Summary
Transient receptor potential channel subfamily A member 1 (TRPA1) is a Ca2+-permeable cation channel that serves as one of the primary sensors of environmental irritants and noxious substances. Many TRPA1 agonists are electrophiles that are recognized by TRPA1 via covalent bond modifications of specific cysteine residues located in the cytoplasmic domains. However, a mechanistic understanding of electrophile sensing by TRPA1 has been limited due to a lack of high-resolution structural information. Here, we present the cryoelectron microscopy (cryo-EM) structures of nanodisc-reconstituted ligand-free TRPA1 and TRPA1 in complex with the covalent agonists JT010 and BITC at 2.8, 2.9, and 3.1 Å, respectively. Our structural and functional studies provide the molecular basis for electrophile recognition by the extraordinarily reactive C621 in TRPA1 and mechanistic insights into electrophile-dependent conformational changes in TRPA1. This work also provides a platform for future drug development targeting TRPA1.
eTOC blurb:
Here Suo et al. unravel the molecular mechanism by which the “wasabi receptor” TRPA1 ion channel senses noxious chemicals. TRPA1 contains a highly-sophisticated binding site for electrophile agents that undergoes a conformational change following covalent agonist binding, triggering TRPA1 activation.
Introduction
The ability to sense noxious chemicals is an important survival mechanism in all mammals, including humans. Mammalian transient receptor potential channel subfamily A member 1 (TRPA1), a member of the transient receptor potential (TRP) channel superfamily, is a tetrameric nonselective cation channel expressed by nociceptive neurons that serves as one of the primary sensors of noxious chemicals that elicit pain and neurogenic inflammation. These noxious chemicals range from pungent irritants such as mustard, garlic and cinnamaldehyde to the environmental irritant acrolein and endogenous algogens including 4-hydroxynonenal (Bandell et al., 2004; Bautista et al., 2006; Bautista et al., 2005; Jordt et al., 2004; Macpherson et al., 2007b; McNamara et al., 2007; Taylor-Clark et al., 2009; Taylor-Clark et al., 2008; Trevisani et al., 2007). TRPA1 is associated with inflammatory pain and acute and chronic itch syndromes, and is therefore a validated therapeutic target for treatment of these disease conditions (Han et al., 2018; Koivisto et al., 2018; Nassini et al., 2014; Skerratt, 2017; Weyer-Menkhoff and Lotsch, 2018; Wilson et al., 2011; Wilson et al., 2013; Xie and Hu, 2018).
Because of the physiological and therapeutic importance of TRPA1, great efforts have been made to understand the mechanisms by which noxious chemicals bind and activate the TRPA1 channel (Bahia et al., 2016; Benedikt et al., 2009; Hinman et al., 2006; Macikova et al., 2019; Macpherson et al., 2007a; Wang et al., 2008; Ye et al., 2018; Zimova et al., 2018). Studies have shown that many TRPA1 agonists are electrophiles which activate the channel via covalent modification of specific cysteine residues in the cytoplasmic domains of TRPA1 (Bahia et al., 2016; Hinman et al., 2006; Macpherson et al., 2007a). Notably, TRPA1 electrophile agonists are structurally and chemically diverse and they elicit both reversible (thiol-Michael adduct formation) and irreversible (nucleophilic SN2 reaction and thiol-α,β-unsaturated aldehyde reaction) cysteine modification reactions (Hinman et al., 2006; Macpherson et al., 2007a).
Two notable independent studies identified cysteine residues important for electrophile activation of TRPA1: C619, C639 and C663 (C621, C641, and C665 according to the human TRPA1 sequence in Uniport entry O75762) were identified by mutagenesis studies (Hinman et al., 2006) and C415,C422 and C622 (C414, C421, and C621 according to the human TRPA1 sequence in Uniport entry O75762) were identified by mass spectrometry and mutagenesis (Macpherson et al., 2007a). The cysteine residues found by these two studies did not overlap entirely, but both confirmed the critical role of C621 in TRPA1 channel gating by covalent-modification (Hinman et al., 2006; Macpherson et al., 2007a). The importance of C621 has been further highlighted by recent studies of pain sensitivity in multiple African rodents (Eigenbrod et al., 2019).
The discovery that electrophile sensing by TRPA1 takes place through covalent modification of cysteine residues on the cytoplasmic side of the TRPA1 posed an intriguing question. The eukaryotic cytosol is rich in cysteine-containing antioxidants (e.g. ~ 5 mM glutathione) that react with electrophiles to prevent oxidative stress (Halliwell, 1999), suggesting that the process of electrophile detection by TRPA1 must be highly efficient in order to compete with the high concentrations of cysteine-containing antioxidants in cytosol (Bahia et al., 2016). Therefore, TRPA1 must possess a high-performing and sophisticated electrophile sensing apparatus, which is critical for nociceptive signaling in humans. Notably, recent mass spectrometry studies have revealed that C621 reacts with electrophiles at a speed that is >1,000 times faster than a canonical cysteine residue, suggesting that it is an extraordinarily reactive site which likely serves as a hot spot for electrophile sensing in TRPA1 (Bahia et al., 2016). However, the molecular basis for its high reactivity remains unclear. It is also unclear how C621 can undergo different types of covalent modifications by a range of structurally diverse electrophiles and how these covalent modifications might trigger channel activation.
In 2015, a cryo-electron microscopy (cryo-EM) structure of the ligand-free TRPA1 offered the first glance of the overall architecture of TRPA1 (Paulsen et al., 2015). However, the overall resolution (~4 Å) of the cryo-EM map that was used for model building was not sufficient for gaining mechanistic insights into electrophile recognition by TRPA1. In order to dissect the molecular mechanisms of TRPA1 we determined the cryo-EM structures of the human TRPA1 (hTRPA1) in its ligand-free state, and also in complex with the reversible covalent agonist benzyl isothiocyanate (BITC) and the irreversible covalent agonist 2-Chloro-N-(4-(4-methoxyphenyl)thiazol-2-yl)-N-(3-methoxypropyl)-acetamide (JT010) (Figures 1A–1F). Our structural and functional studies identify a distinct binding site for these electrophiles and unveil the structural basis for electrophile recognition by TRPA1. Moreover, structural analyses provide insights into the conformational changes elicited by covalent-modifications that could lead to TRPA1 channel activation.
Figure 1. Overview of human TRPA1 structures.

(A and B) 3D reconstruction (A) and model (B) of the ligand-free TRPA1C621S-Apo structure. TMD, CD, and ARD stand for transmembrane domain, coupling domain, and ankyrin repeat domain, respectively.
(C and D) 3D reconstruction (C) and model (D) of the TRPA1JT010 structure. The JT010 molecule is indicated in red.
(E and F) 3D reconstruction (E) and model (F) of the TRPA1BITC structure. BITC is indicated in red. The reconstruction and coordinates are colored by chain in panels A to F.
(G and H) The structure (G) and topology overview (H) of a single TRPA1 protomer with a close-up of the structural arrangement elements within the coupling domain (box).
See also Supplementary Table 1, Figures S2 and S3.
Results
Structure determination of ligand-free and electrophile-bound human TRPA1
In this study, we used the human ortholog of TRPA1 (hTRPA1) for both cryo-EM, electrophysiological and calcium imaging experiments. In order to study the activation mechanisms of hTRPA1 by structurally and chemically different ligands, we conducted cryo-EM experiments in the presence of a reversible covalent agonist BITC, found in the seeds of the papaya plant (Goosen et al., 2001), which modifies cysteine residues by reversible thiol-Michael adduct formation and the synthetic JT010 compound which selectively modifies C621 through an irreversible SN2 reaction (Takaya et al., 2015) (Figure S1). BITC was chosen because it activates hTRPA1 in a similar manner to allyl isothiocyanate (AITC), but unlike AITC it contains a benzyl ring and can therefore be more easily identified in cryo-EM maps (Hinman et al., 2006).
The purified wild-type (WT) TRPA1 channels were reconstituted in nanodiscs and incubated with ligands before being frozen onto grids. The structures were determined by single particle cryo-EM (see Methods, Figure S2). To obtain a ligand-free state, we introduced the C621S mutation (TRPA1C621S) to silence the highly reactive electrophile site. The final 3D reconstructions of ligand-free TRPA1C621S and the wild type TRPA1 in complex with JT010 (TRPA1WT-JT010) and BITC (TRPA1WT-BITC), were resolved to 2.8, 2.9, and 3.1 Å, respectively (Figures 1A to 1F and Table S1). The overall quality of the 3D reconstructions in the transmembrane region (Figures S3) is excellent, revealing cryo-EM densities corresponding to glycans (Figure 2) and annular lipid molecules (Figure 3). Consistent with the previous cryo-EM study (Paulsen et al., 2015), a significant part of the N-terminal region (AR1-AR11) was not resolved in the cryo-EM reconstructions.
