Abstract
In experiment 1 we investigated the accuracy of transrectal ultrasonography (TUS) to assess the number (OR) and diameter of corpora lutea (CL) in 45 and 25 sows, respectively, at 23.4 ± 2.9 d of pregnancy. The diameter was calculated as the average diameter of 10 biggest CL. Sows were subsequently slaughtered and OR was assessed by dissection of CL from both ovaries (n = 45) and average diameter of the 10 biggest CL was also calculated after measurement of CL with the caliper rule (n = 25). There was a weak relationship between OR counted after dissection of the ovaries and OR counted with TUS (β = 0.28 ± 0.01 CL/CL, P = 0.01), but there was a strong relationship between the average CL diameter measured with the caliper rule after dissection and the average CL diameter based on TUS (β = 1.0 ± 0.1 mm/mm, P < 0.0001). This shows that TUS is not a valid method to assess OR in pregnant sows but it is a valid method to assess average CL diameter. In experiment 2, we investigated the relationship between the average CL diameter assessed by TUS (n = 100) at 23.8 ± 2.4 d of pregnancy and average piglet birth weight (BW) and observed an increase of 37.6 ± 17.8 g in piglet BW per mm increase in average CL diameter measured by TUS (P = 0.04). This relationship is probably because larger CL develop from bigger follicles at ovulation, which might have ovulated oocytes of higher quality that developed into embryos with higher growth potential and thus higher birth weight.
Keywords: corpora lutea, piglet birth weight, pregnancy, sows, transrectal ultrasonography
INTRODUCTION
Transrectal ultrasonography (TUS) is an established technique for assessing ovulation rate (OR) by counting the number of pre-ovulatory follicles in sows during oestrus (Soede et al., 1992; Soede et al., 1998; Lucy et al., 2001; Hazeleger et al., 2005; Madej et al., 2005), and it was used by Gonzalez-Añover et al. (2009) to assess OR by counting the number of corpora lutea (CL) in Iberian sows at 9 to 11 d after estrus with an accuracy of 86.7%. However, TUS has not yet been used to OR in Western commercial sows during pregnancy. So far, most assessments of OR in pregnant sows to study its relationship with embryonic characteristics have been based on post-mortem findings (Vonnahme et al., 2002; Town et al., 2005; Da Silva et al., 2016; Da Silva et al., 2017). It was observed that an increase in OR is related with vital embryos at 35 d of pregnancy with lower placental length in sows (Da Silva et al., 2016) and with a higher variation in weight in gilts (Da Silva et al., 2017). This might decrease fetal survival and consequently litter size, but might also lead to a decrease in piglet birth weight and birth weight uniformity. Additionally, average CL weight decreased with the increase in OR in gilts (Da Silva et al., 2017), indicating a compromised follicular growth, with oocytes and possibly embryos of lower quality (Ding and Foxcroft, 1994), compromising embryonic and fetal survival and development. Thus, we hypothesized that the number and size of CL in pregnant sows might be related with litter characteristics at birth, and the objectives of this study were first to investigate the accuracy of TUS to assess the number and average diameter of CL in modern crossbred sows in early pregnancy, and second to investigate the relationship between the CL characteristics evaluated by TUS and subsequent litter characteristics at birth.
MATERIAL AND METHODS
The experiments and all measurements were approved by the Animal Welfare Committee of Wageningen University and Research Centre in compliance with the Dutch Law on Animal Experimentation. Experiment 1 was conducted at Schothorst Feed Research B.V. (Lelystad, The Netherlands), and at a commercial farm (Nijmegen, The Netherlands). Experiment 2 was conducted at the Pig Innovation Centre, Sterksel (VIC, Sterksel, The Netherlands).
Animals and Housing
Experiment 1.
The study included a total of 45 pregnant multiparous (parity 7.3 ± 3.2, ranging from 2 to 13) crossbred sows (Yorkshire × Landrace; Topigs Norsvin, Vught, The Netherlands), at 2 different farms (farm 1, n = 20 and farm 2, n = 25) in 3 batches each.
Experiment 2.
The study included a total of 100 pregnant multiparous (parity 5.0 ± 1.9, ranging from 2 to 9) crossbred sows (Yorkshire × Landrace; Topigs Norsvin, Vught, The Netherlands) at 1 farm, which were used in 6 batches. At the first day after weaning, that took place at d 27.0 ± 3.8 (mean ± SD) of lactation, the sows were group housed with individual feeding stalls, where they received a commercial lactation diet (NE = 9.50 MJ/kg; CP = 149 g/kg and ileal digestible lysine = 7.6 g/kg) ad libitum. From the second day post weaning to the day of first insemination, sows were housed in individual crates, and received 4.5 kg of the lactation diet per day. The light schedule consisted of 16 consecutive hours of light (100 lux) and 8 h of darkness. After insemination, sows were moved to a gestation group housing system in groups of 11 to 45 sows, where they received a commercial diet (NE = 9.06 MJ; CP = 119 g/kg, ileal digestible lysine = 4.6 g/kg); at 2.8 kg/d from 1 to 34 d of pregnancy, 2.7 kg/d from 35 to 76 d, and 3.3 kg/d from 76 d to farrowing. The light schedule during gestation consisted of 12 consecutive hours of light (100 lux) and 12 h of darkness. Sows had free access to water at all times.
Transrectal Ultrasonography
A list of the abbreviations is provided in Appendix 1. In experiments 1 and 2 transrectal real time B-mode ultrasonography (TUS) of the ovaries was performed using an Aquila MyVet30 LAB with a convex transducer at 7.5 MHz (Pie Medical/Esaote, Maastricht, The Netherlands). Sows were scanned in early pregnancy (21.7 ± 1.1 d of pregnancy in experiment 1, and 23.8 ± 2.4 d in experiment 2). To perform the ultrasonography, sows were placed in individual crates. The scanning procedure involved manual cleaning of the rectum and rectal insertion of a transducer covered with a disposable glove containing scanning gel to prevent the presence of air bubbles in contact with the probe. During the entire procedure lubricated disposable transrectal examination gloves were used, to minimize animal discomfort. Ovulation rate (ORTUS) was considered as the total number of CL counted on both ovaries with transrectal ultrasonography. In experiment 1, the CL counting was performed by 2 examiners separately in a subset of sows (E1 and E2, n = 29), to check for inter-examiner agreement in assessment of ORTUS.
Also, a movie clip of the examination of each ovary was saved (25 sows in experiment 1 and 100 sows in experiment 2) and the diameter of the 5 biggest CL on each ovary (10 per sow) was later assessed, and the average CL diameter (mm) measured by TUS was calculated (DIAMTUS). An example of ultrasound images of the ovaries can be seen in Fig. 1, and a movie of ovarian examination has been provided as supplementary material.
Figure 1.
