To the Editor:
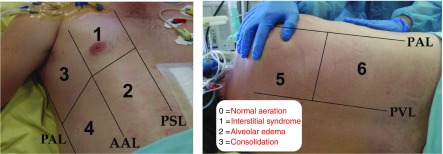
Bedside lung ultrasound is widely used in critically ill and emergency patients (1). Its role in pulmonary imaging was recently reviewed (2). A lung ultrasound score (LUS) based on examination of 12 regions of interest has been proposed to assess lung aeration changes after various therapeutic interventions in mechanically ventilated patients (3, 4). As shown in Figure 1, the LUS is based on the regional aeration of each examined region, which is graded between 0 and 3 depending on the degree of aeration loss. The LUS is a semiquantitative assessment of pulmonary aeration loss and can vary between 0 and 36.
Figure 1.
Lung ultrasound score (LUS) assessment. Six lung regions of interest (numbered in the figure), delineated by a parasternal line, anterior axillary line, posterior axillary line, and paravertebral line, are examined on each side. Each lung region is carefully examined in the longitudinal plane, and each intercostal space present in the region is examined in the transversal plane. The worst ultrasound pattern characterizes the region (regional LUS) using the following grading: 0 = normal aeration; 1 = moderate loss of aeration (interstitial syndrome, defined by multiple spaced B lines, or localized pulmonary edema, defined by coalescent B lines in less than 50% of the intercostal space examined in the transversal plane, or subpleural consolidations); 2 = severe loss of aeration (alveolar edema, defined by diffused coalescent B lines occupying the whole intercostal space); and 3 = complete loss of lung aeration (lung consolidation defined as a tissue pattern with or without air bronchogram). The LUS is calculated as the sum of the 12 regional scores. AAL = anterior axillary line; PAL = posterior axillary line; PSL = parasternal line; PVL = paravertebral line.
In anesthetized patients scheduled for abdominal surgery, lung ultrasound detects intraoperative atelectasis and the LUS correlates with perioperative oxygenation impairment (5). In patients with acute respiratory distress syndrome, the LUS is correlated with disease severity and predicts mortality (6). It provides a comprehensive monitoring of regional lung aeration changes resulting from prone positioning (7), fluid loading (4), positive end-expiratory pressure (8), and drainage of large pleural effusions (9). In ventilated critically ill patients with ventilator-associated pneumonia, a rapid decrease in the LUS indicates successful antimicrobial therapy–induced lung reaeration, whereas an increase in the LUS indicates antibiotic failure (10). During weaning from mechanical ventilation, an LUS >13 measured at the end of a clinically successful spontaneous breathing trial is predictive of extubation failure (3).
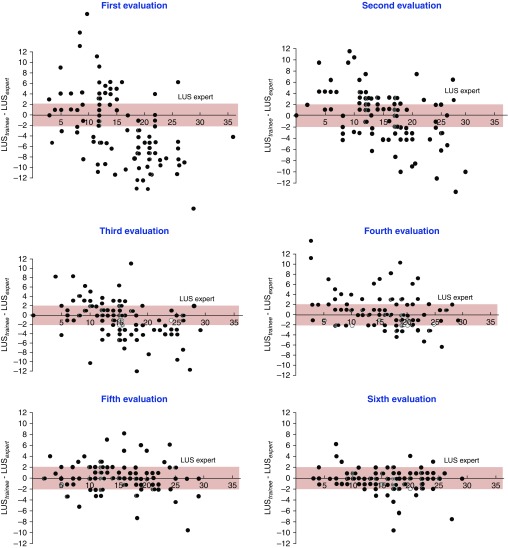
Despite increased interest in the LUS, training methods to acquire the appropriate skills for LUS measurement vary among centers and are not codified. Based on clinical experience accumulated over 10 years of resident training, including the acquisition of skills in lung ultrasound, we hypothesized that 25 LUS determinations supervised by experts would be enough for trainees without expertise in lung ultrasound to appropriately assess the LUS. A multicenter, prospective, and educational study focusing on the acquisition of basic skills for bedside lung ultrasound was conducted in 10 ICUs in Brazil, China, France, and Uruguay. The training course started with a 2-hour video lecture. First, ultrasound patterns characterizing normal aeration, moderate aeration loss (interstitial syndrome, localized alveolar edema, and subpleural consolidations), severe aeration loss (diffuse alveolar edema), and complete aeration loss (consolidation) were described. Second, the method for assessing the LUS was carefully described. One of the objectives of the training program was to reach an agreement in LUSs between trainees and experts. Each trainee had to perform 25 bedside determinations of the LUS supervised by an expert. The experts who participated in the training protocol were staff members in critical care or emergency medicine with at least a 2-year daily lung ultrasound practice. After every five supervised lung ultrasound examinations, the trainee and the expert assessed the LUS in the same patient separately. Concordance was considered as clinically acceptable when the LUS assessment did not differ by more than 2 points between trainees and experts. A total of 610 comparative LUS measurements were performed by 100 trainees and 18 experts in 233 mechanically ventilated and 137 spontaneously breathing critically ill patients. As shown in Figure 2, concordance between trainees and experts was obtained on the sixth evaluation.
