Introduction
Radiopharmaceutical therapy (RPT) with α-particle emitters (αRPT) has drawn the attention of the RPT field and the pharmaceutical industry because α-particle emitter RPT is impervious to the many cancer resistance mechanisms that diminish the effectiveness of all other cancer therapies. Radiopharmaceutical therapy is a cancer treatment modality that delivers radiation to tumor cells in a targeted manner. The radiation is delivered, not as a beam of photons or protons (or carbon ions) from the outside, but as emissions from a radioactive element (a radionuclide) that is conjugated to agents that bind to tumor cells or to elements of the tumor microenvironment. These radiopharmaceuticals are administered to patients systemically or locoregionally. Almost all of the radionuclides used for radiopharmaceutical therapy emit photons that may be imaged using nuclear medicine imaging modalities, radionuclides used for radiopharmaceutical therapy also emit beta particles and α-particles. The latter are particularly effective in causing largely irreparable DNA double-strand breaks. The ability to deliver α-particles directly to tumor cells using tumor targeting molecules is unique to RPT. As α-particles traverse tissue they deposit DNA damaging (ionizing) energy at a density that is two to three orders of magnitude greater relative to that achieved by photons or beta-particles. This high energy deposition density is also delivered over a very short, 50 to 100 μm, range. These properties give αRPT its high potency against tumors that are resistant to other forms of cancer therapy. The high potency and short range can also lead to toxicity. To anticipate or reduce toxicity while optimizing efficacy, early phase αRPT trials should be designed with an understanding of the dosimetry and radiobiology of the αRPT under investigation. Due to the nature of the DNA damage that it induces, αRPT also has a strong potential to be enhanced by inhibiting elements of DNA DSB repair pathways (Fig. 1) in a synthetic lethality-like strategy that would enhance kill only to those cells subjected to DSB damage. This review draws on material from a MIRD Committee monograph on α-particle emitter dosimetry published in 2015 [1] and also covers recent developments and provides an updated perspective on the subject.
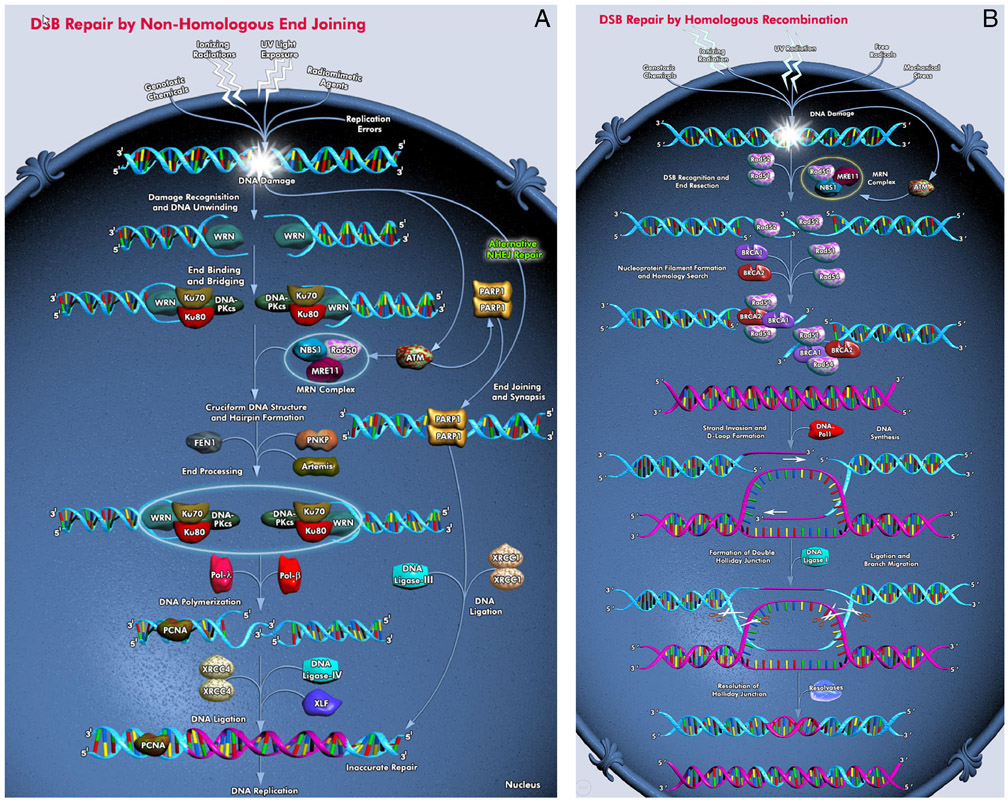
Figure 1.
DNA double-strand break repair pathways (figures from Qiagen). A. Nonhomologous end-joining (NHEJ). B. Homologous recombination (HR).
Radiobiology and Synthetic Lethality
The majority of systemically administered cancer therapy currently used in patients, including targeted or biologic therapy targets tumor cells only in the sense that tumor cells are preferentially sensitive to the therapeutic agent.
William G Kaelin’s review of synthetic lethality [2] states that:
Two genes are synthetic lethal if mutation of either alone is compatible with viability but mutation of both leads to death. So, targeting a gene that is synthetic lethal to a cancer relevant mutation should kill only cancer cells and spare normal cells.
