Abstract
Objectives
Non-pharmacological treatments are an important aspect of dementia care. A wide range of interventions have been trialled for mild dementia and mild cognitive impairment (MCI). However, the variety of outcome measures used in these trials makes it difficult to make meaningful comparisons. The objective of this study is to map trends in which outcome measures are used in trials of non-pharmacological treatments in MCI and mild dementia.
Design
Scoping review.
Data sources
EMBASE, PsychINFO, Medline and the Cochrane Register of Controlled Trials were searched from inception until February 2018. An additional search was conducted in April 2019
Eligibility
We included randomised controlled trials (RCTs) testing non-pharmacological interventions for people diagnosed with MCI or mild dementia. Studies were restricted to full RCTs; observational, feasibility and pilot studies were not included.
Charting methods
All outcome measures used by included studies were extracted and grouped thematically. Trends in the types of outcome measures used were explored by type of intervention, country and year of publication.
Results
91 studies were included in this review. We extracted 358 individual outcome measures, of which 78 (22%) were used more than once. Cognitive measures were the most frequently used, with the Mini-Mental State Examination being the most popular.
Conclusions
Our findings highlight an inconsistency in the use of outcome measures. Cognition has been prioritised over other domains, despite previous research highlighting the importance of quality of life and caregiver measures. To ensure a robust evidence base, more research is needed to highlight which outcome measures should be used over others.
PROSPERO registration number
CRD42018102649.
Keywords: dementia, old age psychiatry, statistics & research methods
Strengths and limitations of this study.
This scoping review has systematically mapped which outcome measures have been used by randomised controlled trials testing non-pharmacological treatments in mild dementia and mild cognitive impairment.
This review has explored how the use of outcome measures varies by diagnosis, type of intervention, country and year of publication.
The papers included in this review were limited to full randomised controlled trials, other study designs may be using different types of outcome measures.
Further research is needed to establish which measures should be used over others.
Introduction
Delivery of both pharmacological and non-pharmacological treatment in the early stages of dementia has been identified as a global priority.1 2 Current pharmacological treatments for the cognitive symptoms of dementia have been found to have a greater effect when delivered as early as possible.3 However, the benefits of delivering non-pharmacological treatments early are less well understood. Non-pharmacological treatments are an important clinical tool for managing dementia as they are more acceptable to some and less prone to side effects, making them a safe alternative to drug treatments.4 Those diagnosed earlier in the disease have more cognitive abilities available to engage with non-pharmacological treatments and bolster their own methods for coping with the disease.5 Previous systematic reviews have found non-pharmacological treatments can improve outcomes; however, these reviews were restricted to a small number of outcome measures.6 7
Mild cognitive impairment (MCI) has been identified as a potential prodrome for dementia, with approximately 10% of people with MCI converting to a diagnosis of dementia per annum.8 There is an interest in MCI, as a diagnosis of MCI can facilitate an early diagnosis of dementia and therefore earlier access to dementia services and treatment.9 MCI is a potentially reversible condition, with many people with MCI reverting back to normal levels of cognition.9 Therefore, it is important treatments are available. However, it is not clear which treatments can reverse MCI or prevent conversion to dementia.3 No drug treatments for MCI have been found to be effective10 11 and acetylcholinesterase inhibitors are not recommended, however, there is some limited evidence that non-pharmacological interventions may be beneficial.3 12
Randomised controlled trials (RCTs) testing non-pharmacological treatments in dementia and MCI are becoming more common. However, they are highly heterogeneous in terms of participants recruited, quality of the study and the types of interventions they are testing, making it difficult to establish the effectiveness of one treatment over another.6 12 13 Compounding these issues is the inconsistent use of outcome measures in this area of work.9 14
Systematic reviews have identified possible benefits of non-pharmacological treatment, yet meta-analyses are difficult to conduct due to the variation in outcome measures used by studies and typically yield small-to-moderate effect sizes.6 7 It is possible that these small effect sizes are due to the selection of outcome measures which either lack sensitivity or the change following the intervention not being in the area covered by the outcome measure. It is important researchers are clear on which domains their interventions are targeting, and which measures are best able to capture this change.15 Pharmacological treatments target specific biological pathways underlying the disease; therefore, outcome measures have been chosen to reflect this and typically focus on cognitive and functional decline.16 Non-pharmacological treatments generally do not target the underlying biological pathway of the disease therefore, outcome measures should theoretically differ between pharmacological and non-pharmacological treatments.17 However, a review on non-pharmacological approaches to treating found that studies tended to pay little attention to the mechanisms of change underlying the intervention.4 The expected mechanisms of change should affect which outcomes are used in non-pharmacological treatments for mild dementia and MCI.
In addition to being clear on how change arises in non-pharmacological treatments, there needs to be a more coherent use of outcomes and the measures used to capture these between studies to ensure a broad and robust evidence base.15 In 2008, the INTERDEM group, a consortium of dementia researchers across Europe, did work to draw a consensus on which outcome measures should be used when evaluating non-pharmacological treatments. They recommended 22 measures across 9 domains including quality of life, mood, global functioning, behaviour, daily living skills, caregiver mood, caregiver burden and staff morale.15 This guidance does not explore outcomes by the stage of the disease. The outcome measures were selected based on their applicability to European research. The utility of outcome measures may vary by culture,16 previous reviews exploring the use of outcome measures in dementia research have not investigated how this differs by country.17
It is not understood which outcome measures are currently being used in non-pharmacological treatments for early dementia and MCI. Scoping reviews present the opportunity to map the evidence on a topic,18 unlike a systematic review scoping reviews can be used to summarise the evidence in a heterogeneous body of literature. Therefore, the aim of this scoping review is to map trends in which outcome measures are being used in RCTs for non-pharmacological treatments in MCI and mild dementia.
Objectives
The specific objectives of this scoping review are to:
Chart which outcomes measures have been used to assess the effectiveness of non-pharmacological treatments in mild dementia and MCI.
Highlight which types of measures have been used most frequently.
Explore whether the outcome measures used differ depending on the type of intervention, study population and country the research was conducted in.
Methods
Protocol registration
The protocol for this review was developed following the guidelines set out by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Extension (PRISMA) statement19 and the PRISMA guidelines for Scoping Reviews.18
Eligibility criteria
We included RCTs testing non-pharmacological interventions for people diagnosed with MCI or mild dementia. Studies were restricted to full RCTs; observational, feasibility and pilot studies were not included.
Studies were included if they met the following criteria:
Testing non-pharmacological interventions. Studies were not excluded if participants were also treated with acetylcholinesterase inhibitors.
Participants had a diagnosis of MCI or mild dementia, which was either diagnosed in clinical practice, or met standardised diagnostic criteria, such as the International Statistical Classification of Diseases or The Diagnostic Statistical Manual of Mental Disorders, The National Institute of Communicative disorders and Stroke and the Alzheimer’s Disease and Related Disorders, the International working group on MCI criteria, The Consortium to Establish a Registry for Alzheimer’s Disease, The National Institute on Aging-Alzheimer’s Associating Diagnostic Guidelines for Alzheimer’s Disease, the Petersen Criteria; or was defined by a standardised clinical measure, such as scores between 24 and 18 on the Mini-Mental State Examination (MMSE); scores ≤26 on the Montreal Cognitive Assessment, scores between 15 and 27 on the St Louis University Mental Status, a Clinical Dementia Rating score of 1 (for dementia) or 0.5 (for MCI); or a 4 (for dementia) or 3 (for MCI) on the Global Deterioration Scale. Studies which include a mix of participants with early dementia and MCI were included, however, studies which included healthy participants and participants with dementia at the later stages of the disease were excluded.
The intervention was targeted for the person living with dementia or MCI. Dyadic interventions, interventions delivered to both the person living with dementia and their caregivers, were included. Interventions delivered solely to caregivers or healthcare professionals were excluded.
Participants were living in long-term care facilities or the community.
Written in English.
Studies were excluded if:
Only pharmacological interventions were tested.
The participants were diagnosed with vascular cognitive impairment, young-onset dementia, Parkinson’s disease dementia or MCI with Parkinson’s disease.
Participants were living in a psychiatric inpatient or acute hospital setting.
The intervention had the primary aim of treating major depressive disorder.
The study tested palliative care interventions or advanced care planning.
The only outcome measures used were economic outcomes, such as cost-effectiveness, etc.
Information sources and search strategy
To identify potentially relevant studies, we searched EMBASE, PsychINFO, Medline and the Cochrane Register of Controlled Trials from inception until 22 February 2018. An additional search was conducted on 2 April 2019. See online supplementary table 1 for the final search strategy for MEDLINE, which was adapted for the other databases. The final search results were exported into EndNote where duplicates were removed.
bmjopen-2019-035980supp001.pdf (58.9KB, pdf)
Additional papers were identified by searching the references of included papers and other systematic reviews. Conference abstracts and publications were not included.
Selection of sources of evidence
Study selection was managed in Rayyan, where citations were screened against the inclusion and exclusion criteria. Rayyan is an online app for systematic reviews which allows researchers to create their own coding system for decision making.20 References were first screened by title and abstract, followed by a full-text screening. A second reviewer (MC) screened 10% of the articles at each stage of the review. Disagreements were resolved by discussions with a third reviewer (AMP).
A critical appraisal or assessment of the risk of bias is not necessary for a scoping review.18 This scoping review is not aiming to critically appraise the cumulative literature of outcome measures for non-pharmacological treatment in MCI and mild dementia, therefore we did not conduct a critical appraisal or risk of bias assessment for this review.
