 2016) and study selection were previously described.14 Two OCS‐sparing studies, ZONDA and SIRIUS,6, 7 were identified but not included in the previous analysis because of differences in study design compared with other trials.14 A third OCS‐sparing study, LIBERTY ASTHMA VENTURE,8 published after August 2016, was also identified. These three studies assessed OCS dosage reduction for patients treated with benralizumab (ZONDA), mepolizumab (SIRIUS), and dupilumab (LIBERTY ASTHMA VENTURE).6, 7, 8 We examined all three therapies at their indicated dosages for patients with asthma, which meant that only the benralizumab Q8W arm of the ZONDA trial was included in this analysis. Only patients receiving high‐dosage inhaled corticosteroids (ICS), defined according to benralizumab studies (fluticasone propionate or equivalent > 500 µg/day), are presented in this analysis. Trial characteristics are summarized in Appendix S1: Table S1.
2.2. Data analysis
Benralizumab was compared with mepolizumab and with dupilumab for three outcomes: OCS dosage reduction (at 24 weeks and at the end of the trial), percentage of patients able to eliminate OCS treatment at 24 weeks, and annual rate of clinically significant exacerbations. End‐of‐trial values were from week 28 for ZONDA and week 24 for SIRIUS and LIBERTY ASTHMA VENTURE. Odds ratios for elimination of OCS treatment were calculated based on the overall trial populations, not just the subsets of patients eligible for complete OCS reduction based on baseline OCS dosage. Annual rates of clinically significant exacerbations were estimated as rate ratios for active treatments vs. placebo.
All analyses were conducted with SAS version 9.1 (SAS Institute Inc, Cary, NC, USA) and R version 3.0.3 (R Foundation for Statistical Computing, Vienna).
2.3. Matching‐adjusted indirect comparison analysis
We compared study population differences between ZONDA7 and SIRIUS6 and between ZONDA and LIBERTY ASTHMA VENTURE8 as well as differences in variables known to modify treatment effects. Study arms included patients receiving benralizumab 30 mg Q8W or placebo in ZONDA, patients receiving mepolizumab 100 mg Q4W or placebo in SIRIUS, and patients receiving dupilumab 300 mg every 2 weeks or placebo in LIBERTY ASTHMA VENTURE. Patients in ZONDA were then weighted to reflect the treatment effect–modifying characteristics in the comparator populations. An anchoring method was used for population‐adjusted indirect comparisons18 (Appendix S2: Figure S1). Matching variables were selected for their clinical and statistical importance in explaining variability in the outcomes of interest and their demonstrated imbalance between ZONDA and SIRIUS or LIBERTY ASTHMA VENTURE. Success of weighting techniques was assessed by comparing adjusted baseline characteristics for patients in ZONDA with characteristics of populations from SIRIUS and LIBERTY ASTHMA VENTURE.17, 18
2.4. Data adjustments
ZONDA7 individual patient data were weighted based on relevant aggregate baseline characteristics from SIRIUS or LIBERTY ASTHMA VENTURE. Variables were adjusted with a logistic propensity score model that was conditional on the identified treatment‐effect modifiers. Individuals were weighted by the inverse of their propensity scores (Appendix S3: Supplemental Methods).13 For comparison between ZONDA and SIRIUS, an additional sensitivity analysis was performed after including two additional variables (history of omalizumab use and Asthma Control Questionnaire 5 [ACQ‐5] score) to assess robustness of the model. For each treatment comparison, we determined the effective sample size (ESS).13
2.5. Treatment comparisons
Relative treatment effects of benralizumab vs. mepolizumab and vs. dupilumab were estimated with standard ITC methodologies.19 Treatment differences for each active treatment compared with placebo were used to derive the anchored ITCs for the outcomes in this MAIC analysis.
2.6. Variability assessment
To identify important variability across study methods, we examined study characteristics including sample size and patient selection criteria (Appendix S1: Table S1). All patients in ZONDA, SIRIUS, and LIBERTY ASTHMA VENTURE were receiving maintenance OCS treatment at study baseline. Details for baseline ICS dosage across these populations were deemed irrelevant, as the effect of ICS treatment would be overtaken by the effect of OCS treatment. Therefore, only one definition of high‐dosage ICS was considered for the analysis of OCS‐sparing trials: fluticasone propionate or equivalent > 500 µg daily.
Evidence networks were generated from the benralizumab ZONDA trial and the mepolizumab SIRIUS trial for placebo‐anchored comparison of benralizumab vs. mepolizumab, and from the ZONDA trial and the dupilumab LIBERTY ASTHMA VENTURE trial for placebo‐anchored comparison of benralizumab vs. dupilumab8 (Appendix S2: Figure S1).