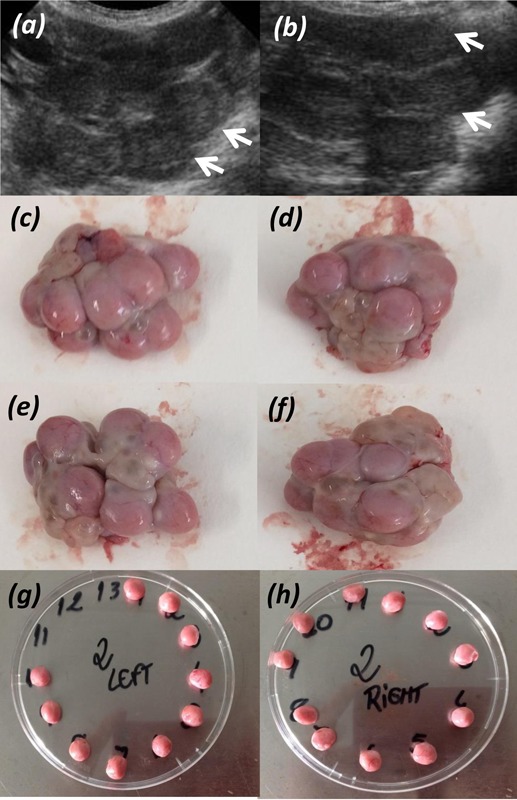
Example of ultrasound image of the ovaries (a and b, left and right ovary respectively), with the white arrows indicating individual corpus luteum. Panels c and d show the left ovary, and e and f show the right ovary, before dissection. Panels g and h show the individual corpus luteum dissected from left and right ovary, respectively.
Dissection of the Ovaries: Ex Vivo Examination of the Ovaries
Sows from experiment 1 (n = 45) were slaughtered at a local abattoir at 29.8 ± 1.9 d of pregnancy and the uterus and ovaries were collected. Ovulation rate was assessed by dissection of each individual corpus luteum present on left and right ovaries (ORDIS).
In the 25 sows in which CL diameter was measured with TUS (farm 2), each individual corpus luteum was cleaned of connective tissue, their individual diameter was measured using a caliper rule and the average and SD of CL diameter were assessed. Further, each individual CL was weighed and the average and SD of CL weight per sow was calculated. Total luteal mass was calculated as the sum of all corpora lutea weights. The diameter (mm) of the 5 biggest CL in each ovary (10 per sow) was used to estimate the average CL diameter measured after dissection (DIAMDIS), and the weight of the 5 CL with the highest diameter in each ovary (10 per sow) was used to assess average CL weight (g) after dissection (WTDIS). All CL measurements were done by the same person.
Litter Characteristics
Sows from experiment 2 (n = 100) farrowed and the length of gestation, number of piglets born alive (live born), number of piglets born dead (stillborn), and number of mummified piglets were assessed. Total number of piglets born (litter size) was defined as the sum of the number of piglets born alive and dead. Piglets were weighed within 24 h after birth and from this, average piglet birth weight (total born and live born), within litter SD of piglet birth weight (total born and live born), and total litter birth weight were calculated.
Statistical Analyses
All analyses were performed using PROC MIXED in SAS version 9.3 (SAS Inst. Inc., Cary, NC).
Experiment 1.
Preliminary analyses showed that there was a farm difference in average OR assessed after dissection [least square means of ORDIS for farm 1 was 27.5 ± 1.0 and for farm 2 was 21.4 ± 0.9, P < 0.0001]. Thus, all statistical models for accuracy of CL counting included the fixed class effect of farm, and the random class effect of batch to account for possible environmental variation. Statistical models for accuracy of CL diameter measurements did not have farm in the model since the measurements were done in for a subset of sows coming from the same farm (n = 25). There was no relationship between the parity of the sows and the CL measurements done by TUS and after ovarian dissection (P > 0.05), so parity was not included in the models. In all models, fixed class effects were corrected with Bonferroni and if nonsignificant were removed from the models. Interactions were never significant and were therefore removed from the models.
First, the relationship between the ORTUS as assessed by the 2 examiners separately (E1 and E2) was investigated to check for inter-examiner agreement. For this, the continuous linear effect of ORTUS counted by E2 was assessed on ORTUS counted by E1.
Analyses on the accuracy of TUS were done using the data of E1 (n = 45). First, the difference between ORTUS and ORDIS in number of CL counted was calculated. Further, to check the accuracy of TUS in assessing OR (i.e., how does ORTUS relates with ORDIS) the continuous linear effect of ORDIS was assessed on ORTUS. To check if the accuracy of TUS was affected by ORDIS the continuous fixed effect of ORDIS was assessed on the difference between ORTUS and ORDIS.
Regarding CL diameter measurements, the difference between average CL diameter estimated based on TUS and after ovarian dissection were assessed (i.e., DIAMTUS – DIAMDIS). Further, to check the accuracy of TUS in measuring the diameter of the 10 biggest CL, the continuous fixed effect of the average diameter of the 10 biggest CL at dissection (DIAMDIS) was assessed on the average diameter of the 10 biggest CL by TUS (DIAMTUS). To check if the accuracy of TUS in measuring CL diameter was affected by ORDIS, the continuous fixed effect of ORDIS was assessed on the difference between DIAMTUS and DIAMDIS.
Also, to investigate the relationship between OR and CL size, the continuous linear and quadratic effect of ORDIS was assessed on DIAMTUS, DIAMDIS and on average CL weight after dissection (WTDIS).
Moreover, aiming to check the relationship between OR and characteristics of all CL dissected, the continuous linear and quadratic effect of ORDIS was assessed on the average and standard deviation of CL diameter and weight of all CL, and on total luteal mass.
Residuals of all models approximated normality based on skewness and kurtosis. Results are presented as the regression coefficients (β) with their standard errors (SE) for the continuous linear and quadratic fixed effects and as least square means and their SE for fixed class effects. Results are considered significant at P ≤ 0.05.
Experiment 2.
To assess the effect of sow parity on CL, gestation and litter characteristics, parity was divided into 3 categories: class 1 (parities 2 and 3, n = 27), class 2 (parities 4 and 5, n = 35), and class 3 (parities 6 to 9, n = 38) and the fixed class effect of parity (parity class 1, 2, and 3) was included in the model together with the random effect of batch.
To assess relationships between the measurements of CL done by TUS and sow and litter characteristics at birth, the continuous fixed effect of DIAMTUS, together with the fixed class effect of parity (1, 2, and 3) and the fixed class effect of litter size [class 1 (9 to 16 piglets born, n = 34); class 2 (17 to 19 piglets born, n = 36); and class 3 (20 to 26 piglets born, n = 30)] and their interaction, were assessed on litter characteristics. In all models, batch was included as a random class effect to account for possible environmental variation. If nonsignificant, the interactions and the fixed class effects were removed from the models. Residuals from all models approximated normality based on skewness and kurtosis. Results are presented as the regression coefficients (β) with their SE for continuous fixed effects and as least squares means and their SE for fixed class effects. Results are considered significant at P ≤ 0.05.