The review goes on to say:
Synthetic lethality therefore provides a conceptual framework for the development of cancerspecific cytotoxic agents. This paradigm has not been exploited in the past because there were no robust methods for systematically identifying synthetic lethal genes. This is changing as a result of the increased availability of chemical and genetic tools for perturbing gene function in somatic cells.
In the context of cancer therapy wherein the therapeutic agent interacts with both cancer and normal cells, synthetic lethality and related strategies to induce this are essential to obtaining the needed therapeutic ratio to eradicate cancer without prohibitive normal tissue toxicity. In the context of radiopharmaceutical therapy (RPT), wherein the therapeutic ratio is obtained by specifically targeting and delivering radiation to cancer cells, synthetic lethality is not essential but enhancing. This is particularly the case for RPT using alpha-particle emitters wherein highly potent, short-range radiation induces preponderant DNA double strand breaks (DSBs) that stress already compromised DSB repair machinery in tumor cells that often have deficiencis in their DSB repair machinery. Alpha-particle emitter RPT can utilize a synthetic lethality approach to either enhance therapeutic efficacy or identify patients that are most likely to show the greatest sensitivity to αRPT.
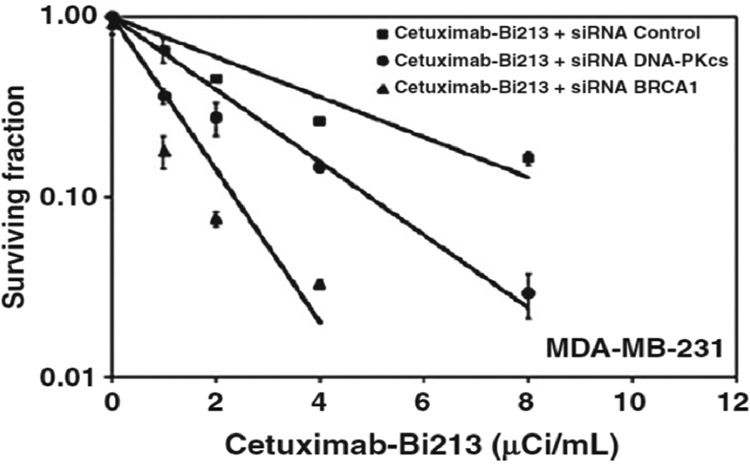
The potential and degree to which synthetic lethality could enhance alpha-particle emitter RPT (αRPT) has been evaluated pre-clinically using MDA-MB-231, a triple-negative (ER−, PR−, HER2−), and EGFR+ breast cancer cell line. Knockdown of genes involved in the non-homologous end-joining (NHEJ) DNA DSB repair pathway (Fig. 1a) by siRNA increased the radiosensitivity of MDA-MB-231 to alpha-particle induced damage by 8-fold. This enhanced sensitivity was measured as an 8-fold increase in RBE over wild type cells (table 1 and Fig. 2 taken from ref. [3]). RBE in these studies was defined as the absorbed dose of conventional external beam radiotherapy radiation required to achieve 37% cell kill divided by the absorbed dose of alpha-particle radiation required to achieve the same biologic end-point. Accordingly, an increase in RBE requires that the radiosensitivity to conventional (or reference) radiation remain unchanged while the sensitivity (=1/D0) to alpha-particle radiation increase. An alpha-emitter, which is already 3 to 5 times more potent per unit absorbed dose than conventional radiotherapy, can be 8-fold more deadly to tumor cells that have compromised DSB repair than to normal tissue with no DSB repair defect.
Table 1.
Radiosensitivity to α-particles (D0) and RBE of MDA-MB-231 cells under different exposure and DNA repair pathway inhibition conditions.
| Agent, manipulation | D0 (Gy) |
RBEa |
|---|---|---|
| 213Bi-Rituximab (irrelevant Ab) | 0.84 | 3.8 |
| 213Bi-Cetuximab | 0.87 | 3.7 |
| 213Bi-Cetuximab, siRNA scrambled control | 0.69 | 4.7 |
| 213Bi-Cetuximab, siRNA DNA-PKcs-/DNA-PKcs- | 0.37 | 8.6 |
| 213Bi-Cetuximab, siRNA BRCA1-/BRCA1- | 0.21 | 15.6 |
RBE is reported using 37% cell survival as the biologic endpoint and Cs-137 gamma rays as the reference radiation.
Figure 2.
Cell survival curves corresponding to the last three conditions shown on table 1; D0 is the slope of each curve (in Gy).