3. RESULTS
3.1. Benralizumab vs. Mepolizumab
3.1.1. Study design and baseline characteristics
The ZONDA and SIRIUS studies were broadly similar in overall design, inclusion/exclusion criteria, trial setting, blinding procedures (Appendix S1: Table S1), and patient characteristics (Table 1). The blood eosinophil count inclusion criteria differed between trials (ZONDA: ≥ 150 cells/µL; SIRIUS: ≥ 150 cells/µL at baseline or ≥ 300 cells/µL in the past year), resulting in a greater mean baseline eosinophil count for patients in ZONDA (583 cells/µL) vs. SIRIUS (381 cells/µL) (Table 2). ZONDA inclusion criteria included use of OCS therapy equivalent to prednisolone or prednisone 7.5‐40.0 mg/d.7 In SIRIUS, the range was 5‐35 mg/d.6 Both trials included an OCS optimization phase before beginning biologic treatment (Appendix S1: Table S1). In ZONDA, this phase lasted 8 weeks, with potential for dosage reduction every 2 weeks.7 In SIRIUS, the optimization phase dosage reduction occurred weekly for 3‐8 weeks.6 Patients were eligible for OCS elimination if they had a baseline optimized OCS dosage ≤ 12.5 mg/d (n = 84, 56.7%) in ZONDA7 and < 25 mg/d (n = 128, 94.8%) in SIRIUS.6
Table 1.
Comparison of baseline characteristics of patients included in benralizumab (ZONDA) and mepolizumab (SIRIUS) studies
| Characteristics | ZONDA | SIRIUS | ||
|---|---|---|---|---|
| Benralizumab 30 mg Q8W | Placebo | Mepolizumab 100 mg Q4W | Placebo | |
| Age, years | 52.9 (10.1) | 49.9 (11.7) | 49.8 (14.1) | 49.9 (10.3) |
| Male (%) | 35.6 | 36.0 | 36.0 | 55.0 |
| BMI, kg/m2 | 30.2 (6.5) | 28.7 (5.2) | 27.8 (5.9) | 29.5 (6.1) |
| Pre‐bronchodilator FEV1 predicted, % | 59.0 (17.9) | 62.0 (16.5) | 59.6 (17.0) | 57.8 (18.5) |
| Pre‐bronchodilator FEV1/FVC, % | 59.0 (12.0) | 62.0 (13.0) | 63.0 (12.4)a | 61.0 (11.7)a |
| Pre‐bronchodilator FEV1, L | 1.8 (0.6) | 1.9 (0.7) | 1.9 (6.6) | 2.0 (8.2) |
| Reversibility, % | 25.1 (19.0) | 23.2 (18.0) | 24.9 (19.3) | 23.7 (18.6) |
| ACQ‐5 score | 2.4 (1.2) | 2.7 (0.9) | 2.2 (1.3) | 1.99 (1.2) |
| Exacerbations in previous year | 3.1 (2.8) | 2.5 (1.8) | 3.3 (3.4) | 2.9 (2.8) |
| 0 exacerbations (%) | 0 | 0 | 17.0 | 15.0 |
| 1 exacerbation (%) | 28.8 | 32.0 | 16.0 | 17.0 |
| ≥ 2 exacerbations (%) | 71.2 | 68.0 | 67.0 | 68.0 |
| Never‐smokers (%) | 83.6 | 77.3 | 59.0 | 62.0 |
| OCS dosage, prednisolone equivalent, mg/d | 14.3 (7.8) | 14.2 (6.4) | 12.4 (7.2) | 13.2 (6.3) |
| Blood eosinophil count, cells/µL | 509.0 (320.2) | 656.0 (589.0) | 413.0 (386.2) | 347.0 (303.3) |
| Omalizumab use (%) | 12.3 | 10.7 | 33.0 | 33.0 |
| Nasal polyps (%) | 27.4 | 37.3 | 23.0 | 26.0 |
| Atopic (%) | 39.7 | 49.3 | – | – |
Data presented as mean (SD) unless otherwise indicated.
Abbreviations: ACQ‐5: Asthma Control Questionnaire 5; BMI: body mass index; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; OCS: oral corticosteroid; Q4W: every 4 weeks; Q8W: every 8 weeks (first 3 doses Q4W); SD: standard deviation.
Data extracted from the respective publication. All other values are extracted from the respective clinical study reports.
Table 2.
Baseline and sensitivity analysis characteristics of ZONDA patients before and after adjusting to SIRIUS patients for the analysis of percentage reduction in OCS dosage, percentage of patients with OCS elimination, and annual rate of clinically significant exacerbations
| Characteristics | ZONDAa (before adjusting) | SIRIUS (aggregate reported data) | ZONDA (after adjusting to SIRIUS) |
|---|---|---|---|
| Benralizumab 30 mg Q8W + placebo N = 148b | Mepolizumab 100 mg Q4W + placebo N = 135 | Base‐case ESS = 72 Sensitivity ESS = 44 | |
| Maintenance OCS dosage, prednisolone equivalent, mg/d | 14.21 (7.06) | 12.79 (6.74) | 12.79 (5.39) |
| Blood eosinophil count, cells/µL | 583.05 (478.99) | 380.73 (348.15) | 380.73 (278.68) |
| Exacerbations in the previous year | 2.78 (2.36) | 3.10 (3.10) | 3.10 (2.48) |
| Nasal polyps (%) | 32.43 | 24.50 | 24.50 |
| BMI, kg/m2 | 29.47 (5.94) | 28.66 (5.97) | 28.66 (4.78) |
| ACQ‐5 score | 2.69 (1.15) | 2.07 (1.22) | 2.07 (0.83) |
| History of omalizumab use (%) | 11.49 | 33 | 33 |
Data presented as mean (SD) unless otherwise indicated. Data in bold indicate variables used only in the sensitivity analysis.