Data charting process and data items
Data from eligible studies were charted using a standardised extraction tool designed for this study. Items deemed most relevant to the review objectives were the diagnosis of the study participants, description of interventions being tested, the number of intervention groups and outcome measures used with references.
Synthesis of results
The charted data were mapped to reflect the objectives of this review. Following data charting, outcome measures which were used more than once across the included studies were grouped by domain. We grouped the interventions thematically by the type of intervention being tested.
We explored which types of outcome measures were used by intervention type, by tabulating the type of intervention against the domain of the outcome measure. We excluded interventions which were only used once from this summary. Results were presented in tables and summarised narratively.
Patient and participant involvement
The South London and Maudsley MALADY group, of current and former carers of people living with dementia, were consulted in the planning of this study.
Results
Included studies
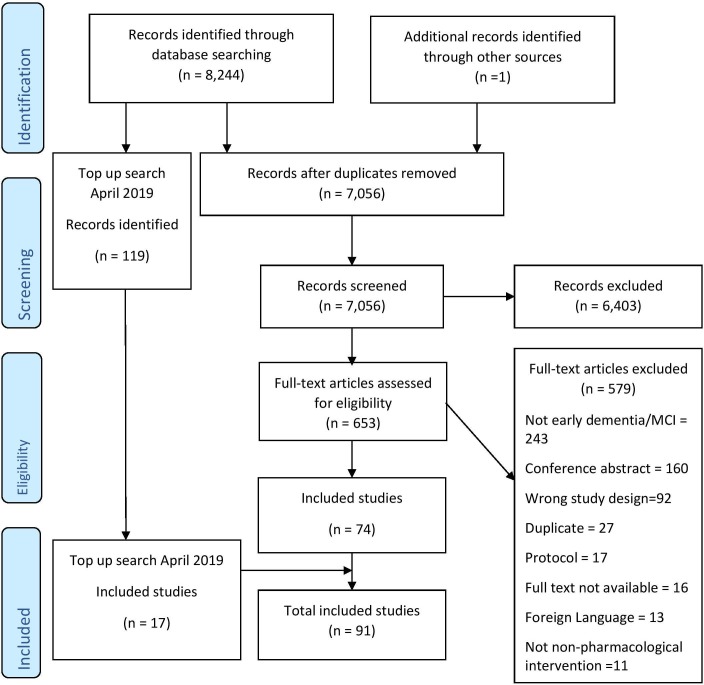
After duplicates were removed, a total of 7056 citations were screened for inclusion, 653 were screened at full text and 74 papers were initially identified. A top-up search in April 2019 identified 119 new citations, 17 were included making the total number of included studies 91 (figure 1).
Figure 1.
Flow chart of included studies.
The studies included in this review are described in table 1, including diagnosis of included participants, number of intervention groups, details on the interventions and comparisons tested and the number of outcomes measures used. The included studies were published between 2002 and 2019.
Table 1.
Included studies
| Study | Country | Diagnosis | Number of groups | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Number of measures |
| Amjad et al37 | Pakistan | MCI | 2 | Aerobic exercise | Non-aerobic exercise | – | – | – | 4 |
| Bae et al38 | Japan | MCI | 2 | Multi-intervention programme | Active control | – | – | – | 10 |
| Baker et al39 | USA | MCI | 2 | Aerobic exercise | Stretching | – | – | – | 11 |
| Belleville et al40 | Canada | MCI | 3 | Cognitive training | Psychosocial intervention | Control | – | – | 7 |
| Biasutti and Mangiacotti41 | Italy | MCI | 2 | Cognitive training | Gym activities | – | – | – | 4 |
| Bono et al42 | Italy | MCI | 2 | Animal assisted therapy | Control | – | – | – | 4 |
| Burgio et al43 | Italy | MCI | 2 | Numerical training | Executive training | – | – | – | 13 |
| Buschert et al44 | Germany | MCI | 2 | Cognitive training | Active control | – | – | – | 5 |
| Carretti et al45 | Italy | MCI | 2 | Cognitive training | Active control | – | – | – | 16 |
| Cavallo et al46 | Italy | Dementia | 2 | Cognitive training | Active control | – | – | – | 3 |
| Chan et al47 | Hong Kong | MCI | 2 | Chinese calligraphy | Computer activities | – | – | – | 13 |
| Chan et al48 | Hong Kong | MCI | 2 | Chinese calligraphy | Computer activities | – | – | – | 8 |
| Choi and Lee49 | South Korea | MCI | 2 | Ground kayaking | Home exercise education | – | – | – | 7 |
| Combourieu Donnezan et al50 | France | MCI | 4 | Physical training | Cognitive training | Simultaneous cognitive and physical training | Control | – | 4 |
| DiNapoli et al51 | USA | MCI | 2 | Individualised social activities | Control | – | – | – | 4 |
| Doi et al52 | Japan | MCI | 2 | Exercise | Active control | – | – | – | 4 |
| Doi et al53 | Japan | MCI | 3 | Dance | Playing musical instruments | Health education group | – | – | 4 |
| Drumond Marra et al54 | Brazil | MCI | 2 | TMS | Sham TMS | – | – | – | 6 |
| Emsaki et al55 | Iran | MCI | 2 | Cognitive training | Active control | – | – | – | 9 |
| Eyre et al56 | USA | MCI | 2 | Yoga | Cognitive training | – | – | – | 10 |
| Feng et al57 | China | MCI | 2 | Single component cognitive training | Multiple component cognitive training | – | – | – | 3 |
| Fernández-Calvo et al58 | Spain | Dementia | 2 | Multi-intervention programme | Control | – | – | – | 21 |
| Fiatarone Singh et al59 | Australia | MCI | 4 | Progressive resistance training and sham cognitive training | Progressive resistance training and cognitive training | Cognitive training | Control | – | 12 |
| Finn and McDonald60 | Australia | MCI | 2 | Repetition-lag training | Control | – | – | – | 6 |
| Fogarty et al61 | Canada | MCI | 2 | Memory intervention programme and tai chi | Memory intervention programme | – | – | – | 5 |
| Förster et al62 | Germany | Both | 2 | Cognitive training | Control | – | – | – | 10 |
| Galante et al63 | Italy | Dementia | 2 | Cognitive training | Active control | – | – | – | 12 |
| Greenaway et al21 | USA | MCI | 2 | Memory support system (memory rehabilitation) with training | Memory support system without training | – | – | – | 15 |
| Hagovská et al64 | Czech Republic | MCI | 2 | Cognitive training (computer based) | Cognitive training | – | – | – | 0 |
| Hagovská et al65 | Czech Republic | MCI | 2 | Cognitive training and dynamic balance training | Balance training | – | – | – | 4 |
| Han et al66 | South Korea | MCI | 2 | Ubiquitous spaced retrieval-based memory advancement and rehabilitation training | Control | – | – | – | 4 |
| Han et al67 | South Korea | Both | 2 | Multimodal cognitive enhancement therapy | Active control | – | – | – | 7 |
| Hattori et al29 | Japan | Dementia | 2 | Art therapy | Active control | – | – | – | 4 |
| Ho et al68 | Hong Kong | Both | 3 | Dance movement therapy | Physical exercise | Control | – | – | 7 |
| Horie et al69 | Brazil | MCI | 2 | Group weight loss programme | Control | – | – | – | 10 |
| Hyer et al70 | USA | MCI | 2 | Cognitive training (computer based) | Active control | – | – | – | 3 |
| Jansen et al22 | The Netherlands | Dementia | 2 | Case management | Control | – | – | – | 5 |
| Jean et al71 | Canada | MCI | 2 | Cognitive training | Active control | – | – | – | 10 |
| Jelcic et al72 | Italy | Dementia | 2 | Lexical-semantic treatment | Cognitive stimulation | – | – | – | 11 |
| Jeong et al73 | South Korea | MCI | 2 | Cognitive intervention (group based) | Cognitive intervention (home based) | – | – | – | 8 |
| Kinsella et al23 | Australia | MCI | 2 | Cognitive intervention | Control | – | – | – | 4 |
| Kohanpour et al74 | Iran | MCI | 4 | Aerobic exercise | Lavender extract | Aerobic exercise and lavender extract | Control | – | 14 |
| Koivisto et al24 | Finland | Dementia | 2 | Psychosocial intervention | Control | – | – | – | 7 |
| Kovács et al75 | Hungary | MCI | 2 | Multimodal exercise | Control | – | – | – | 1 |
| Küster et al76 | Germany | MCI | 3 | Cognitive training | Physical training | Control | – | – | 7 |
| Kwok et al77 | Hong Kong | MCI | 2 | Cognitive training | Active control | – | – | – | 5 |
| Lam et al78 | Hong Kong | MCI | 2 | Tai Chi | Stretching | – | – | – | 4 |
| Lam et al79 | Hong Kong | MCI | 4 | Cognitive training | Cognitive and physical training | Physical training | Social groups | – | 2 |
| Lam et al25 | Hong Kong | Dementia | 2 | Case management | Control | – | – | – | 2 |
| Langoni et al80 | Brazil | MCI | 2 | Group exercise | Control | – | – | – | 14 |
| Law et al81 | Hong Kong | MCI | 2 | Functional tasks exercise programme | Cognitive training | – | – | – | 7 |
| Lazarou et al82 | Greece | MCI | 2 | Ballroom dancing | Control | – | – | – | 5 |
| Li et al83 | China | MCI | 2 | Computerised cognitive training | Control | – | – | – | 4 |
| Lim et al84 | Singapore | MCI | 2 | Mindfulness | Health education | – | – | – | 5 |
| Logsdon et al26 | USA | Dementia | 2 | Early stage memory loss support group | Control | – | – | – | 10 |
| Luijpen et al85 | The Netherlands | MCI | 2 | TENS | Sham TENS | – | – | – | 6 |
| Maffei et al86 | Italy | MCI | 2 | Multidomain training | Control | – | – | – | 10 |
| İnel Manav and Simsek87 | Turkey | Dementia | 2 | Reminiscence therapy | Social interview | – | – | – | 6 |
| Melendez et al88 | Spain | Both | 2 | Reminiscence therapy | Control | – | – | – | 6 |
| Nagamatsu et al89 | Canada | MCI | 2 | Aerobic exercise | Resistance training | – | – | – | 13 |
| Olsen et al90 | Norway | Both | 2 | Animal-assisted therapy | Control | – | – | – | 9 |
| Pantoni et al91 | Italy | MCI | 2 | Attention process training | Control | – | – | – | 4 |
| Park and Park92 | South Korea | MCI | 2 | Cognition-specific computer training | Non-specific computer training | – | – | – | 5 |
| Poinsatte et al93 | USA | MCI | 2 | Aerobic exercise | Stretching | – | – | – | 3 |
| Pongan et al94 | France | Dementia | 2 | Choral singing | Painting | – | – | – | 14 |
| Poptsi et al95 | Greece | MCI | 5 | Paper language tasks | Computer language tasks | Oral language tasks | Active control | Control | 4 |
| Qi et al96 | China | MCI | 2 | Aerobic exercise | Control | – | – | – | 3 |