Figure 2. S1–S2 linker and pore structures of TRPA1.

(A) Cryo-EM density surrounding the S1–S2 linker in the TRPA1WT-JT010 3D reconstruction. The density is shown at 0.024 thresholding. The linker adopts a three-stranded β-sheet conformation. Residue N747 is glycosylated. The glycans are shown in stick representation. NAG, N-acetylglucosamine.
(B) The three-stranded β-sheet (cartoon and yellow surface representation) and the NAG molecules (magenta surface representation) plug the extracellular crevice of the VSLD domain.
(C) Residue F476 from the S1–S2 linker is nestled into the hydrophobic crevice formed by aromatic and hydrophobic residues at the extracellular face of the VSLD helical bundle. Residues L736 (S1), I740, P742, F746 (S1–S2 linker), I770, M774 (S2), F818 (S3), and W832 (S4) are shown in stick and orange sphere representation.
(D) Top-down view of the TRPA1 channel pore. The pore of TRPA1WT-JT010 is shown. The side chains of the acidic residue D915 line the entry to the pore. Interactions are observed between P2 segments of neighboring protomers. R919 forms interactions with E920 and S921 from the neighboring protomer. All three residues reside in the π-helical turn in P2. D915 is shown in stick and sphere representation. R919, E920, and S921 are shown in stick representation. Dashed lines are drawn between residues which are within interaction distance.
(E) Side view of the pore of TRPA1WT-JT010, the residues that form the selectivity filter and the gate are shown in stick representation.
(F) Pore radii calculated using the HOLE program for TRPA1C621S-Apo (light green), TRPA1WT-JT010 (light blue), and TRPA1WT-BITC (salmon). Dashed line represents the approximate radius of a water molecule (1.4 Å).
Figure 3. Lipids in TRPA1.

(A) Lipids captured in the TRPA1 WT-JT010 structure. Lipids are numbered. A phospholipid molecule (lipid 7) is observed in the interfacial cavity between the pre-S1, S4, the S4-S5 linker, and the IFH. The lipid 7 binding site is indicated by the box. A close-up of the binding site is shown in (C). The cryo-EM map is shown at 0.02 thresholding.
(B) Lipids captured in the TRPA1WT-BITC structure. In the TRPA1WT-BITC, the interfacial cavity contains no lipid density (boxed region). The cryo-EM map is shown at 0.018 thresholding.
(C) A close-up view of the interfacial cavity in TRPA1WT-JT010 shows that the phospholipid interacts with residues W711 in the Pre-S1 and E854 in the loop connecting the S4 to the S4–S5 linker. Residue N855, the location of a gain-of-function disease mutation, is also located at this interface. W711, E854, and N855 are shown in stick representation and the phospholipid molecule is shown in stick and sphere representation.
(D) A close-up of the interfacial cavity in TRPA1WT-BITC shows that the space occupied by phospholipid in TRPA1WT-JT010 is filled with aromatic residues from IFH and Pre-S1. In this structure, a direct interaction is observed between N855 and the backbone carbonyl of C1024 in the IFH. Residues W711, E854, N855, F1017, F1020, C1021, C1024, and F1025 are shown in stick representation.
(E) An overlay of the interfacial cavities of TRPA1WT-JT010 (blue) and TRPA1WT-BITC (salmon) shows that the IFH in the TRPA1WT-BITC moves closer to the Pre-S1, S4 and the S4–S5 linker, thereby reducing the size of the cavity and making it incompatible with lipid binding.
(F) A close-up view of the interfacial cavity in TRPM8 with a PI(4,5)P2 molecule bound. The presence of a large number of positively charged residues, absent in the interfacial cavity in TRPA1, facilitates coordination of PI(4,5)P2. PI(4,5)P2 is shown in stick and sphere representation and the interacting residues (R688, Y683, N692, R850, and R997) are shown in stick representation.
The overall structure of the homotetrameric TRPA1 channel can be divided into three layers. The top layer is composed of the transmembrane domain (TMD), while the middle layer contains the coupling domain and the bottom layer consists of the ankyrin repeat domain (ARD). The transmembrane domain is composed of the voltage-sensor-like domain (VSLD; S1–S4) with the pore domain (S5, S6, and PH) arranged in a domain-swapped manner. The N- and C-terminal regions form a two-layered cytoplasmic assembly. The coupling domain, which forms the middle layer, is composed of 8 short helices (H1–H7 and pre-S1), a β sheet (βCD) composed of three β strands (β1.1–β1.3), and the TRP-like helix (Figures 1G and 1H). Ankyrin repeats 12–16 (AR12-AR16) from four subunits surround the central tetrameric C-terminal coiled coil, resulting in the formation of the ARD (Figure 1).
Unique Structural Features in the Transmembrane Channel Domain
Our 3D reconstructions of hTRPA1 reconstituted in nanodiscs allowed us to visualize several unique structural features that were not resolved in the previous structural study. First, the S1–S2 linker region, which was previously thought to be a flexible loop (Marsakova et al., 2017), adopts a three-stranded β sheet (β2) motif (Figures 2A–C). This region appears to have functional roles (Marsakova et al., 2017) and is the binding site of the spider toxin Pro-toxin 1 (ProTx-I) (Gui et al., 2014). Furthermore, we observe an additional cryo-EM density at the C-terminal of the first β strand (N747 in β2.1), which we assigned as two N-acetylglucosamine (GlcNAc) molecules (Figure 2A). The β2 and the two sugar moieties appear to act as a single entity to plug the extracellular crevice of VSLD (Figures 2B and 2C). Consistent with our structure, previous studies have shown that the mutation of F746 in β2 to alanine substantially affects voltage-dependence of TRPA1 (Marsakova et al., 2017) and mutation of N747 reduces the channel’s sensitivity to various types of agonists (Egan et al., 2016). Second, our structures reveal the intricate selectivity filter architecture in TRPA1 (Figures 2D–F). The selectivity filter, composed of backbone carbonyls of residues L913 and G914 and the side chain of D915, is wider than that of the previously reported TRPA1 structure (9.2–10.5 Å distance between the backbone carbonyls and the aspartate side chains of diagonally opposing subunits) and large enough to accommodate a hydrated Ca2+ ion. hTRPA1 contains two pore helices (P1 and P2), similar to the pores of TRPML channels (Hirschi et al., 2017, Chen et al., 2017). Interestingly, we found that the P2 of TRPA1 adopts a π helical turn at its N-terminal end, and that the π-helix is apparently maintained by the interactions between the R919 on P2 from one subunit and E920 and S921 on P2 from the adjacent subunit (Figure 2D). Although the role of the π-helix in S6 and the S4–S5 linker has been well studied in other TRP channels (Zubcevic and Lee, 2019), the π helical turn in P2 has not been observed before, and thus its role is not clear at this point.
Lipids and the interfacial helix
In our 3D reconstructions, we identified a protein density consistent with a helix at the cytosol-membrane interface located near the side of S1 and S4 of the VSLD (Figures 3A and 3B), which was not modelled in the previous study (Paulsen et al., 2015). We term this structural motif the interfacial helix (IFH). Our cryo-EM maps show that the IFH is part of the C-terminal region which connects the βCD (β1.3) and the coiled coil (CC) (Figures 1, 3A, and 3B). The cryo-EM density around this region is well resolved in the TRPA1WT-BITC reconstruction and allows unambiguous placement and register assignment of the IFH as well as the linker between β1.3 and IFH. However, in the ligand-free TRPA1C621S and TRPA1WT-JT010 reconstructions the corresponding cryo-EM density is less well defined, and thus IFHs in these two models were built as polyalanine chains (Figures S3, and S4).
