Abstract
Exosomes are small extracellular microvesicles that are secreted by cells when intracellular multivesicular bodies fuse with the plasma membrane. We have previously demonstrated that Nischarin inhibits focal adhesion formation, cell migration, and invasion, leading to reduced activation of focal adhesion kinase. In this study, we propose that the tumor suppressor Nischarin regulates the release of exosomes. When cocultured on exosomes from Nischarin-positive cells, breast cancer cells exhibited reduced survival, migration, adhesion, and spreading. The same cocultures formed xenograft tumors of significantly reduced volume following injection into mice. Exosomes secreted by Nischarin-expressing tumors inhibited tumor growth. Expression of only one allele of Nischarin increased secretion of exosomes, and Rab14 activity modulated exosome secretions and cell growth. Taken together, this study reveals a novel role for Nischarin in preventing cancer cell motility, which contributes to our understanding of exosome biology.
Significance
Regulation of Nischarin-mediated exosome secretion by Rab14 seems to play an important role in controlling tumor growth and migration.
Introduction
Nischarin, or imidazoline receptor antisera-selected (IRAS) protein, is a protein involved in a number of biological processes. The Nisch gene is located on chromosome 3p21, which is frequently lost in cancers (1). Most notably, Nischarin is an integrin α5β1 binding protein known to affect cell migration by antagonizing the actions of cell signaling proteins that contribute to tumor cell migration and invasion (2). Furthermore, Nischarin has also been shown to affect cytoskeletal reorganization, mainly by inhibiting Rac-induced lamellipodia formation (2). Consistent with this, Nischarin’s inhibition of cell migration has been linked to other proteins (3–5).
During cell migration, cells adhere to its extracellular environment through focal adhesions. These complexes use integrins to attach to extracellular matrix (ECM) proteins (6, 7). Each integrin has designated ligand(s), and decreased expression of the ligand or receptor affects focal adhesion number. Integrins also bind to fibronectin-coated exosomes (8). Exosomes are smaller microvesicles (30–200 nm in diameter) secreted from cells when multivesicular bodies (MVB) fuse with the plasma membrane (9–12). Although Nischarin’s role has yet to be linked to exosomes, previous studies have shown that the Nischarin-Rab14 interaction promotes the maturation of CD63+ endosomes (13). Nischarin is an effector of the GTPase Ras-related protein Rab-14 (13). Although Rab14 is involved in vesicle sorting and trafficking (14), only one report has identified Rab14 function in breast cancer exosomes (15). Nischarin directly interacts with Rab14 to effect intracellular Salmonella survival (13). In the presence of Nischarin, there is triple colocalization between the late endosome and exosome marker CD63, Rab14, and Nischarin (13).
While it is known that MVBs fuse with the plasma membrane just before exosome release, the physiologic consequences of this have yet to be determined in the breast cancer microenvironment. Furthermore, the proteins responsible for the MVB-plasma membrane fusion are not well characterized. We hypothesized that Nischarin may affect the migration of cancer cells by controlling exosome release. Exosomes from 231 cells promoted migration of 231 cells, while exosomes from 231 Nisch cells inhibited migration. These effects were due to the decreased number of exosomes released by 231 Nisch cells. In contrast, active Rab14 promotes exosome secretion and cell growth. In summary, our study highlights the antitumoral function of Nischarin expression mediated by exosome-dependent secretions in breast cancer.
Materials and Methods
Cell culture
MDA-MB-231, MDA-MB-231 Nischarin, MDA-MB-231 GFP, MDA-MB-231 Rab14, MDA-MB-231 Rab14 S25N, MDA-MB-231 Rab14 Q70L, MCF7, BT20, T47D, MDA-MB-468, and SUM185, and MCF7 cells were cultured in DMEM at 37°C, 5% CO2 supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco). MCF10A cells were cultured in DMEM/F12 supplemented with 5% horse serum EGF, hydrocortizone, choleratoxin, insulin, and penicillin/streptomycin. MDA-MB-231 Nisch, MDA-MB-231 GFP, MDA-MB-231 Rab14, MDA-MB-231 Rab14 S25N, and MDA-MB-231 Rab14 Q70L were prepared as described previously (4). Briefly, 231 Nisch cells were generated by amplifying human NISCH. The 4545 base pair PCR product was then cloned into a pCDH-CMV-MCS-EF1-copGFP vector. The viral particles were generated in HEK-293T cells along with the pCDH-GFP-Nisch plasmid. The supernatants containing the lentiviral particles were collected 48 hours later, concentrated, then reconstituted in serum-free media. The MDA-MB-231 GFP cells were generated similarly, except there was no cloning of NISCH into the pCDH-CMV-MCS-EF1-copGFP vector. The cells were sorted by FACS for GFP selection. Low passage cells were used for all the experiments and the cell line purity was verified every two months using appropriate markers of the cell type. Transfected cells were selected using the antibiotic puromcyin.
Nischarin WT (+/+), Nischarin HET (+/−), and Nischarin Null (−/−) animals were generated as described previously (16). Briefly, exons 7 to 10 of Nischarin were deleted, and the resulting animals were intercrossed with animals expressing the mouse mammary tumor virus-polyma middle T transgene. For mouse genotyping, mouse tail genomic DNA was extracted and amplified by PCR and electrophoresed on 2% agarose gels. Primary WT-PyMT (Nisch+/+), HET-PyMT (Nisch+/−), and Null-PyMT (Nisch−/−) cells were isolated as described previously (17). Briefly, the mammary tumors were isolated and cut into small pieces with a razor blade and scissors. The tissues were incubated with collagenase for 2 hours to allow for enzymatic dissociation of the tissue. The resulting material was ultracentrifuged to remove debris and blood. The following conditions were used for cell culture experiments of cells that were seeded on ECM proteins, 10 μg/mL of fibronectin (BD Biosciences) was prepared in PBS. Bovine collagen 1 (BD Biosciences) was added to each well at 0.16 mL/cm2. The ECM proteins were added to the wells and placed on a rocker for 2 hours at room temperature then washed two times with warm PBS. The cells were seeded onto the wells immediately after washing with PBS.
Cell line authentication
MDA-MB-231 cells were obtained from ATCC and Nischarin expression in these cells was maintained by puromycin selection. Nischarin expression was monitored by immunoblotting using anti-Nischarin antibody. Cells were not used beyond passage five and Mycoplasma was tested for all cell lines at least once every 6 months. The primary cells prepared from PyMT tumors were tested every time for Nischarin truncation genotype, PyMT expression by genomic PCR approach. The primary cells were never used beyond passage three.
Animal studies
All mouse experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center (New Orleans, LA). Four- to 10-week-old female Prkdc scid mice were used in the xenograft studies (4 mice per group). The exosomes used for all mouse coinjections were isolated from 1.8 g of mouse mammary tumor. Therefore, the approximate number of Nisch+/+ exosomes isolated were 1 × 108 while the number of Nisch+/− exosomes was 1.9 × 108. After exosome isolation, animals were either injected with exosomes only, 1 × 106 viable Nisch+/+ or Nisch+/− cells alone, or Nisch+/+ or Nisch+/− cells that were cocultured with the Nisch+/+ or Nisch+/− exosomes for 4 days. Tumor growth was assessed every three days with calipers and tumor volume was calculated as π × length × width2/6.
Isolation of exosomes
A total of 3 × 106 cells were seeded in T175 flasks and cultured for 48 hours. The medium was centrifuged at 300 × g for 10 minutes, and then the supernatant was collected and centrifuged again at 2,000 × g for 20 minutes. This was repeated again for two more centrifugations both at 10,000 × g for 30 minutes and 1 × 100,000 × g for 70 minutes. The supernatant was then discarded and the pellet was centrifuged with PBS for 70 minutes at 1 × 100,000 × g. The pellet was finally resuspended in PBS for subsequent studies. To isolate exosomes from whole tumors, we harvested breast tumors from Nisch+/+ and Nisch+/− mice as described previously (17). Tumors were disintegrated and incubated with collagenase for 3 hours. The samples were centrifuged for 5 minutes at 200 × g. The supernatant was then used for the exosome isolation procedure by ultracentrifugation.
The Beckman Couter DelsaNanoC instrument was used to measure diameter and molecular weight. The samples were diluted in ddH2O, added to a cuvette, and inserted into the device. This instrument uses Photon Correlation Spectroscopy (PCS) to analyze the molecular weight of the sample as it relates to the α and β ion particles in the solution. Basically, when laser light is directed toward the particle, light will scatter in different directions. The intensities observed by the machine are a result of the movement of ion particles due to Brownian motion. For particle counting, the Malvern NanoSight, a Nanoparticle Tracking Analysis instrument was used. The samples were diluted in ddH2O and added to the flow system with a syringe. As the samples pass through the flow system, the exosomes are visualized, counted, and characterized.
Labeling of exosomes
Exosomes were isolated from Nisch+/+ and Nisch+/− tumors and resuspended in PBS. For CD63 labeling, the CD63 antibody was added at a concentration of 1:100 and incubated for 30 minutes at 37°C as described previously (18). The exosomes were rinsed in 0.5% BSA-PBS and centrifuged at 100,000 × g for 1 hour at 4°C. The secondary antibody (Alexa Fluor 594) was added at a 1:200 concentration for 30 minutes at 37°C. The exosomes were washed again by centrifugation, and the pellet was resuspended with PBS. Exosome images were captured using the Leica DMi8 confocal microscope.