| Rapp et al97 | USA | MCI | 2 | Memory enhancement training (multicomponent) | Control | – | – | – | 9 |
| Rojas et al98 | Argentina | MCI | 2 | Cognitive intervention | Control | – | – | – | 8 |
| Rozzini et al99 | Italy | MCI | 2 | Cognitive training and AChEIs | AChEIs | – | – | – | 7 |
| Savulich et al100 | UK | MCI | 2 | Cognitive training | Control | – | – | – | 9 |
| Scherder et al101 | The Netherlands | MCI | 3 | Walking | Hand and face exercises | Control | – | – | 11 |
| Shimada et al102 | Japan | MCI | 2 | Physical and cognitive training | Health education group | – | – | – | 7 |
| Shimizu et al103 | Japan | MCI | 2 | Movement music therapy | Single training task | – | – | – | 4 |
| Simon et al104 | Brazil | MCI | 2 | Memory training | Active control | – | – | – | 8 |
| Song et al105 | China | MCI | 2 | Aerobic exercise | Active control | – | – | – | 4 |
| Suzuki et al106 | Japan | MCI | 2 | Multicomponent exercise group | Active control | – | – | – | 6 |
| Tappen and Hain27 | USA | Both | 2 | Cognitive training (home based) | Life story interview | – | – | – | 11 |
| Troyer et al107 | Canada | MCI | 2 | Multicomponent intervention | Control | – | – | – | 6 |
| Tsai et al108 | Taiwan | MCI | 3 | Aerobic exercise | Resistance training | Control | – | – | 7 |
| Tsantali et al109 | Greece | Dementia | 3 | Cognitive training | Cognitive stimulation | Control | – | – | 5 |
| van Uffelen et al110 | The Netherlands | MCI | 4 | Walking | Placebo activity | Folic acid/Vitamin b supplements | Placebo pills | – | 3 |
| Waldorff et al28 | Denmark | Dementia | 2 | Multifaceted counselling, education and support | Control | – | – | – | 2 |
| Wei et al111 | China | MCI | 2 | Handball training | Control | – | – | – | 8 |
| Yang et al112 | USA | MCI | 2 | Memory enhancement training | Yoga | – | – | – | 3 |
| Yoon et al113 | South Korea | MCI | 2 | High-speed power strength training | Low-speed strength training | – | – | – | 5 |
| Young et al114 | Hong Kong | Dementia | 2 | Support groups | Control | – | – | – | 4 |
| Young et al115 | Hong Kong | MCI | 2 | Holistic health group | Control | – | – | – | 4 |
| Yun et al116 | South Korea | MCI | 2 | TDS | Sham TDS | – | – | – | 1 |
| Zhao et al117 | China | MCI | 2 | Creative expression therapy | Cognitive training | – | – | – | 7 |
| Zhu et al118 | China | MCI | 2 | Dance | Control | – | – | – | 7 |
MCI, mild cognitive impairment; TDS, transcranial direct current stimulation; TENS, transcutaneous electrical nerve stimulation; TMS, transcranial magnetic stimulation.
The majority of studies included in this review were conducted in the USA (n=10), Hong Kong (n=10) and Italy (n=11), followed by mainland China (n=7), Japan (n=8), South Korea (n=8) and Canada (n=6). Studies were also conducted in: Argentina, Australia, Brazil, Czech Republic, Denmark, France, Finland, Germany, Greece, Hungary, Iran, Norway, Pakistan, Singapore, Spain, Taiwan, The Netherlands, Turkey and the UK; these countries had fewer than five included studies each.
Most studies only recruited participants with MCI (n=71), followed by mild dementia only (n=14), and six studies recruited both participants with MCI and mild dementia.
Results of individual sources of evidence
We extracted 358 individual outcome measures from the included studies, of these 78 (22%) were used more than once. Out of the 78 measures used more than once, 70 (88%) were measures of participants living with dementia (PLWD), 6 measures were used in both the PLWD and their caregiver, 2 measures were only of the caregiver. The number of outcome measures used by each study ranged between 1 and 21 with an average of 6.85.
Types of non-pharmacological interventions
We grouped the interventions thematically by type. The most frequently tested type of intervention was cognitive training (n=37) followed by physical activity (n=25), combined physical activity and cognitive training (n=4), multicomponent psychosocial interventions (n=4) and support groups (n=3). Animal-assisted therapies, art-based therapies, case management, Chinese calligraphy, music-based interventions and reminiscence therapy were each tested in two studies.
A group weight loss programme, mindfulness, social activities, transcranial direct current stimulation, transcutaneous electrical nerve stimulation and Transcranial magnetic stimulation were each trialled once. These interventions were not included in the analysis of trends in outcome measures.
PLWD outcome measures
Table 2 presents the PLWD-specific outcome measures grouped by domain. The most frequently measured domain in PLWD was cognition/memory, which was measured 219 times across the 93 included studies. The most frequent measure of cognition was the MMSE, which was measured 37 times. In addition to measures of memory performance, knowledge of memory strategies was measured 3 times in PLWD.
Table 2.
Outcome measures by domain and subdomains
| Person living with dementia measures Domain and subdomain |
Outcome measure | N |
| Cognition/Memory | 219 | |
| Cognition | MMSE | 37 |
| Trail Making Test | 27 | |
| Digit Span Test | 12 | |
| ADAS-Cog | 10 | |
| Rey Auditory Test | 9 | |
| Rivermead Behavioural Memory Test | 9 | |
| Stroop Test | 7 | |
| MMQ | 7 | |
| Novelli Lexical Test | 7 | |
| MoCA | 6 | |
| CDR | 6 | |
| Verbal Fluency | 6 | |
| CERAD-NB | 5 | |
| Addenbrooke's Cognitive Examination | 4 | |
| Boston Naming Test | 4 | |
| Rey Osterrieth Complex Figure Task | 4 | |
| Montreal Cognitive Test | 3 | |
| Attentional Matrices Test | 3 | |
| California Verbal Learning Test | 3 | |
| Digit Symbol Coding Test | 3 | |
| Hopkins Verbal Learning Test | 3 | |
| The Wechsler Memory Scale | 3 | |
| CAMcog | 2 | |
| Cognitive Failures Test | 2 | |
| Colour Trails Test | 2 | |
| Dementia Rating Scale-2 | 2 | |
| DSM IV Test | 2 | |
| Auditory Verbal Learning Test | 2 | |
| Corsi's Block Tapping Test | 2 | |
| Frontal Assessment Test | 2 | |
| Fuld Object Memory Evaluation | 2 | |
| Logical Memory (Subtest of Wechsler Memory Scale) | 2 | |
| Prospective and Retrospective Memory Questionnaire | 2 | |
| Pyramids & Palm Trees | 2 | |
| Questionnaire d’Auto Evaluation de la Memoire | 2 | |
| Raven’s Coloured Matrices | 2 | |
| Repeatable Battery Test | 2 | |
| The verbal learning and memory test | 2 | |
| Visual Memory Span | 2 | |
| Wechsler Adult Intelligence Scale | 2 | |
| Knowledge of memory strategies | Memory Strategy Toolbox | 2 |
| Strategy Knowledge Repertoire | 1 | |
| Attention | Test of Everyday Attention | 2 |
| Behavioural and psychological symptoms of dementia | 51 | |
| Anxiety/Depression | Geriatric Depression Scale* | 21 |
| Cornell Scale for Depression in Dementia* | 7 | |
| Hospital Anxiety and Depression Scale | 4 | |
| Beck Depression Inventory | 1 | |
| Other | Neuropsychiatric Inventory* | 12 |
| Apathy Evaluation Scale | 3 | |
| Revised memory and behaviour problem checklist* | ||
| Everyday living | 20 | |
| Activities of daily living | Instrumental Activities of Daily Living* | 8 |
| Bayer Activities of Daily Living Scale | 3 | |
| Alzheimer's Disease Cooperative Study Activities of Daily Living Scale | 2 | |
| Barthel Index | 2 | |
| Functional ability | Functional Activities Questionnaire | 3 |
| Functional and Cognitive Assessment Test and Functional Rating Scale for Dementia | 2 | |
| Physical outcomes | 19 | |
| Physical performance | Timed Up and Go Test | 7 |
| Gait | 3 | |
| Handgrip strength | 3 | |
| Stride | 2 | |
| Walking Speed | 2 | |
| Physical measures | Weight | 2 |
| Quality of life/Well-being | 15 | |
| Quality of life | QoL in Alzheimer’s disease* | 7 |
| Dementia Quality of Life Instrument* | 3 | |
| EuroQoL EQ 5D* | 2 | |
| EQ-VAS | 1 | |
| Stress | Perceived Stress Scale | 1 |
| General Well-being | SF-36 | 1 |
| Biological outcome | 9 | |
| Brain activity | EEG | 4 |
| MRI | 2 | |
| Biomarker | BDNF | 3 |
| Adherence to intervention | 2 | |
| Adherence to intervention | Adherence | 2 |
|
Caregiver measures domain |
Outcome measure | N |
| Depression | 5 | |
| The Center for Epidemiological Studies Depression Scale* | 3 | |
| Geriatric Depression Scale | 1 | |
| Beck Depression Inventory | 1 | |
| Caregiver burden | 2 | |
| Zarit caregiver burden interview* | 2 | |
| General well-being | 1 | |
| SF-36* | 1 | |
| Knowledge of memory strategies | 1 | |
| Strategy Knowledge Repertoire | 1 | |
| Quality of life | 1 | |
| EQ-VAS | 1 | |
| Stress | 1 | |
| Perceived Stress Scale | 1 | |
*Measure recommended by INTERDEM Consensus.14
CDR, Clinical Dementia Rating; CERAD-NB, Consortium to Establish a Registry for Alzheimer’s Disease- Neuropsychological Battery; DSM, Diagnostic Statistical Manual of Mental Disorders; EEG, electroencephalogram; EQ-VAS, EuroQoL Visual Analogue Scales; EuroQoL EQ 5D, EuroQoL 5-dimension; MMQ, Multifactorial Memory Questionnaire; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment; SF-36, 36-Item Short Form Survey.