Abbreviations: ACQ‐5: Asthma Control Questionnaire 5; BMI: body mass index; ESS: effective sample size; OCS: oral corticosteroid; Q4W: every 4 weeks; Q8W: every 8 weeks (first three doses Q4W); SD: standard deviation.
Data for the ZONDA population are calculated from individual patient data.
One patient was missing a baseline blood eosinophil count; six patients were missing information on 100% OCS reduction.
3.1.2. Population adjustment
In comparing benralizumab vs. mepolizumab, the following variables were selected for matching in the base‐case model: eosinophil count, exacerbations in the previous year, OCS dosage, body mass index (BMI), and presence of nasal polyps. These variables, plus history of omalizumab use and ACQ‐5 score, were used in the sensitivity analysis. After adjustment for SIRIUS population characteristics, ZONDA baseline characteristics were well‐matched to the mepolizumab population (Table 2). ESS was 72 for the base‐case analysis and 44 for the sensitivity analysis. Variables were removed individually from the model to assess their effects on the ESS. Removal of only one variable, history of exacerbations, notably increased the ESS. However, as a treatment‐effect modifier, this variable was too relevant to leave out of the model.
3.1.3. OCS dosage reduction
Comparisons were performed at week 24. Because ZONDA (28 weeks) was longer than SIRIUS (24 weeks), additional analyses were conducted for end of study. From baseline to week 24, in the base‐case analysis, reduction of mean OCS dosage was 36% (95% CI 19‐54) greater with benralizumab treatment compared with placebo in ZONDA before matching adjustment and 22% (95% CI 4‐40) greater after matching adjustment to the mepolizumab population. From baseline to end of study, reduction of mean OCS dosage was 37% (95% CI 21‐53) greater with benralizumab treatment compared with placebo before matching and 21% (95% CI 5‐38) greater after matching to the mepolizumab population. Mepolizumab treatment reduced mean OCS dosage by 16% (95% CI −6‐38) more than placebo from baseline to week 24, which was also the end of study. After matching, mean differences in OCS reduction between benralizumab and mepolizumab at 24 weeks and at study end were 6.08% (95% CI −22.22‐34.38; P = .67) and 5.06% (95% CI −22.39‐32.52; P = .72), respectively (Figure 1).
Figure 1.
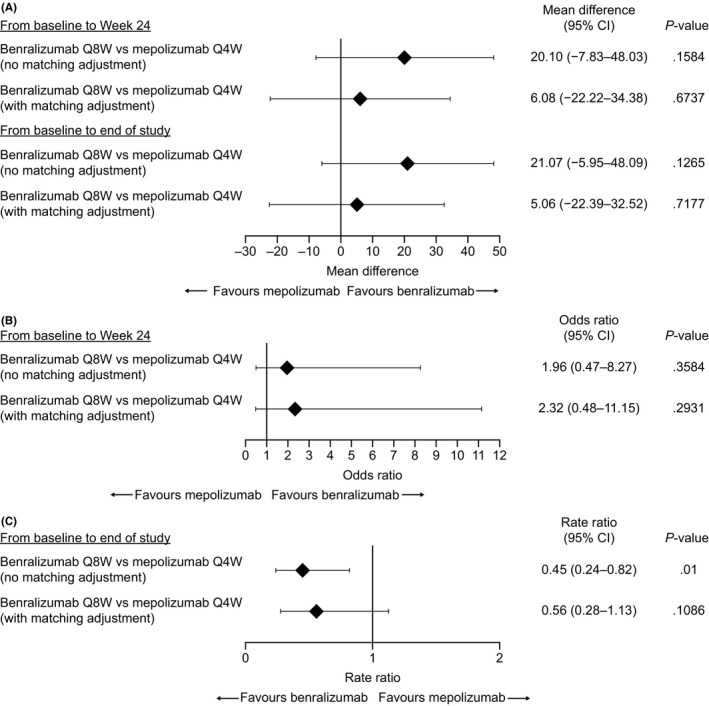
Indirect treatment comparisons of benralizumab and mepolizumab for (A) percentage reduction in oral corticosteroid (OCS) dosage, (B) percentage of patients with OCS elimination, and (C) reduction in annual rate of clinically significant exacerbations. CI: confidence interval; Q4W: every 4 wk; Q8W: every 8 wk (first three doses every 4 wk)
In a sensitivity analysis where adjustments for ACQ‐5 score and omalizumab use were added to the model as matching variables, reduction of mean OCS dosage was 28.14% (95% CI 8.94‐47.33) and 31.00% (95% CI 14.93‐47.07) greater with benralizumab treatment compared with placebo at week 24 and end of study, respectively. Mean differences in OCS reduction between benralizumab and mepolizumab at 24 weeks and at study end were 11.94% (95% CI −17.20‐41.08; P = .42) and 14.80% (95% CI −12.38‐41.98; P = .29), respectively. The wide CIs suggest high uncertainty in these results.