Antibodies
Antibodies and dilutions were used as follows: mouse monoclonal anti-vinculin (Sigma; 1:5,000), mouse monoclonal anti-paxillin (BD Biosciences; 1:5,000), rabbit monoclonal anti-flotillin (Cell Signaling Technology; 1:100–500), mouse monoclonal anti-CD63 (Santa Cruz Biotechnology; 1:500), mouse monoclonal anti-Rab14 (Santa Cruz Biotechnology; 1:100–1,000), mouse monoclonal anti-Nischarin (BD Biosciences; 1:1,000), and phalloidin (Sigma; 1:100).
Cell proliferation assay
For MTT cell viability assays, cells were seeded onto 96-well plates for 24–48 hours with the various coatings. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent (5mg/mL in PBS) was added to each well. After a 3.5-hour incubation, the media/MTT was aspirated and a HCl/isopropanol solution (0.1% NP-40) was added to each well. The plate was incubated for 20 minutes and then the absorbance was measured at 565 nm. The results were normalized to medium alone control.
For cell counting experiments, 5,000 231 GFP, 231 GFP Nisch, Nisch+/+, and Nisch+/− cells were seeded onto 96-well dishes and incubated for 24 hours. After 24 hours, representative images were captured at 10× with the EVOS XL Cell Imaging System. The number of cells per milliliter were calculated using the Bio-Rad TC20 Automated Cell Counter.
Live-cell migration
For live-cell imaging, the cells were seeded onto 12-well plates and cultured at 37° C with 5% CO2 overnight. The next day, a small wound was created on the corner of the well with a 10 μL pipette tip to stimulate migration, and live cell migration was captured every hour for 19 hours. Max distance and mean velocity were calculated using the ImageJ plugins MTrackJ and Chemotaxis Tool. Migration tracks were created by using the X and Y coordinates from the MTrackJ plugin. These coordinates were tracked by the microscope and registered at subpixel precision as the inverse of the zooming factor. For data representation, the subsequent X and Y points were subtracted from the previous X and Y points. The represented axis boundaries were selected on the basis of the cell that traveled the most distance and standardized for each cell type. For example, the 231 cell that traveled the furthest distance on the −x axis reached (−313.333,−97.333), therefore the −x axis for all 231 cells was set to −350 μm. Images were captured at 10× using the Olympus IX81 and Nikon Eclipse Ti-S.
Immunofluorescence
Cells were washed two times with PBS and fixed in 3.7% formaldehyde for 10 minutes. Then, the cells were washed three times with PBS for 5 minutes each. The cells were then permeabilized with 0.5% Triton-X100 for five minutes. The cells were washed again three times with PBS then blocked for 1 hour with 2% BSA in PBS. After blocking, the coverslips were incubated upside down on the primary antibody overnight (vinculin or CD63) at 4°C. After primary antibody staining, the cells were washed three times for ten minutes each. The cells were then incubated with the secondary antibody for 1 hour. Prior to mounting, the cells were washed twice with PBS for 10 minutes, then once with water. The coverslips were mounted with Fluoromount-G (Southern Biotech).
For phalloidin staining, cells were washed two times with PBS, and fixed in 3.7% formaldehyde for five minutes. The cells were washed three times with PBS for five minutes each and were stained with 50μg/mL of a fluorescently conjugated phalloidin (Sigma) for 30 minutes. After staining, the cells were washed three times in PBS for 10 minutes each. The coverslips were mounted with DAPI Fluoromount-G (Southern Biotech).
CellProfiler was used to determine the number of focal adhesions per cell and the percentage of area covered by focal adhesions. After incubating the cells overnight on fibronectin, the cells were fixed and stained with vinculin. Images of vinculin-stained cells were uploaded into CellProfiler and the Enhance or Suppress Features and Indentify Primary Objects modules were used to assess the focal adhesions. For the Identify Primary Objects module, the typical diameter used was 10–50. The threshold strategy was Global, the thresholding method was Robust Background. The lower bounds on threshold was then changed to 0.001. Images were captured at 60× using the Nikon Eclipse Ti-S.
Real-time PCR
Total RNA was isolated from cultured cells with TRIzol reagent (Invitrogen). cDNA was generated from 2 μg of RNA using the Invitrogen/Applied Biosystems/ABl High Capacity cDNA Reverse Transcriptase Kit. For gel detection, samples were run on a 3% agarose gel.
Stiffness with indentation
A cylindrical sample with 6-mm diameter was cut out from the tumor tissue and constrained by a PDMS mold. The sample was mounted inside a petri dish on top of an electronic scale (SPX622, Ohaus), which is located on a motorized stage (H101E1F, PRIOR Scientific). The motorized stage brings the sample up and pushes it through a fixed circular indenter with 1-mm diameter, which produces a known surface displacement of the sample. The resulting force is measured simultaneously using the scale and the force versus strain curve is extracted. To calculate the stiffness, an indentation model is used considering the geometry of the indenter (19). The stage is lifted with step size of 10 μm until a force of 5 mN is applied on the sample. To avoid the adhesion effect (20), the average slope over the region between 50% and 100% of the maximum indentation force is defined as contact stiffness, k. The effective elastic modulus, Eeff can be defined using the standard relation (21) as shown in Eq. A.
| (A) |
where, A is the projected contact area underneath the indenter and d is the diameter of the circular indenter.
Generally, the effective elastic modulus is a function of elastic modulus of both indenter and sample. However, because the elasticity of used indenter, Ei, is much larger than the elasticity of the sample, Es, this relation can be simplified as shown in Eq. B
| (B) |
where, vi and vs are Poisson’s ratio of the indenter and the sample. Assuming that the sample is incompressible (vs = 0.5), the elastic modulus of the sample is extracted from contact stiffness as shown in Eq. C.
| (C) |
Statistical analysis
All experiments were repeated at least three times. Statistical analyses involving only one factor were performed using Graph-Pad Prism 5.0 software using either a two-sample t test or one-way ANOVA. Experiments involving two factors were analyzed in SAS (Version 9.4) with two-factor ANOVA with interaction. Pairwise mean comparisons were performed using the PDIFF option on the LSMEANS statement of PROC GLM in SAS. The error bars indicate the SD from the mean (*, P < 0.05; **, P < 0.01; ***, P < 0.001; and ****, P < 0.0001). All experiments were repeated at least three times.
Results
Nischarin regulates cell attachment
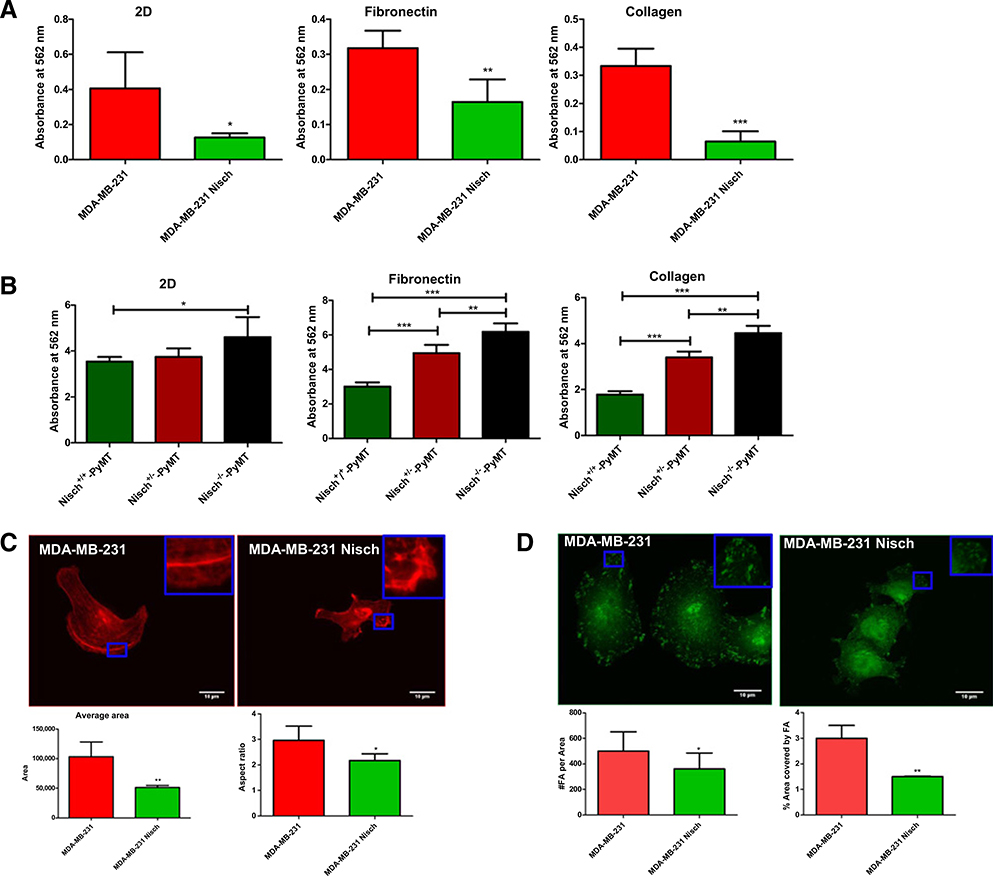
Detachment of cells from a matrix leads to anoikis, cell death due to the loss of adhesion (22, 23). Because Nischarin reduces the activation of proteins, such as FAK and Rac that contribute to cell adhesion, we examined whether there was a decrease in cell attachment in Nischarin-positive breast cancer cells seeded on different matrices. To perform this, we used our previously published MDA-MB-231 cells stably expressing Nischarin (hereafter, 231 Nisch) and Nisch+/+-Polyoma Middle T (PyMT), Nisch+/−- Polyoma Middle T (PyMT) and Nisch−/−- Polyoma Middle T (PyMT) cells (4). To determine whether Nischarin affects cell proliferation, we seeded MDA-MB-231 (hereafter, 231) and 231 Nisch cells on 2D (directly on the tissue culture plate), Fibronectin, and collagen and measured cell proliferation using the MTT assay and automated cell counting. The human breast cancer cells stably expressing Nischarin had a decrease in cell proliferation on 2D, fibronectin, and collagen (Fig. 1A; Supplementary Fig S1A). Similarly, we checked cell proliferation of Nisch+/+-PyMT, Nisch+/−-PyMT and Nisch−/−-PyMT mouse tumor cells on 2D, fibronectin, and collagen. Hereafter, these tumor cells are referred to as Nisch+/+, Nisch+/−, and Nisch−/− cells. On 2D, only Nisch−/− had a significant increase in proliferation. On fibronectin and collagen, Nisch+/− cells had significantly greater proliferation compared with Nisch+/+ (Fig. 1B; Supplementary Fig. S1B). Overall, Nisch−/− cells had the greatest amount of proliferation compared with Nisch+/+ and Nisch+/− cells. In all of the cell lines we tested, the presence of Nischarin in cancer cells led to a significant reduction in cell proliferation regardless of the matrix (Fig. 1A and B).