This implies that patients treated with αRPT agents are more likely to have a better response if they harbor an inactivating somatic or germline mutation in a DNA DSB repair pathway. Evidence for this was obtained from a retrospective analysis of 190 patients with bonepredominant metastatic castrate-resistant prostate cancer (mCRPC), 28 of whom received standard of care 223Ra. Ten of the 28 patients had germline or somatic inactivating mutations in the Homologous Recombination (HR) DNA DSB repair pathway (Fig. 1b) (table 2, from reference [4])
Table 2 –
List of inactivating HR mutations
| Sample ID | Gene | Origin of mutation | Amino acid change | Nucleotide change | Mutation mechanism | Type of analysis |
|---|---|---|---|---|---|---|
| 02 | BRCA2 | Germline | D3095E | c.9285C>G | Missense | Germline + somatic |
| 03 | BRCA2 | Somatic | E164Qfs*23 | c.4936_4939delGAAA | Frameshift deletion | Germline + somatic |
| 14 | CHEK2 | Somatic | R519X* | c.1555C>T | Nonsense | Germline + somatic |
| 15 | ATM | Somatic | E2014X* | c.6040G>T | Nonsense | Germline + somatic |
| 18 | ATR | Germline | - | c.2634-1G>A | Splicing | Germline only |
| 19 | FANCI | Germline | K808X* | c.2422A>T | Nonsense | Germline only |
| 20 | FANCL | Somatic | T372Nfs*4 | c.1114_1117insATTA | Frameshift insertion | Germline + somatic |
| 29 | PALB2 | Somatic | - | c.212-2A>G | Splicing | Germline + somatic |
| 31 | FANCG | Germline | L53Afs*4 | c.156insG | Frameshift insertion | Germline + somatic |
| 32 | BRCA2 | Somatic | S3147Cfs*2 | c.9435_9436delGT | Frameshift deletion | Germline + somatic |
The response to 223Ra of these 10 HR deficient (HRD+) patients was compared to that of patients with no inactivating HR mutations (HRD−), and was found to be superior. These results are summarized in table 3.
Table 3 –
PSA and ALP responsesa in HRD(+) and HRD(−) patients
| HRD(+) N = 10 |
HRD(−) N = 18 |
p value | |
|---|---|---|---|
| PSA (≥50%) response | 0% (0) | 0% (0) | >0.99 |
| ALP (≥30%) response | 80% (8) | 39% (7) | 0.04 |
| Patients with ALP normalization (if baseline ALP was elevated) | 100% (5) | 33% (3) | 0.03 |
ALP = alkaline phosphatase; HRD = homologous recombination deficiency; PSA = prostate-specific antigen.
Response rate is defined as a decrease in PSA of ≥50% and in ALP of ≥30% from baseline within 12 wk.
As noted in this table [4], the time to alkaline phosphatase (ALP) progression for HRD+ patients was 10.4 months; it was 5.8 months for HRD− patients (hazard ratio [HR] 6.4, 95% CI, 1.5–28.9; p = 0.005). Time to clinically indicated next systemic therapy was 9.7 and 7.2 months for HRD+ and HRD− patients, respectively (HR 1.5, 95% CI, 0.5–5.3; p = 0.39). Finally, overall survival was also greater in HRD+ versus HRD− patients (median 36.9 vs 19.0 months, HR 3.3, p = 0.11).
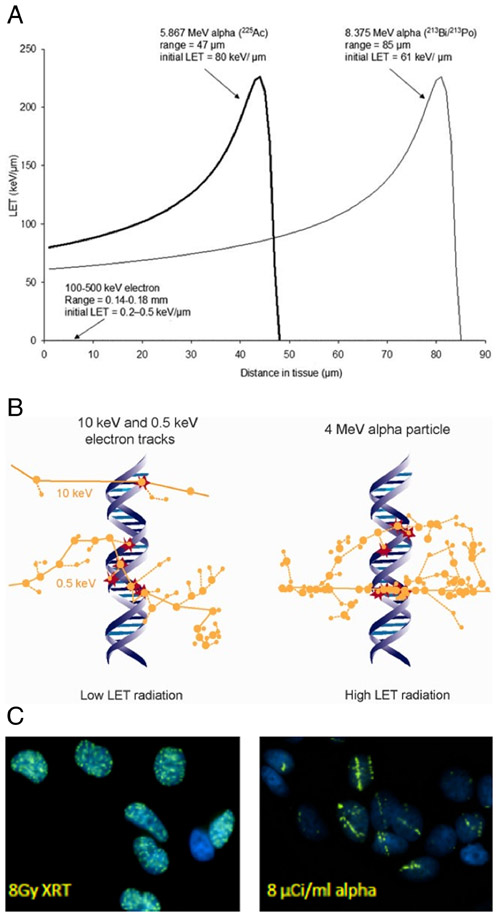
Much of our understanding of the molecular basis of DNA repair, along with the synthetic lethality concept that is derived from it, did not exist when the first studies investigating the radiobiology of alpha-emitters were published by Barendsen [5]. Barendsen found that 110 keV of energy is deposited in tissue by alpha-particles per micron distance traveled through the tissue. If the alpha-particles originate from radionuclides, this linear energy transfer (LET) ranges from 60 to more than 200 keV/μm; corresponding LET values for other radionuclide emissions such as beta-particles or photons are in the 0.2-0.5 keV/μm range (Fig. 3A). The difference in the energy deposition pattern is illustrated in a biologic context in Figs 3B and 3C.
Fig. 3.