Our 3D reconstructions of TRPA1 embedded in lipid-nanodiscs also allowed us to resolve annular phospholipids bound to the TRPA1 channel. Six to seven phospholipids per subunit were resolved in the 3D reconstructions (lipids 1–7, Figure 3A and 3B). Four phospholipids (lipids 1–4) located at the site of the extracellular leaflet of the membrane mostly occupy the interfaces between the S5 and S6 helices of adjacent subunits and between the VSLD and the pore (Figure 3A). Several phospholipids (lipids 5–7) are also observed in the putative location of the cytoplasmic leaflet of the plasma membrane. In the 3D reconstructions of both TRPA1WT-JT010 and TRPA1C621S, we also identified a phospholipid (lipid 7) located in the cavity formed by S4, the S4–S5 linker, pre-S1, S1, and IFH (termed interfacial cavity, Figure 3C). Interestingly, the location of this phospholipid in TRPA1 is reminiscent of the PI(4,5)P2 binding site recently identified in TRPM8 (Figure 3F) (Yin et al., 2019). In TRPM8, PI(4,5)P2 binding at this position is essential for channel function (Rohacs et al., 2005; Yin et al., 2019). However, in TRPA1, the bound lipid 7 molecule is unlikely to be PI(4,5)P2 given the absence of positively charged residues in the interfacial cavity. The lipid 7 is most consistent with phosphatidylcholine (PC) with its choline head group sandwiched between E854 of the S4–S5 linker and W711 of pre-S1. Notably, the conformation of the interfacial cavity in the TRPA1WT-BITC is different to those observed in the TRPA1WT-JT010 and TRPA1C621S and the density corresponding to the lipid 7 is missing in the TRPA1WT-BITC 3D reconstruction (Figures 3B and 3D). In the TRPA1WT-BITC structure the IFH is positioned closer to the VSLD, resulting in a reduced size of the cavity and elimination the lipid binding site (Figures 3D and 3E). Intriguingly, N855, when mutated to serine (N855S), causes the familial episodic pain syndrome in humans (Kremeyer et al., 2010). This residue is located at the junction between S4 and the S4–S5 linker and interacts with IFH in the TRPA1WT-BITC structure (Figure 3D). Furthermore, C1021, which was proposed to be the binding site for the potent TRPA1 activator zinc, is located in the IFH (Hu et al., 2009). Our structures now reveal that the IFH is a distinct structural motif which forms a part of a dynamic interfacial cavity that can be occupied by lipids and contains the potential binding site for zinc. The dynamic movement of IFH appears to be a determinant for phospholipid binding in the interfacial cavity as well as for the interactions between the IFH and the N855 residue which is a site for a disease-inducing gain-of-function mutation, suggesting a role of IFH in TRPA1 gating. Consistent with this idea, we found that deletion of IFH substantially affects hTRPA1 channel function (Figures S6–S7).
JT010-binding pocket
In the 2.88 Å reconstruction of the TRPA1-JT010 complex, we observed a strong EM density located in the coupling domain at the C621 residue (Figures 4 and S5). We assigned this density to JT010 because it matches the size and shape of the JT010 molecule and is absent in the reconstruction of TRPA1C621S (Figures S5A and S5D). The cryo-EM density peaks for JT010 and C621 are connected, consistent with formation of a covalent bond between JT010 and C621 (Figure S5).
Figure 4. JT010 and BITC binding in TRPA1.

(A and B) The electrophile JT010 (A) and BITC (B) binding pocket is located in the coupling domain of TRPA1. The binding site is shaped as a clamshell, with H1, H2, and the H1–H2 loop forming the bottom part and the region between β1.2 and H5 forming the upper part. The pocket is shown in cartoon and surface representation, and the modeled JT010 (A) and BITC (B) molecules are shown in sphere representation.
(C and D) A close-up view of the JT010 binding site. JT010 is covalently bound to the C621 residue via its acetamide group. Its thiazol group forms CH-π and sulphur-π interactions with F612 and Y680, respectively. In addition, the methoxyphenyl group interacts with H614, P666, and F669. Residues L609, F612, H614, C621, P622, I623, Y662, C665, P666, F669, Y680, and T684 are shown in stick representation.
(E and F) A close-up view of the BITC binding site (E) and a view of the binding site rotated counter-clockwise by 45°(F). BITC is covalently bound to C621. Residues L609, F612, H614, C621, P622, I623, Y662, C665, P666, F669, Y680, and T684 are shown in stick representation.
See also Figure S5
When applied to HEK293 cells expressing wild-type hTRPA1, JT010 elicits robust inward and outward currents (Figures 5A). Based on our dose-response curves, the EC50 of JT010 is ~7.6 nM (Fig. S6B) which is slightly higher than the previously reported value measured by a cell-based calcium uptake assay (Takaya et al., 2015). To confirm that C621 in hTRPA1 is indeed the site for covalent modification by JT010, we introduced a C621S mutant (TRPA1C621S) and observed that the HEK293 cells expressing TRPA1C621S do not exhibit detectable currents upon application of 100 nM of JT010 (Figures 5B).
Figure 5. Functional characterization of TRPA1.
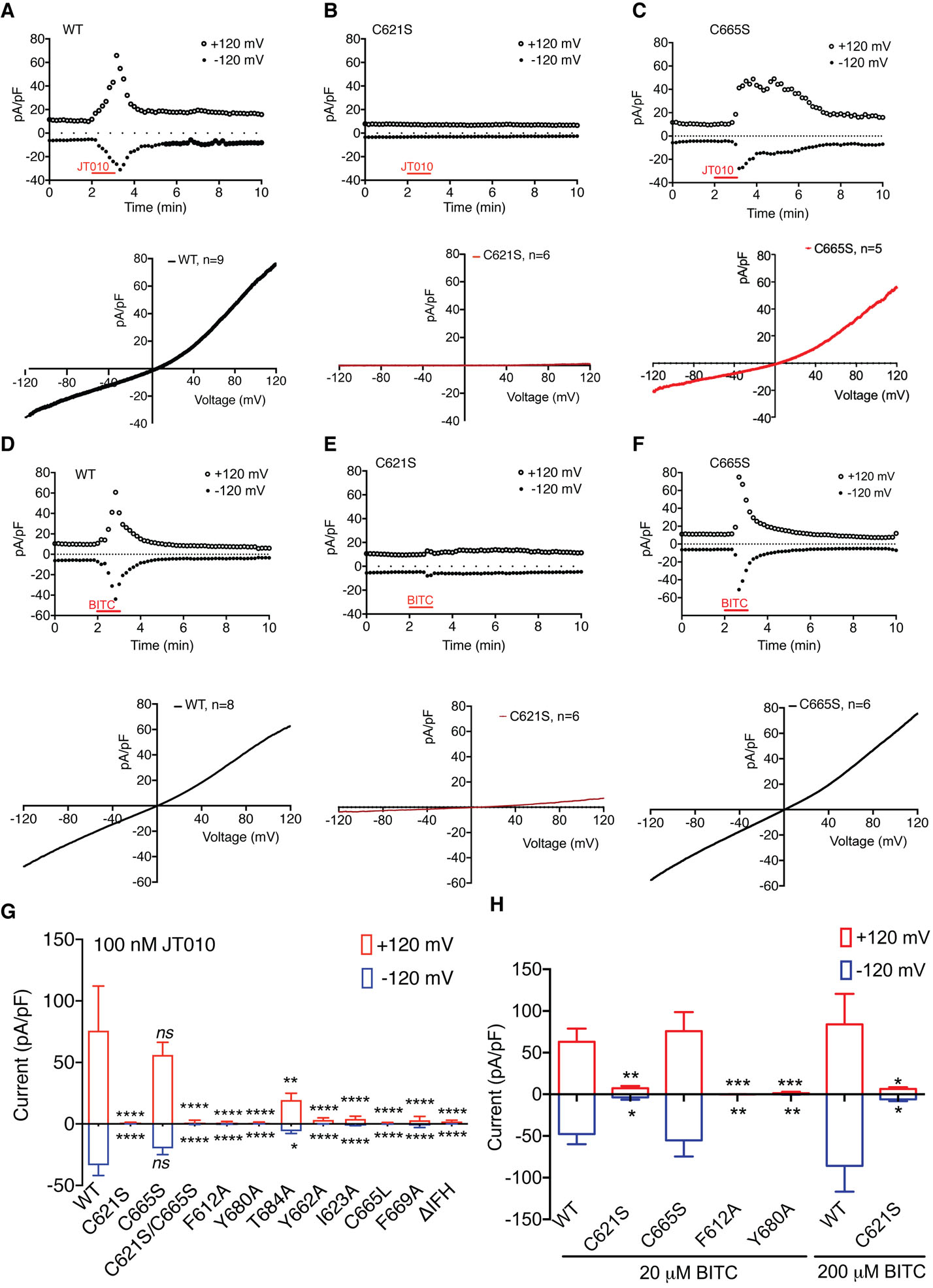
(A-C) Current recordings of hTRPA1 wild-type (A), C621S (B) and C665S (C) mutants elicited by JT010. Wild-type hTRPA1 responds robustly to application of JT010. However, the JT010-induced current is abolished in HEK293T cells transfected with the C621S mutant. Upper panel represents outward and inward currents recorded at +120 mV and −120 mV, respectively, lower panel represents current-voltage relationship after JT010 application (100 nM).
(D-F) Current recordings of hTRPA1 wild-type (D), C621S (E) and C665S (F) constructs elicited by BITC. The BITC-induced current is abolished in HEK293T cells transfected with the C621S mutant while C665S mutant exhibits a similar response to BITC as wild-type. Upper panel represents outward and inward currents recorded at +120 mV and −120 mV, respectively. Lower panel represents current-voltage relationship after BITC application (20 μM).