Figure 1.
Nischarin decreases the attachment of breast cancer cells. A, The 231 (n = 6) and 231 Nisch (n = 6) cells were seeded onto a standard tissue culture surface (2D), fibronectin, or collagen for 48 hours. Cell proliferation was determined by the MTT assay. B, Nisch+/+, Nisch+/−, and Nischr−/− cells were seeded onto a tissue culture surface (2D), fibronectin, or collagen for 48 hours. Cell proliferation was determined by the MTT assay. C, The 231 (n = 18) and 231 Nisch (n = 21) cells were seeded on 10 μg/mL fibronectin for 24 hours and stained with phalloidin. The average area and aspect ratio were calculated using ImageJ (inset with a zoom is shown). Aspect ratio = major axis/minor axis. D, Vinculin immunofluorescence staining of 231 and 231 Nisch cells (inset with a zoom is shown). Number of vinculin-positive focal adhesions (FA) per area and percentage of area covered by focal adhesion was calculated using CellProfiler. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
There are many possible contributors to reduced cell proliferation. To determine whether the reduction in cell proliferation of Nischarin-positive breast cancer cells was due to cell attachment, we assessed cell spreading and focal adhesion dynamics. 231 and 231 Nisch cells were seeded onto Fibronectin, stained with phalloidin, and captured by immunofluorescence microscopy. Cell area and aspect ratio were quantified with ImageJ. Nischarin decreased the average cell area and aspect ratio (Fig. 1C). This “shrinking” of cells is an indicator of cancer cell death. To determine whether this reduction of cell spreading is due to a decrease in focal adhesions, we stained cells with the focal adhesion marker vinculin, then determined the number of focal adhesions and the area covered by focal adhesions using CellProfiler. 231 Nisch cells had a reduction in focal adhesion number and in the area covered by focal adhesions (Fig. 1D). This suggests that the decrease in cell area is due to destabilized cell-matrix attachment. Several intracellular signaling pathways have been elucidated, but whether Nischarin regulates attachment to specific components of the ECM remains elusive.
Nischarin alters exosomes’ properties
The ECM is a diverse mesh network of proteins that support cell attachment. The ECM stimulates PI3K activity, which results in cancer phenotypes, such as anchorage-independent growth on soft agar and protection from apoptosis (24, 25). Previous reports show that culturing noncancerous cells in conditioned media from cancer cells promotes proliferation and migration of noncancerous cells (26, 27). We previously determined that incubating noncancerous MCF10A cells with media from 231 cells promotes cell migration (28). Incubating the cells with media from 231 Nisch cells promotes a moderate amount of cell migration, but significantly less than media from 231 cells (28). These results suggest that Nischarin is regulating the cancer cell secretome. The possible factors involved in this regulation could be matrix metalloproteases, cytokines, or secreted exosomes, to name a few. Thus, we first examined whether Nischarin is regulating the release of exosomes.
Although each cell type secretes unique exosomes, CD63 and flotillin1 are commonly used exosomal markers for mammary cells. Flotillin proteins function in a number of contexts, such as endocytosis and cell signaling. We isolated exosomes from 231 and 231 Nisch cells by differential ultracentrifugation. We validated flotillin1 expression in exosomes and cells by Western blotting (Fig. 2A). Previous studies have shown that flotillin1 is expressed in whole-cell lysates, as well as, in exosomes from 231 cells (29). RNAs are also present in exosomes (30). We observed the expression of HSP70, ITGA5, ITGA11, ITGAL, and ITGAV mRNA in the exosomes (Fig. 2B). This further confirms that we have isolated exosomes. Very little amounts of Nisch mRNA and protein were found in the exosomes (Fig. 2B; Supplementary Fig. S2A) and Rab27a is not detected in exosomes (Supplementary Fig. S2B).
Figure 2.
Characterization of exosomes from breast cancer cells. A, Western blot detection of flotillin and vinculin (control) in 231 exosomes, 231 cells, 231 Nisch exosomes, and 231 Nisch cells. B, RT-PCR of NISCHARIN, ACTIN, HSP70, ITGA5, ITGA11, ITGAL, and ITGAV mRNA in 231 and 231 Nisch exosomes, as well as 231 and 231 Nisch cells. C, Diameter and molecular weight analysis of exosomes from 231 (n = 9) and 231 Nisch (n = 9) cells with the DelsaNanoC. D, Number of particles per frame and per mL of 231 (n = 6) and 231 Nisch exosomes (n = 15) with the Nanosight Nanoparticle Tracking Analysis. E, Nisch+/+ and Nisch+/− exosomes were labeled with CD63 (inset with a zoom is shown). Scale bars, 10 μm. F, Western blot detection of CD63 and vinculin (control) in Nisch+/+ tumor exosomes, Nisch+/+ tumors, Nisch+/− tumor exosomes, and Nisch+/− tumor cells. G, Diameter and molecular weight (Mw) analysis of exosomes from Nisch+/+ (n = 3) and Nisch+/− (n = 3) tumors with the DelsaNanoC. H, Number of particles per frame and per mililiter of Nisch+/+ (n = 6), Nisch+/− exosomes (n = 11), and Nisch−/− exosomes (n = 3) with the Nanosight Nanoparticle Tracking Analysis. ***, P < 0.001.
We examined the mean diameter of our exosomes (9–12). Photon correlation spectroscopy (PCS) indicated that our exosomes are between 30 and 200 nm. Although not statistically significant, exosomes from 231 cells had a mean diameter of 189 nm while those from 231 Nisch cells have a lower mean diameter of 172 nm (Fig. 2C). Because only small variations were observed in vesicle size between 231 and 231 Nisch samples, we next assessed changes in exosome quantity. Using PCS, we were able to analyze the molecular weight of the sample as it relates to α and β ion particles in the solution. Although not statistically significant, exosomes from 231 cells had a molecular weight of 1.49 × 109 while those from 231 Nisch cells had a weight of 1.24 × 109 (Fig. 2C). Because we did not see significant differences in the diameter and molecular weight of 231 and 231 Nisch cells, we assessed particle number using the Nanoparticle Tracking Analysis instrument (Malvern NanoSight). 231 Nisch cells had a decreased number of particles per frame (counted by the software) and per milliliter (Fig. 2D). These results suggest that Nischarin-positive cells produce less exosomes than Nischarin-reduced cells. Furthermore, we performed rescue experiments in Nisch knockdown cells to see whether we can reduce particle counts. To do this, we introduced Nisch into previously published MCF7 cells (4), which have a stable knockdown of Nisch. Introducing Nisch to knockdown cells reduced exosome numbers to control levels (Supplementary Fig. S2C and S2D). These data indicate that Nischarin-positive 231 cells secrete significantly fewer exosomes while Nischarin-positive MCF7 cells secrete slightly fewer exosomes when compared with their Nisch-negative counterparts.
We also examined exosome number using whole mouse tumor tissues. Exosomes secreted from Nisch+/+ and Nisch+/− tumors are also CD63 positive (Fig. 2E and F). We assessed the diameter and molecular weight of exosomes. Nisch+/+ tumor exosomes had an average diameter of 67nm, which was significantly lower than the Nisch+/− exosome size (98 nm; Fig. 2G). Furthermore, we found that for the same sized tumor, Nisch+/− exosomes had an average weight of 3.99 × 108, while Nisch+/+ exosome samples had a significantly lower weight (1.86 × 108; Fig. 2G). Finally, Nisch+/− andNisch−/− exosomes have a slightly greater number of particles per frame, as well as number of particles per ml, although not statistically significant (Fig. 2H). Taken together, our results suggest that the presence of Nischarin in cells promotes secretion of fewer and smaller exosomes compared with cells that have nonfunctional Nischarin.