Biophysical properties of radiation with different linear energy transfers (LET). A. α-particle LET as a function of distance for α emissions from Actinium-225. The plot shows the LET vs distance track for the 5.9 MeV α-particle emitted directly from 225Ac and also the 8.4 MeV α-particle emitted by bismuth-213, the 45.6 min half-life daughter of 225Ac; a plot of the 0.2 to 0.4 keV/μm LET of electrons is barely visible on the scale of this plot. B. Ionization events (circles) within the DNA molecule (red circles) are illustrated for low LET radiation (10 and 0.5 keV electrons) and for high LET radiation (4 MeV α-particle); figure taken from Ref. [6]. Note that the LET of high energy electrons is lower and would have even fewer DNA interactions than depicted in panel B. C. γH2AX staining in the nuclei of MCF7 cells showing the fluorescence associated with localization of the DNA DSB repair machinery for: left-20 min after 8 Gy (137Cs-irradiator) photon irradiation and right-20 min after incubation with 8 μCi/ml 213Bi-labeled antibody. (from Hong Song, unpublished)
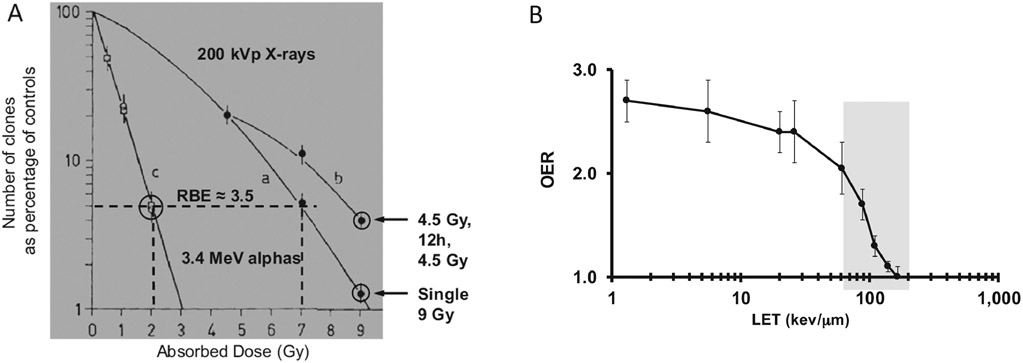
The biological effects of the higher ionization density arising from alpha-emitters is manifest in terms of a relative biologic effectiveness (RBE), a diminished repair during fractionation and reduced hypoxia-induced radioresistance (Fig. 4). Accordingly, cellular resistance to alpha-particles has not been observed [7-9]
Figure 4.
Illustration of the biological effects associated with α-particles due to their high LET. A. Cell survival curve for T1 cells irradiated with 200 kVp X-rays (curves a and b) and alpha-particles from 210Po (curve c). The figure shows an RBE of approximately 3.5 for 4% cellular survival for these two radiation types. The figure also shows increased survival (curve b) when a 12 h delay is introduced between 2 4.5 Gy fractions compared to the survival associated with a single 9 Gy irradiation. Cell survival remained unchanged when a 12h delay between alphabeam irradiation was introduced (two encircled data points at 2 Gy for curve c). B. The oxygen enhancement ratio (OER), defined as the absorbed dose ratio required to achieve the same level of cell kill for cells irradiated under different oxygen conentrations. The plot shows that the absorbed dose delivered must be increased by a factor of 2.5 to 3 for low LET radiations. In the range of LET values for α-particles the factor needed depends on LET and ranges from approximately 2 to 1 (where OER=1 means no hypoxia-induced radioresistance). Figures adapted from references [10] and [1].
Dosimetry
In radiotherapy absorbed dose may be directly measured. Treatment is delivered by specifying a configuration of external beams that delivers a pre-specified absorbed dose distribution to the tumor while respecting dose constraints to adjacent normal tissues. Since the beams originate outside the body, the patient may be replaced by an appropriate configuration of materials to measure the absorbed dose to the target region. In this case, the measurable quantity is absorbed dose with the unit gray (Gy). In RPT, the energy is deposited within tissue from radiation sources distributed within the tissue. Measurement devices of the type used in radiotherapy will perturb the activity distribution. Rather, the measurable quantity in RPT is the administered activity. In RPT, the pharmacokinetics of the administered RPT are required to estimate the total number of radionuclide disintegrations in different tissues. This information is coupled with radionuclide properties and information regarding patient anatomy (either from imaging or from a reference representation) to convert the distributions of radionuclide disintegrations to an absorbed distribution. The low amount of activity administered for αRPT and the low photon yield for some of the α-emitters have been seen as obstacles to collecting quantitative images for accurate αRPT dosimetry. This problem may be overcome by using validated surrogate imaging agents that mimic the pharmacokinetics and biodistribution of the αRPT. Quantitative planar imaging methods have been developed [11, 12] and quantitative SPECT imaging is becoming increasingly available for direct imaging of αRPT agents [13-16].