The next most frequently measured domain in PLWD was behavioural and psychological symptoms of dementia (BPSD), within this depression was the most commonly measured BPSD. The Geriatric Depression Scale was the most used measure in this domain, followed by the Neuropsychiatric Inventory which examines a greater number of symptoms. Other BSPDs measured were apathy and agitation resulting from memory problems.
Quality of life and well-being were measured 15 times across the included studies. Quality of life was measured 15 times using four different instruments, the most popular of which was Logsdon’s Quality of Life in Alzheimer’s disease scale which was used 7 times.
Measures of everyday living, physical ability, biological outcomes and adherence to the intervention delivered in the study were measured <20 times across the included studies.
Caregiver measures
Eight interventions in this study were dyadic,21–28 all included outcome measures specific to the caregiver in addition to the PLWD. One study of an intervention solely delivered to the PLWD also included a caregiver-specific measure.29
Table 2 also presents the outcome measures administered to caregivers grouped by domain. The Center for Epidemiological Studies Depression Scale and the Zarit Caregiver Burden interview were the only measures which were administered solely to caregivers. The other caregiver measures were also administered to PLWD. The most frequently measured domain in caregivers was depression, followed by caregiver burden. General well-being, knowledge of memory strategies, quality of life and stress were each measured once.
Use of outcome measures over time
RCTs of non-pharmacological treatments in mild dementia and MCI have become more frequent over recent years. Almost half (48%) of studies included in this review were published between 2016 and 2018.
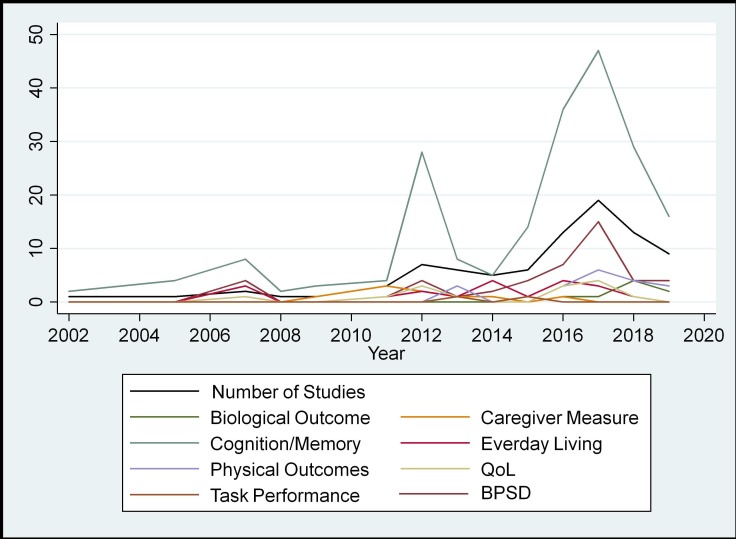
Figure 2 charts trends in outcome measure domains over time. As the number of studies in this area has increased over time, so too has the use of outcome measures in all domains. Cognition/memory has consistently been measured over other domains from the beginning of this sample. The only noticeable trend change is in measures of BPSD, which was generally in line with other domains until around 2012, when it overtakes other domains.
Figure 2.
Trends in outcome measures over time. BPSD, behavioural and psychological symptoms of dementia; QoL, quality of life.
Nearly all studies in 2014 included a measure of everyday living; however, since then, the number of studies including these measures has declined. Where measures of everyday living are being used less, measures of BPSD are being used more.
Similarly, caregiver measures were consistently used until 2011, when in 2010 and 2011 all studies included a caregiver measure, however since then the use of such measures has declined.
Use of outcome measures by intervention
Table 3 presents diagnosis and type of intervention by the domains measured. Cognition/memory was the most measured domain across all diagnostic groups, followed by BPSD. The third most common domain for MCI studies was physical performance, whereas caregiver measures were the third most common type of measures used in studies of early dementia.
Table 3.
Outcome measure domain by diagnosis and intervention
| Number of studies | BPSD | Biological outcome | Caregiver measure | Cognition/Memory | Everyday living | Physical measures | Physical performance | Quality of life/Well-being | Task performance | |
| Diagnosis | ||||||||||
| Both | 6 | 5 | – | 1 | 12 | 1 | – | – | – | – |
| Dementia | 14 | 16 | – | 7 | 42 | 6 | – | – | 6 | – |
| MCI | 71 | 30 | 9 | 3 | 163 | 12 | 2 | 17 | 9 | 2 |
| Type of intervention | ||||||||||
| Animal-assisted therapy | 2 | 2 | – | – | 2 | 1 | – | – | – | – |
| Art-based therapy | 2 | 1 | – | 1 | 6 | 1 | – | – | – | – |
| Case management | 2 | 2 | – | 3 | 1 | – | – | – | 1 | – |
| Chinese calligraphy | 2 | 1 | 1 | – | 4 | – | – | – | – | – |
| Cognitive training | 37 | 23 | 2 | 3 | 103 | 11 | – | 1 | 6 | 2 |
| Cognitive training and physical activity | 4 | – | – | – | 14 | 2 | – | 2 | – | – |
| Multicomponent psychosocial intervention | 4 | 6 | – | 3 | 10 | 2 | – | 2 | 3 | – |
| Music-based intervention | 2 | 1 | – | – | 7 | – | 1 | 2 | 1 | – |
| Physical activity | 25 | 11 | 6 | – | 53 | 3 | 1 | 10 | 2 | – |
| Reminiscence therapy | 2 | 1 | – | – | 2 | – | – | – | – | – |
| Support group | 3 | 3 | – | 1 | 1 | – | – | – | 1 | – |
BPSD, behavioural and psychological symptoms of dementia; MCI, mild cognitive impairment.
Cognition/memory was measured in all types of intervention. Measures of BPSD were most common in cognitive training interventions and physical activity interventions, however, they were not used by combined cognitive and physical training interventions. Quality of life was measured by studies of case management, cognitive training, psychosocial interventions, physical activity and support groups.
Caregiver measures were used in five types of interventions: case management, cognitive training and psychosocial interventions; followed by arts-based therapy and support groups.
Use of outcome measures by country
Table 4 presents the country the research was conducted in by outcome measure domain. Generally, there was not much variability in the domain of outcome measures used by country. Cognition/memory was the domain most frequently measured by all countries, followed by BPSD. The majority of studies were conducted in China (including Hong Kong and Taiwan), these studies focused on cognition/memory, BPSD and biological outcome measures. Other than China, only three other countries included biological measures (Iran, Pakistan and the USA). The USA had the second largest number of studies included in this review, these studies favoured cognition/memory, BPSD, caregiver measures and quality of life. Out of the 24 countries with studies included in this review, less than half (n=9) included measures of quality of life.
Table 4.