Exosomes from Nischarin-positive cells reduce breast cancer cell motility
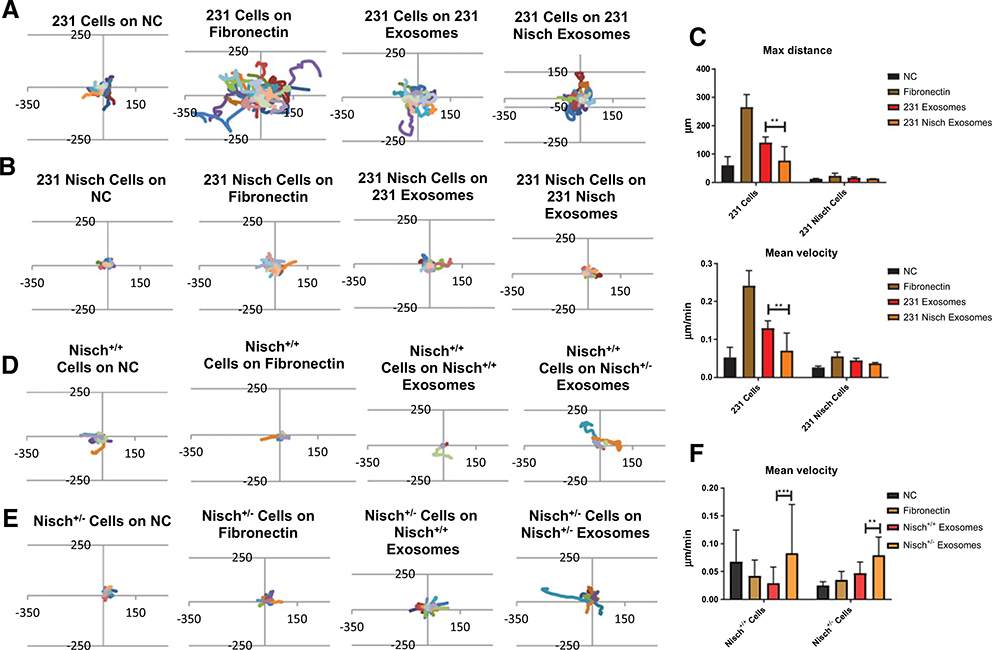
Previous reports show that exosomes are fibronectin coated and cancer cells migrate on them (8, 31). Thus, we investigated whether the altered dynamics of exosomes from Nischarin-positive cells affect cell motility. We have previously shown that the presence of Nischarin in 231 cells inhibits cell migration on gelatin and on matrices from NIH3T3 fibroblasts (28). MDA-MB-231 or 231 Nisch cells were seeded on a dish coated with no coating (NC), fibronectin (as a positive control), exosomes from 231 cells, and exosomes from 231 Nisch cells and migration was tracked every hour for 19 hours (Fig. 3A and B). Each individual line corresponds to one tracked cell. The 231 cells had more cells venture past 150 and −150 mm on the x- and y-axes when seeded on fibronectin compared with the other substrates (Fig. 3A). The 231 Nisch cells have a much lower migratory capacity and do not reach 150 and −150 μm on the axes (Fig. 3B). There were more 231 Nisch cells that reached 50 and −50 on the x- and y-axes when the cells were placed on Fibronectin as well. Interestingly, migration on exosomes from 231 cells is higher than the migration on exosomes from 231 Nisch cells. The live cell tracking was further analyzed on ImageJ to detect the maximum distance and mean velocity. Maximum distance and mean velocity were significantly reduced in 231 Nisch cells regardless of the coating but more pronounced on 231 exosomes. Interestingly, 231 cell migration on 231 Nisch exosomes is significantly lower than the on 231 exosomes (Fig. 3C). Altogether, the cells migrated at a greater distance and velocity on fibronectin, followed by on 231 exosomes, then on 231 Nisch exosomes.
Figure 3.
Exosomes from Nischarin cells reduce cell motility. The 231 (A) and 231 Nisch cells (B) were seeded onto NC, fibronectin, 231 exosomes, or 231 Nisch exosomes and live imaging was captured every hour for 19 hours (n = 27) using an Olympus IX81 light microscope. Migration tracks were created using the X and Y coordinates from the MTrackJ plugin. C, Max distance and mean velocity were calculated using the ImageJ plugins MTrackJ and Chemotaxis Tool. D, Migration tracks of Nisch+/+ cells were seeded on NC (n = 4), fibronectin (n = 7), Nisch+/+ exosomes (n = 11), or Nisch+/− exosomes (n = 9). E, Migration tracks of Nisch+/− cells were seeded on NC (n = 14), fibronectin (n = 19), Nisch+/+ exosomes (n = 27), or Nisch+/− exosomes (n = 10). F, Mean velocity of Nisch+/+ and Nisch+/− cells on NC, fibronectin, Nisch+/+ exosomes, and Nisch+/− exosomes. **, P < 0.01; ***, P < 0.001.
Next, we examined migration using mouse tumor exosomes from animals with different Nischarin profiles. We investigated whether the altered exosome characteristics from Nischarin-positive cells affect cell motility in mouse tumors. Fewer Nisch−/− animals are viable; therefore, to maintain significant replicates for our experiments, we only used Nisch+/+ and Nisch+/− tumor cells. Nisch+/+ or Nisch+/− tumor cells were seeded on tissue culture dishes coated with no substrate, fibronectin, exosomes from Nisch+/+ tumors, or exosomes from Nisch+/− tumors and migration was tracked every hour for 19 hours. The migration was most dramatic for Nisch+/+ and Nisch+/− cells on Nisch+/− exosomes (Fig. 3D and E). When mean velocity was assessed, there was a slight increase in the migration of Nisch+/− cells on fibronectin, although not statistically significant. Furthermore, Nisch+/+ cell migration on Nisch+/− exosomes is significantly higher than on Nisch+/+ exosomes (Fig. 3F). Nisch+/− exosomes promoted greater cell velocity when compared with fibronectin and Nisch+/+ exosome coating. The most significant changes between Nisch+/+ and Nisch+/− cells were seen with the exosome coatings (3F). Generally, exosomes from Nischarin-reduced cells increased cell migration compared with exosomes from Nischarin-positive cells. The data suggest that the absence of Nischarin may lead the cells to increase release of exosomes for an added migratory advantage.
Exosomes from Nischarin-positive cells reduce cell adhesion
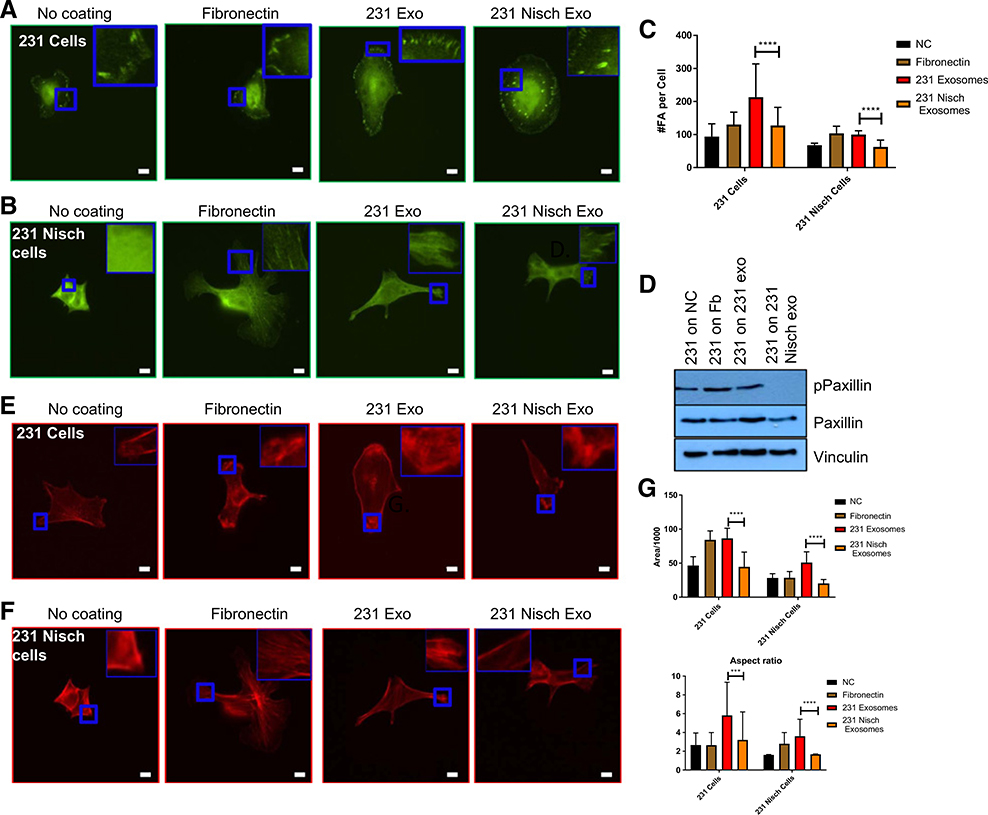
From the above data, we have established a potential role for exosomes in cell migration. Cells seeded on fibronectin have greater dynamic adhesions and cell spreading. The presence of dynamic adhesions determines that whether focal adhesion machinery is able to disassemble and assemble in a highly motile cell. To understand how these exosomes reduce cell migration, we examined cell adhesion to the exosomes and changes in focal adhesion numbers. MDA-MB-231 and 231 Nisch cells were seeded onto NC, fibronectin, 231 exosomes and 231 Nisch exosomes, and visualized the focal adhesion complexes. In addition, we analyzed the number of focal adhesions per cell using CellProfiler. MDA-MB-231 cells displayed an increased number of focal adhesions on all matrices, while the focal adhesions on 231 exosomes showed further increase in number. However, focal adhesions on 231 Nisch cells are significantly lower in number (Fig. 4A–C). Furthermore, 231 cells seeded on 231 Nisch exosomes showed less number of focal adhesions. As stated before, Nischarin is an Integrin α5β binding protein that is known to affect cell migration by antagonizing the actions of cell signaling proteins that contribute to tumor cell migration and invasion (2). The data in Fig. 4A–C is also consistent with previously published data showing that Nischarin affects cytoskeletal reorganization, mainly by inhibiting Rac-induced lamellipodia formation (2).
Figure 4.