3.1.4. OCS treatment elimination
From baseline to week 24, odds ratios for OCS elimination with benralizumab Q8W vs. placebo were 4.06 (95% CI 1.67‐9.88) before matching and 4.80 (95% CI 1.62‐14.26) after matching adjustment to the mepolizumab population. Odds ratio for complete OCS dosage reduction for mepolizumab vs. placebo was 2.07 (95% CI 0.67‐6.44) from baseline to week 24. After matching, patients receiving benralizumab were not statistically significantly different (odds ratio 2.32 [95% CI 0.48‐11.15]) from those receiving mepolizumab for achieving OCS elimination (Figure 1).
In the sensitivity analysis, before matching, the odds ratio for benralizumab vs. placebo was 6.25 (95% CI 1.63‐23.96). After matching, benralizumab had an odds ratio of 3.02 (95% CI 0.52‐17.49) vs. mepolizumab for achieving OCS elimination.
3.1.5. Annual rate of clinically significant exacerbations
Benralizumab reduced the annual rate of clinically significant exacerbations vs. placebo by 70% (rate ratio [RR] 0.30, 95% CI 0.19‐0.49) in ZONDA before matching adjustment and by 62% (RR 0.38, 95% CI 0.21‐0.69) after matching adjustment to the mepolizumab population. Mepolizumab reduced the exacerbation rate by 32% (RR 0.68, 95% CI 0.47‐0.99) vs. placebo (Figure 1).
3.2. Benralizumab vs. Dupilumab
3.2.1. Study design and baseline characteristics
The ZONDA and LIBERTY ASTHMA VENTURE studies were broadly similar in overall design, inclusion/exclusion criteria, trial setting, blinding procedures (Appendix S1: Table S1), and patient characteristics (Table 3). Blood eosinophil count inclusion criterion differed between ZONDA (≥ 150 cells/µL) and LIBERTY ASTHMA VENTURE (no restriction). Exacerbation history required for study enrolment was also different (ZONDA: ≥ 1 exacerbation in the past year; LIBERTY ASTHMA VENTURE: no restriction). As a result, baseline mean blood eosinophil count and mean exacerbations in the previous year were greater for patients in ZONDA (592 cells/µL and 2.82, respectively) than in LIBERTY ASTHMA VENTURE (347 cells/µL and 2.09, respectively) (Table 4). ZONDA inclusion criteria included use of OCS therapy equivalent to prednisolone or prednisone 7.5‐40.0 mg/d.7 In LIBERTY ASTHMA VENTURE, the range was 5‐35 mg/d.8 Both trials included an OCS optimization phase before beginning biologic treatment (Appendix S1: Table S1). In ZONDA, this phase lasted 8 weeks, with potential for dosage reduction every 2 weeks.7 In LIBERTY ASTHMA VENTURE, the optimization phase was 16 weeks, with potential for dosage reduction every 4 weeks.8 Ratios of OCS dosage at the beginning and end of the optimization phase were similar for both trials (benralizumab 0.96; dupilumab 0.95). The maintenance OCS dosage cutoff that defined patients eligible for OCS dosage elimination also differed. Patients were eligible for OCS elimination if they had a baseline optimized OCS dosage ≤ 12.5 mg/d (n = 84, 56.7%) in ZONDA7 and ≤ 30 mg/d in LIBERTY ASTHMA VENTURE.8
Table 3.
Comparison of baseline characteristics of patients included in benralizumab (ZONDA) and dupilumab (LIBERTY ASTHMA VENTURE) studies
| Characteristics | ZONDA | LIBERTY ASTHMA VENTURE | ||
|---|---|---|---|---|
| Benralizumab 30 mg Q8W | Placebo | Dupilumab 300 mg Q2W | Placebo | |
| Age, years | 52.9 (10.1) | 49.9 (11.7) | 51.9 (12.5) | 50.7 (12.8) |
| Male (%) | 35.6 | 36.0 | 39.8 | 39.3 |
| BMI, kg/m2 | 30.2 (6.5) | 28.7 (5.2) | 28.88 (5.91) | 29.77 (6.00) |
| Pre‐bronchodilator FEV1 predicted, % | 59.0 (17.9) | 62.0 (16.5) | 51.64 (15.28) | 52.69 (15.14) |
| Pre‐bronchodilator FEV1, L | 1.8 (0.6) | 1.9 (0.7) | 1.53 (0.53) | 1.63 (0.61) |
| ACQ‐5 score | 2.4 (1.2) | 2.7 (0.9) | 2.42 (1.24) | 2.58 (1.09) |
| Exacerbations in previous year | 3.1 (2.8) | 2.5 (1.8) | 2.01 (2.08) | 2.17 (2.24) |
| OCS dosage, prednisolone equivalent, mg/d | 14.3 (7.8) | 14.2 (6.4) | 10.75 (5.90) | 11.75 (6.31) |
| Blood eosinophil count, cells/µL | 509.0 (320.2) | 656.0 (589.0) | 370.0 (316) | 325 (298) |
| Blood eosinophil count < 150 cells/µL (%) | 0 | 0 | 21.4 | 35.5 |
| Blood eosinophil count ≥ 150 to < 300 cells/µL (%) | 16.4 | 14.7 | 32 | 26.2 |
| Blood eosinophil count ≥ 300 cells/µL (%) | 83.6 | 85.3 | 46.6 | 38.3 |
| Nasal polyps (%) | 27.4 | 37.3 | 32.0 | 35.5 |
| Chronic rhinosinusitis (%) | 35.6 | 38.7 | 22.3 | 28 |
| Omalizumab use (%) | 12.3 | 10.7 | – | – |
| Atopic status (%) | 39.7 | 49.3 | – | – |
Data presented as mean (SD) unless otherwise indicated.