RESULTS
Experiment 1
The averages and SD of the CL, litter and sows characteristics are presented in Table 1. In experiment 1, the total number of CL (mean ± SD) assessed by TUS (ORTUS) was 23.4 ± 5.8 (ranging from 13 to 34) and the number counted after dissection of the ovaries (ORDIS) was 24.1 ± 5.3 (ranging from 13 to 37). The average diameter of the 10 biggest CL measured with TUS (DIAMTUS) was 10.3 ± 0.7 mm and after dissection (DIAMDIS) was 10.3 ± 0.6 mm. The average diameter of all CL dissected was 9.8 ± 0.7 mm with an SD of 0.8 ± 0.4 mm. The average weight of the 10 biggest CL (WTDIS) was 0.40 ± 0.07 g, and the average weight of all CL dissected was 0.38 ± 0.07g. Average total luteal mass per sow was 7.9 ± 1.5g.
Table 1.
Summary statistics of sows, corpus luteum and litter characteristics
| Variables | n | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Parity | 45 | 7.3 | 3.2 | 2 | 13 |
| TUS pregnancy age, d | 45 | 23.4 | 2.9 | 20 | 28 |
| Slaughter pregnancy age, d | 45 | 29.8 | 1.9 | 27 | 32 |
| Ovulation rate by TUS | 45 | 23.4 | 5.8 | 13 | 34 |
| Ovulation rate after dissection | 45 | 24.1 | 5.3 | 13 | 37 |
| Average CL diameter TUS1, mm | 25 | 10.3 | 0.73 | 8.4 | 11.7 |
| Average CL diameter DISS2, mm | 25 | 10.3 | 0.64 | 8.7 | 11.5 |
| Average CL weight DISS3, g | 25 | 0.40 | 0.07 | 0.22 | 0.56 |
| Total average CL diameter4, mm | 25 | 9.8 | 0.7 | 7.8 | 11.0 |
| Total average CL weight4, g | 25 | 0.38 | 0.1 | 0.20 | 0.51 |
| Total luteal mass5, g | 25 | 7.9 | 1.5 | 5.2 | 10.7 |
| Experiment 2 | |||||
| Parity | 100 | 5.0 | 1.9 | 2 | 9 |
| TUS pregnancy age, d | 100 | 23.8 | 2.4 | 21 | 29 |
| Gestational length, d | 100 | 115 | 1.7 | 111 | 120 |
| Average CL diameter TUS1, mm | 100 | 8.4 | 0.8 | 5.5 | 10.5 |
| Litter size6 | 100 | 17.9 | 3.0 | 9 | 26 |
| Number of live born | 100 | 16.3 | 2.9 | 6 | 23 |
| Number of stillborn | 100 | 1.53 | 1.8 | 0 | 9 |
| Number of mummies | 100 | 0.42 | 0.8 | 0 | 5 |
| Average piglet BW7, g | 100 | 1277 | 165 | 914 | 1618 |
| SD BW7, g | 100 | 303 | 76 | 161 | 555 |
| Average live born piglets BW7, g | 100 | 1299 | 167 | 933 | 1674 |
| SD BW live born7, g | 100 | 292 | 76 | 115 | 492 |
| Litter BW, kg | 100 | 23 | 4.0 | 15 | 30 |
1Average calculated based on the diameter of the 5 biggest corpora lutea in each ovary (10 per sow) measured by Transrectal ultrasonography (TUS).
2Average calculated based on the diameter of the 5 biggest corpora lutea in each ovary (10 per sow) measured with caliper rule after slaughter of the sows and ovarian dissection.
3Average weight of the 5 corpora lutea in each ovary (10 per sow) that had the highest diameter measured after ovarian dissection.
4Average diameter and weight calculated based on the measurement of all corpora lutea dissected from each ovary.
5Sum of the weight of all the corpora lutea dissected from the ovaries.
6Litter size is the sum of piglets born alive (live born) and stillborn piglets.
7Piglets were weighed within 24 h after birth.
Relationship between CL Characteristics after Ovarian Dissection and with TUS
Results show that there was a strong relationship between ORTUS assessed by the 2 examiners, E2 and E1 (β = 0.87 ± 0.09 CL E1/CL E2, P < 0.0001, R2 = 0.93, Fig. 2A), showing that there was inter-observer agreement in the assessment of OR using TUS.
Figure 2.
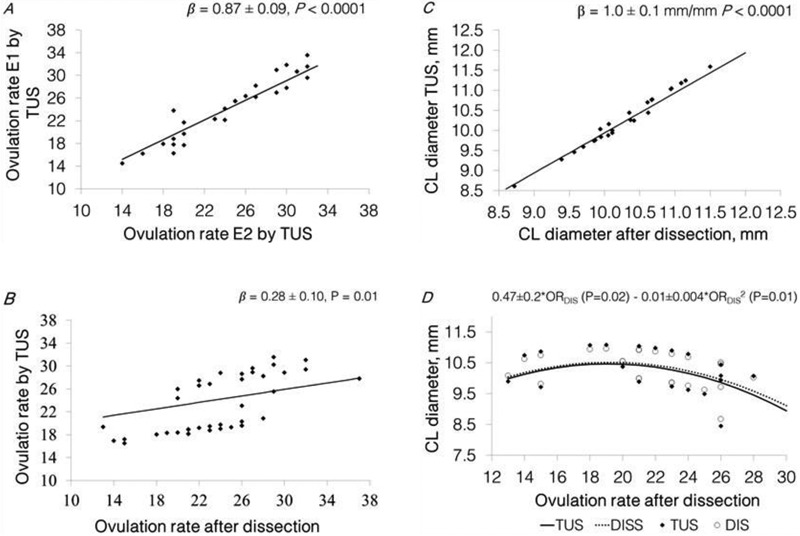
Panel A, predicted relationship between OR assessed by TUS performed by examiner 2 and the OR assessed by TUS performed by examiner 1 [(n = 29), β = 0.87 ± 0.09 CLTUS E1/CLTUS E2, P < 0.0001, farm P = 0.12]. Panel B, predicted relationship between the OR assessed by E1 by TUS and OR after dissection [β = 0.28 ± 0.01 TUS/DIS, P = 0.01, farm P = 0.0002, (n = 45)]. Panel C; predicted relationship between the average diameter of the 10 biggest CL assessed after ovarian dissection and the average diameter of the 10 biggest CL assessed by TUS [(n = 25), β = 1.0 ± 0.07 mm TUS/mm dissected, P < 0.0001]. Panel D, predicted relationship between the OR after dissection and the average CL diameter [TUS = 0.49 ± 0.19 (P = 0.02) – 0.01 ± 0.005 (P = 0.01) and DISS = 0.47 ± 0.18 (P = 0.02) – 0.01 ± 0.004 (P = 0.01)]. Statistical models included the fixed class effect of farm (n = 2, for panels a and b), and the random effect of batch (n = 6). Data points (♦) represent the predicted values estimated as the difference with the model residuals.