Exosomes from Nischarin cells reduce focal adhesions. A and B, Vinculin Immunofluorescence of 231 cells on NC (n = 9), flbronectln (n = 9), 231 exosomes (n = 10), or 231 Nisch exosomes (n = 9; A) and 231 Nisch cells on NC (n = 30), fibronectin (n = 26), 231 exosomes (n = 25), or 231 Nisch exosomes (n = 30; inset with a zoom is shown; B). C, The number of focal adhesions (FA) per cell was determined by CellProfiler. D, Western blot analysis of pPaxillin, paxillin, and vincilin in 231 cells grown on NC, fibronectin, 231 exosomes, and 231 Nisch exosomes. E and F, Phalloidin immunofluorescence of 231 cells on NC (n = 9), fibronectin (n = 9), 231 exosomes (n = 8), or 231 Nisch exosomes (n = 11; E) and 231 Nisch cells on NC (n = 23), fibronectin (n = 25), 231 exosomes (n = 25), or 231 Nisch exosomes (n = 23; inset with a zoom is shown; F). G, Area and aspect ratio were acquired by ImageJ. Images were captured at 60× using a Nikon Eclipse Ti-S fluorescent microscope. Scale bars, 10 mm. ****, P < 0.0001.
To determine the effects of exosomes from Nischarin-reduced cells biochemically, we seeded cells on different matrices, and collected the lysates for protein detection. Paxillin is a focal adhesion scaffold protein and one of the first proteins to be recruited to focal adhesions during activation (32–36). We observed an increase in paxillin expression when 231 cells were attached to 231 exosomes (Fig. 4D). Furthermore, the phosphorylation of paxillin was visibly decreased in 231 cells on 231 Nisch exosomes (Fig. 4D). Similarly, Nisch+/+ and Nisch+/− cells were also analyzed for focal adhesion dynamics. Consistent with 231 cells data, Nisch+/− cells had greater number of focal adhesions on all coatings when compared with Nisch+/+ cells (Supplementary Fig. S3A and S3B). Increased number of focal adhesions were seen when cells plated on Nisch+/− exosomes. These results suggest that there is a reduction in focal adhesion signaling when cells are attached to exosomes from Nischarin-positive cells and increased signaling in Nisch+/− cells.
Increased focal adhesion number often leads to increased cell spreading. Thus, we wished to determine whether the increased number of focal adhesions induced by exosomes from Nischarin-reduced cells led to increased cell spreading. MDA-MB-231 and 231 Nisch cells were seeded onto NC, fibronectin, 231 exosomes and 231 Nisch exosomes, and imaged. Visually, the cells appeared to be enlarged when seeded onto fibronectin and 231 exosomes (Fig. 4E and F). Using ImageJ, we analyzed the average area of each cell and found that the 231 cells had a greater average area on all coatings compared with 231 Nisch cells (Fig. 4G). Among 231 cells, the cell areas on fibronectin and 231 exosomes was similar, while the areas on control (NC) and 231 Nisch exosomes was almost identical (Fig. 4G). The differences in aspect ratio (cell length:breadth) between 231 and 231 Nisch cells seeded on 231 and 231 Nisch exosomes were significant. The area and aspect ratio of 231 cells seeded on 231 Nisch exosomes are significantly low compared with the cells seeded on 231 exosomes (Fig. 4G). Similar results were obtained when the 231 Nisch cells seeded on 231 Nisch exosomes. These results indicate that 231 exosomes enhance cell spreading.
Furthermore, we examined the effect of Nischarin-positive exosomes on cell spreading using our mouse tumor cells. Nisch+/+ and Nisch+/− cells were seeded onto NC, fibronectin, Nisch+/+ exosomes and Nisch+/− exosomes, and stained with phalloidin. Our primary mouse tumor cells are significantly larger than the Nisch+/− cells, but their size expands even more when they are on Nisch+/− exosomes (Supplementary Fig. S3C and S3D). ImageJ analysis revealed an increased area of Nisch+/− cells compared with Nisch+/+ cells on all coatings (Supplementary Fig. S3C and S3D). Furthermore, we noticed the greatest spreading of Nisch+/− cells on fibronectin and Nisch+/− exosomes (Supplementary Fig. S3C and S3D). These data reveal that attachment of cancer cells to exosomes from Nischarin-reduced cells enhances adhesion and cell spreading.
Active Rab14 is involved in exosomal trafficking
Our data indicate that the exosomes from Nischarin-expressing cells are less effective than the exosomes from cells lacking Nischarin expression in promoting migration and spreading. However, the underlying mechanism for this remains unknown. Nischarin interacts with the trafficking GTPase Rab14 (13). On the basis of Rab14’s role in vesicle trafficking, we hypothesized that Nischarin’s interaction with Rab14 determines the fate of exosomes. Our hypothesis is supported by previous evidence from our laboratory demonstrating that Nischarin, Rab14, and the exosome marker CD63 colocalize (13). To determine whether this triple colocalization affects exosome trafficking, we created Rab14 stably expressing MDA-MB-231 cell lines (Fig. 5A). A Q70L Rab14 single point mutation yields a constitutively active protein, while the S25N mutation yields a dominant-negative protein (37–39). First, we examined the role of Rab14 in cell proliferation using our novel cell lines. Introducing the dominant-negative Rab14 did not have a significant effect on cell proliferation, while the WT and constitutively active Rab14 increased cell proliferation (Fig. 5B). Very little is known about the role Rab14 plays in breast cancer. To determine the further implications of Rab14 in breast cancer, we surveyed other breast cancer cell lines and found that Rab14 is expressed in MCF7, BT20, T47D, MDA-MB-468, and SUM185 cells (Fig. 5C).
Figure 5.
Active Rab14 is involved in the intracellular trafficking of exosomes. A, Western blot detection of Rab14 and vinculin (control) in 231, 231 Rab14 S25N, 231 Rab14 Q70L, and 231 Rab14 WT cells. B, Cell proliferation of 231, 231 Rab14 S25N, 231 Rab14 Q70L, 231 Rab14 WT, and 231 Nisch cells by MTT (n = 5 each). C, Western blot detection of Rab14 and vinculin (control) in MCF7, BT20, T47D, MDA-MB-468, and SUM185 cells. D, RT-PCR detection of Rab27a, Rab14, and GAPDH in 231, 231 Rab14 S25N, 231 Rab14 Q70L, 231 Rab14 WT cells (left) and exosomes (right). E, Western blot detection of Rab14 and vinculin (control) in protein lysates and exosomes from 231 and 231 Nisch cells. F, Diameter and molecular weight analysis of exosomes from 231, 231 Rab14 S25N, 231 Rab14 Q70L, and 231 Rab14 WT with the DelsaNanoC. G and H, Relapse-free survival of human patients with high and low expression of NISCHARIN (G) and RAB14 (H). *, P < 0.05; ***, P < 0.001.
Because we hypothesized that Rab14 contributes to exosome trafficking, we assessed whether Rab14 is present in exosomes. Insignificant amounts of Rab14 RNA were found in the exosomes from the various 231 Rab14 cells, while the exosomal RNA marker Rab27a was clearly present (Fig. 5D). Rab14 protein is also not expressed in exosomes (Fig. 5E) as indicated by lysates that are positive for Rab27a (Supplementary Fig. S2B). Even though Rab14 is not present in exosomes, it may still have an indirect effect that alters the sizes of exosomes. To examine this, we isolated exosomes from 231 Rab14 cell lines. S25N Rab14 overexpression significantly decreased exosome diameter and molecular weight, while the constitutively active Rab14 has a significant increase (Fig. 5F), suggesting that the presence of active Rab14 leads to increased exosome diameter and number (sample molecular weight). These results indicate that Rab14 plays a significant role in exosome biogenesis and thus we propose that active Rab14 in 231 cells is responsible for the “oncogenic” exosomal phenotype.
This interaction between Nischarin and Rab14 has been well established in our previous studies (13), but we have yet to demonstrate the biological significance of these genes in breast cancer. A study of human patients with breast cancer with variable levels of Nisch determined that those with high Nisch have a greater probability of relapse-free survival (Fig. 5G). In contrast, patients with lower RAB14 have a greater probability of relapse-free survival than those with high levels of RAB14 (Fig. 5H).These data agree with our previous experiments that showed an increase in the proliferation of Rab14-transfected cells (Fig. 5B). These studies demonstrate the importance of Nischarin and Rab14 in breast cancer.
Exosomes from Nischarin cells reduce tumor volume
Exosomes play an important role on tumor growth and thus it is necessary to understand the affect that they have on tumors. To explore the effects of Nischarin-reduced exosomes in vivo, we isolated exosomes from Nisch+/+ and Nisch+/− and cocultured them with Nisch+/+ or Nisch+/− cells for 4 days, then injected the cells into SCID mice (Fig. 6A). As a negative control, the exosomes alone were injected and, as a positive control, the cells alone (without coculturing) were injected. Tumor volumes were measured every 3 days until the humane endpoint of the whole study. Nisch+/− exosomes significantly increased the tumor volume of Nisch+/+ cells, while coculturing with Nisch+/+ exosomes did not produce tumors by the end of the study (Fig. 6B). Concurrently, Nisch+/− exosomes also significantly increased the tumor volume of Nisch+/− cells, while Nisch+/− cells cocultured with Nisch+/+ exosomes did not produce any tumors (Fig. 6C). This does not suggest that Nisch+/+ exosomes are not capable of stimulating tumorigenesis, but they may do so at a delayed time compared with Nisch+/− exosomes because the study had reached its humane endpoint. At 44 days postinjection, we were able to conclude that Nisch+/− exosomes increase the tumor volume of both Nischarin-positive and reduced cells. We further isolated the tumors and confirmed this visually (Fig. 6D). To visualize the tumor architecture, we sectioned and stained the tumors with H&E (Fig. 6E). To confirm the number of proliferating cells, we stained tissue sections with Ki67. Nisch+/+ and Nisch+/− cells that were previously cocultured with Nisch+/− exosomes produced the greatest number of proliferative cells per area (Fig. 6F).