(G) Effect of binding site mutations on the TRPA1 JT010 response. (WT: n = 9, C621S: n=6, C665S: n=5, C621S/C665S: n=7, F612A: n=5, Y680Aa: n=5, T684A: n=5, Y662A: n=9, I623A: n=6, C665L: n=7, F669A: n=9, and ΔIFH: n=8 cells) **P < 0.01, ***P < 0.01, ****P < 0.0001, WT vs. mutants, two-way ANOVA, followed by Bonferroni’s post hoc test. Only one cell was recorded from each cover slip. Data represent mean ± S.E.M.
(H) Inward and outward current density of WT and mutant hTRPA1 elicited by 20 μM BITC at −120 mV and +120 mV (WT: n = 8, C621S: n=6, C665S: n=6, Y680A: n=8, F612A: n=8 cells), and by 200 μM BITC, (WT: n=5, C621S: n=8). *P < 0.05, **P < 0.01, ***P < 0.001, WT vs. mutants, two-way ANOVA, followed by Bonferroni’s post hoc test. Data represent mean ± S.E.M.
See also Figures S6 and S7
The JT010 binding site is reminiscent of a clam shell, with H1, H2, and the H1–H2 loop forming the bottom half and the region between β1.2 and H5 forming the top half (Figures 1G, 4A, an 4D). Residue C621 is located at the base of the bottom half of the binding site (Figure 4A). Four aromatic residues (F612, H614, F669 and Y680) from both halves of the pocket participate in sealing the lateral entries to the binding site and complete the pocket (Figures 4C and 4D). The acetamide group of JT010 is covalently linked to the thiol moiety of C621 while its methoxyphenyl thiazol group interacts with above-mentioned aromatic amino acids. Specifically, the thiazol group interacts with F612 and Y680 through CH-π and sulphur-π interactions, respectively (Figures 4C and 4D) and the methoxyphenyl group is surrounded by and interacts with H614, P666, F669 (Figure 4D). To test the role of these observed interactions in JT010-dependent TRPA1 activation, we performed site-directed mutagenesis and electrophysiological as well as calcium imaging studies, and found that mutations of the aromatic amino acids have significant effects on JT010-induced TRPA1 currents (Figures 5G, S6, S7) without affecting channel expression (Figure S7).
BITC-binding in TRPA1
BITC is an isothiocyanate that reacts with thiols via a reversible thiol-Michael addition reaction. To capture the BITC bound conformation, we incubated the nanodisc-reconstituted wild type TRPA1 with a high concentration of BITC (1 mM) before sample vitrification. The 3D reconstruction of the TRPA1-BITC complex was resolved to an overall resolution of 3.1 Å and we observed a strong EM density located in the pocket of the coupling domain and connected to C621 (Figures 4B and S5C). This density matches the size and shape of a BITC molecule and is also absent from the reconstruction of TRPA1C621S (Figure S5A and S5D). We therefore assigned this EM density to BITC. Interestingly, we observed an additional weaker cryo-EM density of a similar shape adjacent to C665 in the upper half of the binding site that might correspond to a second BITC molecule (Figures S5G). However, because this weaker density connected to C665 does not allow for unambiguous ligand placement, we did not model a BITC molecule at this site. C665 has previously been implicated in AITC sensing in mouse TRPA1 (Hinman et al., 2006). Our electrophysiological data shows that the C621S mutation does not exhibit significant BITC-dependent hTRPA1 currents while the C665S mutant responds robustly to BITC treatment, indicating that BITC modification of C665 is not necessary for hTRPA1 activation (Figures 5D–5F, 5H). However, it is possible that C665 can be covalently modified by BITC without contributing to hTRPA1 activation, and therefore, we speculate that the high concentration of BITC used might have forced covalent modification of C665. Indeed, our structure suggests that the binding pocket may accommodate simultaneous binding of two BITC molecules (Figures S5C, S5F and S5G). The benzyl ring of BITC fits snugly into the groove generated by the loop between β1.2 and H4, H2, and H1 (Figures 4B, 4E and 4F). Notably, the region containing residues P666, F669, and Y680 in the BITC-binding pocket adopts a similar conformation to the one observed in TRPA1WT-JT010. The Y680A mutation also significantly affects the BITC-dependent TRPA1 response (Figure 5). The turn of the H4–H5 loop appears to differ slightly in the two structures but given that the density is poorly resolved in TRPA1WT-BITC, we refrain from drawing conclusions from this observation. Taken together, our structural and functional data show that the electrophile-sensing site in human TRPA1 centers around C621 and that covalent modification of C621 by electrophiles JT010 and BITC is critical for TRPA1 activation. Therefore, these findings provide the structural basis for electrophile recognition in TRPA1.
Structural basis of high reactivity of Cys621 in TRPA1
Although the first structure of hTRPA1 (Paulsen et al., 2015) has been invaluable for our understanding of the channel architecture, the low local resolution resulted in uncertain register assignments of a substantial part of the coupling domain including the position of C621, which in turn might have precluded detailed insights into the structural basis for the high reactivity of C621 in TRPA1 (Figure S8).
In all of our ligand-free and ligand-bound structures, the bottom half of the electrophile binding pocket maintains a similar conformation. C621 is located near the base of H2 and is separated from the helix by P622 which positions the N-terminus of H2 toward the thiol group of C621 (Figure 6). In this arrangement, the helical dipole (N cap) exerts electrostatic force on the thiol group of C621. Furthermore, directly opposite C621 in the H1–H2 loop, F612 is positioned facing the C621 thiol moiety and engages it in a thiol-π interaction (Figure 6). Notably, H1 is oriented perpendicular to H2, and its C-terminus with its helical dipole (C cap) forms an electrostatic interaction with the quadrupole of F612. This apparently stabilizes the C621-F612 thiol-π interaction by fixing the F612 side chain in the optimal rotamer conformation for this interaction. We suggest that this unique structural arrangement serves two roles. First, the pKa of C621 is lowered synergistically by interaction between the N cap and C621 and by the thiol-π interaction that exists between C621 and F612. However, as was previously discussed (Bahia et al., 2016), lowering of the pKa alone is not sufficient to account for the extraordinary reactivity of C621 and other factors are likely to contribute to its unique chemical properties. Our data suggest that the second role of the structural arrangement in this binding pocket is to fix the orientation of the aromatic ring of F612 via the quadrupole-C cap interactions. The rigidified aromatic ring of F612 reduces the entropy of the thiol group of C612 and thus fixes it in the optimal orientation for covalent modification by electrophiles. Interestingly, the H1–H2 loop also contains residue K620 which has previously been identified as important in the electrophile-dependent TRPA1 response (Bahia et al., 2016). In our structure, K620 forms salt bridge interactions with residues E625 and E628 located within the H2 and appears to maintain the structural integrity of the electrophile binding site by fixing the plane of the H2 helix (Figure 6D). The combined effect of these interactions contributes to the high reaction rate of C621 in hTRPA1 by enhancing the effective collision of the electrophile-thiol reaction. Consistent with this observation, our functional data shows that F612 is important for both JT010- and BITC-dependent activation of TRPA1 (Fig. 5G, 5H). In addition, one side of the binding pocket in the coupling domain is decorated with four aromatic residues (F612, H614, F669, and Y680) and the other side with two additional ones (Y662 and W605). We suggest that the role of these electron-rich aromatic amino acids surrounding C621 is to facilitate the entry of electrophiles into this pocket and increase their local concentration.
Figure 6. The architecture of the electrophile binding pocket endows C621 with high reactivity.

(A and B) The environment in the electrophile binding pocket of TRPA1C621S-Apo (A) and the view of the binding pocket rotated by 90°(B). Residue 621 is surrounded by helical dipoles of H1 and H2 helices (shown in cylinder and stick-and-ball representation).
(C) The close-up of the interactions between H1, H2, F612 and C621. The helical dipole of H2 exerts electrostatic force on the thiol group of C621, lowering its pKa. The conformation of the F612 residue is held in place by the helical dipole of H1, which exerts electrostatic force on the positively charged edge of the F612 quadrupole. The combination of these interactions results in stabilization of the C621-F612 thiol-π interaction, which in turn reduces the entropy of the C621 side chain. The lowering of the pKa by the helical dipole of H2 and the stabilization of the thiol-π interaction by the helical dipole of H1 contributes to the high reactivity of C621. The high reactivity is also aided by the electron-attracting aromatic residues in the pocket.
(D) The close-up interactions showing that the residue K620 forms salt bridge interactions with E625 and E628 located within the H2 and appears to maintain the structural integrity of the electrophile binding site.