Dosimetry for RPT has its roots in the formalism established by the MIRD Committee to address the potential risks associated with the diagnostic use of radionuclides in nuclear medicine imaging. The formalism was updated in MIRD Pamphlet 21 [17] and adapted for α-emitter dosimetry in the MIRD Monograph- Radiobiology and Dosimetry for Radiopharmaceuical Therapy with Alpha-Particle Emitters [1]. This formalism is summarized by the following set of equations:
| (1) |
| (2) |
| (3) |
| (4) |
with:
| Dx(rT,TD) | absorbed dose to target region, rT from emission type x. |
| DRBE(rT,TD) | RBE-weighted dose to target region, rT. |
| rT, rs | target, source region (or tissue), respectively. |
| time-integrated activity or total number of nuclear transitions in target region, rT. | |
| M(rT) | mass of target region. |
| mean energy emitted per nuclear transition for ith emission of particle type x (= alpha, electron or photon). | |
| fraction of energy emitted per nuclear transition in source region, rS, that is absorbed in target region, rT by the ith emission of particle type x that is emitted with initial energy, E. | |
| RBEα, RBEe RBEph | relative biological effectiveness for alpha-particles (α), electrons (e) and photons (ph) , respectively, where RBEe = RBEph = 1 |
Absorbed fractions for α-particles were not generated for the Cristy-Eckerman phantom [18]. Accordingly, for human tissue dimensions, the following assumption has been used in αRPT calculations that pre-date the most recent ICRP phantoms and absorbed fractions [19, 20]:
| (5) |
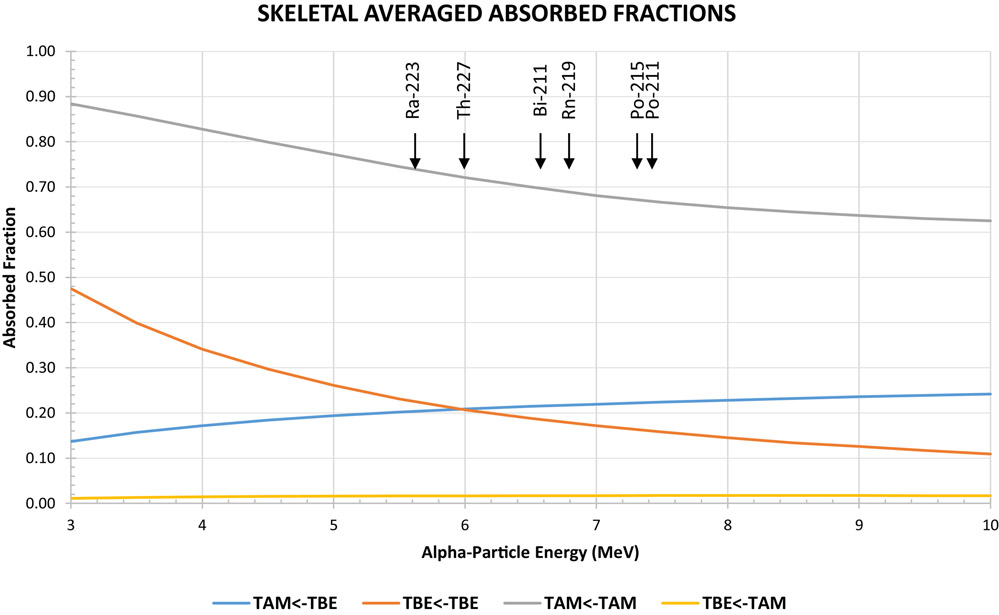
Although this assumption is reasonable for most tissues it is inaccurate, even at the macroscopic scale for selected organs. This is illustrated in the figure below for the bone marrow and for α-particle emissions arising from the decay of thorium-227 and its daughters.
The energy of the alpha particles emitted by thorium-227 and its daughters is between 5.5 and 7.5 MeV. As demonstrated by the arrows, the corresponding skeletal average absorbed fraction for decays originating in the trabecular bone surface (taken as a 10 μm-thick endosteal layer, denoted, TBE) irradiating the trabecular active marrow (TAM) ranges from 0.20 to 0.22. The absorbed fractions reported by Watchman et al. [21] were used to obtain figure 5. The most recently published absorbed fractions have explicitly modeled energy deposition for alpha particles and electrons for all tissues listed in the International Commission on Radiological Protection (ICRP) publication 110 voxelized phantom series [19, 20].
Figure 5 --.
Absorbed fraction vs alpha-particle energy. The figure shows the that the self-dose absorbed fractions (TAM←TAM) are less than 1 and the cross dose absorbed fractions (TAM←TBE) are less than 0.5 but greater than zero. The energy of alpha particles (with a yield greater than 20%) and corresponding alpha-emitter is shown by the arrows.
(TAM = Trabecular Active Marrow, TBE = Trabecular Bone Endosteum)
The MIRD Committee has recommended that αRPT absorbed doses be reported individually for each emission type, along with the RBEα. Based upon a review of experimental literature, an RBE value of between 3 and 5 was recommended for cell killing by an expert scientific panel convened by the Department of Energy in 1996 [22]. Since human studies using alpha-particle emitters have yet to be analyzed for deterministic effects, an RBE of 5 was recommended for projecting the possible deterministic biological effects associated with an estimated alpha-particle absorbed dose.