Outcome measure domain by country
| Country | Number of studies | BPSD | Biological outcome | Caregiver measure | Cognition/Memory | Functional ability | Physical measures | Physical performance | Quality of life/Well-being | Task performance |
| Argentina | 1 | 1 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 |
| Australia | 4 | 0 | 0 | 1 | 5 | 1 | 0 | 0 | 0 | 0 |
| Brazil | 5 | 1 | 1 | 0 | 14 | 0 | 0 | 1 | 0 | 0 |
| Canada | 6 | 2 | 0 | 0 | 16 | 0 | 0 | 2 | 0 | 0 |
| Mainland China, Hong Kong and Taiwan | 20 | 10 | 5 | 1 | 35 | 2 | 0 | 0 | 0 | 1 |
| Czech Republic | 3 | 0 | 0 | 0 | 3 | 2 | 0 | 1 | 0 | 0 |
| Denmark | 1 | 2 | 0 | 2 | 1 | 1 | 0 | 0 | 2 | 0 |
| Finland | 1 | 1 | 0 | 1 | 3 | 1 | 0 | 0 | 1 | 0 |
| France | 3 | 1 | 0 | 0 | 6 | 0 | 0 | 2 | 1 | 0 |
| Germany | 4 | 1 | 0 | 0 | 10 | 0 | 0 | 0 | 1 | 0 |
| Greece | 4 | 3 | 0 | 0 | 18 | 2 | 0 | 0 | 1 | 0 |
| Hungary | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Iran | 3 | 1 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 |
| Italy | 11 | 8 | 0 | 0 | 32 | 6 | 0 | 0 | 1 | 0 |
| Japan | 8 | 2 | 0 | 1 | 16 | 1 | 1 | 6 | 0 | 0 |
| Norway | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Pakistan | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Singapore | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| South Korea | 8 | 5 | 0 | 0 | 14 | 1 | 0 | 4 | 3 | 0 |
| Spain | 3 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| The Netherlands | 5 | 0 | 0 | 2 | 10 | 0 | 0 | 0 | 2 | 0 |
| Turkey | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| UK | 1 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| USA | 10 | 6 | 1 | 3 | 19 | 2 | 0 | 0 | 3 | 1 |
BPSD, behavioural and psychological symptoms of dementia.
Discussion
In this study, we used a scoping review to map which outcome measures had been used in trials for non-pharmacological treatments of mild dementia and MCI. We extracted 358 individual outcome measures used in 91 trials, only 22% of which were used more than once. We grouped the outcome measures which had been used more than once and examined differences in their use over time, by diagnostic group, country the research was set in and by the type of intervention they were being used to evaluate. Measures of cognition and BPSDs were the most frequently used across all studies and types of intervention.
Perhaps unsurprisingly, measures of cognition or memory are the most prevalent across all countries, diagnostic groups and types of intervention with the MMSE being the most frequently used outcome measure, despite the ADAS-cog having been validated as the gold-standard measure of cognition.15 30 31 Measuring cognition is central to measuring the progression of dementia and is a clinically and empirically useful outcome to measure in dementia research.31 However, in this review, we charted 40 different measures of cognition. This indicates that while cognition has been prioritised as an outcome in studies of non-pharmacological interventions, there is no consensus between researchers on which specific measures should be used. In addition to measures of cognitive performance, three studies have also measured participant’s knowledge or retention of memory strategies, indicating an interest in long-term coping strategies for memory loss.
Measures of the BPSD have become more common over time, becoming in 2017 the most measured outcome after cognition. There is not much variety in the BPSDs which have been measured. Generally, depression was measured over other BPSDs. Other BPSDs such as agitation were measured less, perhaps because they are more associated with the later stages of the disease and depression is associated with the earlier stages.32
Quality of life and well-being were not among the most measured domains. Four measures of quality of life were used 15 times across the included studies and all but one of these measures were dementia-specific measures. It is surprising quality of life has not been measured more, as previous research has stated that in the absence of a cure, healthcare providers have a greater ability to improve quality of life than alter the progression of the disease.33 Furthermore, both people with MCI and caregivers rated quality of life of the patient as the most important outcome to measure, followed by caregiver quality of life/burden.34 Indicating while quality of life has been identified as a priority by PLWD, people diagnosed with MCI and their caregivers in previous research, the findings of this study shows this is not being translated into trials of non-pharmacological treatments for early dementia and MCI.
Likewise, caregiver measures had consistent low use across the studies included in this review. We charted eight caregiver measures which were used 11 times across the included studies. Caregiver measures were more commonly used in studies of PLWD, rather than MCI. Previous research has highlighted the profound effect of dementia on their caregivers, with around half of caregivers experiencing high levels of burden.35 However, a third of caregivers of people with MCI also report extreme levels of burden,36 yet the findings of this study show this is less investigated.
There was great variability in the types of outcomes being used to evaluate the different types of intervention. All studies measured cognition and all but one measured BPSD. A lack of clarity in how change occurs as a result of non-pharmacological treatments is a fundamental weakness in this area of work.4 It is unlikely that all interventions being tested in this review could hope to improve cognition, however this is the most prevalent domain of outcome measures. There are a number of practical reasons as to why certain outcomes, and therefore outcome measures are used over others, In the past, pharmacological treatments have been required to include some measure of cognition, functional or global assessment,17 it is possible that this approach has influenced the choice in outcomes used in non-pharmacological studies. Furthermore, some measures may be used over others for more practical reasons. For example, measures which are short to administer and free to use may be priorities over others.31 Several interventions in this review comprise more than one component, for example, physical activity and cognitive training. In these cases, it may take multiple measures over many domains to accurately capture change. It is vital that outcome measures are selected depending on the domains the intervention is seeking to address.31
In 2008, the INTERDEM group recommended 22 outcome measures for use across 9 domains.15 We found 11 of these 22 measures (50%) were used by the studies included in this review, one of the recommended domains (staff carer morale) was not applicable to the studies included in this review. All measures recommended for measuring patient mood, and patient quality of life were charted in this review. Only one of the recommended measures for the activities of daily living, caregiver mood, caregiver burden and caregiver quality of life domains were charted and no measures under the global measures domain were charted in this review. This indicates that there is some consistency between which measures are recommended and which measures are used, this is largely for patient measures and there is less consistency for caregiver measures.
In this study, we found that the use of outcome measures did not vary much by the country the study was conducted in. In each country, cognition/memory was the most commonly tested domain, followed by BPSD. The importance of outcomes may vary between cultures; therefore, it is important that the outcomes and measures used reflect this.16 However, due to the limitations of the methodology used we cannot comment on the cultural relevance of the outcome measures charted in this review. Furthermore, articles were only included if they were published in English. It is possible that more culturally appropriate outcomes were used in articles published in the same language as the population under investigation. This is an important area for future research.
Limitations
The findings of this review must be interpreted in the context of the study. To make this review feasible we only included full RCTs, other outcome measures may have been used in different types of studies. Due to time constraints, some subtypes of dementia and cognitive impairment (young-onset, Parkinson’s disease dementia and vascular cognitive impairment) were excluded from this review, which limits the applicability of these findings. Further research is needed to explore whether the pattern in the use of outcomes and outcome measures is similar in these groups, compared with the ones included in this review. Furthermore, only outcome measures which were published could be included in this review. The studies included in this study were heterogeneous in terms of participants recruited, interventions tested and outcome measures used, making it difficult to group them thematically. It is possible some nuance is lost in the exploration of broader themes. As with the nature of scoping reviews, we are only able to present which outcome measures have been used in previous research, we are unable to draw conclusions as to which outcome measures should be used over others. Future research should explore which population measures have been validated for and what constitutes a clinically useful change.
Implications and recommendations for future research
The findings of this review indicate there is very little consistency in outcome measures used in RCTs for non-pharmacological interventions in MCI and mild dementia, however we are not able to conclude which measures should be used over others. To create a strong evidence base for non-pharmacological treatments more research, with the involvement of PLWD and their carers, is needed to determine which measures are preferable over a greater number of domains. Additionally, the prevalence of cognitive measures found in this study suggests that researchers are including such measures because there is an expectation to do so. Researchers should be clear on the theory behind how their intervention creates change and use the appropriate outcome measures.
Conclusions
In summary, this study has found RCTs for non-pharmacological treatments in mild dementia and MCI use a broad range of outcome measures, with a small proportion being used more than once. Excepting measures of cognition, there is very little commonality between studies. Where previous research has set priorities on outcomes preferred by PLWD, people with MCI and caregivers, quality of life, for example, this has not yet translated into studies measuring new treatments. Further research is needed to understand which outcomes should be prioritised and how they should be measured.
Supplementary Material
Footnotes
Contributors: EC designed the study, carried out the literature review, the data charting and synthesis, data interpretation, article preparation, article review and correspondence. MP and VL contributed to the study design, data interpretation and article review. MC contributed to the data charting.