Differences in CL number measured with TUS and after ovarian dissection are presented in Fig. 3. The average ± SD of the difference in OR assessed by TUS and OR assessed after ovarian dissection was 0.7 ± 4.7 CL (ranging from –8 up to +12 CL difference). Results show that in 24.4% of the sows ORTUS differed from ORDIS in by 0 or 1 CL; in 28.9% of the sows the difference was 2 or 3, in 24.4% 4 or 5 and in 22.2% of the sows the difference was 6 or more. So, there was not a close relationship between ORDIS and ORTUS [β = 0.28 ± 0.01 TUS/DISS, P = 0.01, farm 1 = 28.0 ± 1.3 and farm 2 = 20.5 ± 1.2; P = 0.0002, R2 = 0.79; Fig. 2B]. Furthermore, there was a positive linear relationship between ORDIS and the difference in OR assessed by TUS and after dissection (β = 0.72 ± 0.10 difference/CL dissected, P < 0.0001, farm P = 0.0002, R2 = 0.68). So, TUS is not an accurate method to estimate OR in sows at early pregnancy, and there is an increase in the inaccuracy of TUS with an increase in OR.
Figure 3.
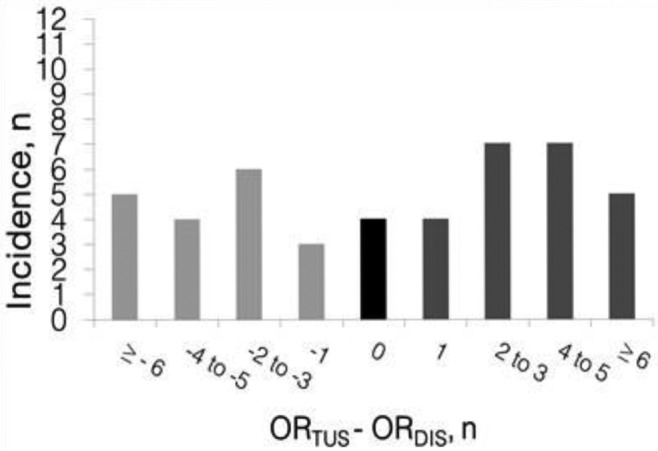
Summary of the differences between the number of corpora lutea (CL) counted with transrectal ultrasonography (ORTUS) and the number of CL counted after slaughter and dissection of the ovaries (ORDIS) in 45 multiparous sows at early pregnancy. Underestimations are shown in light gray and overestimations are shown in dark gray.
Differences in the average CL diameter measured by TUS and the average CL diameter measured after ovarian dissection (DIAMTUS – DIAMDIS) were assessed. The average ± SD of the difference between the DIAMTUS and DIAMDIS was 0.02 ± 0.20 mm (ranging from –0.36 up to 0.34 mm difference). There was a positive linear relationship between the DIAMDIS and DIAMTUS (β = 1.00 ± 0.07 mm TUS/mm DIS; P < 0.0001, R2 = 0.96; Fig. 2C). Moreover, there was no relationship between ORDIS and the difference between DIAMTUS and DIAMDIS (β = 0.005 ± 0.01 mm difference/CL dissected, R2 = 0.44; P = 0.58). So, TUS is an accurate method to estimate the average diameter of the 10 biggest CL in sows in early pregnancy and the accuracy of the measurements done by TUS is not related with an increase in OR.
Regarding the relationship between ORDIS and the average CL diameter and weight of the 10 biggest CL, there was a quadratic relationship between ORDIS and the DIAMTUS [0.49 ± 0.2 × ORDIS (P = 0.02)– 0.01 ± 0.0004 × ORDIS2 (P = 0.01), R2 = 0.76; Fig. 2D] and DIAMDIS [0.47 ± 0.2 × ORDIS (P = 0.02) – 0.01 ± 0.0004 × ORDIS2 (P = 0.01), R2 = 0.69; Fig. 2D], which shows a maximum average diameter of the 10 biggest CL of 10.5 mm at 19 ovulations for measurements done with TUS and also after dissection (DIAMTUS and DIAMDIS, respectively). Also, there was a negative linear relationship between ORDIS and the average weight of the 10 biggest CL (β = - 0.01 ± 0.003 g/CL dissected, R2 = 0.51; P = 0.01). So, the average diameter and weight of the 10 biggest CL is lower in sows with a higher OR.
There was a quadratic relationship between ORDIS and the average diameter of all CL measured after dissection [0.43 ± 0.2 × ORDIS (P = 0.05)– 0.01 ± 0.01 × ORDIS (P = 0.03)], which shows a maximum average CL diameter of 10.1 mm at 18 ovulations. There was a negative linear relationship between ORDIS and total average CL weight (β = - 0.01 ± 0.03 g/CL dissected, P = 0.002). However, ORDIS was not related with the SD in CL diameter (P = 0.85) and with the SD in CL weight (P = 0.12). Furthermore, there was a positive linear relationship between ORDIS and total luteal mass (β = + 0.19 ± 0.1 g/CL dissected, P = 0.003). So, the total average diameter and weight of the CL is lower in sows with a higher OR, but there is no increase in variation in CL size with the increase in OR. Also, the total luteal mass increases linearly with the increase in OR, despite the decrease in average CL weight.
Experiment 2
The averages and SD of sows, CL and litter characteristics are presented in Table 1. The average CL diameter measured with TUS was 8.4 ± 0.8 mm, ranging from 5.5 to 10.5 mm. The average total number of piglets born was 17.9 ± 3.0, and 16.3 ± 2.9 were born alive. The average birth weight (BW) of the total piglets born was 1,277 ± 165 g and the average within litter BW variation was 303 ± 76 g, the average BW of the piglets born alive was 1,299 ± 167 g and average within litter BW variation was 292 ± 76 g.
Effect of Parity on Gestation Length, CL and Litter Characteristics
Parity did not affect the gestation length, the average diameter of the 10 biggest CL measured by TUS (DIAMTUS), litter size, average and SD piglet birth weight or litter weight (P > 0.05). Sows in parity 6 up to 9 had a higher (2.13 ± 0.3, P = 0.02) number of stillborn piglets than sows in parity 2 up to 3 (0.86 ± 0.4) and 4 up to 5 (1.34 ± 0.3).
Relationship between CL Diameter and Litter Characteristics
Relationships between DIAMTUS and litter characteristics are presented in Table 2. There was a positive linear relationship between DIAMTUS and average piglet BW [(β = + 37.6 ± 17.8 g/mm; P = 0.04, Fig. 4A) + c value dependent on litter size (P < 0.0001): 9 to 16 piglets = 1367 ± 25 g, 17 to 19 piglets = 1,280 ± 24 g, and 20 to 26 piglets = 1,171 ± 26 g]. There was also a positive linear relationship between DIAMTUS and the SD in BW of the total born piglets (β = 24.3 ± 10.4 g/mm; P = 0.02, Fig. 4B) and the SD in BW of the piglets born alive (β = 27.3 ± 10.5 g/mm, P = 0.01). There were no interactions between DIAMTUS, litter size classes and parity classes. So, an increase in the average CL diameter in early pregnancy is related with an increase in average piglet birth weight and in within litter birth weight variation.