See also Figure S6
Conformational change associated with electrophile binding
In all of our TRPA1 structures, the I957 and V961 in S6 form the gate (Figure 2F). Despite being bound to ligand, both the TRPA1WT-JT010 and TRPA1WT-BITC structures apparently adopt non-conducting states, suggesting that our cryo-EM reconstructions have captured conformations that either precede or follow the open state. Because prolonged exposure of TRPA1 to JT010 is followed by desensitization (Fig S6C), our ligand-bound TRPA1 might sample multiple conformational states. However, it is also possible, as is often observed in cryo-EM structures of TRP channels (Paulsen et al., 2015; Yin et al., 2019; Zubcevic et al., 2019; Zubcevic et al., 2018), that we have captured energetically favored conformational states of the channel, as closed or desensitized states are more stable than the open state.
Despite the fact that the ligand-bound structures adopt non-conducting states, we observed substantial conformational changes that are associated with binding of electrophile agonists. As discussed earlier, the bottom half of the coupling domain binding pocket remains unchanged in all of our TRPA1 structures. However, the top half of this pocket appears to possess a large degree of plasticity and undergoes ligand-dependent conformational changes. In the ligand-free TRPA1C621S-Apo, the top half of the pocket adopts a conformation that allows P666 to interact with Y680 through CH-π interactions (Figure 7C). Upon binding of JT010, the thiazole group of JT010 displaces P666 and interacts with Y680, resulting in a large rotation of the region containing C665 and P666 (Figures 7D and 7E). This JT010-dependent conformational change in the binding pocket is propagated laterally to the βCD which swings outwards and in turn causes a change in the position of the IFH (Figures 7F–H). Consistent with our observation, previous limited proteolysis and mass-spectrometry studies suggested conformational changes elicited by ligand binding occur on the segment including β1.3 of the βCD and the loop between β1.3 and IFH (Samanta et al., 2018). The conformational change is also propagated vertically to result in a ~3° counterclockwise rotation of ARD and coupling domain when viewed from the extracellular side (Figure 7B). Interestingly, binding of BITC leads to similar global conformational changes in the coupling domain and the ARD (Figures 7A, 7B and 7F–H). Notably, BITC elicits a rotation of C665 and P666 as well as an outward swing motion of βCD similar to that observed in the JT010-bound structure (Figures 7B and 7F–H). The comparison of these structures has led us to speculate that the local conformational changes in the top half of the binding pocket would be similar regardless of the type of covalent agonist used to activate the channel. This might explain why TRPA1 can be activated by so many structurally and chemically diverse covalent agonists. Based on this idea, we hypothesize that the top half of the binding pocket acts as a conformational switch that senses covalent modification of C621. We posit that C665, P666, Y680 serve as the determinants for conformational switching in the top-half of the binding pocket and that C665 is not the main target of covalent modification. We found that introduction of the isosteric C665S mutation results in functional channels that can be activated by either JT010 or BITC while introduction of a larger sidechain (C665L) results in channels with no detectable JT010-dependent activity (Figures 5G and S6D). The lack of iodoacetamide-dependent TRPA1 C665L activation was also previously observed (Bahia et al., 2016). These observations, in addition to mutational effect on the position in Y680 in both JT010 and BITC dependent TRPA1 gating, are consistent with our hypothesis that the top half of the binding pocket acts as a conformational switch in TRPA1(Figure 8).
Figure 7. Conformational changes in the electrophile binding pocket and the CD upon JT010 and BITC binding.

(A) Orthogonal view of the channels TRPA1C621S-Apo (light green), TRPA1WT-JT010 (light blue), and TRPA1WT-BITC (salmon) overlaid via their transmembrane domains. The boxes indicate the views shown in (B)–(H).
(B) The ARDs of the ligand-bound structures also undergo a counter-clockwise rotation. Rotation angles are measured between Cα atoms in H570. In all panels, TRPA1C621S-Apo is shown in green, TRPA1WT-JT010 in blue, and TRPA1WT-BITC in salmon.
(C) In the ligand-free TRPA1C621S structure, P666 and Y680 engage in a CH-π interaction.
(D) Upon binding of JT010, the thiazole moiety of the molecule displaces P666 and engages in interactions with Y680. This causes a large conformational change in the region containing C665 and P666. The JT010 molecule is shown in stick representation.
(E) Overlay of the electrophile binding pockets in TRPA1C621S-Apo (green) and TRPA1WT-JT010 (blue). The conformational change in the region containing C665 and P666 is indicated with a red arrow. The JT010 molecule is shown in stick representation.
(F) To observe the local conformational changes in the coupling domain upon ligand binding, the coupling domains of TRPA1C621S-Apo (green), TRPA1WT-JT010 (blue), and TRPA1WT-BITC (salmon) are aligned. The alignment shows that only the upper half of the binding site undergoes a conformational change.
(G) Relative to the transmembrane domain, the coupling domain undergoes a ~3.5 Å outward swing upon ligand binding. TRPA1C621S-Apo is shown in green, TRPA1WT-JT010 in blue, and TRPA1WT-BITC in salmon.
(H) The β- sheet of the coupling domain undergoes a counter-clockwise rotation when viewed from the extracellular space. The IFH is also displaced during ligand binding. In the TRPA1WT-JT010 (blue) structure, the IFH moves away from the Pre-S1. In the TRPA1WT-BITC (salmon) structure the IFH moves closer to the Pre-S1.
Figure 8.

Proposed electrophile sensing mechanism for TRPA1. The electrophile binding site adopts a clam shell conformation. The region that forms the top part of the binding site flips when an electrophile covalently modifies C621, triggering a swing of the β-sheet (β1.1–β1.3) and a change in the position of the IFH.
Interestingly, the IFH in JT010- and BITC-bound structures adopt different positions: the helix moves away from the S1 in the presence of JT010 but moves towards it and causes displacement of lipid 7 in the presence of BITC. Because the IFH in the TRPA1WT-BITC structure is more closely engaged with the interfacial cavity and is in a position to exert a force on the S4–S5 linker to open the S6 gate, it is possible that TRPA1WT-BITC might represent a pre-open state while TRPA1WT-JT010 might reflect a desensitized state. We speculate that the reversibility of BITC-dependent modification of C621 might have allowed us to capture a functional state of TRPA1 different from the one observed in the presence of JT010. However, in the absence of a structure in an open state, it is difficult to unambiguously assign functional states to our structures. The assignment is further complicated by the possibility that multiple dynamic functional states could exist in the sample in the presence of ligand.
Discussion
Our structural and functional analyses revealed that TRPA1 adopts a distinct design principle and a unique mechanisms of ligand sensing which stand in stark contrast to the menthol sensor TRPM8 (Yin et al., 2019; Yin et al., 2018) and the capsaicin sensor TRPV1 (Cao et al., 2013; Gao et al., 2016; Liao et al., 2013). First, unlike TRPV1 and TRPM8, the electrophile sensing site is located at the cytoplasmic side. The highly-orchestrated design of helical dipoles as well as π interactions within the coupling domain confers high reactivity onto C621. Furthermore, the ability of TRPA1 to react with various types of electrophiles could be explained by the unique design of the “clam shell” binding pocket where the bottom half contains the highly reactive C621 that is modified by electrophiles and the top half acts as a conformational switch (Figure 8). To the best of our knowledge, this design is unique and different from active sites in highly substrate-specific enzymes that utilize reactive cysteines. Interestingly, this configuration of the binding site also appears to be important for the binding of the cell-penetrating scorpion toxin WaTx. WaTx activates the human TRPA1 but not the rat snake ortholog, which carries an alanine instead of a proline in position 622 (A627 in rat snake TRPA1) (Fig. 6). An alanine-to-proline substitution at this position confers WaTx-sensitivity to the rat snake TRPA1 (Lin King et al., 2019).