Figure 2.
Difference between lung ultrasound scores (LUSs) measured by trainees and experts over six successive evaluations. The first evaluation was performed 2 hours after a lecture describing the method for measuring the LUS. Further evaluations were each separated by five ultrasound examinations performed by the trainee and supervised by the expert. The pink zone indicates the limit of agreement between trainees and experts.
The median (interquartile range) duration of training was 51 (23–69) days. At the end of the training, the median time required to measure LUS was 8 (3–14) minutes for experts and 10 (4–17) minutes for trainees.
This study shows that residents and senior physicians without expertise in lung ultrasound can acquire the skills required to measure the LUS after 25 supervised measurements. The training should include appropriate recognition of normal aeration, interstitial syndrome, alveolar edema, and lung consolidation—all of which have ultrasound patterns that are necessary to calculate the LUS. Two issues that could affect accurate determination of the LUS deserve specific comments. Alveolar edema, characterized by the presence of coalescent B lines, can remain localized, as in acute respiratory distress syndrome, or be diffuse, as in cardiogenic pulmonary edema. When an examined region is characterized by “focal” alveolar edema, corresponding to a “ground-glass area” on lung computed tomography, the loss of lung aeration is moderate, and the region should be graded 1. When the examined region is characterized by diffuse alveolar edema, the loss of lung aeration is severe, and the region should be graded 2. As recently recommended (11), when coalescent B lines occupy less than 50% of the intercostal space in the transversal plane, the grade should be 1, and when coalescent B lines occupy more than 50% of the intercostal space, the grade should be 2. Systematically grading “2” in the presence of coalescent B lines without considering their extension could lead to overestimation of the LUS and aeration loss. The same reasoning also applies for subpleural consolidations that characterize ventilator-associated pneumonia (12). These small subpleural consolidations, representative of foci of bronchopneumonia, have dimensions varying between 5 and 15 mm, are limited by spaced or coalescent B lines, and are associated with moderate loss of lung aeration (13). For this reason, regions characterized by subpleural consolidations should be graded 1 and not 3.
In conclusion, measurement of the LUS as a tool for monitoring lung aeration in critically ill patients requires a short and easy-to-implement training program based on 25 ultrasound examinations supervised by a physician with expertise in bedside lung ultrasound.
Acknowledgments
Acknowledgment
The authors thank Professor Pascaline Faure, Ph.D. (Director of the Medical English Department, Pierre and Marie Curie School of Medicine, Paris 6, Sorbonne University, Paris, France) for reviewing the English in the article.
The APECHO (Apprentissage de l’ECHOgraphie pulmonaire) Study Group members, listed according to their institution, include Charlotte Arbelot, Jean-Jacques Rouby, Hélène Brisson, Romain Deransy, Corinne Vezinet, Pierre Garçon, Nabil El Hadj Kacem, Denis Lemesle, Antoine Monsel, Qin Lu, and Olivier Langeron (Multidisciplinary Intensive Care Unit, Department of Anesthesiology and Critical Care Medicine, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Sorbonne University, Paris, France); Frédérick Gay (Department of Parasitology-Mycology, La Pitié-Salpêtrière Hospital, Assistance Publique Hôpitaux de Paris, Sorbonne University, Paris, France); Bruno Lucena, Luiz Malbouisson, and Maria José Carvalho Carmona (Surgical and Trauma Intensive Care Unit, Hospital Das Clinicas, University of São Paulo, São Paulo, Brazil); Julio Neves (Multidisciplinary Intensive Care Unit, Hospital da Bahia, Salvador, Brazil); Paulo de Tarso Roth Dalcin (Intensive Care Unit, Ernesto Dornelles Hospital, Hospital Moinhos de Vento and Programa de Pós Graduação em Ciências Pneumológicas, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil); Guilherme de Paula Pinto Schettino (Multidisciplinary Intensive Care Unit, Hospital Albert Einstein, São Paulo, Brazil); Alberto Biestro (Intensive Care Unit, Hospital de Clínicas Dr Manuel Qintela, Faculdade de Medicina, Universidad de la Republica, Montevideo, Uruguay); and Davi Cristovao and Jorge Salluh (Multidisciplinary Intensive Care Unit, Hospital Copa D’Or, Rio de Janeiro, Brazil).