Table 2.
Relationship between average corpora lutea diameter measured by transrectal ultrasonography (DIAMTUS) and litter characteristics at birth
| DIAMTUS1, mm | Litter size | Parity | ||
|---|---|---|---|---|
| Dependent variables | β (SEM) | P-value | P-value | P-value |
| Gestational length, d | 0.48 (0.26) | 0.06 | ns2 | ns |
| Litter size3 | –0.098 (0.43) | 0.82 | – | ns |
| Number of live born | –0.084 (0.43) | 0.84 | – | ns |
| Number of stillborn4 | –0.015 (0.22) | 0.95 | 0.02 | 0.01 |
| Number of mummies | –0.013 (0.10) | 0.90 | ns | ns |
| Average piglet BW5, g | 37.57 (17.84) | 0.04 | < 0.0001 | ns |
| SD BW5, g | 24.25 (10.37) | 0.02 | ns | ns |
| Average live born piglets BW5, g | 32.98 (18.41) | 0.08 | < 0.0001 | ns |
| SD BW live born5, g | 27.33 (10.52) | 0.01 | ns | ns |
| Litter BW, kg | 0.81 (0.47) | 0.09 | < 0.0001 | ns |
1Average calculated based on the diameter of the 5 biggest corpora lutea in each ovary (10 per sow) measured by TUS.
2ns = P > 0.05.
3Litter size is the sum of piglets born alive (live born) and stillborn piglets.
4Interaction between parity (PC) and litter size (LS) classes (P = 0.002): PC1 × LS1 (n = 9): 0.67 ± 0.55b; PC2 × LS1 (n = 12): 0.48 ± 0.48b; PC3 × LS1 (n = 6): 1.54 ± 0.47ab; PC2 × LS2 (n = 12): 1.02 ± 0.49b; PC2 × LS2 (n = 12): 2.01 ± 0.49b; PC3 × LS2 (n = 11): 0.92 ± 0.49ab; PC1 × LS3 (n = 13): 0.81 ± 0.66b; PC2 × LS3 (n = 12): 1.58 ± 0.51ab; PC3 × LS3 (n = 13): 3.83 ± 0.47a.
5Piglets were weighed within 24 h after birth (BW).
Figure 4.
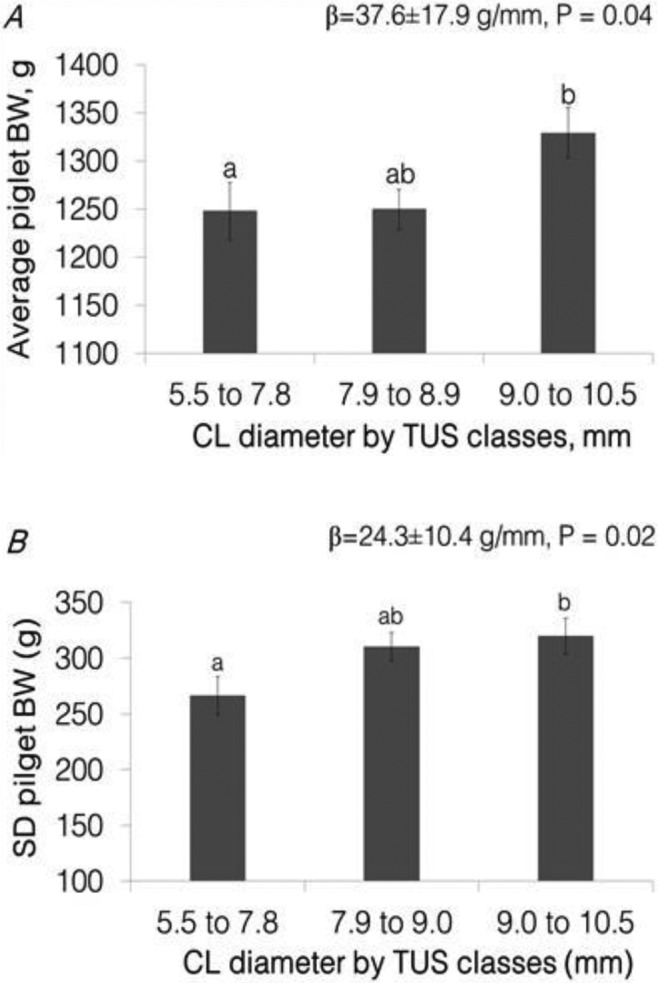
Panel A: estimated least square means for the effect of average CL diameter classes [5.5 to 7.8 mm (n = 23); 7.9 to 8.9 mm (n = 47); and 9.0 to 10.5 mm (n = 30)] on BW of total piglets born [P = 0.04; litter size class P < 0.0001]. Panel B: estimated least square means for the effect of CL diameter classes on standard deviation (SD) of BW of the total piglets born (P = 0.02). Statistical models included the fixed class effect of litter size [LS, 8 to 18 piglets born (n = 35); 17 to 19 piglets born (n = 36) and 20 to 26 piglets born (n = 30)] and its interactions, which were excluded from the models when not significant (P > 0.05). Significant differences between classes are indicated by letters above the columns and the error bars indicate the SE of the estimates.
DISCUSSION
This is, to our knowledge, the first study that investigated the accuracy of transrectal ultrasonography to assess CL number and diameter in sows in early pregnancy and investigated the relationship between CL diameter measured by transrectal ultrasonography and litter characteristics at birth.
Transrectal ultrasonography did not provide an accurate estimation of the number of corpora lutea (OR) in sows in early pregnancy. The difference between the OR assessed after ovarian dissection and with TUS was on average 0.7 ± 4.7 CL, ranging from an underestimation of 8 CL up to an overestimation of 12 CL. Moreover, only in 24.4% of the sows, the difference between OR assessed with TUS and after ovarian dissection was of only 1 CL, with 46.7% of the TUS estimations differing with more than 4 CL. This inaccuracy occurred almost equally due to under and over estimations of the number of CL assessed after dissection of the ovaries (33.3 and 42.2%, respectively). Underestimations are probably related with the difficulty in visualizing all individual CL. Ultrasound machines uses high frequency sound waves and their echo to produce images (Pierson et al., 1988) and different tissues have different abilities to reflect the sound waves (Pierson et al., 1988). Thus, characteristics of a tissue determine the proportion of the sound wave that will be reflected, which will then be represented on the ultrasound image display by dots of different shades of gray, varying from black to white. Liquids, like follicular fluid, do not reflect sound waves (non-echogenic) and therefore the image appears as black on the screen, in contrast with the surrounding ovarian tissue, that reflects part of the sound waves (echogenic) and can be seen as different shades of gray (Pierson et al., 1988). This contrast facilitates visualization of individual follicles and explains the high accuracy of transrectal ultrasonography in assessing the number of pre-ovulatory follicles in sows. Soede et al. (1992) described a difference of only 0.4 ± 1.8 between the number of follicles counted by transrectal ultrasonography and the number of CL counted after slaughter of the sows (18.6 ± 3.5). Also, Bolarin et al. (2009) observed a significant correlation between the number of pre-ovulatory follicles (6 to 10 mm) counted per ovary with transrectal ultrasonography and with laparoscopy (r = 0.98, P < 0.001). Corpora lutea, however, are tissue filled glands and are echogenic, surrounded by the also echogenic tissue of the ovarian stroma, which makes visualization of each individual CL more difficult. Moreover, despite the fact that transrectal ultrasonography is believed to provide clearer images of the ovaries due to less interference of intestinal tissues (Kauffold and Althouse, 2007), is still possible that the ovaries were only partially visible due to interference of the intestinal and uterine tissue of the pregnant sows.