Second, the conformational step that is required for TRPA1 activation is different from that required by TRPV1 and TRPM8. The binding of covalent agonists to the C621 in the coupling domain leads to the conformational change in the top half of the binding pocket that relays to a conformational change in βCD, which may in turn reposition the IFH to interact with the S4–S5 linker to open the S6 gate (Figure 8). This is in stark contrast to TRPV1 and TRPM8 where agonist binding to the S4–S5 linker or VSLD cavity and the TRP domain, respectively, leading to opening of the S6 gate (Cao et al., 2013; Yin et al., 2019). It is worth noting that the residue K708 (K710 in human TRPA1), located in the pre-S1 helix, has previously been reported to play a role in the irreversible AITC-evoked TRPA1 response (Hinman et al., 2006). These data might be explained by our current structural studies, as residue K710 is downstream of the conformational switch and may be involved in transducing the wave of conformational changes from the electrophile binding site to the transmembrane domains of the channel. Third, the role of lipids in TRPA1 gating appears to be different to the mechanisms identified in TRPV1 and TRPM8. Even though we observe a phospholipid in the interfacial cavity, a site that is analogous to the PI(4,5)P2 site in TRPM8, the cavity is electrostatically incompatible with PI(4,5)P2. We therefore speculate that the dynamic interactions between IFH and the phospholipid in the interfacial cavity may play a role in TRPA1 gating which is yet to be illuminated. In order to understand the true unliganded state of TRPA1, we neutralized the highly reactive cysteine by introducing the mutation C621S and used this structure to gain insights into the structural changes elicited by covalent agonists. However, given the high reactivity of C621, it is possible that TRPA1 has a basal activity under physiological conditions and that introduction of exogenous algogens shifts the complex equilibrium further toward an open state of TRPA1, thereby eliciting the pain response.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Seok-Yong Lee (seok-yong.lee@duke.edu). Plasmid constructs generated in this are available from the Lead Contact without restriction.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HEK293T cells were used for patch-clamp electrophysiology, calcium imaging and immunostaining. Cells were obtained from Duke University Cell Culture Facility and were not further authenticated. Cells were maintained in DMEM containing 10% fetal bovine serum (FBS, Sigma), 1% penicillin and streptomycin (Gibco) and supplemented with 5% CO2 at 37 °C HEK293S GnTI-cells were used for recombinant protein expression for cryo-EM studies. Cells were obtained from ATCC and were not further authenticated. Cells were tested for mycoplasma contamination regularly at Duke University Cell Culture Facility. Cells were maintained in Freestyle 293 media (Life Technologies) supplemented with 2% (v/v) FBS (Gibco) at 8% CO2. Cells were passaged twice a week and were used until 25th passage.
METHOD DETAILS
Protein expression and purification
The Homo sapiens full-length human TRPA1 was synthesized (BioBasic) and cloned into the pEG BacMam vector (Goehring et al., 2014), in-frame with a FLAG-tag followed by 10× His-tag in C-terminus. The wild type human TRPA1 (hTRPA1) was used to obtain the JT010- and BITC-bound structures. To obtain the ligand-free state, the C621S mutation was introduced using the QuikChange site-directed mutagenesis kit (Agilent). Baculovirus was generated according to manufacturer’s protocol (Bac-to-Bac, Invitrogen). For TRPA1 protein expression, HEK293S GnTI− cells (ATCC) was cultured in Freestyle 293 media (Life Technologies) supplemented with 2% (v/v) FBS (Gibco) at 8% CO2. Cells were infected with 8% (v/v) P3 baculovirus at 3×106 ml−1 density and supplied with 3μM Ruthenium Red. After 20 hours shaking incubation at 37°C in the presence of 8% CO2, 10 mM sodium butyrate (Sigma-Aldrich) was added to cell culture and the incubation temperature was lowered to 30°C to boost protein expression. The cells were harvested after an additional 40–44 hours by centrifugation at 550× g, and were subsequently resuspended in lysis buffer (20 mM Tris pH8, 150 mM NaCl, 12 μg mL−1 leupeptin, 12 μg mL−1 pepstatin, 12 μg mL−1 aprotinin, DNase I, 1 mM phenylmethylsulphonyl fluoride, 1 mM inositol hexaphosphate (InsP6), 5 mM TCEP-HCl and 1% (w/v) digitonin). Protein extraction was performed at 4°C for 1 hour, followed by centrifugation at 13000× g for 30 min to remove insoluble material. The supernatant was subsequently incubated with anti-FLAG M2 resin (Sigma-Aldrich) at 4°C for 1 hour. The resin was then packed onto a gravity-flow column (BioRad), and washed with 10 column volumes of wash buffer (20 mM Tris pH 8, 150 mM NaCl, 0.07% digitonin, 1 mM InsP6, 1 mM TCEP). The TRPA1 protein was then eluted with 5 column volumes of elution buffer (20 mM Tris pH 8, 150 mM NaCl, 0.07% digitonin, 3 mM TCEP, 1 mM InsP6, 100 μg mL−1 FLAG peptide). The eluted protein was concentrated and further purified on a Superose 6 size-exclusion column equilibrated with gel filtration (GF) buffer (20 mM Tris pH 8, 150 mM NaCl, 0.07% digitonin, 3 mM TCEP, 1 mM InsP6). The peak fraction was collected and subjected to nanodiscs reconstitution.
Nanodisc Reconstitution
MSP2N2 was purified according to the previously published protocol (Ritchie et al., 2009). Detergent-solubilized TRPA1 was concentrated to 1–1.5 mg mL−1, and mixed with purified MSP2N2 and extruded lipid mix (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (POPG), Avanti Polar Lipids; POPC:POPE:POPG=3:1:1) at 1:3:200 molar ratio. The mixture was incubated in 4° C for 30 min with constant rocking. 100 mg mL−1 Bio-Beads SM2 (Bio-Rad) was then added to the mixture to initialize the reconstitution reaction. The Bio-Beads were exchanged after two hours (100 mg mL−1), and the mixture incubated with constant rocking in 4° C for 12–15 hours. The mixture was then subjected to size exclusion chromatography on a Superose 6 column pre-equilibrated with detergent-free GF buffer (20 mM Tris pH 8, 150 mM NaCl, 3 mM TCEP, 1 mM InsP6).
Cryo-EM sample preparation and data collection
The peak fractions from the final size exclusion chromatography containing nanodisc-reconstituted TRPA1 were concentrated to ~0.5 mg ml−1. For the TRPA1WT-JT010 data, the protein was incubated with 100 μM JT010 (Sigma-Aldrich) on ice for 30 minutes before freezing. For the TRPA1WT-BITC data, 1 mM BITC (Sigma-Aldrich) was added to the protein sample on ice for ~100 minutes before freezing. The TRPA1WT in the presence of JT010 and TRPA1C621S samples were plunge frozen using Leica EM GP Automatic Plunge Freezer operated at 24° C and ~95 % humidity. A sample volume of 3 μl was applied to a freshly glow-discharged UltrAuFoil R1.2/1.3 300mesh (Quantifoil), blotted with Whatman No. 1 filter paper for 3 seconds followed by plunge-freezing in liquid-ethane cooled by liquid nitrogen. The TRPA1WT sample in the presence of BITC was plunge frozen using Vitrobot Mk IV (FEI) Plunge Freezer operated at 24° C and ~100 % humidity. As before, a sample volume of 3 μl sample was applied to a freshly glow-discharged UltrAuFoil R1.2/1.3 300 mesh (Quantifoil), blotted with Whatman No. 1 filter paper for 2 seconds followed by plunge-freezing in liquid-ethane cooled by liquid nitrogen.
TRPA1WT-JT010 dataset was collected using a Titan Krios (Thermo Fisher) transmission electron microscope operating at 300 kV equipped with a Falcon III detector in counting mode, using the EPU automated data acquisition program. 2313 movies were collected at a nominal magnification of 75,000× with a pixel size of 1.08 Å/pix at specimen level. Each movie contains 30 frames over 60.0 s exposure time, using a dose rate of 0.8 e−/pix/s, resulting a total accumulated dose of ~42 e−/Å2. The nominal defocus range was set from −1.25 to −3 μm. TRPA1C621S-Apo and TRPA1WT-BITC datasets were collected using a Titan Krios operating at 300 kV equipped with a K3 (Gatan) detector operated in counting mode, using Latitude automated data acquisition program. 3,544 movies for the TRPA1C621S-Apo dataset and 4,418 movies for the TRPA1WT BITC dataset were collected at a nominal magnification of 22,500× with a pixel size of 1.07 Å/pix at specimen level. Each movie contains 60 frames over 4.6 s exposure time, using a dose rate of ~15 e−/pix/s, resulting a total accumulated dose of ~60 e−/Å2. The defocus range was set from −0.75 to −2.5 μm.
Cryo-EM data processing
TRPA1WT JT010
Beam-induced motion correction and dose-weighing was performed using MotionCor2 (Zheng et al., 2017), followed by CTF estimation using Gctf (Zhang, 2016). Micrographs were subsequently selected based on CTF fit quality, yielding 2,031 good quality micrographs. 1,035 particles were manually picked and subjected to a reference-free 2D classification (k=8, T=2), from which the best six classes were selected as reference for automated particle picking in RELION 3.0 (Zivanov et al., 2018). A total of 752,104 particles were picked from 2,031 micrographs and extracted (4×4 Fourier binned, 4.32 Å/pix pixel size and 64 pix box size). Reference free 2D classification (k=50, T=2) was performed in RELION resulting in 21 classes (571,201 particles) which showed clear secondary structure features of TRPA1. These particles were subsequently subjected to 3D auto-refine in RELION, using previously published TRPA1 map (EMD-6267, low-passed filtered to 30 Å) as reference, without masking. Refined particles were re-extracted, re-centered and un-binned (1.08 Å/pix) and subjected to another round of 3D refinement, using the result of the previous 3D refinement as a reference. A 3D classification without image alignment was then performed using the output from the un-binned 3D refinement (k=6, T=8), with a soft solvent mask covering the best resolved region of the channel (AR12 to CC). One class, containing 137,605 particles and displaying the most high-resolution features of the channel was isolated and subjected to 3D refinement, producing a 3.06 Å map. The particles form this refinement were then subjected to another round of 2D classification (k=15, T=2), and the best 8 classes were utilized as a template for automated particle picking, resulting in 380,605 particles. 2D classification, 3D refinement and 3D classification were repeated for this set of particles. One class from this new round of 3D classification, which displayed the most high-resolution features, was subjected to 3D refinement yielding a 3.04 Å map. Focused 3D classification did not reveal alternative conformations of transmembrane domain or coupling domain. CTF refinement and Bayesian polishing were subsequently carried out on this set of particles using RELION, which yielded a final map with 2.88 Å resolution. Local resolution was calculated using RELION 3.0 (Zivanov et al., 2018).