Abbreviations: ACQ: Asthma Control Questionnaire 5; BMI: body mass index; FEV1: forced expiratory volume in 1 s; OCS; oral corticosteroid; Q2W: every 2 wk; Q8W: every 8 wk (first three doses every 4 wk); SD: standard deviation.
Table 4.
Baseline characteristics of ZONDA patients before and after adjusting to LIBERTY ASTHMA VENTURE patients for the analysis of mean percentage reduction in OCS dosage and percentage of patients with OCS elimination
| Characteristics | ZONDAa (before adjusting) | LIBERTY ASTHMA VENTURE (aggregate reported data) | ZONDA (after adjusting to LIBERTY ASTHMA VENTURE) |
|---|---|---|---|
| Benralizumab 30 mg Q8W + placebo N = 148b | Dupilumab 300 mg Q2W + placebo N = 210 | Benralizumab 30 mg Q8W SC + placebo ESS = 36 | |
| BMI, kg/m2 | 29.47 (6.06) | 29.34 (5.96) | 29.34 (3.66) |
| ACQ‐5 score | 2.67 (1.16) | 2.50 (1.16) | 2.5 (0.71) |
| Exacerbations in previous year | 2.82 (2.39) | 2.09 (2.16) | 2.09 (1.33) |
| OCS dosage adjusted at baseline, mg/d | 14.2 (7.05) | 11.26 (6.12) | 11.26 (3.76) |
| Blood eosinophil count, cells/µL | 592.22 (483.84) | 347 (307) | 347 (188.45) |
| Nasal polyps (%) | 32.39 | 33.80 | 33.81 |
Data presented as mean (SD) unless otherwise indicated.
Abbreviations: ACQ‐5: Asthma Control Questionnaire 5; BMI: body mass index; ESS: effective sample size; OCS: oral corticosteroid; Q2W: every 2 wk; Q8W: every 8 wk (first three doses every 4 wk); Abbreviation: SD, standard deviation.
Data for the ZONDA population are calculated from individual patient data.
One patient was missing a baseline blood eosinophil count; six patients were missing information on 100% OCS reduction.
3.2.2. Population adjustment
For the benralizumab vs. dupilumab comparison of mean percentage OCS dosage reduction and percentage of patients with OCS elimination, the following variables were selected for matching: BMI, ACQ‐5 score, exacerbations in the previous year, OCS dosage, and presence of nasal polyps. After adjustment for LIBERTY ASTHMA VENTURE population characteristics, ZONDA baseline characteristics were well‐matched to the dupilumab population (Table 4). For the comparison of annual exacerbation rate, the following were selected for matching: BMI, ACQ‐5 score, exacerbations in the previous year, OCS dosage, blood eosinophil counts, and presence of nasal polyps. After adjustment for LIBERTY ASTHMA VENTURE population characteristics, ZONDA baseline characteristics were well‐matched to the dupilumab population (Table 5). ESS for these analyses was 36. Variables were removed individually from the model to assess their effects on the ESS. Removal of only one variable, history of exacerbations, notably increased the ESS. However, this variable was too relevant to leave out of the model.
Table 5.
Baseline characteristics of ZONDA patients before and after adjusting to LIBERTY ASTHMA VENTURE patients for the analysis of annual exacerbation rate
| Characteristics | ZONDAa (before adjusting) | LIBERTY ASTHMA VENTURE (aggregate reported data) | ZONDA (after adjusting to LIBERTY ASTHMA VENTURE) |
|---|---|---|---|
| Benralizumab 30 mg Q8W + placebo N = 148b | Dupilumab 300 mg Q2W + placebo N = 210 | Benralizumab 30 mg Q8W + placebo ESS = 36 | |
| BMI, kg/m2 | 29.47 (5.94) | 29.34 (5.96) | 29.34 (3.72) |
| ACQ‐5 score | 2.69 (1.15) | 2.50 (1.16) | 2.50 (0.72) |
| Mean number of exacerbations in previous year | 2.78 (2.36) | 2.09 (2.16) | 2.09 (1.35) |
| OCS dosage adjusted at baseline, prednisolone equivalent, mg/d | 14.21 (7.06) | 11.26 (6.12) | 11.26 (3.82) |
| Blood eosinophil count, cells/µL | 583.05 (478.99) | 347 (307) | 347 (191.48) |
| Nasal polyps (%) | 32.43 | 33.8 | 33.8 |
Data presented as mean (SD) unless otherwise indicated.