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201802-0227LE on March 20, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the APECHO Study Group, Charlotte Arbelot, Jean-Jacques Rouby, Hélène Brisson, Romain Deransy, Corinne Vezinet, Pierre Garçon, Nabil El Hadj Kacem, Denis Lemesle, Antoine Monsel, Qin Lu, Olivier Langeron, Frédérick Gay, Bruno Lucena, Luiz Malbouisson, Maria José Carvalho Carmona, Julio Neves, Paulo de Tarso Roth Dalcin, Guilherme de Paula Pinto Schettino, Alberto Biestro, Davi Cristovao, and Jorge Salluh
References
- 1.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International Liaison Committee on Lung Ultrasound (ILC-LUS) for International Consensus Conference on Lung Ultrasound (ICC-LUS). International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38:577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 2.Bellani G, Rouby JJ, Constantin JM, Pesenti A. Looking closer at acute respiratory distress syndrome: the role of advanced imaging techniques. Curr Opin Crit Care. 2017;23:30–37. doi: 10.1097/MCC.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 3.Soummer A, Perbet S, Brisson H, Arbelot C, Constantin JM, Lu Q, et al. Lung Ultrasound Study Group. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med. 2012;40:2064–2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 4.Caltabeloti F, Monsel A, Arbelot C, Brisson H, Lu Q, Gu WJ, et al. Early fluid loading in acute respiratory distress syndrome with septic shock deteriorates lung aeration without impairing arterial oxygenation: a lung ultrasound observational study. Crit Care. 2014;18:R91. doi: 10.1186/cc13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monastesse A, Girard F, Massicotte N, Chartrand-Lefebvre C, Girard M. Lung ultrasonography for the assessment of perioperative atelectasis: a pilot feasibility study. Anesth Analg. 2017;124:494–504. doi: 10.1213/ANE.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Yang Q, Li L, Guan J, Liu Z, Han J, et al. [The value of lung ultrasound score on evaluating clinical severity and prognosis in patients with acute respiratory distress syndrome] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:579–584. doi: 10.3760/cma.j.issn.2095-4352.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Haddam M, Zieleskiewicz L, Perbet S, Baldovini A, Guervilly C, Arbelot C, et al. CAR’Echo Collaborative Network; AzuRea Collaborative Network. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 8.Bouhemad B, Brisson H, Le-Guen M, Arbelot C, Lu Q, Rouby JJ. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 9.Chinardet B, Brisson H, Arbelot C, Langeron O, Rouby JJ, Lu Q. Ultrasound assessment of lung consolidation and reaeration after pleural effusion drainage in patients with acute respiratory distress syndrome: a pilot study. Acta Anaesthesiol Belg. 2016;67:29–35. [PubMed] [Google Scholar]
- 10.Bouhemad B, Liu ZH, Arbelot C, Zhang M, Ferarri F, Le-Guen M, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med. 2010;38:84–92. doi: 10.1097/CCM.0b013e3181b08cdb. [DOI] [PubMed] [Google Scholar]
- 11.Mongodi S, Bouhemad B, Orlando A, Stella A, Tavazzi G, Via G, et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med. 2017;38:530–537. doi: 10.1055/s-0042-120260. [DOI] [PubMed] [Google Scholar]
- 12.Mongodi S, Via G, Girard M, Rouquette I, Misset B, Braschi A, et al. Lung ultrasound for early diagnosis of ventilator-associated pneumonia. Chest. 2016;149:969–980. doi: 10.1016/j.chest.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Bouhemad B, Mongodi S, Via G, Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122:437–447. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]