TRPA1WT BITC
Beam-induced motion correction and dose-weighing was performed using MotionCor2 (Zheng et al., 2017), followed by CTF estimation using Gctf (Zhang, 2016). Good micrographs were selected based on quality of CTF fit, resulting in 2,186 good micrographs. 1,435 particles were manually selected and used in a reference-free 2D classification (k=8, T=2). From this classification, the six classes showing the best-defined TRPA1 features were selected as template for automated reference-based particle picking in RELION. A total of 396,597 particles were extracted (4×4 Fourier binned, 4.28 Å/pix pixel size and 64 pix box size). Reference free 2D classification (k=50, T=2) was performed in RELION and 8 classes containing a total of 283,493 particles which showed clear, crisp TRPA1 features were selected. These particles were then subjected to 3D auto-refinement without masking in RELION, using the 3D reconstruction of TRPA1WT-JT010 low-pass filtered to 30 Å as reference map. Refined particles were re-extracted, re-centered and un-binned (1.07 Å/pix, 256 pix box size) and subjected to another round of 3D refinement, using the map generated from previous 3D refinement as a reference. Particles and the refined coordinates from the 3D refinement were then subjected to a no-alignment 3D classification (k=4, T=8), using a soft solvent mask covering the best resolved region of the channel (AR12 to CC). One class, containing 74,677 particles and displaying the most high-resolution features of the channel, was selected and subjected to 3D refinement, producing a 3.37 Å map. Focused 3D classification did not reveal alternative conformations of transmembrane domain or coupling domain. CTF refinement (with per-micrograph astigmatism estimation and beam tilt fitting) and Bayesian polishing (using first 40 frames only) was subsequently carried out using RELION, which yielded a final map with 3.06 Å resolution. Local resolution was calculated using RELION 3.0 (Zivanov et al., 2018).
TRPA1C621S
Beam-induced motion correction and dose-weighing was performed using MotionCor2 (Zheng et al., 2017), followed by CTF estimation using Gctf (Zhang, 2016). 2,856 micrographs were selected for further data processing based on CTF fit quality. 1,362 particles were manually picked and subjected to a reference-free 2D classification (k=10, T=2), from which the best six classes, showing highly defined TRPA1 features were selected as template for automated reference-based particle picking in RELION. A total of 1,150,312 particles were boxed and then extracted (4×4 Fourier binned, 4.28 Å/pix pixel size and 64 pix box size). Reference free 2D classification (k=50, T=2) was performed in RELION and 14 classes containing 639,334 particles which showed well-defined TRPA1 features were selected. A 3D auto-refinement without masking was then performed with the selected particles, using the TRPA1WT-JT010 map low-pass filtered to 30 Å as reference map. The refined particles were re-extracted, re-centered and un-binned (1.07 Å/pix, 256 pix box size) and subjected to another round of 3D refinement, using the map generated from the previous 3D refinement. Particles and refined coordinates from this 3D refinement were then subjected to a no-alignment 3D classification (k=4, T=8), using a soft solvent mask covering the best resolved region of the channel (AR12 to CC). One best class, containing 119,697 particles, displayed the best-defined features of TRPA1 and was selected and subjected to 3D refinement, producing a 3.13 Å map. Focused 3D classification did not reveal alternative conformations for TMD or CD. Two iterative rounds of CTF refinement (with per-micrograph astigmatism estimation and beam tilt fitting) and Bayesian polishing was subsequently carried out using RELION, which yielded a final map with 2.81 Å resolution. Local resolution was calculated using RELION 3.0 (Zivanov et al., 2018).
Model building and refinement
The previously published TRPA1 structure (PDB 3J9P) (Paulsen et al., 2015) was used as a reference for building TRPA1WT-JT010 and the coupling domain, the interfacial helix (IFH), the S1–S2 linker, and P2 were built de novo. During model building the register assignment was guided by the presence of large aromatic side chains. The structure was refined manually with ideal secondary structure geometry restrains in Coot (Emsley and Cowtan, 2004) and using the phenix-real_space_refine function with global minimization and secondary structure restrains as implemented in the Phenix suite (Adams et al., 2010). The models for the TRPA1WT-BITC and TRPA1C621S-Apo were built using the TRPA1WT-JT010 coordinates as a reference. The placement of individual structural elements was performed by rigid body fitting, and the structures were refined in Coot, with ideal secondary structure geometry imposed, as well as using phenix-real_space_refine with global minimization and secondary structure restrains in Phenix. The restraints for lipids, sugars and ligands, including POPC, POPE, POPG, N-acetylglucosamine, JT010 and BITC, were calculated in Elbow (as implemented in Phenix (Adams et al., 2010)) from canonical SMILES strings and optimized using the REEL QM2 method (as implemented in the Phenix suite (Adams et al., 2010)). These were then inspected manually to ensure correct stereochemistry before being fitted into the experimental densities in Coot. The MolProbity server (http://molprobity.biochem.duke.edu) was utilized to identify errors and problematic regions in the models, which were then corrected manually in Coot. The Fourier shell correlation of the half- and full-maps against the model, calculated in Phenix, were in good agreement, indicating that the models were not over-refined. The model of TRPA1WT-JT010 contains residues 446–1079 with ARD1–11, C-terminus and three loops missing (residues 1–445, 754–760, 1013–1014, 1026–1038, 1079–1119 missing); TRPA1WT-BITC contains residues 446–1079 with ARD111, C-terminus and two loops missing (residues 1–445, 754–760, 1027–1038, 1079–1119 missing); TRPA1C621S-Apo contains residues 446–1079 with ARD1–11, C-terminus and four loops missing (residues 1–445, 754–760, 669–676, 1013–1014, 1026–1038, 1079–1119 missing). Structural analysis and illustrations were performed using Pymol and UCSF Chimera (Goddard et al., 2007).
Patch-clamp Electrophysiology in HEK-293 cells
HEK-293 cells were obtained from Duke Cell Culture Facility and maintained in DMEM containing 10% fetal bovine serum (FBS, Sigma), 1% penicillin and streptomycin (Gibco) and supplemented with 5% CO2 at 37 °C. Cells grown in a 6-well plate reaching 40–60% confluency were transiently transfected using Lipofectamine 2000 (Invitrogen). Generally, 2.5 μg of TRPA1 or TRPA1 mutant’s DNA and 1 μg of pEGFP DNA were used for each well. At 24 h after the transfections, the cells were digested with 0.05% trypsin and plated on poly-L-lysine coated glass coverslips.
Whole-cell patch clamp recordings were performed at room temperature using an Axopatch-200B amplifier with a Digidata 1440A, acquired with Clampex 10.6, and analyzed with Clampfit 10.6 (Axon Instruments, Union City, CA). The patch pipettes were pulled from borosilicate capillaries (World Precision Instruments) using a P-97 Flaming/Brown micropipette puller (Sutter Instrument). Pipettes with the resistance of 4–6 MΩ were used for the whole-cell recordings. Whole cell recordings were performed in an extracellular solution that contained (in mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH and osmolality to 300–310 mOsm. The internal solution contained (in mM): 140 CsCl, 10 EGTA, 10 HEPES, and 2 Mg-ATP, adjusted to pH 7.3 with CsOH. TRPA1-expressing HEK293 cells were identified by co-expressed GFP. The cell was held at −70 mV, and the membrane potential was ramped from −120 mV to 120 mV over 200 ms, as previously reported (Macpherson et al., 2007a). The interval of each sweep is 10 s. The TRPA1 agonists were perfused for 1 min following a 2 min baseline recording. The whole cell current densities (pA/pF) after drug perfusion at ± 120 mV were baseline subtracted and used for generating the statistics shown in the figures.
Calcium Imaging and Immunostaining in HEK-293 cells
Ca2+ imaging was measured after loading cells with 5 μM Fura2-AM (Invitrogen) for 40 min in the Ca2+ imaging buffer (in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, adjusted to pH 7.4 with NaOH). The Ca2+ imaging protocol was a ratio-metric method with 340/380-nm wavelength light for dual excitation. Data were presented as ΔR/R0, determined as the fraction ΔR (Rt-R0) of the increase of a given ratio over baseline ratio (R0) (Han et al., 2018).