Abbreviations: ACQ‐5: Asthma Control Questionnaire 5; BMI: body mass index; ESS: effective sample size; OCS: oral corticosteroid; Q2W: every 2 wk; Q8W: every 8 wk (first three doses every 4 wk); SD: standard deviation.
Data for the ZONDA population are calculated from individual patient data.
One patient was missing a baseline blood eosinophil count.
3.2.3. OCS dosage reduction
From baseline to week 24 in ZONDA, reduction in mean OCS dosage was 36% (95% CI 19‐54) greater with benralizumab compared with placebo before matching and 27% (95% CI 12‐43) greater with benralizumab compared with placebo after matching adjustment to the dupilumab population. Mean OCS dosage reduction was 28% (95% CI 16‐41) greater from baseline to week 24 with dupilumab treatment compared with placebo. After matching, mean difference in OCS reduction between benralizumab and dupilumab was −0.71% (95% CI −20.56‐19.15) (Figure 2).
Figure 2.
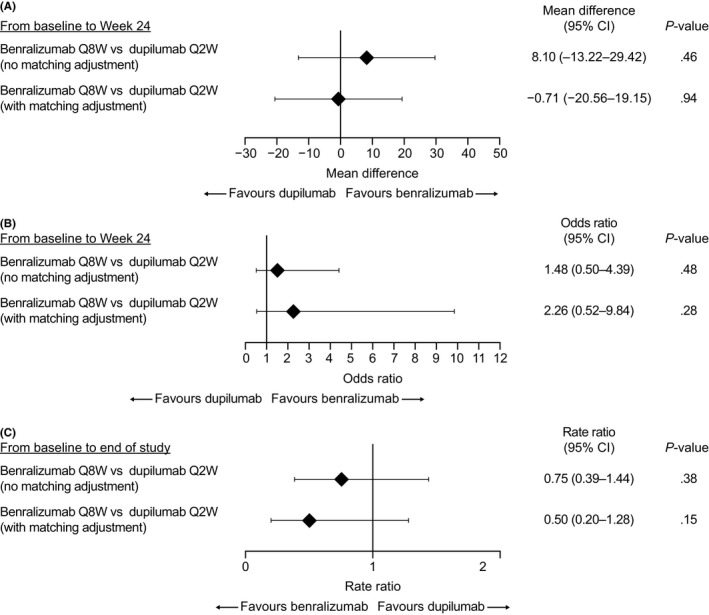
Indirect treatment comparisons of benralizumab and dupilumab for (A) percentage reduction in oral corticosteroid (OCS) dosage, (B) percentage of patients with OCS elimination, and (C) reduction in annual rate of clinically significant exacerbations. CI: confidence interval; Q2W: every 2 wk; Q8W: every 8 wk (first three doses every 4 wk)
3.2.4. OCS treatment elimination
Odds ratios for OCS elimination for benralizumab vs. placebo were 4.06 (95% CI 1.67‐9.88) before matching and 6.19 (95% CI 1.63‐23.49) after matching. Odds ratio for OCS elimination for dupilumab vs. placebo was 2.74 (95% CI 1.47‐5.10). After matching to the LIBERTY ASTHMA VENTURE population, odds ratio for benralizumab vs. dupilumab was not statistically significant (2.26 [95% CI 0.52‐9.84]) for achieving OCS elimination (Figure 2).
3.2.5. Annual rate of clinically significant exacerbations
Benralizumab treatment reduced annual exacerbation rate vs. placebo by 70% (RR 0.30, 95% CI 0.19‐0.49) in ZONDA before matching adjustment and by 79% (RR 0.21, 95% CI 0.09‐0.47) after matching adjustment to the dupilumab population (Figure 2). Dupilumab reduced the exacerbation rate in LIBERTY ASTHMA VENTURE by 59% (RR 0.41, 95% CI 0.26‐0.63) vs. placebo.
4. DISCUSSION
Our study used the MAIC technique to evaluate OCS dosage reduction and exacerbation outcomes of benralizumab treatment compared with those of mepolizumab and dupilumab, two other biologics for the treatment of severe, uncontrolled asthma. After matching adjustment to balance baseline characteristics between the ZONDA (benralizumab) and SIRIUS (mepolizumab) populations, there were no statistically significant differences between the two treatments. No significant differences were observed between benralizumab vs. mepolizumab and vs. dupilumab before matching.
ESS ranged from 25% to 49% of the original sample size in these analyses. This represented a substantial reduction from the original trial populations (ZONDA, N = 148; SIRIUS, N = 135; LIBERTY ASTHMA VENTURE, N = 210). Greater differences between original study population size and ESS for a MAIC comparison indicate greater differences in characteristics of patients in the trials being compared.12 ESS from these analyses indicates that the overall population in ZONDA was markedly different from the populations in SIRIUS and LIBERTY ASTHMA VENTURE in ways that could be expected to impact treatment effect, including baseline OCS dosage, blood eosinophil count, history of exacerbations, nasal polyposis, and BMI. This reinforces the need for matching patients through MAIC methodology to compare data between clinical trials and demonstrates that the use of less robust ITC methods could provide biased results.