Funding: EC is supported by a studentship from the ESRC LISS-DTP.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting, dissemination plans of this research. Refer to the 'Methods' section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Organization WH Global action plan on the public health response to dementia 2017–2025, 2017. [Google Scholar]
- 2.Prince M, Bryce R, Ferri C. World Alzheimer report 2011: the benefits of early diagnosis and intervention, 2018. [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. The Lancet 2017;390:2673–734. 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 4.Douglas S, James I, Ballard C. Non-Pharmacological interventions in dementia. Adv. psychiatr. treat 2004;10:171–7. 10.1192/apt.10.3.171 [DOI] [Google Scholar]
- 5.Kasl-Godley J, Gatz M. Psychosocial interventions for individuals with dementia: an integration of theory, therapy, and a clinical understanding of dementia. Clin Psychol Rev 2000;20:755–82. 10.1016/s0272-7358(99)00062-8 [DOI] [PubMed] [Google Scholar]
- 6.Olazarán J, Reisberg B, Clare L, et al. . Nonpharmacological therapies in Alzheimer's disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161–78. 10.1159/000316119 [DOI] [PubMed] [Google Scholar]
- 7.Brodaty H, Arasaratnam C. Meta-Analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012;169:946–53. 10.1176/appi.ajp.2012.11101529 [DOI] [PubMed] [Google Scholar]
- 8.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr 2004;16:129–40. 10.1017/S1041610204000092 [DOI] [PubMed] [Google Scholar]
- 9.Portet F, Ousset PJ, Visser PJ, et al. . Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI Working group of the European Consortium on Alzheimer's disease. J Neurol Neurosurg Psychiatry 2006;77:714–8. 10.1136/jnnp.2005.085332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allain H, Bentué-Ferrer D, Akwa Y. Treatment of the mild cognitive impairment (MCI). Hum Psychopharmacol 2007;22:189–97. 10.1002/hup.838 [DOI] [PubMed] [Google Scholar]
- 11.Petersen RC. Mci treatment trials: failure or not? Lancet Neurol 2007;6:473–5. 10.1016/S1474-4422(07)70113-8 [DOI] [PubMed] [Google Scholar]
- 12.Teixeira CVL, Gobbi LTB, Corazza DI, et al. . Non-Pharmacological interventions on cognitive functions in older people with mild cognitive impairment (MCI). Arch Gerontol Geriatr 2012;54:175–80. 10.1016/j.archger.2011.02.014 [DOI] [PubMed] [Google Scholar]
- 13.Harding AJE, Morbey H, Ahmed F, et al. . Developing a core outcome set for people living with dementia at home in their neighbourhoods and communities: study protocol for use in the evaluation of non-pharmacological community-based health and social care interventions. Trials 2018;19:247. 10.1186/s13063-018-2584-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulino Ramirez Diaz S, Gil Gregório P, Manuel Ribera Casado J, et al. . The need for a consensus in the use of assessment tools for Alzheimer's disease: the feasibility study (assessment tools for dementia in Alzheimer centres across Europe), a European Alzheimer's disease Consortium's (EADC) survey. Int J Geriatr Psychiatry 2005;20:744–8. 10.1002/gps.1355 [DOI] [PubMed] [Google Scholar]
- 15.Moniz-Cook E, Vernooij-Dassen M, Woods R, et al. . A European consensus on outcome measures for psychosocial intervention research in dementia care. Aging Ment Health 2008;12:14–29. 10.1080/13607860801919850 [DOI] [PubMed] [Google Scholar]
- 16.Katona C, Livingston G, Cooper C, et al. . International psychogeriatric association consensus statement on defining and measuring treatment benefits in dementia. Int Psychogeriatr 2007;19:345–54. 10.1017/S1041610207005145 [DOI] [PubMed] [Google Scholar]
- 17.Harrison JK, Noel-Storr AH, Demeyere N, et al. . Outcomes measures in a decade of dementia and mild cognitive impairment trials. Alzheimers Res Ther 2016;8:48. 10.1186/s13195-016-0216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, et al. . PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, et al. . Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenaway MC, Duncan NL, Smith GE. The memory support system for mild cognitive impairment: randomized trial of a cognitive rehabilitation intervention. Int J Geriatr Psychiatry 2013;28:402–9. 10.1002/gps.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen APD, van Hout HPJ, Nijpels G, et al. . Effectiveness of case management among older adults with early symptoms of dementia and their primary informal caregivers: a randomized clinical trial. Int J Nurs Stud 2011;48:933–43. 10.1016/j.ijnurstu.2011.02.004 [DOI] [PubMed] [Google Scholar]
- 23.Kinsella GJ, Mullaly E, Rand E, et al. . Early intervention for mild cognitive impairment: a randomised controlled trial. J Neurol Neurosurg Psychiatry 2009;80:730–6. 10.1136/jnnp.2008.148346 [DOI] [PubMed] [Google Scholar]
- 24.Koivisto AM, Hallikainen I, Välimäki T, et al. . Early psychosocial intervention does not delay institutionalization in persons with mild Alzheimer disease and has impact on neither disease progression nor caregivers' well-being: ALSOVA 3-year follow-up. Int J Geriatr Psychiatry 2016;31:273–83. 10.1002/gps.4321 [DOI] [PubMed] [Google Scholar]
- 25.Lam LCW, Lee JSW, Chung JCC, et al. . A randomized controlled trial to examine the effectiveness of case management model for community dwelling older persons with mild dementia in Hong Kong. Int J Geriatr Psychiatry 2010;25:395–402. 10.1002/gps.2352 [DOI] [PubMed] [Google Scholar]
- 26.Logsdon RG, Pike KC, McCurry SM, et al. . Early-Stage memory loss support groups: outcomes from a randomized controlled clinical trial. J Gerontol B Psychol Sci Soc Sci 2010;65:691–7. 10.1093/geronb/gbq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tappen RM, Hain D. The effect of in-home cognitive training on functional performance of individuals with mild cognitive impairment and early-stage Alzheimer's disease. Res Gerontol Nurs 2014;7:14–24. 10.3928/19404921-20131009-01 [DOI] [PubMed] [Google Scholar]
- 28.Waldorff FB, Buss DV, Eckermann A, et al. . Efficacy of psychosocial intervention in patients with mild Alzheimer's disease: the multicentre, rater blinded, randomised Danish Alzheimer intervention study (daisy). BMJ 2012;345:e4693. 10.1136/bmj.e4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattori H, Hattori C, Hokao C, et al. . Controlled study on the cognitive and psychological effect of coloring and drawing in mild Alzheimer's disease patients. Geriatr Gerontol Int 2011;11:431–7. 10.1111/j.1447-0594.2011.00698.x [DOI] [PubMed] [Google Scholar]
- 30.Rockwood K, Fay S, Gorman M, et al. . The clinical meaningfulness of ADAS-Cog changes in Alzheimer's disease patients treated with donepezil in an open-label trial. BMC Neurol 2007;7:26. 10.1186/1471-2377-7-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehan B. Assessment scales in dementia. Ther Adv Neurol Disord 2012;5:349–58. 10.1177/1756285612455733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccininni M, Di Carlo A, Baldereschi M, et al. . Behavioral and psychological symptoms in Alzheimer's disease: frequency and relationship with duration and severity of the disease. Dement Geriatr Cogn Disord 2005;19:276–81. 10.1159/000084552 [DOI] [PubMed] [Google Scholar]
- 33.Brod M, Stewart AL, Sands L, et al. . Conceptualization and measurement of quality of life in dementia: the dementia quality of life instrument (DQoL). Gerontologist 1999;39:25–36. 10.1093/geront/39.1.25 [DOI] [PubMed] [Google Scholar]
- 34.Barrios PG, González RP, Hanna SM, et al. . Priority of treatment outcomes for caregivers and patients with mild cognitive impairment: preliminary analyses. Neurol Ther 2016;5:183–92. 10.1007/s40120-016-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz R, O'Brien AT, Bookwala J, et al. . Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist 1995;35:771–91. 10.1093/geront/35.6.771 [DOI] [PubMed] [Google Scholar]
- 36.Bruce JM, McQuiggan M, Williams V, et al. . Burden among spousal and child caregivers of patients with mild cognitive impairment. Dement Geriatr Cogn Disord 2008;25:385–90. 10.1159/000122587 [DOI] [PubMed] [Google Scholar]
- 37.Amjad I, Toor H, Niazi IK, et al. . Therapeutic effects of aerobic exercise on EEG parameters and higher cognitive functions in mild cognitive impairment patients. Int J Neurosci 2019;129:551–62. 10.1080/00207454.2018.1551894 [DOI] [PubMed] [Google Scholar]
- 38.Bae S, Lee S, Lee S, et al. . The effect of a multicomponent intervention to promote community activity on cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Complement Ther Med 2019;42:164–9. 10.1016/j.ctim.2018.11.011 [DOI] [PubMed] [Google Scholar]
- 39.Baker LD, Frank LL, Foster-Schubert K, et al. . Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol 2010;67:71–9. 10.1001/archneurol.2009.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belleville S, Hudon C, Bier N, et al. . MEMO+: efficacy, durability and effect of cognitive training and psychosocial intervention in individuals with mild cognitive impairment. J Am Geriatr Soc 2018;66:655–63. 10.1111/jgs.15192 [DOI] [PubMed] [Google Scholar]
- 41.Biasutti M, Mangiacotti A. Assessing a cognitive music training for older participants: a randomised controlled trial. Int J Geriatr Psychiatry 2018;33:271–8. 10.1002/gps.4721 [DOI] [PubMed] [Google Scholar]
- 42.Bono A, Benvenuti C, Buzzi M, et al. . Effects of animal assisted therapy (AAT) carried out with dogs on the evolution of mild cognitive impairment. G Gerontol 2015;63:32–6. [Google Scholar]
- 43.Burgio F, Delazer M, Meneghello F, et al. . Cognitive training improves ratio processing and decision making in patients with mild cognitive impairment. J Alzheimers Dis 2018;64:1213–26. 10.3233/JAD-180461 [DOI] [PubMed] [Google Scholar]
- 44.Buschert VC, Giegling I, Teipel SJ, et al. . Long-Term observation of a multicomponent cognitive intervention in mild cognitive impairment. J Clin Psychiatry 2012;73:e1492–8. 10.4088/JCP.11m07270 [DOI] [PubMed] [Google Scholar]