Overestimations, on the other hand might be explained by counting CL from part of an ovary 2 times, which might happen due to the proximity of the 2 ovaries during the examination and the difficulty in distinguishing between the 2 separate ovaries. The lack of accuracy of transrectal ultrasonography in assessing the number of CL in pregnant sows might also be related with the high number of CL present in each ovary (high ovulation rate), where crowding of the ovaries makes it more difficult to differentiate the CL. Gonzalez-Añover et al. (2009) investigated the accuracy of transrectal ultrasonography in accessing the number of CL in Iberian sows in the mid luteal phase (average ovulation rate of 6.0 ± 1.3), and achieved accuracy close to 100% in ovaries with 5 CL or less, which decreased with the increase in number of CL present per ovary. A decrease in accuracy with the increase in OR was also observed by Soede et al. (1992) and Bolarin et al. (2009) when counting the number of pre-ovulatory follicles. In the present study (average OR of 24.1 ± 5.3) there was also a decrease in accuracy of TUS (difference between OR counted by TUS and after slaughter) with the increase in OR after ovarian dissection. This could be related with the decrease in CL size with the increase in ovulation rate. Average CL diameter in sows with 13 up to 22 ovulations was predicted to be 10.3 mm, decreasing to 8.7 mm in sows with more than 23 ovulations. The same is true for the average CL weight, which can be 0.13g smaller in sows with more than 23 ovulations in comparison with sows with 13 up to 22 ovulations. A decrease in average CL weight (and thus size, this study) with the increase in ovulation rate was also observed in gilts at 35 d of pregnancy (Da Silva et al., 2017). This indicates that with a higher OR, not only the ovaries are more crowded, but also individual CL are smaller, which might increase the difficulty in visualization of the individual CL, thus decreasing the accuracy of counting CL with TUS. The reason for the decrease in CL size with the increase in ovulation rate is not known. During follicular growth, recruited follicles respond to FSH by increasing the production of estradiol-17β (Britt and Findlay, 2002; Drummond, 2006). The increase in estradiol-17β (E2) production leads to an increase in granulosa cell number and therefore to further follicular development (Drummond and Findlay, 1999). So, together with the increase in E2 production, follicles increase in size. E2 production increases until it reaches a certain systemic threshold concentration that triggers a pre-ovulatory GnRH/LH surge that subsequently triggers ovulation (Drummond and Findlay, 1999; Drummond, 2006). With more follicles recruited from the pool (i.e., higher OR), and therefore more follicles producing E2, the threshold of E2 necessary to trigger ovulation might occur when follicles are of smaller size than in sows with a lower OR. Indeed, Knox et al. (2003) observed that systemic E2 levels were the same at days –1 to +1 relative to the LH peak (d 0) in gilts selected for high OR (OR 18.8 ± 0.4, E2 37.9 ± 3.4 ng/mL) and in gilts of the control line (OR 14.3 ± 0.6, E2 44.7 ± 3.6 ng/mL), indicating that the threshold of E2 that precedes ovulation is independent of OR. Soede et al. (1998) observed that the average volume of pre-ovulatory follicles at ovulation per sow was significantly correlated with the average CL weight at 5 d of pregnancy (r = 0.28, P < 0.01), and Wientjes et al. (2012) observed that each mm increase in follicle diameter at ovulation was related with 1.23 mm increase in CL diameter (P = 0.03) in multiparous sows at 10 d of pregnancy. Thus, high OR sows may have smaller follicles at ovulation that develop into smaller CL.
We also investigated the validity of TUS to measure CL diameter in sows. Results show that TUS is an accurate method to assess CL diameter in sows at 3 to 4 wk of pregnancy. There was a strong relationship between the CL diameter measured by transrectal ultrasonography and the CL diameter measured after dissection of the ovaries. Further, we investigated the relationship between average CL diameter assessed by transrectal ultrasonography in early pregnancy and litter characteristics at birth and observed that there is an increase in average piglet birth weight with an increase in average diameter of the 10 biggest CL. This might indicate that sows with a higher average CL diameters ovulated oocytes of better quality, that developed into embryos with higher growth potential and consequently into piglets with higher birth weight. Indeed, in a recent study with 390 gilts slaughtered at 35 d of pregnancy we found that there is an increase of 2.3 g in the weight of the vital embryos per gram of increase in average CL weight [P = 0.001; C. L. A. Da Silva, unpublished results]. Heavier embryos at d 35 of pregnancy might develop into heavier piglets at birth. Larger/heavier CL develop from larger follicles at ovulation, as discussed above. Larger follicles at ovulation are known to release oocytes with superior quality due to a more advanced maturational status (Hunter, 2000; Gandolfi et al., 2005), which might lead to the development of embryos with higher growth potential (Krisher, 2004). At d 11 and 12 of pregnancy, pig conceptuses transition from spherical to tubular and filamentous blastocysts in a process called elongation (Geisert et al., 1982a; Geisert et al., 1982b). Timing of rapid conceptus elongation is established by the conceptus (Geisert et al., 2014) and more advanced embryos [derived from the more developed oocytes, Pope et al. (1990)] elongate earlier and will have an increased uterine implantation length (Geisert et al., 1982a). The uterine implantation length is related with the placental length of vital embryos at 35 d of pregnancy in sows [β = 0.98 ± 0.14 cm/cm, P < 0.0001. C. L. A. Da Silva, unpublished results], which will possibly favor further fetal development and consequently piglet birth weight.
Corpora lutea produce progesterone, which is of primary importance for maintenance of the pregnancy in the pig (Spencer et al., 2004). So, it could be hypothesized that higher CL size in pregnant sows is related with higher progesterone production favoring embryonic growth and piglet birth weight. However, systemic progesterone levels in 238 gilts at 35 d of pregnancy (C. L. A Da Silva, unpublished results), were not related with average CL weight (P = 0.69). It could also be possible that average CL weight and piglet birth weight have a common origin in early pregnancy. Corpora lutea regression occurs on d 15 to 16 of the oestrus cycle due to the increase in pulsatile endometrial secretion of prostaglandin F2-ɑ (Moeljono et al., 1976). Thus, pregnancy recognition requires the development and maintenance of CL beyond the luteal phase, and is a result of oestrogens (mainly estradiol-17β, E2) secretion by the conceptuses on d 11 and 12, followed by a second peak between d 15 and 25 through 30 of pregnancy (Geisert et al., 1990). Conceptuses E2 increases luteal LH receptor concentration, and together with Il-β1, favors the production of luteoprotective PGE2, which stimulates the expression of Vascular Endothelial Growth Factor (VEGF) in luteal cells, increasing luteal permeability and delivery of cholesterol to the luteal cells (Ziecik et al., 2011), which might favor CL growth. So, conceptus development at the time of elongation and maternal recognition of pregnancy may influence luteal vascularization (through the effects of PGE2 and VEGF), and thereby increase CL size. However, further investigations are needed to understand the mechanisms underlying the relationship between CL size, embryonic growth and piglets birth weight.