These results extend our previous baseline‐adjusted analysis of the Phase III benralizumab and mepolizumab exacerbation studies. In those studies, reduction in asthma exacerbation rates was similar for both treatments, and improvements in FEV1 were numerically greater with benralizumab, but the differences were not statistically significant.17 Similarly, we conducted MAIC analysis of results from the pivotal Phase III benralizumab trials with exacerbation reduction as the primary end‐point (SIROCCO and CALIMA9, 10) vs. the Phase III exacerbation trial of dupilumab (LIBERTY ASTHMA QUEST20) (data not provided). Unfortunately, given the substantial heterogeneity and substantial lack of overlap between trial populations, matching adjustment was not possible. For example, nearly 51% of patients in the dupilumab trial had experienced only one exacerbation in the previous 12 months, while all patients in the benralizumab trials had had more than one exacerbation in the previous year.
A recently published report by Busse et al used ITC analysis to suggest that mepolizumab was associated with significantly greater improvements than benralizumab in clinically significant exacerbations and asthma control.21 However, their methodology was imperfect.22 Studies included in the analysis were not similar enough to justify cross‐study comparisons. Moreover, one of the key clinical trials used to register mepolizumab was omitted from the analysis. These findings could not be replicated through MAIC analysis.17 ITC and network meta‐analysis also allow indirect comparisons and have better statistical power than MAIC, but they may use nonhomogenous populations and introduce bias.
The three OCS‐sparing studies of monoclonal antibody treatments for severe, uncontrolled asthma examined in this analysis varied in important ways, including differences in inclusion/exclusion criteria and baseline patient characteristics that would have biased standard ITCs. However, by matching individual patient data from the benralizumab ZONDA trial to important aggregate baseline characteristics from the comparator trial via MAIC analysis, the re‐weighted, matching‐adjusted data can provide an estimate of what the outcome would have been if the comparator trial had included a benralizumab arm. MAIC is a more powerful tool than meta‐regression in adjusting for the impact of treatment‐effect modifiers, because the use of individual patient data for adjustment offers more information about patient‐level associations than does the aggregate‐level adjustments used in standard ITCs.18 In situations with few trials and no head‐to‐head data, as with these OCS‐sparing studies, MAIC analysis provides a way to address gaps in evidence and provide more complete information to payers, health technology assessment authorities, and healthcare professionals.18
5. LIMITATIONS
These OCS‐sparing trials varied in defining which patients were eligible for OCS elimination. In the ZONDA trial, patients had to have a baseline optimized OCS dosage ≤ 12.5 mg/d; in the SIRIUS and LIBERTY ASTHMA VENTURE trials, the thresholds were < 25 mg/d and ≤ 30 mg/d, respectively. This component could not be adjusted with MAIC methodology, so the results should be interpreted with caution. Moreover, patient characteristics that were not measured in these trials, and could therefore not be accounted for in matching, may have played an undetermined role in these outcomes. ESS was substantially reduced from the original trial populations (ZONDA, N = 148; SIRIUS, N = 135; LIBERTY ASTHMA VENTURE, N = 210). Moreover, the optimization and OCS‐tapering schemes also differed. Faster schemes inherently led to better OCS‐sparing effect in a given (and presently quite short) time, usually at the price of greater exacerbation rates in the placebo arm. Undoubtedly, from a patient perspective, longer term OCS‐sparing effects of new treatments are a welcome development, as OCS dependence usually lasts years. Adrenal insufficiency is a frequent cause of OCS weaning failure, and this event was not specifically addressed in these trials. This limitation could have potentially impacted the size of the treatment effect observed in this MAIC analysis.
6. CONCLUSIONS
MAIC provides a more reliable comparison of benralizumab vs. mepolizumab and vs. dupilumab than aggregate data alone. Matching eliminates biases that might arise in a standard ITC because of cross‐trial differences. This MAIC analysis demonstrated that, after adjustment for differences in baseline population characteristics, reductions in OCS dosage, percentages of patients achieving OCS elimination, and annual asthma exacerbation rates were comparable between mepolizumab, dupilumab, and benralizumab.
CONFLICTS OF INTEREST
A. Bourdin received personal fees, nonfinancial support, and other support from AstraZeneca, Novartis, Chiesi Pharmaceuticals, and Actelion; grants, personal fees, and other support from GSK; grants, personal fees, nonfinancial support, and other support from Boehringer Ingelheim; personal fees and other support from Teva and Regeneron; other support from Gilead; and personal fees and nonfinancial support from Roche, outside the submitted work. D. Husereau is a board or advisory committee member for GSK and AstraZeneca and has received financial support from AstraZeneca. N. Molinari has nothing to declare. S. Golam, L. Lindner, and X. Xu are full‐time employees of AstraZeneca. MK Siddiqui is an employee of PARAXEL International and performed the analysis on behalf of AstraZeneca.
PRIOR DISCLOSURE
An abstract describing these results was presented as a poster at the European Respiratory Society (ERS) International Congress 2019.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Lance Brannman, PhD, James G. Zangrilli, MD, and Ian Hirsch, PhD, of AstraZeneca for conceptual input in the early stages of this work and Pragya Shukla, MS, of PARAXEL International for contributions to the design and conduct of the analyses. Editorial support was provided by Jennie G. Jacobson, PhD, CMPP, and Caryne Craige, PhD, of JK Associates, Inc, and Michael A. Nissen, ELS, of AstraZeneca. This support was funded by AstraZeneca.