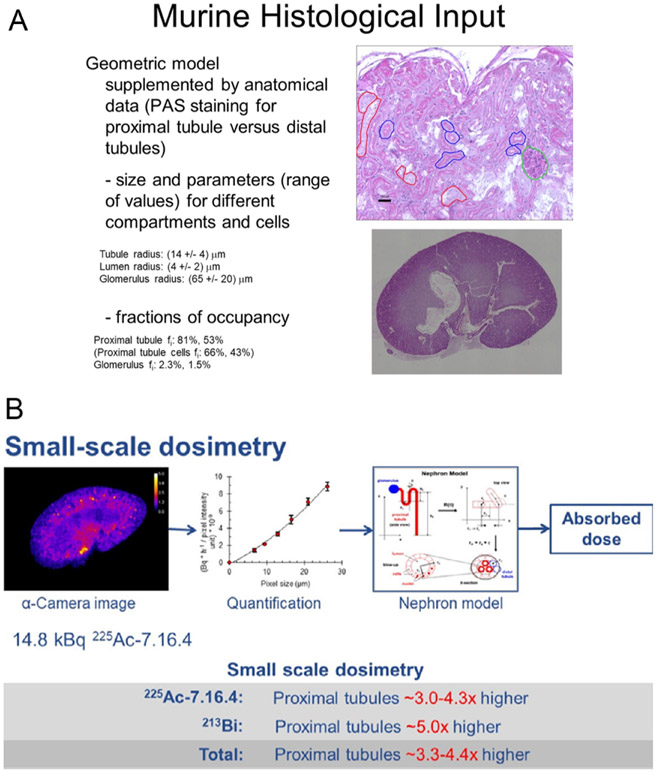
In some cases, the microscale distribution of the αRPT must be considered to arrive at a biologically relevant absorbed dose calculation. An example of this is provided by comparing the kidney absorbed dose calculated macroscopically and then also by accounting for the microscale distribution of an 225Ac-labeled antibody and it’s 45.6 min α-particle emitting daughter, 213Bi. The process for doing this is illustrated in figure 6.
Figure 6.
Microscale absorbed dose calculation for a pre-clinical model evaluation of a 225Ac-labeled antibody [23-26]. A. The dimensions of the sub-structures within the kidney were measured using histopathology and their fractional occupancy within the entire kidney volume was calculated. B. This information was used with alpha camera images and an idealized representation of the kidney substructure to calculate the ratio of whole kidney absorbed dose to that for the relevant critical structure.
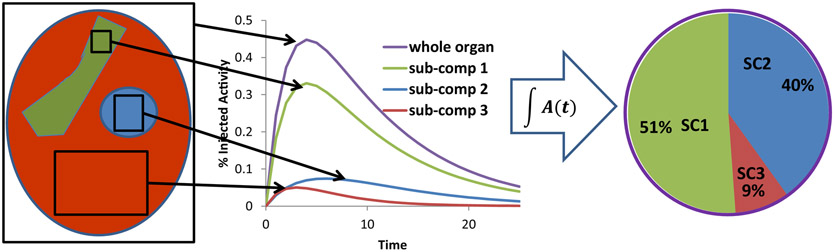
Clearly, it is not possible to directly obtain the data illustrated in Figure 6 from human studies. However, the methodology illustrated in figure 6 may be adapted to a clinical scenario as illustrated in the macro to micro scheme summarized in figure 7 and references [24, 27].
Figure 7.
Illustration of the macro to micro methodology. The methodology is founded on the ability to collect pharmacoknetics for an entire organ as well as relevant critical subcompartments of the organ in a preclinical model. This information may be used to establish apportionment factors for whole-organ measurements made in humans by quantitative imaging. The approach may be validated by assessing the variability of the apportionment factors for different pre-clinical models and also by showing within any particular model that the approach can be used to predict toxicity induced by sub-compartmental localization of the αRPT.
The absorbed dose values for αRPT should be expressed in Gy and not as effective or equivalent doses in Sv. Although effective dose can be calculated, it does not apply to the therapeutic use of radiopharmaceuticals. A detailed explanation for this is provided in MIRD Pamphlet 21 [17] and in the MIRD Committee’s monograph on alpha-particle dosimetry [1]. Briefly, the conversion of absorbed dose to effective dose is a two step process. In the first step, the absorbed dose D, for each organ is converted to equivalent dose H, by multiplying the organ absorbed dose by a radiation weighting factor, WR, which adjusts for radiation type R, (previously referred to as radiation “quality”). In the second step the difference in tissue sensitivity to induction of cancer and other detrimental effects is taken into account. In this step, the tissue equivalent doses are multiplied by tissue-specific weighting factors WT. The product H and WT, summed over all organs gives the effective dose E. The effective dose is, therefore, not defined for individual organs or tissues. It is a weighted sum over all tissues intended to give a single value that represents the risk of stochastic effects (cancer and other detrimental effects) due to radiation exposure. The following equations summarize this two-step process:
| (6) |
| (7) |
In equation 6, DR(rT) is the absorbed dose to tissue rT from radiation type R. The weighted sum of absorbed dose over all radiation types gives the equivalent dose to tissue rT. In equation 7, the sum is over all tissues. Effective dose does not correspond to any one tissue. It is also not the whole-body absorbed dose.