Figure 6.
Exosomes from Nischarin cells reduce tumor volume. A, Prkdcscid mice injected with Nisch+/+ or Nisch+/− cells, and Nisch+/+ or Nisch+/− cells previously cocultured with Nisch+/− exosomes. B, Tumor volumes of mice injected with Nisch+/+ (n = 3) exosomes, Nisch+/+ (n = 3) cells, or Nisch+/+ cells previously cocultured with Nisch+/+ (n = 3) or Nisch+/− (n = 3) exosomes. C, Tumor volumes of cells injected with Nisch+/− (n = 3) exosomes, Nisch+/− (n = 4) cells, or Nisch+/− cells previously cocultured with Nisch+/+ (n = 4) or Nisch+/− exosomes (n = 4). Red asterisks indicate statistical significance to the exosome groups. Black asterisks indicate statistical significance between the cells only group. D, Isolated mammary tumors from mice injected with Nisch+/+ or Nisch+/− cells, and Nisch+/+ or Nisch+/− cells previously cocultured with Nisch+/− exosomes. E, H&E staining of tumors. F, Ki67 staining of tumors with quantitation of the number of Ki67-positive cells per area (inset with a zoom is shown). G, Tumor stiffness (in kPa) of Nisch+/+ and Nisch+/− tumors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
As reported above, our data showed that exosomes from Nisch+/− tumors have greater diameter and molecular weight (Fig. 2E and F). A physiological consequence of this would be an increase in the overall stiffness of the tumor due to a greater exosomal burden. Changes in stiffness during cancer progression are important in understanding the pathophysiology of cancer cells and metastatic mechanisms of cancer. Tumor progression is characterized by gradual stiffening of the tissue. We measured the stiffness of tumors from Nisch+/+ and Nisch+/− animals using a novel identification model system we developed (see Materials and Methods for details). We compared the mechanical properties of both types of tumors by measuring Young’s moduli. We found that Nisch+/− animals produce stiffer tumors (Fig. 6G). We propose that this increased stiffness is partly due to the exosomes. An important step in metastasis formation is cancer cell invasion through tissues. It has been shown that metastatic cells indent stiff polyacrylamide gels to promote invasion, whereas benign cells do not indent (40), suggesting that this approach can be used for tumors to assess their potential metastatic potential. The mechanical properties of cancer cells change depending on the stiffness of the ECM. This stiffness depends on stable focal adhesion proteins, such as Talin 1. For example, suppression of Talin-1 reduces cell stiffness, which suggests that focal adhesion signaling increases cell stiffness. In this study, for the first time, we show that Nischarin-negative tumors are much stiffer than Nischarin-positive tumors.
Nischarin-reduced cells confer resistance to cell-cycle control by exosomes
So far, we have shown that coculturing cells with Nischarin-positive exosomes significantly delays tumor growth. To determine whether these Nischarin-positive exosomes increase apoptosis, we performed apoptosis assays on the cocultured cells from Fig. 6 prior to their injection into the mice. By staining the cells with Annexin and propidium iodide and counting with flow cytometry, we were able to determine the percentage of necrotic and apoptotic cells. Coculturing Nisch+/+ cells with Nisch+/+ exosomes decreased the percentage of live cells (Fig. 7A). For the Nisch+/+ cells, the percentage of cells in apoptosis (early and late apoptosis) rose from 5.4% in control conditions, to 10.8% after coculturing with the Nisch+/+ exosomes (Fig. 7A). These findings might explain why there was a decrease in the tumor growth of the Nisch+/+ cells cocultured with Nisch+/+ exosomes. In contrast, coculturing Nisch+/+ cells with Nisch+/− exosomes decreased the percentage of cells in apoptosis to 5.3%, which was lower than Nisch+/+ cells (Fig. 7A). This was further confirmed by caspase-3 staining of the mouse tumors from Fig. 6D. Nisch+/+ cells cocultured with Nisch+/− exosomes had the greatest amount of caspase-3 staining, while Nisch+/− cells cocultured with Nisch+/− exosomes had the least amount of caspase-3–positive cells (Supplementary Fig. S4A and S4B). The data in Fig. 7A also match the data in Fig. 6B that show an increase in tumor cell growth of Nisch+/+ cells cocultured with Nisch+/− exosomes. Overall, these data show that Nisch+/+ exosomes increase apoptosis in both cell lines, while Nisch+/− exosomes do not induce apoptosis.
Figure 7.
Nischarin-reduced cells confer resistance to cell-cycle control by exosomes. A and B, Annexin/propidium iodide apoptosis assay of Nisch+/+ and cells previously cocultured with no exosomes, and Nisch+/+ or Nisch+/− exosomes (A) and Nisch+/− cells previously cocultured with no exosomes, and Nisch+/+ or Nisch+/− exosomes (B). C, MTT assay of Nisch+/+ and Nisch+/− cells previously cocultured with no exosomes, Nisch+/+ exosomes, or Nisch+/− exosomes. D, Western blot analysis of cyclin D1 and vinculin in 231 and 231 Nisch cells. E-G, Flow cytometric analysis of the cell cycle with propidium iodide in 231 and 231 Nisch cells (E), as well as Nisch+/+ (F) and Nisch+/− cells with Nisch+/+ exosomes or Nisch+/− exosomes (G). ***, P < 0.001.
The apoptosis experiments with Nisch+/− cells corroborated the data in Fig. 6C. For the Nisch+/− cells, total apoptosis increased from 1.1% to 20.1% after coculturing with the Nisch+/+ exosomes, also explaining why we saw delayed growth at the humane endpoint of the study (Fig. 7B). Furthermore, when the Nisch+/− cells were cocultured with the Nisch+/− exosomes, the percentage of live cells returned to 97.9%, which was the same as the Nisch+/− control (Fig. 7B). As with the Nisch+/+ cells, Nisch+/+ exosomes increased apoptosis, while Nisch+/− exosomes had the opposite effect. We next assessed cell proliferation using a different approach by performing a MTT assay on the cells cocultured with Nisch+/+ exosomes prior to injection into the SCID mice. Coculturing Nisch+/+ cells with Nisch+/+ exosomes slightly reduced cell proliferation, while culturing the same cells with Nisch+/− exosomes increased proliferation (Fig. 7C). Interestingly, coculturing Nisch+/− cells with Nisch+/+ exosomes still increased proliferation, while culturing them with Nisch+/− exosomes increased proliferation even more (Fig. 7C). These results further confirmed that Nisch+/− exosomes increase cell proliferation.
Although Nisch+/− exosomes increase cell proliferation, Nisch+/+ exosomes slightly decrease WT cell proliferation. We then determined whether these exosomes are halting cell-cycle control. Nischarin decreases the expression of cyclin D1 (Fig. 7D), a regulator of G1–S-phase progression (41). Further FACS analysis of propidium iodide-stained cells showed that 231 Nisch cells have an increase in G1 cell-cycle arrest, which increased from 49% in 231 cells to 65% in 231 Nisch cells (Fig. 7E). While this confirms that Nischarin regulates the cell cycle, we wanted to determine whether Nischarin-reduced or positive exosomes also contribute to cell-cycle regulation. To determine whether there was cell-cycle arrest after coculturing with the exosomes, we performed cell-cycle analysis again. Coculturing Nisch+/+ cells with Nisch+/+ exosomes increased the percentage of cells in G1 phase from 32.2% to 65.4%, while decreasing the percentage of cells in S-phase from 29% to 21.1% (Fig. 7F). Furthermore, coculturing Nisch+/+ cells with Nisch+/− exosomes increases the percentage of cells in S-phase from 29% in controls to 44.3% (Fig. 7F). These results suggest that coculturing Nisch+/+ cells with Nisch+/+ exosomes increased G1 cell-cycle arrest, while coculturing with Nisch+/− exosomes with other types of cells enhances progression through S-phase.
Furthermore, our mouse tumor cells showed 29% of Nisch+/+ cells in S-phase compared with 34.7% of Nisch+/− cells (Fig. 7G). After coculturing Nisch+/− cells with Nisch+/+ exosomes, the number of cells in G1 phase increased from 23.4% to 85% (Fig. 7G). This significant increase may explain why these cells had not formed tumors at the endpoint of the study. Furthermore, coculturing Nisch+/− cells with Nisch+/− exosomes yielded percentages of cells in G1 and S-phase close to control cells (Fig. 7G). Taken together, our cell-cycle data show that coculturing Nisch+/+ and Nisch+/− cells with Nisch+/+ exosomes induces G1 cell-cycle arrest.
Discussion
Our data demonstrate that exosome production is decreased in Nischarin-expressing cells, while Nisch+/− cells enhance the production of exosomes. Exosomes from Nischarin-positive cells promote migration and in vivo tumor growth of orthotopic breast cancer cells. Although Nischarin functions as a tumor suppressor, its role in exosome release is unknown. Exosomes are released by cancer cells and are known to promote cancer progression. In this article, we report that the decrease of Nischarin expression augments exosome shedding. Coculturing of naïve breast cancer cells with exosomes secreted by Nischarin-reduced (Nisch+/−) cells promote focal adhesion formation, cell migration, tumor growth, and metastasis. Colocalization of Rab14 with Nischarin appears to be important for the regulation of exosome production and release. In patients with breast cancer, Rab14 mRNA overexpression in the primary tumor is associated with decreased overall survival and, in an orthotopic mouse model, Rab14 inhibition impairs breast cancer progression.