- 45.Carretti B, Borella E, Fostinelli S, et al. . Benefits of training working memory in amnestic mild cognitive impairment: specific and transfer effects. Int Psychogeriatr 2013;25:617–26. 10.1017/S1041610212002177 [DOI] [PubMed] [Google Scholar]
- 46.Cavallo M, Hunter EM, van der Hiele K, et al. . Computerized structured cognitive training in patients affected by early-stage Alzheimer's disease is feasible and effective: a randomized controlled study. Arch Clin Neuropsychol 2016;31:868–76. 10.1093/arclin/acw072 [DOI] [PubMed] [Google Scholar]
- 47.Chan SCC, Lam TLH, Fong KNK, et al. . Generalization of context-specific training in individuals with mild cognitive impairment: an event-related potential study. Dement Geriatr Cogn Dis Extra 2016;6:568–79. 10.1159/000453546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan SCC, Chan CCH, Derbie AY, et al. . Chinese calligraphy writing for augmenting attentional control and working memory of older adults at risk of mild cognitive impairment: a randomized controlled trial. J Alzheimers Dis 2017;58:735–46. 10.3233/JAD-170024 [DOI] [PubMed] [Google Scholar]
- 49.Choi W, Lee S. Ground kayak paddling exercise improves postural balance, muscle performance, and cognitive function in older adults with mild cognitive impairment: a randomized controlled trial. Med Sci Monit 2018;24:3909–15. 10.12659/MSM.908248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Combourieu Donnezan L, Perrot A, Belleville S, et al. . Effects of simultaneous aerobic and cognitive training on executive functions, cardiovascular fitness and functional abilities in older adults with mild cognitive impairment. Ment Health Phys Act 2018;15:78–87. 10.1016/j.mhpa.2018.06.001 [DOI] [Google Scholar]
- 51.DiNapoli EA, Scogin F, Bryant AN, et al. . Effect of individualized social activities on quality of life among older adults with mild to moderate cognitive impairment in a geriatric psychiatry facility. Aging Ment Health 2016;20:262–70. 10.1080/13607863.2015.1008990 [DOI] [PubMed] [Google Scholar]
- 52.Doi T, Makizako H, Shimada H, et al. . Effects of multicomponent exercise on spatial-temporal gait parameters among the elderly with amnestic mild cognitive impairment (aMCI): preliminary results from a randomized controlled trial (RCT). Arch Gerontol Geriatr 2013;56:104–8. 10.1016/j.archger.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 53.Doi T, Verghese J, Makizako H, et al. . Effects of cognitive leisure activity on cognition in mild cognitive impairment: results of a randomized controlled trial. J Am Med Dir Assoc 2017;18:686–91. 10.1016/j.jamda.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 54.Drumond Marra HL, Myczkowski ML, Maia Memória C, et al. . Transcranial magnetic stimulation to address mild cognitive impairment in the elderly: a randomized controlled study. Behav Neurol 2015;2015:1–13. 10.1155/2015/287843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emsaki G, NeshatDoost HT, Tavakoli M, et al. . Memory specificity training can improve working and prospective memory in amnestic mild cognitive impairment. Dement Neuropsychol 2017;11:255–61. 10.1590/1980-57642016dn11-030007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyre HA, Siddarth P, Acevedo B, et al. . A randomized controlled trial of Kundalini yoga in mild cognitive impairment. Int Psychogeriatr 2017;29:557–67. 10.1017/S1041610216002155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng W, Wang D, Tang L, et al. . Effects of different cognitive Trainings on amnestic mild cognitive impairment in the elderly: a one-year longitudinal functional magnetic resonance imaging (MRI) study. Med Sci Monit 2018;24:5517–27. 10.12659/MSM.908315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fernández-Calvo B, Contador I, Ramos F, et al. . Effect of unawareness on rehabilitation outcome in a randomised controlled trial of multicomponent intervention for patients with mild Alzheimer's disease. Neuropsychol Rehabil 2015;25:448–77. 10.1080/09602011.2014.948461 [DOI] [PubMed] [Google Scholar]
- 59.Fiatarone Singh MA, Gates N, Saigal N, et al. . The study of mental and resistance training (smart) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J Am Med Dir Assoc 2014;15:873–80. 10.1016/j.jamda.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 60.Finn M, McDonald S. Repetition-lag training to improve recollection memory in older people with amnestic mild cognitive impairment. A randomized controlled trial. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015;22:244–58. 10.1080/13825585.2014.915918 [DOI] [PubMed] [Google Scholar]
- 61.Fogarty JN, Murphy KJ, McFarlane B, et al. . Taoist tai Chi® and memory intervention for individuals with mild cognitive impairment. J Aging Phys Act 2016;24:169–80. 10.1123/japa.2014-0062 [DOI] [PubMed] [Google Scholar]
- 62.Förster S, Buschert VC, Teipel SJ, et al. . Effects of a 6-month cognitive intervention on brain metabolism in patients with amnestic MCI and mild Alzheimer's disease. J Alzheimers Dis 2011;26 Suppl 3:337–48. 10.3233/JAD-2011-0025 [DOI] [PubMed] [Google Scholar]
- 63.Galante E, Venturini G, Fiaccadori C. Computer-Based cognitive intervention for dementia: preliminary results of a randomized clinical trial. G Ital Med Lav Ergon 2007;29:B26–32. [PubMed] [Google Scholar]
- 64.Hagovská M, Dzvoník O, Olekszyová Z. Comparison of two cognitive training programs with effects on functional activities and quality of life. Res Gerontol Nurs 2017;10:172–80. 10.3928/19404921-20170524-01 [DOI] [PubMed] [Google Scholar]
- 65.Hagovská M, Takáč P, Dzvoník O. Effect of a combining cognitive and balanced training on the cognitive, postural and functional status of seniors with a mild cognitive deficit in a randomized, controlled trial. Eur J Phys Rehabil Med 2016;52:101–9. [PubMed] [Google Scholar]
- 66.Han JW, Son KL, Byun HJ, et al. . Efficacy of the ubiquitous spaced Retrieval-based memory advancement and rehabilitation training (USMART) program among patients with mild cognitive impairment: a randomized controlled crossover trial. Alzheimers Res Ther 2017;9:39. 10.1186/s13195-017-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han JW, Lee H, Hong JW, et al. . Multimodal Cognitive Enhancement Therapy for Patients with Mild Cognitive Impairment and Mild Dementia: A Multi- Center, Randomized, Controlled, Double-Blind, Crossover Trial. J Alzheimers Dis 2017;55:787–96. 10.3233/JAD-160619 [DOI] [PubMed] [Google Scholar]
- 68.Ho RTH, Fong TCT, Chan WC, et al. . Psychophysiological effects of dance movement therapy and physical exercise on older adults with mild dementia: a randomized controlled trial. J Gerontol B Psychol Sci Soc Sci 2020;75:560-570. 10.1093/geronb/gby145 [DOI] [PubMed] [Google Scholar]
- 69.Horie NC, Serrao VT, Simon SS, et al. . Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab 2016;101:1104–12. 10.1210/jc.2015-2315 [DOI] [PubMed] [Google Scholar]
- 70.Hyer L, Scott C, Atkinson MM, et al. . Cognitive training program to improve working memory in older adults with MCI. Clin Gerontol 2016;39:410–27. 10.1080/07317115.2015.1120257 [DOI] [PubMed] [Google Scholar]
- 71.Jean L, Simard M, Wiederkehr S, et al. . Efficacy of a cognitive training programme for mild cognitive impairment: results of a randomised controlled study. Neuropsychol Rehabil 2010;20:377–405. 10.1080/09602010903343012 [DOI] [PubMed] [Google Scholar]
- 72.Jelcic N, Cagnin A, Meneghello F, et al. . Effects of lexical-semantic treatment on memory in early Alzheimer disease: an observer-blinded randomized controlled trial. Neurorehabil Neural Repair 2012;26:949–56. 10.1177/1545968312440146 [DOI] [PubMed] [Google Scholar]
- 73.Jeong JH, Na HR, Choi SH, et al. . Group- and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychother Psychosom 2016;85:198–207. 10.1159/000442261 [DOI] [PubMed] [Google Scholar]
- 74.Kohanpour MA, Peeri M, Azarbayjani MA. The effects of aerobic exercise with lavender essence use on cognitive state and serum brain-derived neurotrophic factor levels in elderly with mild cognitive impairment. J Herbmed Pharmacol 2017;6. [Google Scholar]
- 75.Kovács E, Sztruhár Jónásné I, Karóczi CK, et al. . Effects of a multimodal exercise program on balance, functional mobility and fall risk in older adults with cognitive impairment: a randomized controlled single-blind study. Eur J Phys Rehabil Med 2013;49:639–48. [PubMed] [Google Scholar]
- 76.Küster OC, Fissler P, Laptinskaya D, et al. . Cognitive change is more positively associated with an active lifestyle than with training interventions in older adults at risk of dementia: a controlled interventional clinical trial. BMC Psychiatry 2016;16:315. 10.1186/s12888-016-1018-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kwok TCY, Bai X, Li JCY, et al. . Effectiveness of cognitive training in Chinese older people with subjective cognitive complaints: a randomized placebo-controlled trial. Int J Geriatr Psychiatry 2013;28:208–15. 10.1002/gps.3812 [DOI] [PubMed] [Google Scholar]
- 78.Lam LCW, Chau RCM, Wong BML, et al. . A 1-year randomized controlled trial comparing mind body exercise (tai chi) with stretching and toning exercise on cognitive function in older Chinese adults at risk of cognitive decline. J Am Med Dir Assoc 2012;13:568. 10.1016/j.jamda.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 79.Lam LC-W, Chan WC, Leung T, et al. . Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: a cluster randomized controlled trial. PLoS One 2015;10:e0118173. 10.1371/journal.pone.0118173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langoni CdaS, Resende TdeL, Barcellos AB, CdS L, TdL R, et al. . The effect of group exercises on balance, mobility, and depressive symptoms in older adults with mild cognitive impairment: a randomized controlled trial. Clin Rehabil 2019;33:439–49. 10.1177/0269215518815218 [DOI] [PubMed] [Google Scholar]