For radiation protection purposes, the International Commission on Radiological Protection (ICRP 60) has described the effectiveness of radiations of differing qualities by a series of Radiation Weighting Factors (ICRP 92) [28, 29]. The Radiation Weighting Factors currently being used in the ICRP's system of radiation protection purposes for alpha particles is 20 versus a value of 1 for all radiations having low energy transfer (sparsely ionizing), including x-ray and gamma radiations of all energies. The number 20 for alpha particles is a conservative estimate presumed to account for the increased risk of cancer or possible hereditary endpoints. Likewise, the ICRP has specified a series of tissue weighting factors to reflect the radiation sensitivity of each tissue. Using the methodology embodied in equations 14 and 15, and the radiation and tissue weighting factors, the effective dose may be calculated. The appropriateness of doing so for patients is addressed by the ICRP in publication 92 which states:
“Finally, it is important to remember that effective dose is a quantity intended for use in radiological protection and was not developed for use in epidemiological studies or other specific investigations of human exposure. For these other studies, absorbed dose in the organs of interest and specific data relating to the RBE of the radiation type in question are the most relevant quantities to use.”
This ICRP statement is made with regard to stochastic long-term effects, therefore a factor of 20 would be inappropriate even regarding long term effects for a therapeutic agent.
The question of the most appropriate weighting factor and unit for reporting alpha-particle absorbed doses that reflect acute (deterministic effect) as opposed to stochastic biological effects is a source of substantial discussion [30].
Some text from a recent MIRD Committee publication suggesting the use of a relative biological effectiveness (RBE) rather than the weighting factor for the therapeutic application of the high-LET radiation is quoted below [30]:
“Unlike the situation for stochastic effects, no well-defined formalism and associated special named quantities have been widely adopted for deterministic effects. Rather, scientific organizations have recommended that the relative biological effectiveness (RBE) of the high-LET radiation for specific deterministic effects be used to weight the absorbed dose.… In this context, the RBE is analogous to the weighting factor wR used to define the equivalent dose except that in this case, the RBE is a measured quantity for a specific deterministic endpoint rather than a value established by a review committee’s consensus of RBE values for relevant stochastic end-points.”
These passages emphasize that the effective dose concept is innapropriate and potentially meaningless for RPT, in general, and αRPT, in particular. Even, in terms of assessing long-term stochastic effects the tissue weighting factor of 20 used for protection is not appropriate since this factor was derived in a context where no benefit to the patient may be expected as a result of the exposure. In the absence of human data, a recommended RBE value of 5 has been suggested for deterministic effects (tissue reactions) in αRPT dosimetry. Rigorous dosimetry applied to αRPT trials, coupled with the resulting clincal experience, and a priori knowledge from radiotherapy will be essential to assessing RBE for different tissues and also for different agents. Experience with proton beam therapy suggests, RBE is important in avoiding toxicity [31]. On the other hand, since αRPT is targeted at the cellular level and α-particles emitted from the targeted radionuclide have a 50 to 100 μm range, the experience in proton beam therapy may not be directly relevant to αRPT.
Conclusions
Radiopharmaceutical therapy with α-particle emitters has emerged as a promising and unique treatment modality. αRPT is enhanced by synthetatic lethality approaches that utilize DNA DSB repair inhibitors. Alternatively, mutations in these pathways may be used as biomarkers to identify patients whose cancer will be especially responsive to αRPT. The radiobiology of αRPT along with studies that have examined potential resistance mechanisms suggest that αRPT is impervious to resistance mechanism that render other cancer therapeutics ineffective. The dosimetry of αRPT presents greater challenges than RPT with beta-particle-emitters. The challenges relate to collecting the pharmacokinetics and biodistribution data needed for dosimetry and also the scale at which the calculations must be performed to arrive at absorbed dose estimates that are more likely to predict biologic effects. Progress in these areas is being made and moving forward it will be critical to standardize dosimetry methods for αRPT so that they may be implemented in clinical trials, initially to collect dose-response date and subsequently for treatment planning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sgouros G, et al. , MIRD Monograph: Radiobiology and Dosimetry for Radiopahrmaceutical Therapy with Alpha-Particle Emitters, Sgouros G (editor). MIRD Monographs, ed. Sgouros G. 2015, Reston VA: SNMMI. [Google Scholar]
- 2.Kaelin WG Jr., The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer, 2005. 5(9): p. 689–98. [DOI] [PubMed] [Google Scholar]
- 3.Song H, et al. , Targeting aberrant DNA double strand break repair in triple negative breast cancer with alpha particle emitter radiolabeled anti-EGFR antibody. Mol Cancer Ther, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isaacsson Velho P, et al. , Efficacy of Radium-223 in Bone-metastatic Castration-resistant Prostate Cancer with and Without Homologous Repair Gene Defects. Eur Urol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barendsen GW, Impairment of the Proliferative Capacity of Human Cells in Culture by Alpha-Particles with Differing Linear-Energy Transfer. Int J Radiat Biol Relat Stud Phys Chem Med, 1964. 8(5): p. 453–466. [DOI] [PubMed] [Google Scholar]