We have demonstrated that human breast cancer cells that express Nischarin have reduced cell proliferation. We suggest that this might be due to insufficient cell attachment. This hypothesis was also based on previous findings that linked Nischarin to decreasing the function of key cell attachment proteins, such as FAK (1, 42). Also, it has been shown by other investigators that Nischarin regulates apoptosis and metastasis of breast cancer (43), loss of Nischarin promotes cell proliferation metastasis of ovarian cancer (42), and suppression of Nischarin promotes neuronal migration (44), suggesting that Nischarin has significant role in other cancers as well. We found that Nischarin-reduced cells had increased proliferation regardless of the substrate they were seeded on. Nischarin is one of many tumor suppressors whose down-regulation leads to cancer cell death resistance. PTEN and Tropomyosin-1 are examples of tumor suppressors that induce cancer cell death upon expression in breast cancer cells and primary breast tumors (45, 46). When a cell is undergoing cell death, it detaches from the ECM. We also confirmed that Nischarin decreases cell spreading and focal adhesion number. This is a novel role for Nischarin in regulating the number of adhesion points between a cell and its external matrix.
We showed that Nischarin alters the size of exosomes and further explored the implications of these changes. We first assessed the migration of cells on exosomes. We previously published that Nischarin reduces cell migration of 231 cells when stably transfected (4). Fibronectin itself promotes cell migration in many cell models (47–49). In fact, increased total fibronectin expression correlates with poorer prognosis in patients with cancer (50–52). Coating tissue culture dishes with fibronectin significantly increased the maximum distance and mean velocity. Sometimes it increased these parameters to a greater extent than the exosomes did. Nischarin-reduced human and mouse exosomes promoted a greater distance and velocity than Nischarin-positive exosomes. Because cancer cell migration is generally regarded as a prerequisite for metastasis, we propose a novel mechanism by which Nischarin exerts its tumor-suppressive functions.
Exosomes have been shown to promote the invasion and migration of cancer cells. Also, multiple studies indicated that exosomes migrate and localize to future sites of metastasis, which will establish a metastatic niche into which cancer cells will spread. Our results demonstrate that exosomes promote or inhibit metastasis through their migration regulation depending on the Nischarin expression levels. This study demonstrates that exosomes have an important effect on focal adhesion signaling through their effect on paxillin phosphorylation. Furthermore, we propose that Nischarin’s interaction with active Rab14 regulates exosome production, which in turn affects cell adhesion, cell migration, tumor growth, and metastasis. In contrast, exosomes from cells with reduced levels of Nischarin expression may migrate and establish a metastatic niche, which favors enhanced metastatic growth. In conclusion, our work shows that the secretion of exosomes by breast tumors in vivo can regulate tumor progression. In addition, Nischarin-expressing tumor-derived exosomes decrease tumor progression. These data may or may not be applicable to all tumors as the idiosyncrasies of each tumor vary.
In this study, we characterized the effects of Nischarin on cell motility. In the presence of Nischarin, there is a triple co-localization between Nischarin, Rab14, and CD63 that reduces the release of exosomes (Supplementary Fig. S5). Furthermore, cells that are seeded on exosomes from Nischarin-reduced cells have increased phosphorylation of the focal adhesion scaffold protein, paxillin. Our findings identified the presence of HSP70, Flot1, RAB27A, ITGA5, ITGA11, ITGAL, and ITGAV mRNA in exosomes. The cargo present in exosomes is poorly characterized; however, our results contribute significantly to the understanding of exosome biology. An additional contribution of our findings is that exosomes have the ability to transform cells into highly metastatic agents. This novel role for the tumor suppressor Nischarin not only increases our understanding of exosome biology, but can be translated to identifying new targets for modulating cancer metastasis.
Supplementary Material
Supplementary Figure 1. Nischarin Decreases the Attachment of Breast Cancer Cells. A) Representative images of 231 GFP and 231 GFP Nisch cells seeded on 2D, Fibronectin and Collagen captured at 10x with the EVOS XL Cell Imaging System. The number of cells per milliliter were calculated using the Bio-Rad TC20 Automated Cell Counter. B) Representative images of Nisch+/+, Nisch+/−, and Nisch−/− cells seeded on 2D, Fibronectin and Collagen captured at 10x with the EVOS XL Cell Imaging System. The quantitation is shown in the bottom panels. The number of cells per milliliter were calculated using the Bio-Rad TC20 Automated Cell Counter. *p<0.05 **p<0.01 and ***p<0.001.
Supplementary Figure 2. Characterization of Exosomes in Human Cell Lines. Nischarin (A) and Rab27A (B) protein expression in exosomes and total cell lysates of MDA-MB-231 and MDA-MB-231 Nisch cell lines. C) Number of particles per frame and per ml of MCF7scramble (n=3), MCF7siNisch (n=3) and MCF7siNisch +Nisch exosomes (n=3) with the Nanosight NTA. D) Western blot showing the rescue of Nischarin expression in MCF7 si+Nisch cells.
Supplementary Figure 3. Exosomes from Nischarin Tumors Reduce Focal Adhesions and Cell Spreading. A) Vinculin immunofluorescence of Nisch+/+ cells (n=11) and Nisch+/− cells on NC (n=27), Fibronectin (n=27), Nisch+/+ exosomes (n=24), and Nisch+/− exosomes (n=28). Images were captured at 60X using a Nikon Eclipse Ti-S fluorescent microscope. B) The number of FA’s per cell was determined by CellProfiler. C) Phalloidin immunofluorescence of Nisch+/+ cells on NC (n=20), Fibronectin (n=20), Nisch+/+ exosomes (n=20), and Nisch+/− exosomes (n=20); and Nisch+/− cells on NC (n=29), Fibronectin (n=31), Nisch+/+ exosomes (n=27), and Nisch+/− exosomes (n=29). D) Cell area was analyzed with ImageJ. Scale bars indicate 10μm. *p<0.05 **p<0.01 ***p<0.001 and ****p<0.0001.
Supplementary Figure 4. Caspase 3 Staining of Mouse Tumors From Exosome Studies. A) Representative images of Caspase 3 staining of mouse tumors from Nisch+/+ and Nisch+/− control cells and those previously co-cultured Nisch+/− exosomes. B) Quantitative data.
Supplementary Figure 5. Schematic Representation of the Effects of Nischarin on Breast Cancer Cell Motility through Exosomes.
Acknowledgments
We would like to thank the laboratory of Dr. Tarun Mandal at Xavier University of Louisiana for allowing us to use the Malvern Nanosight and the Beckman DelsaNanoC. Also, we thank the Fred Brazda Foundation and LSU School of Medicine for financial support.