An increase in average CL diameter was not only related with average piglet birth weight as discussed above, but also with an increase in within litter variation in piglet birth weight. Within litter piglet birth weight variation increases with the increase in litter size (Milligan et al., 2002; Quiniou et al., 2002; Wolf et al., 2008), and is seen as a consequence of uterine crowding on placental development in the early post implantation period (Foxcroft et al., 2006). However, in the current study, we did not observe a higher variation in piglet birth weight in bigger litters. Before uterine implantation, an increase in variation in the length of implantation in the uterus will contribute to variation in timing and capacity to establish an adequate surface area for placentation, thus increasing variation in fetal growth and in birth weight (Yuan et al., 2015). But it is also possible that this increase in variation is caused by a limitation in uterine capacity imposed by the increase competition during fetal growth, i.e., an increase in competition for blood flow and nutrient uptake between littermates, which might also lead to variation in fetal growth and in piglet birth weight (Ford et al., 2002).
In conclusion, transrectal ultrasonography is not a valid method to assess OR in sows in early pregnancy, but it is a valid method to assess CL diameter. Also, a higher average CL diameter measured by transrectal ultrasonography in sows around 25 d of gestation was related with a higher average piglet birth weight and with a higher within litter variation in piglet birth weight. This might be related with the influence of follicular/oocyte quality in embryonic development. However, the mechanisms leading to increase in birth weight and in birth weight variation needs further investigation.
Supplementary Material
APPENDIX 1: LIST OF ABBREVIATIONS
| Abbreviations | Description |
|---|---|
| TUS | Transrectal ultrasonography |
| OR | Ovulation rate, i.e. the total number of CL present in both ovaries |
| CL | Corpora lutea |
| ORTUS | Total number of corpora lutea counted on both ovaries with transrectal ultrasonography |
| E1 | Transrectal ultrasonography examiner one |
| E2 | Transrectal ultrasonography examiner two |
| DIAMTUS | The average diameter of the 10 biggest corpora lutea measured by transrectal ultrasonography in a sow |
| ORDIS | Total number of corpora lutea counted after slaughter and dissection of the ovaries for individual corpora lutea |
| DIAMDIS | The average diameter of the 10 biggest corpora lutea measured with a caliper rule after slaughter and dissection of the ovaries for individual corpora lutea |
| WTDIS | The average weight of the 10 biggest corpora lutea measured after slaughter and dissection of the ovaries for individual corpora lutea |
| LS | Litter size, i.e. the sum of the number of piglets born alive and dead |
| BW | Piglet birth weight |
LITERATURE CITED
- Bolarin A., Vazquez J. M., Parrilla I., Vazquez J. L., Martinez E. A., Roca J. 2009. Validation of trans-rectal ultrasonography for counting preovulatory follicles in weaned sows. Anim. Reprod. Sci. 113:137–142. doi: 10.1016/j.anireprosci.2008.07.002 [DOI] [PubMed] [Google Scholar]
- Britt K. L., Findlay J. K. 2002. Estrogen actions in the ovary revisited. J. Endocrinol 175:269–76. doi: 10.1677/joe.0.1750269 [DOI] [PubMed] [Google Scholar]
- Da Silva C. L. A., van den Brand H., Laurenssen B. F. A., Broekhuijse M. L. W. J., Knol E. F., Kemp B., Soede N. M. 2016. Relationships between ovulation rate and embryonic and placental characteristics in multiparous sows at 35 days of pregnancy. Animal 10:1192–1199. doi: 10.1017/S175173111600015X [DOI] [PubMed] [Google Scholar]
- Da Silva C. L. A., Broekhuijse M. L. W. J., Laurenssen B. F. A., Mulder H. A., Knol E. F., Kemp B., Soede N. M. 2017. Relationship between ovulation rate and embryonic characteristics in gilts at 35 d of pregnancy. J. Anim. Sci.doi: 10.2527/jas.2017.1577 [DOI] [PubMed] [Google Scholar]
- Ding J., Foxcroft G. R. 1994. Conditioned Media Produced by Follicular Shells of Different Maturity Affect Maturation of Pig Oocytes. Biol. Reprod. 50:1377–1384. doi: 10.1095/biolreprod50.6.1377 [DOI] [PubMed] [Google Scholar]
- Drummond A. E. 2006. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 4:16. doi: 10.1186/1477-7827-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. E., Findlay J. K. 1999. The role of estrogen in folliculogenesis. Mol. Cell. Endocrinol. 151:57–64. doi: 10.1016/S0303-7207(99)00038-6 [DOI] [PubMed] [Google Scholar]
- Ford S. P., Vonnahme K. A., Wilson M. E. 2002. Uterine capacity in the pig reflects a combination of uterine environment and conceptus genotype effects. J. Anim. Sci. 80(E-Suppl_1):E66–E73doi: 10.2527/animalsci2002.0021881200800ES10010x [DOI] [Google Scholar]
- Foxcroft G. R., Dixon W. T., Novak S., Putman C. T., Town S. C., Vinsky M. D. 2006. The biological basis for prenatal programming of postnatal performance in pigs. J. Anim. Sci. 84(Suppl):E105–E112. doi: 10.2527/2006.8413_supplE105x [DOI] [PubMed] [Google Scholar]
- Gandolfi F., Brevini T. A., Cillo F., Antonini S. 2005. Cellular and molecular mechanisms regulating oocyte quality and the relevance for farm animal reproductive efficiency. Rev. Sci. Tech. 24:413–423. doi: 10.20506/rst.24.1.1580 [DOI] [PubMed] [Google Scholar]
- Geisert R. D., Renegar R. H., Thatcher W. W., Roberts R. M., Bazer F. W. 1982a. Establishment of pregnancy in the pig: I. Interrelationships between preimplantation development of the pig blastocyst and uterine endometrial secretions. Biol. Reprod. 27:925–939. doi: 10.1095/biolreprod27.4.925 [DOI] [PubMed] [Google Scholar]
- Geisert R. D., Brookbank J. W., Roberts R. M., Bazer F. W. 1982b. Establishment of pregnancy in the pig: II. Cellular remodeling of the porcine blastocyst during elongation on day 12 of pregnancy. Biol. Reprod. 27:941–955. doi: 10.1095/biolreprod27.4.941 [DOI] [PubMed] [Google Scholar]