- 81.Law LLF, Barnett F, Yau MK, et al. . Effects of functional tasks exercise on older adults with cognitive impairment at risk of Alzheimer's disease: a randomised controlled trial. Age Ageing 2014;43:813–20. 10.1093/ageing/afu055 [DOI] [PubMed] [Google Scholar]
- 82.Lazarou I, Parastatidis T, Tsolaki A, et al. . International ballroom dancing against neurodegeneration: a randomized controlled trial in Greek community-dwelling elders with mild cognitive impairment. Am J Alzheimers Dis Other Demen 2017;32:489–99. 10.1177/1533317517725813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li B-Y, He N-Y, Qiao Y, et al. . Computerized cognitive training for Chinese mild cognitive impairment patients: a neuropsychological and fMRI study. Neuroimage Clin 2019;22:101691. 10.1016/j.nicl.2019.101691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim H-W, Saw W-Y, Feng L, et al. . Dataset on gene expression in the elderly after mindfulness awareness practice or health education program. Data Brief 2018;18:902–12. 10.1016/j.dib.2018.03.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Luijpen MW, Swaab DF, Sergeant JA, et al. . Effects of transcutaneous electrical nerve stimulation (TENS) on memory in elderly with mild cognitive impairment. Behav Brain Res 2005;158:349–57. 10.1016/j.bbr.2004.09.017 [DOI] [PubMed] [Google Scholar]
- 86.Maffei L, Picano E, Andreassi M, et al. . Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: the train the brain study. Sci Rep 2017;7:39471. 10.1038/srep39471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.İnel Manav A, Simsek N. The effect of reminiscence therapy with Internet-based Videos on cognitive status and apathy of older people with mild dementia. J Geriatr Psychiatry Neurol 2019;32:104–13. 10.1177/0891988718819864 [DOI] [PubMed] [Google Scholar]
- 88.Melendez JC, Torres M, Redondo R, et al. . Effectiveness of follow-up reminiscence therapy on autobiographical memory in pathological ageing. Int J Psychol 2017;52:283–90. 10.1002/ijop.12217 [DOI] [PubMed] [Google Scholar]
- 89.Nagamatsu LS, Handy TC, Hsu CL, et al. . Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med 2012;172:666–8. 10.1001/archinternmed.2012.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olsen C, Pedersen I, Bergland A, et al. . Effect of animal-assisted interventions on depression, agitation and quality of life in nursing home residents suffering from cognitive impairment or dementia: a cluster randomized controlled trial. Int J Geriatr Psychiatry 2016;31:1312–21. 10.1002/gps.4436 [DOI] [PubMed] [Google Scholar]
- 91.Pantoni L, Poggesi A, Diciotti S, et al. . Effect of attention training in mild cognitive impairment patients with subcortical vascular changes: the RehAtt study. J Alzheimers Dis 2017;60:615–24. 10.3233/JAD-170428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park J-H, Park J-H. Does Cognition-specific computer training have better clinical outcomes than non-specific computer training? A single-blind, randomized controlled trial. Clin Rehabil 2018;32:213–22. 10.1177/0269215517719951 [DOI] [PubMed] [Google Scholar]
- 93.Poinsatte K, Smith EE, Torres VO, et al. . T and B cell subsets differentially correlate with amyloid deposition and neurocognitive function in patients with amnestic mild cognitive impairment after one year of physical activity. Exerc Immunol Rev 2019;25:34-49. [PMC free article] [PubMed] [Google Scholar]
- 94.Pongan E, Tillmann B, Leveque Y, et al. . Can musical or painting interventions improve chronic pain, mood, quality of life, and cognition in patients with mild Alzheimer's disease? Evidence from a randomized controlled trial. J Alzheimers Dis 2017;60:663–77. 10.3233/JAD-170410 [DOI] [PubMed] [Google Scholar]
- 95.Poptsi E, Lazarou I, Markou N, et al. . A comparative single-blind randomized controlled trial with language training in people with mild cognitive impairment. Am J Alzheimers Dis Other Demen 2019;34:176–87. 10.1177/1533317518813554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qi M, Zhu Y, Zhang L, et al. . The effect of aerobic dance intervention on brain spontaneous activity in older adults with mild cognitive impairment: a resting-state functional MRI study. Exp Ther Med 2019;17:715–22. 10.3892/etm.2018.7006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rapp S, Brenes G, Marsh AP. Memory enhancement training for older adults with mild cognitive impairment: a preliminary study. Aging Ment Health 2002;6:5–11. 10.1080/13607860120101077 [DOI] [PubMed] [Google Scholar]
- 98.Rojas GJ, Villar V, Iturry M, et al. . Efficacy of a cognitive intervention program in patients with mild cognitive impairment. Int Psychogeriatr 2013;25:825–31. 10.1017/S1041610213000045 [DOI] [PubMed] [Google Scholar]
- 99.Rozzini L, Costardi D, Chilovi BV, et al. . Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry 2007;22:356–60. 10.1002/gps.1681 [DOI] [PubMed] [Google Scholar]
- 100.Savulich G, Piercy T, Fox C, et al. . Cognitive training using a novel memory game on an iPad in patients with amnestic mild cognitive impairment (aMCI). Int J Neuropsychopharmacol 2017;20:624–33. 10.1093/ijnp/pyx040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scherder EJA, Van Paasschen J, Deijen J-B, et al. . Physical activity and executive functions in the elderly with mild cognitive impairment. Aging Ment Health 2005;9:272–80. 10.1080/13607860500089930 [DOI] [PubMed] [Google Scholar]
- 102.Shimada H, Makizako H, Doi T, et al. . Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients With Mild Cognitive Impairment: A Randomized Clinical Trial. J Am Med Dir Assoc 2018;19:584–91. 10.1016/j.jamda.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 103.Shimizu N, Umemura T, Matsunaga M, et al. . Effects of movement music therapy with a percussion instrument on physical and frontal lobe function in older adults with mild cognitive impairment: a randomized controlled trial. Aging Ment Health 2018;22:1614–26. 10.1080/13607863.2017.1379048 [DOI] [PubMed] [Google Scholar]
- 104.Simon SS, Hampstead BM, Nucci MP, et al. . Cognitive and brain activity changes after mnemonic strategy training in amnestic mild cognitive impairment: evidence from a randomized controlled trial. Front Aging Neurosci 2018;10:342. 10.3389/fnagi.2018.00342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song D, Yu DSF, Doris S. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud 2019;93:97–105. 10.1016/j.ijnurstu.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 106.Suzuki T, Shimada H, Makizako H, et al. . Effects of multicomponent exercise on cognitive function in older adults with amnestic mild cognitive impairment: a randomized controlled trial. BMC Neurol 2012;12:128. 10.1186/1471-2377-12-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Troyer AK, Murphy KJ, Anderson ND, et al. . Changing everyday memory behaviour in amnestic mild cognitive impairment: a randomised controlled trial. Neuropsychol Rehabil 2008;18:65–88. 10.1080/09602010701409684 [DOI] [PubMed] [Google Scholar]
- 108.Tsai C-L, Ukropec J, Ukropcová B, et al. . An acute bout of aerobic or strength exercise specifically modifies circulating exerkine levels and neurocognitive functions in elderly individuals with mild cognitive impairment. Neuroimage Clin 2018;17:272–84. 10.1016/j.nicl.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsantali E, Economidis D, Rigopoulou S. Testing the benefits of cognitive training vs. cognitive stimulation in mild Alzheimer's disease: a randomised controlled trial. Brain Impairment 2017;18:188–96. 10.1017/BrImp.2017.6 [DOI] [Google Scholar]
- 110.van Uffelen JGZ, Chin A Paw MJM, Hopman-Rock M, et al. . The effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: a randomized, controlled trial. Qual Life Res 2007;16:1137–46. 10.1007/s11136-007-9219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wei X-hong, Ji L-li, X-h W, L-l J. Effect of handball training on cognitive ability in elderly with mild cognitive impairment. Neurosci Lett 2014;566:98–101. 10.1016/j.neulet.2014.02.035 [DOI] [PubMed] [Google Scholar]
- 112.Yang H, Leaver AM, Siddarth P, et al. . Neurochemical and neuroanatomical plasticity following memory training and yoga interventions in older adults with mild cognitive impairment. Front Aging Neurosci 2016;8:277. 10.3389/fnagi.2016.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoon DH, Kang D, Kim H-J, Hj K, et al. . Effect of elastic band-based high-speed power training on cognitive function, physical performance and muscle strength in older women with mild cognitive impairment. Geriatr Gerontol Int 2017;17:765–72. 10.1111/ggi.12784 [DOI] [PubMed] [Google Scholar]
- 114.Young DKW, Kwok TCY, Ng PYN. A single blind randomized control trial on support groups for Chinese persons with mild dementia. Clin Interv Aging 2014;9:2105. 10.2147/CIA.S68687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Young K-W, Ng P, Kwok T, et al. . The effects of holistic health group interventions on improving the cognitive ability of persons with mild cognitive impairment: a randomized controlled trial. Clin Interv Aging 2017;12:1543. 10.2147/CIA.S142109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yun K, Song I-U, Chung Y-A. Changes in cerebral glucose metabolism after 3 weeks of noninvasive electrical stimulation of mild cognitive impairment patients. Alzheimers Res Ther 2016;8:49. 10.1186/s13195-016-0218-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao J, Li H, Lin R, et al. . Effects of creative expression therapy for older adults with mild cognitive impairment at risk of Alzheimer's disease: a randomized controlled clinical trial. Clin Interv Aging 2018;13:1313–20. 10.2147/CIA.S161861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y, Wu H, Qi M, et al. . Effects of a specially designed aerobic dance routine on mild cognitive impairment. Clin Interv Aging 2018;13:1691–700. 10.2147/CIA.S163067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035980supp001.pdf (58.9KB, pdf)