- 6.Schipler A and Iliakis G, DNA double-strand-break complexity levels and their possible contributions to the probability for error-prone processing and repair pathway choice. Nucleic acids research, 2013. 41(16): p. 7589–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yard BD, et al. , Cellular and genetic determinants of the sensitivity of cancer to alpha-particle irradiation. Cancer Res, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haro KJ, Scott AC, and Scheinberg DA, Mechanisms of resistance to high and low linear energy transfer radiation in myeloid leukemia cells. Blood, 2012. 120(10): p. 2087–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sgouros G, Alpha-Particle-Emitter Radiopharmaceutical Therapy: Resistance is Futile. Cancer Res, in press. [DOI] [PubMed] [Google Scholar]
- 10.Barendsen GW, Modification of Radiation Damage by Fractionation of Dose Anoxia + Chemical Protectors in Relation to Let. Ann N Y Acad Sci, 1964. 114(A1): p. 96–114. [DOI] [PubMed] [Google Scholar]
- 11.Hindorf C, et al. , Quantitative imaging of 223Ra-chloride (Alpharadin) for targeted alpha-emitting radionuclide therapy of bone metastases. Nucl Med Commun, 2012. 33(7): p. 726–32. [DOI] [PubMed] [Google Scholar]
- 12.Carrasquillo J, et al. , Phase I pharmacokinetic and biodistribution study with escalating doses of 223Ra-dichloride in men with castration-resistant metastatic prostate cancer. European Journal of Nuclear Medicine and Molecular Imaging, 2013. 40(9): p. 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghaly M, Sgouros G, and Frey E, Quantitative Dual Isotope SPECT Imaging of the alpha-emitters Th-227 and Ra-223. Journal of Nuclear Medicine, 2019. 60(supplement 1): p. 41–41.30030338 [Google Scholar]
- 14.He B, et al. , Development and Validation of Methods for Quantitative In Vivo SPECT of Pb-212. Journal of Medical Imaging and Radiation Sciences, 2019. 50(1): p. S33. [Google Scholar]
- 15.Ghaly M, et al. , Quantitative SPECT Imaging of Thorium-227: A phantom experiment. EJNMMI, 2017. 44 (Supp 2): p. S180. [Google Scholar]
- 16.Ghaly M, Sgouros G, and Frey E, Development and evaluation of a quantitative reconstruction method for Th-227 SPECT. J Nucl Med, 2017. 58 (supplement 1): p. 748. [Google Scholar]
- 17.Bolch WE, et al. , MIRD pamphlet No. 21: a generalized schema for radiopharmaceutical dosimetry--standardization of nomenclature. J Nucl Med, 2009. 50(3): p. 477–84. [DOI] [PubMed] [Google Scholar]
- 18.Cristy M and Eckerman KF, Specific absorbed fractions of energy at various ages for internal photon sources. 1987, Oak Ridge National Laboratory: Oak Ridge, TN. [Google Scholar]
- 19.Menzel HG, Clement C, and DeLuca P, ICRP Publication 110. Realistic reference phantoms: an ICRP/ICRU joint effort. A report of adult reference computational phantoms, in Ann ICRP; 2009: England: p. 1–164. [DOI] [PubMed] [Google Scholar]
- 20.Bolch WE, et al. , ICRP Publication 133: The ICRP computational framework for internal dose assessment for reference adults: specific absorbed fractions. Annals of the ICRP, 2016. 45(2): p. 5–73. [DOI] [PubMed] [Google Scholar]
- 21.Watchman CJ, et al. , Absorbed fractions for alpha-particles in tissues of trabecular bone: Considerations of marrow cellularity within the ICRP reference male. J Nucl Med, 2005. 46(7): p. 1171–1185. [PubMed] [Google Scholar]
- 22.Feinendegen LE and McClure JJ, Meeting report - Alpha-emitters for medical therapy - Workshop of the United States Department of Energy - Denver, Colorado, May 30-31,1996. Radiat Res, 1997. 148(2): p. 195–201. [Google Scholar]
- 23.Song H, et al. , Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res, 2009. 69(23): p. 8941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hobbs RF, et al. , A nephron-based model of the kidneys for macro-to-micro alpha-particle dosimetry. Phys Med Biol, 2012. 57(13): p. 4403–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josefsson A, et al. , Pharmacokinetics and Dosimetry for Normal Organs/Tissues of Vector Labeled 225Ac and Daughter Radionuclides. Brachytherapy, 2019. 18(3): p. S21. [Google Scholar]
- 26.Josefsson A, et al. , Small Scale Renal Dosimetry for Alpha Particle Radiopharmaceutical Therapy of Metastatic Breast Cancer With 225Ac-7.16.4. International Journal of Radiation Oncology • Biology • Physics, 2016. 93(3): p. S149–S150. [Google Scholar]
- 27.Hobbs RF, et al. , A bone marrow toxicity model for (2)(2)(3)Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol, 2012. 57(10): p. 3207–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ICRP, Publication 60,1990 Recommendations of the International Commission on Radiological Protection. 1991, International Commission on Radiological Protection: Pergamon, Oxford. [Google Scholar]
- 29.ICRP, Publication 92, Relative biological effectiveness (RBE), quality factor (Q), and radiation weighting factor (WR), 92. 2003: ICRP; [DOI] [PubMed] [Google Scholar]
- 30.Sgouros G, et al. , MIRD commentary: proposed name for a dosimetry unit applicable to deterministic biological effects the barendsen (Bd). J Nucl Med, 2009. 50(3): p. 485–7. [DOI] [PubMed] [Google Scholar]
- 31.Lühr A, et al. , Relative biological effectiveness in proton beam therapy - Current knowledge and future challenges. Clinical and translational radiation oncology, 2018. 9: p. 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]