- Geisert R. D., Lucy M. C., Whyte J. J., Ross J. W., Mathew D. J. 2014. Cytokines from the pig conceptus: Roles in conceptus development in pigs. J. Anim. Sci. Biotechnol. 5:51. doi: 10.1186/2049-1891-5-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisert R. D., Zavy M. T., Moffatt R. J., Blair R. M., Yellin T. 1990. Embryonic steroids and the establishment of pregnancy in pigs. J. Reprod. Fertil. Suppl. 40:293–305. [PubMed] [Google Scholar]
- Gonzalez-Añover P., Encinas T., Gomez-Izquierdo E., Sanz E., Sanchez-Sanchez R., Gonzalez-Bulnes A. 2009. Accuracy of in vivo and ex vivo ultrasonographic evaluation of ovarian follicles and corpora lutea in sows. Theriogenology 71:1433–1439. doi: 10.1016/j.theriogenology.2009.01.020 [DOI] [PubMed] [Google Scholar]
- Hazeleger W., Soede N. M., Kemp B. 2005. The effect of feeding strategy during the pre-follicular phase on subsequent follicular development in the pig. Domest. Anim. Endocrinol. 29:362–370. doi: 10.1016/j.domaniend.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Hunter M. G. 2000. Oocyte maturation and ovum quality in pigs. Rev. Reprod. 5:122–130. doi: 10.1530/ror.0.0050122 [DOI] [PubMed] [Google Scholar]
- Kauffold J., Althouse G. C. 2007. An update on the use of B-mode ultrasonography in female pig reproduction. Theriogenology 67:901–911. doi: 10.1016/j.theriogenology.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Knox R. V., Vatzias G., Naber C. H., Zimmerman D. R. 2003. Plasma gonadotropins and ovarian hormones during the estrous cycle in high compared to low ovulation rate gilts. J. Anim. Sci. 81:249–260. doi: 10.2527/2003.811249x [DOI] [PubMed] [Google Scholar]
- Krisher R. L. 2004. The effect of oocyte quality on development. J. Anim Sci. 82 E-Suppl, E14–23 doi:10.2527/2004.8213_supplE14x [DOI] [PubMed] [Google Scholar]
- Lucy M. C., Liu J., Boyd C. K., Bracken C. J. 2001. Ovarian follicular growth in sows. Reprod. Suppl. 58:31–45. [PubMed] [Google Scholar]
- Madej A., Lang A., Brandt Y., Kindahl H., Madsen M. T., Einarsson S. 2005. Factors regulating ovarian function in pigs. Domest. Anim. Endocrinol. 29:347–361. doi: 10.1016/j.domaniend.2005.02.030 [DOI] [PubMed] [Google Scholar]
- Milligan B. N., Fraser D., Kramer D. L. 2002. Within-litter birth weight variation in the domestic pig and its relation to pre-weaning survival, weight gain, and variation in weaning weights. Livest. Prod. Sci. 76:181–191. doi: 10.1016/S0301-6226(02)00012-X [DOI] [Google Scholar]
- Moeljono M. P., Bazer F. W., Thatcher W. W. 1976. A study of prostaglandin F2ɑ as the luteolysin in swine: I. Effect of prostaglandin F2ɑ in hysterectomized gilts. Prostaglandins 11:737–743. doi: 10.1016/0090-6980(76)90073-3 [DOI] [PubMed] [Google Scholar]
- Pierson R. A., Kastelic J. P., Ginther O. J. 1988. Basic principles and techniques for transrectal ultrasonography in cattle and horses. Theriogenology 29:3–20. doi: 10.1016/0093-691X(88)90028-3 [DOI] [Google Scholar]
- Pope W. F., Xie S., Broermann D. M., Nephew K. P. 1990. Causes and consequences of early embryonic diversity in pigs. J. Reprod. Fertil. Suppl. 40:251–260. [PubMed] [Google Scholar]
- Quiniou N., Dagorn J., Gaudré D. 2002. Variation of piglets' birth weight and consequences on subsequent performance. Livest. Prod. Sci. 78:63–70. doi: 10.1016/S0301-6226(02)00181-1 [DOI] [Google Scholar]
- Spencer T. E., Johnson G. A., Burghardt R. C., Bazer F. W. 2004. Progesterone and Placental Hormone Actions on the Uterus: Insights from Domestic Animals. Biol. Reprod. 71:2–10. doi: 10.1095/biolreprod.103.024133 [DOI] [PubMed] [Google Scholar]
- Soede N. M., Noordhuizen J. P. T. M., Kemp B. 1992. The duration of ovulation in pigs, studied by transrectal ultrasonography, is not related to early embryonic diversity. Theriogenology 38:653–666. doi: 10.1016/0093-691X(92)90028-P [DOI] [PubMed] [Google Scholar]
- Soede N. M., Hazeleger W., Kemp B. 1998. Follicle Size and the Process of Ovulation in Sows as Studied with Ultrasound. Reprod. Domest. Anim. 33:239–244. doi: 10.1111/j.1439-0531.1998.tb01350.x [DOI] [Google Scholar]
- Town S. C., Patterson J. L., Pereira C. Z., Gourley G., Foxcroft G. R. 2005. Embryonic and fetal development in a commercial dam-line genotype. Anim. Reprod. Sci. 85:301–316. doi: 10.1016/j.anireprosci.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Vonnahme K. A., Wilson M. E., Foxcroft G. R., Ford S. P. 2002. Impacts on conceptus survival in a commercial swine herd. J. Anim. Sci. 80:553–559. doi: 10.2527/2002.803553x [DOI] [PubMed] [Google Scholar]
- Wientjes J. G. M., Soede N. M., van den Brand H., Kemp B. 2012. Nutritionally Induced Relationships Between Insulin Levels During the Weaning-to-Ovulation Interval and Reproductive Characteristics in Multiparous Sows: II. Luteal Development, Progesterone and Conceptus Development and Uniformity. Reprod. Domest. Anim. 47:62–68. doi: 10.1111/j.1439-0531.2011.01802.x [DOI] [PubMed] [Google Scholar]
- Wolf J., Žáková E., Groeneveld E. 2008. Within-litter variation of birth weight in hyperprolific Czech Large White sows and its relation to litter size traits, stillborn piglets and losses until weaning. Livest. Sci. 115, 195–205. doi: 10.1016/j.livsci.2007.07.009 [DOI] [Google Scholar]
- Yuan T.-l., Zhu Y.-h., Shi M., Li T.-t., Li N., Wu G.-y., Bazer F. W., Zang J.-j., Wang F.-l., Wang J.-j. 2015. Within-litter variation in birth weight: Impact of nutritional status in the sow. J. Zhejiang Univ. Sci. B 16:417–435. doi: 10.1631/jzus.B1500010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziecik A. J., Waclawik A., Kaczmarek M. M., Blitek A., Jalali B. M., Andronowska A. 2011. Mechanisms for the Establishment of Pregnancy in the Pig. Reprod. Domest. Anim. 46:31–41. doi: 10.1111/j.1439-0531.2011.01843.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