Bourdin A, Husereau D, Molinari N, et al. Matching‐adjusted comparison of oral corticosteroid reduction in asthma: Systematic review of biologics. Clin Exp Allergy. 2020;50:442–452. 10.1111/cea.13561
Funding information
This study was funded by AstraZeneca.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.
REFERENCES
- 1. Chastek B, Korrer S, Nagar SP, et al. Economic burden of illness among patients with severe asthma in a managed care setting. J Manag Care Spec Pharm. 2016;22:848‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lang DM. Severe asthma: epidemiology, burden of illness, and heterogeneity. Allergy Asthma Proc. 2015;36:418‐424. [DOI] [PubMed] [Google Scholar]
- 3. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long‐term observational study. J Asthma Allergy. 2018;11:193‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zazzali JL, Broder MS, Omachi TA, et al. Risk of corticosteroid‐related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc. 2015;36:268‐274. [DOI] [PubMed] [Google Scholar]
- 5. Bloechliger M, Reinau D, Spoendlin J, et al. Adverse events profile of oral corticosteroids among asthma patients in the UK: cohort study with a nested case‐control analysis. Respir Res. 2018;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bel E, Wenzel S, Thompson P, et al. Oral glucocorticoid‐sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189‐1197. [DOI] [PubMed] [Google Scholar]
- 7. Nair P, Wenzel S, Rabe K, et al. Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448‐2458. [DOI] [PubMed] [Google Scholar]
- 8. Rabe KFN, Brusselle P, Maspero G, et al. Efficacy and safety of dupilumab in glucocorticoid‐dependent severe asthma. N Engl J Med. 2018;378:2475‐2485. [DOI] [PubMed] [Google Scholar]
- 9. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): a randomised, multicentre, placebo‐controlled phase 3 trial. Lancet. 2016;388:2115‐2127. [DOI] [PubMed] [Google Scholar]
- 10. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet. 2016;388:2128‐2141. [DOI] [PubMed] [Google Scholar]
- 11. Park HS, Lee SH, Kim MK, et al. Efficacy and safety of benralizumab for Korean patients with severe, uncontrolled eosinophilic asthma. Allergy Asthma Immunol Res. 2019;11:508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Signorovitch JE, Wu EQ, Yu AP, et al. Comparative effectiveness without head‐to‐head trials: a method for matching‐adjusted indirect comparisons applied to psoriasis treatment with adalimumab or etanercept. Pharmacoeconomics. 2010;28:935‐945. [DOI] [PubMed] [Google Scholar]
- 13. Phillippo DM, Ades AE, Dias S, et al. NICE DSU Technical Support Document 18: Methods for population‐adjusted indirect comparisons in submissions to NICE. http://nicedsu.org.uk/wp-content/uploads/2017/05/Population-adjustment-TSD-FINAL.pdf. Accessed October 28, 2019.
- 14. Fox RJ, Chan A, Zhang A, et al. Comparative effectiveness using a matching‐adjusted indirect comparison between delayed‐release dimethyl fumarate and fingolimod for the treatment of multiple sclerosis. Curr Med Res Opin. 2017;33:175‐183. [DOI] [PubMed] [Google Scholar]
- 15. Pocoski J, Li N, Ayyagari R, et al. Matching‐adjusted indirect comparisons of efficacy of BAY 81–8973 vs two recombinant factor VIII for the prophylactic treatment of severe hemophilia A. J Blood Med. 2016;7:129‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Sanden S, Ito T, Diels J, et al. Comparative efficacy of daratumumab monotherapy and pomalidomide plus low‐dose dexamethasone in the treatment of multiple myeloma: a matching adjusted indirect comparison. Oncologist. 2018;23:279‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bourdin A, Husereau D, Molinari N, et al. Matching‐adjusted indirect comparison of benralizumab versus interleukin‐5 inhibitors: systematic review. Eur Respir J. 2018;52:1801393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Signorovitch JE, Sikirica V, Erder MH, et al. Matching‐adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15:940‐947. [DOI] [PubMed] [Google Scholar]
- 19. Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683‐691. [DOI] [PubMed] [Google Scholar]
- 20. Busse WW, Maspero JF, Rabe KF, et al. Liberty Asthma QUEST: phase 3 randomized, double‐blind, placebo‐controlled, parallel‐group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate‐to‐severe asthma. Adv Ther. 2018;35:737‐748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Busse W, Chupp G, Nagase H, et al. Anti‐IL‐5 treatments in patients with severe asthma by blood eosinophil thresholds: indirect treatment comparison. J Allergy Clin Immunol. 2019;143(1):190‐200.e20. [DOI] [PubMed] [Google Scholar]
- 22. Bourdin A, Molinari N. Indirect treatment comparison of asthma biologics fraught with methodology issues. J Allergy Clin Immunol. 2019;143:1266‐1267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described in this manuscript may be requested in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagroup-dt.pharmacm.com/DT/Home.