D.E. Mercante was supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Maziveyi M, Alahari SK. Breast cancer tumor suppressors: a special emphasis on novel protein nischarin. Cancer Res 2015;75:4252–9. [DOI] [PubMed] [Google Scholar]
- 2.Alahari SK, Lee JW, Juliano RL. Nischarin, a novel protein that interacts with the integrin alpha5 subunit and inhibits cell migration. J Cell Biol 2000;151:1141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alahari SK. Nischarin inhibits Rac induced migration and invasion of epithelial cells by affecting signaling cascades involving PAK. Exp Cell Res 2003;288:415–24. [DOI] [PubMed] [Google Scholar]
- 4.Baranwal S, Wang Y, Rathinam R, Lee J, Jin L, McGoey R, et al. Molecular characterization of the tumor-suppressive function of nischarin in breast cancer. J Natl Cancer Inst 2011;103:1513–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain P, Baranwal S, Dong S, Struckhoff AP, Worthylake RA, Alahari SK. Integrin-binding protein nischarin interacts with tumor suppressor liver kinase B1 (LKB1) to regulate cell migration of breast epithelial cells. J Biol Chem 2013;288:15495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci 2006;119:3901–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem 2000;275:21785–8. [DOI] [PubMed] [Google Scholar]
- 8.Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun 2015;6:7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol 2002;2:569–79. [DOI] [PubMed] [Google Scholar]
- 10.Liu C, Guo J, Tian F, Yang N, Yan F, Ding Y, et al. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano 2017;11:6968–76. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Contreras M, Shah SH, Tamayo A, Robbins PD, Golberg RB, Mendez AJ, et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration Type 1 diabetes. Sci Rep 2017;7:5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon J, Jo W, Jeong D, Kim J, Jeong H, Park J. Generation of nanovesicles with sliced cellular membrane fragments for exogenous material delivery. Biomaterials 2015;59:12–20. [DOI] [PubMed] [Google Scholar]
- 13.Kuijl C, Pilli M, Alahari SK, Janssen H, Khoo PS, Ervin KE, et al. Rac and Rab GTPases dual effector Nischarin regulates vesicle maturation to facilitate survival of intracellular bacteria. EMBO J 2013;32:713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrasco-Ramirez P, Greening DW, Andres G, Gopal SK, Martin-Villar E, Renart J, et al. Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation. Oncotarget 2016;7:16070–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurwitz SN, Rider MA, Bundy JL, Liu X, Singh RK, Meckes DG Jr. Proteomic profiling of NCI-60 extracellular vesicles uncovers common protein cargo and cancer type-specific biomarkers. Oncotarget 2016;7:86999–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong S, Baranwal S, Garcia A, Serrano-Gomez SJ, Eastlack S, Iwakuma T, et al. Nischarin inhibition alters energy metabolism by activating AMP-activated protein kinase. J Biol Chem 2017;292:16833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong S, Maziveyi M, Alahari SK. Primary tumor and MEF cell isolation to study lung metastasis. J Vis Exp 2015;20:e52609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pospichalova V, Svoboda J, Dave Z, Kotrbova A, Kaiser K, Klemova D, et al. Simplified protocol for flow cytometry analysis of fluorescently labeled exosomes and microvesicles using dedicated flow cytometer. J Extracell Vesicles 2015;4:25530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed Mater 2015;46:318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Carrillo F, Li C, Pruitt L, Puttlitz C. Adhesive forces significantly affect elastic modulus determination of soft polymeric materials in nanoin-dentation. Mater Lett 2007;61:448–51. [Google Scholar]
- 21.Oliver WC, Pharr GM. Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. J Mater Res 2004;19:3–20. [Google Scholar]
- 22.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta 2013;1833:3481–98. [DOI] [PubMed] [Google Scholar]
- 23.Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer 2014;14:632–41. [DOI] [PubMed] [Google Scholar]
- 24.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res 2005;65:10992–1000. [DOI] [PubMed] [Google Scholar]
- 25.Serrano-Gomez SJ, Maziveyi M, Alahari SK. Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Mol Cancer 2016;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Zhou H, Di G, Liu J, Liu Y, Wang Z, et al. Mesenchymal stem cell-conditioned medium promotes MDA-MB-231 cell migration and inhibits A549 cell migration by regulating insulin receptor and human epidermal growth factor receptor 3 phosphorylation. Oncol Lett 2017;13:1581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muranen T, Iwanicki MP, Curry NL, Hwang J, DuBois CD, Coloff JL, et al. Starved epithelial cells uptake extracellular matrix for survival. Nat Commun 2017;8:13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maziveyi M, Dong S, Baranwal S, Alahari SK. Nischarin regulates focal adhesion and Invadopodia formation in breast cancer cells. Mol Cancer 2018;17:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koh M, Yong HY, Kim ES, Son H, Jeon YR, Hwang JS, et al. A novel role for flotillin-1 in H-Ras-regulated breast cancer aggressiveness. Int J Cancer 2016;138:1232–45. [DOI] [PubMed] [Google Scholar]
- 30.Tsang EK, Abell NS, Li X, Anaya V, Karczewski KJ, Knowles DA, et al. Small RNA sequencing in cells and exosomes identifies eQTLs and 14q32 as a region of active export. G3 2017;7:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the surface of myeloma cell-derived exosomes mediates exosome-cell interactions. J Biol Chem 2016;291:1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Colome AM, Lee-Rivera I, Benavides-Hidalgo R, Lopez E. Paxillin: a crossroad in pathological cell migration. J Hematol Oncol 2017;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene 2001;20:6459–72. [DOI] [PubMed] [Google Scholar]
- 34.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol 2000;2:E231–6. [DOI] [PubMed] [Google Scholar]
- 35.Crowe DL, Ohannessian A. Recruitment of focal adhesion kinase and paxillin to beta1 integrin promotes cancer cell migration via mitogen activated protein kinase activation. BMC Cancer 2004;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deakin NO, Turner CE. Paxillin comes of age. J Cell Sci 2008;121:2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Junutula JR, De Maziere AM, Peden AA, Ervin KE, Advani RJ, van Dijk SM, et al. Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol Biol Cell 2004;15:2218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lall P, Lindsay AJ, Hanscom S, Kecman T, Taglauer ES, McVeigh UM, et al. Structure-function analyses of the interactions between Rab11 and Rab14 Small GTPases with their shared effector rab coupling protein (RCP). J Biol Chem 2015;290:18817–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitt KN, Hernandez-Deviez D, Ballantyne SD, Spiliotis ET, Casanova JE, Wilson JM. Rab14 regulates apical targeting in polarized epithelial cells. Traffic 2008;9:1218–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merkher Y, Weihs D. Proximity of metastatic cells enhances their mechanobiological invasiveness. Ann Biomed Eng 2017;45:1399–406. [DOI] [PubMed] [Google Scholar]
- 41.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer 2007;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, He X, Dong R, Wang Y, Yu J, Qiu H. Frequent Loss of NISCH promotes tumor proliferation and invasion in ovarian cancer via inhibiting the FAK Signal Pathway. Mol Cancer Ther 2015;14:1202–12. [DOI] [PubMed] [Google Scholar]
- 43.Chang C, Wei W, Han D, Meng J, Zhu F, Xiao Y, et al. Expression of Nischarin negatively correlates with estrogen receptor and alters apoptosis, migration and invasion in human breast cancer. Biochem Biophys Res Commun 2017;484:536–42. [DOI] [PubMed] [Google Scholar]
- 44.Ding Y, Zhang R, Zhang K, Lv X, Chen Y, Li A, et al. Nischarin is differentially expressed in rat brain and regulates neuronal migration. PLoS One 2013;8:e54563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raval GN, Bharadwaj S, Levine EA, Willingham MC, Geary RL, Kute T, et al. Loss of expression of tropomyosin-1, a novel class II tumor suppressor that induces anoikis, in primary breast tumors. Oncogene 2003;22:6194–203. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Lin YZ, La Pushin R, Cuevas B, Fang X, Yu SX, et al. The PTEN/MMAC1/TEP tumor suppressor gene decreases cell growth and induces apoptosis and anoikis in breast cancer cells. Oncogene 1999;18:7034–45. [DOI] [PubMed] [Google Scholar]
- 47.Ramos Gde O, Bernardi L, Lauxen I, Sant’Ana Filho M, Horwitz AR, Lamers ML. Fibronectin modulates cell adhesion and signaling to promote single cell migration of highly invasive oral squamous cell carcinoma. PLoS One 2016;11:e0151338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MC, Neal DM, Kamm RD, Asada HH. Dynamic modeling of cell migration and spreading behaviors on fibronectin coated planar substrates and micropatterned geometries. PLoS Comput Biol 2013;9:e1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sbaa-Ketata E, Vasse M, Lenormand B, Schneider P, Soria C, Vannier JP. Fibronectin increases the migration induced by stromal cell-derived factor-1 alpha (SDF-1 alpha) in pre-B acute lymphoblastic leukemia cells. Eur Cytokine Netw 2001;12:223–30. [PubMed] [Google Scholar]
- 50.Bae YK, Kim A, Kim MK, Choi JE, Kang SH, Lee SJ. Fibronectin expression in carcinoma cells correlates with tumor aggressiveness and poor clinical outcome in patients with invasive breast cancer. Hum Pathol 2013;44:2028–37. [DOI] [PubMed] [Google Scholar]
- 51.Nishioka A, Ogawa Y, Inomata T, Maeda T, Seguchi H. Fibronectin expression in cancer tissues from patients undergoing radiation therapy. Histol Histopathol 1993;8:457–62. [PubMed] [Google Scholar]
- 52.Fernandez-Garcia B, Eiro N, Marin L, Gonzalez-Reyes S, Gonzalez LO, Lamelas ML, et al. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology 2014;64:512–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Nischarin Decreases the Attachment of Breast Cancer Cells. A) Representative images of 231 GFP and 231 GFP Nisch cells seeded on 2D, Fibronectin and Collagen captured at 10x with the EVOS XL Cell Imaging System. The number of cells per milliliter were calculated using the Bio-Rad TC20 Automated Cell Counter. B) Representative images of Nisch+/+, Nisch+/−, and Nisch−/− cells seeded on 2D, Fibronectin and Collagen captured at 10x with the EVOS XL Cell Imaging System. The quantitation is shown in the bottom panels. The number of cells per milliliter were calculated using the Bio-Rad TC20 Automated Cell Counter. *p<0.05 **p<0.01 and ***p<0.001.
Supplementary Figure 2. Characterization of Exosomes in Human Cell Lines. Nischarin (A) and Rab27A (B) protein expression in exosomes and total cell lysates of MDA-MB-231 and MDA-MB-231 Nisch cell lines. C) Number of particles per frame and per ml of MCF7scramble (n=3), MCF7siNisch (n=3) and MCF7siNisch +Nisch exosomes (n=3) with the Nanosight NTA. D) Western blot showing the rescue of Nischarin expression in MCF7 si+Nisch cells.
Supplementary Figure 3. Exosomes from Nischarin Tumors Reduce Focal Adhesions and Cell Spreading. A) Vinculin immunofluorescence of Nisch+/+ cells (n=11) and Nisch+/− cells on NC (n=27), Fibronectin (n=27), Nisch+/+ exosomes (n=24), and Nisch+/− exosomes (n=28). Images were captured at 60X using a Nikon Eclipse Ti-S fluorescent microscope. B) The number of FA’s per cell was determined by CellProfiler. C) Phalloidin immunofluorescence of Nisch+/+ cells on NC (n=20), Fibronectin (n=20), Nisch+/+ exosomes (n=20), and Nisch+/− exosomes (n=20); and Nisch+/− cells on NC (n=29), Fibronectin (n=31), Nisch+/+ exosomes (n=27), and Nisch+/− exosomes (n=29). D) Cell area was analyzed with ImageJ. Scale bars indicate 10μm. *p<0.05 **p<0.01 ***p<0.001 and ****p<0.0001.
Supplementary Figure 4. Caspase 3 Staining of Mouse Tumors From Exosome Studies. A) Representative images of Caspase 3 staining of mouse tumors from Nisch+/+ and Nisch+/− control cells and those previously co-cultured Nisch+/− exosomes. B) Quantitative data.
Supplementary Figure 5. Schematic Representation of the Effects of Nischarin on Breast Cancer Cell Motility through Exosomes.