Immunostaining was performed to check the expression level of TRPA1 and TRPA1 mutants. The transfected cells were fixed in 4% paraformaldehyde 15 min at room temperature. The cells were then washed 3 times with PBS and blocked with 5% goat serum with 0.1% Triton-X100 in PBS for 1 h at room temperature and then incubated overnight at 4 °C with the primary antibody: anti-Flag antibody (mouse, 1:200, Sigma Aldrich catalog no. F3165). The cover slips were washed in PBS and incubated with the secondary antibody (1:400, Cy3-goat anti-mouse Jackson Immunoresearch) for 2 h at room temperature. DAPI (1:1,000, Thermo Scientific, catalog: 62248) was used to stain the cell nuclei. The cover slips were then washed with PBS and mounted in fluorescent mounting medium and observed under a confocal laser scanning microscope (SP5 Inverted confocal-LSRC, Leica Microsystems). Each group has 3 cover slips and 3 pictures were taken from each cover glass. Image J was used for the quantification.
QUANTIFICATION AND STATISTICAL ANALYSIS
Image quantification were carried out using Image J. Statistical testing was carried out in GraphPad Prism using two-way ANOVA, followed by Bonferroni’s post hoc test. Only one cell was recorded from each cover slip. Data represent mean ± S.E.M, unless otherwise noted. Statistical significance were represented as: **P < 0.01, ***P < 0.01, ****P < 0.0001.
DATA AND SOFTWARE AVAILABILITY
The coordinates are deposited in the Protein Data Bank with the PDB ID 6PQO (TRPA1WT-JT010), 6PQP (TRPA1WT-BITC) and 6PQQ (TRPA1C621S-Apo) the cryo-EM density maps have been deposited in EMDB with the ID EMD-20449 (TRPA1WT-JT010), EMD-20450 (TRPA1WT-BITC) and EMD-20451 (TRPA1C621S-Apo).
Supplementary Material
Highlights:
TRPA1 senses electrophiles via modification of the extraordinarily reactive C621
High resolution cryo-EM structures explain the high reactivity of C621
C621 modification elicits conformational changes that are propagated to the channel
Structures provide a basis of promiscuous but rapid electrophile sensing by TRPA1
Acknowledgments
Cryo-EM data were collected at the Shared Materials Instrumentation Facility at Duke University. We thank Ying Yin for critical manuscript reading as well as help with grid freezing and Alberto Bartesaghi for a pre-processing interface.
Funding: This work was supported by the National Institutes of Health (R35NS097241 to S.-Y.L. and R01DE17794 to R.-R.J.) and by the National Institute of Health Intramural Research Program; US National Institutes of Environmental Health Sciences (ZIC ES103326 to M.J.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors declare no competing interests.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia PK, Parks TA, Stanford KR, Mitchell DA, Varma S, Stevens SM Jr., and Taylor-Clark TE (2016). The exceptionally high reactivity of Cys 621 is critical for electrophilic activation of the sensory nerve ion channel TRPA1. J Gen Physiol 147, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, and Patapoutian A (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, and Julius D (2006). TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, and Zygmunt PM (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102, 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedikt J, Samad A, Ettrich R, Teisinger J, and Vlachova V (2009). Essential role for the putative S6 inner pore region in the activation gating of the human TRPA1 channel. Biochim Biophys Acta 1793, 1279–1288. [DOI] [PubMed] [Google Scholar]
- Cao E, Liao M, Cheng Y, and Julius D (2013). TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, She J, Zeng W, Guo J, Xu H, Bai XC, Jiang Y (2017) Structure of mammalian endolysosomal TRPML1 channel in nanodiscs Nature, 550 (2017), 415–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TJ, Acuna MA, Zenobi-Wong M, Zeilhofer HU, and Urech D (2016). Effects of N-Glycosylation of the human cation channel TRPA1 on agonist-sensitivity. Biosci Rep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod O, Debus KY, Reznick J, Bennett NC, Sanchez-Carranza O, Omerbasic D, Hart DW, Barker AJ, Zhong W, Lutermann H, et al. (2019). Rapid molecular evolution of pain insensitivity in multiple African rodents. Science 364, 852–859. [DOI] [PubMed] [Google Scholar]
- Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. [DOI] [PubMed] [Google Scholar]
- Gao Y, Cao E, Julius D, and Cheng Y (2016). TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard TD, Huang CC, and Ferrin TE (2007). Visualizing density maps with UCSF Chimera. J Struct Biol 157, 281–287. [DOI] [PubMed] [Google Scholar]
- Goehring A, Lee CH, Wang KH, Michel JC, Claxton DP, Baconguis I, Althoff T, Fischer S, Garcia KC, and Gouaux E (2014). Screening and large-scale expression of membrane proteins in mammalian cells for structural studies. Nat Protoc 9, 2574–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosen TC, Mills DE, and Hollenberg PF (2001). Effects of benzyl isothiocyanate on rat and human cytochromes P450: identification of metabolites formed by P450 2B1. J Pharmacol Exp Ther 296, 198–206. [PubMed] [Google Scholar]
- Gui J, Liu B, Cao G, Lipchik AM, Perez M, Dekan Z, Mobli M, Daly NL, Alewood PF, Parker LL, et al. (2014). A tarantula-venom peptide antagonizes the TRPA1 nociceptor ion channel by binding to the S1–S4 gating domain. Curr Biol 24, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (1999). Antioxidant defence mechanisms: from the beginning to the end (of the beginning). Free Radic Res 31, 261–272. [DOI] [PubMed] [Google Scholar]
- Han Q, Liu D, Convertino M, Wang Z, Jiang C, Kim YH, Luo X, Zhang X, Nackley A, Dokholyan NV, et al. (2018). miRNA-711 Binds and Activates TRPA1 Extracellularly to Evoke Acute and Chronic Pruritus. Neuron 99, 449–463 e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, and Julius D (2006). TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 103, 19564–19568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi M, Herzik MA Jr., Wie J, Suo Y, Borschel WF, Ren D, Lander GC, and Lee SY (2017). Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature 550, 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Bandell M, Petrus MJ, Zhu MX, and Patapoutian A (2009). Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 5, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, and Julius D (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265. [DOI] [PubMed] [Google Scholar]
- Koivisto A, Jalava N, Bratty R, and Pertovaara A (2018). TRPA1 Antagonists for Pain Relief. Pharmaceuticals (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, et al. (2010). A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 66, 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M, Cao E, Julius D, and Cheng Y (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin King JV, Emrick JJ, Kelly MJS, Herzig V, King GF, Medzihradszky KF, and Julius D (2019). A Cell-Penetrating Scorpion Toxin Enables Mode-Specific Modulation of TRPA1 and Pain. Cell 178, 1362–1374 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macikova L, Sinica V, Kadkova A, Villette S, Ciaccafava A, Faherty J, Lecomte S, Alves ID, and Vlachova V (2019). Putative interaction site for membrane phospholipids controls activation of TRPA1 channel at physiological membrane potentials. FEBS J 286, 3664–3683. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, and Patapoutian A (2007a). Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445, 541–545. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, and Patapoutian A (2007b). An ion channel essential for sensing chemical damage. J Neurosci 27, 11412–11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsakova L, Barvik I, Zima V, Zimova L, and Vlachova V (2017). The First Extracellular Linker Is Important for Several Aspects of the Gating Mechanism of Human TRPA1 Channel. Front Mol Neurosci 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. (2007). TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 104, 13525–13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini R, Materazzi S, Benemei S, and Geppetti P (2014). The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev Physiol Biochem Pharmacol 167, 1–43. [DOI] [PubMed] [Google Scholar]
- Paulsen CE, Armache JP, Gao Y, Cheng Y, and Julius D (2015). Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 520, 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, and Sligar SG (2009). Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol 464, 211–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CM, Michailidis I, and Logothetis DE (2005). PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8, 626–634. [DOI] [PubMed] [Google Scholar]
- Samanta A, Kiselar J, Pumroy RA, Han S, and Moiseenkova-Bell VY (2018). Structural insights into the molecular mechanism of mouse TRPA1 activation and inhibition. J Gen Physiol 150, 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerratt S (2017). Recent Progress in the Discovery and Development of TRPA1 Modulators. Prog Med Chem 56, 81–115. [DOI] [PubMed] [Google Scholar]
- Takaya J, Mio K, Shiraishi T, Kurokawa T, Otsuka S, Mori Y, and Uesugi M (2015). A Potent and Site-Selective Agonist of TRPA1. J Am Chem Soc 137, 15859–15864. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Ghatta S, Bettner W, and Undem BJ (2009). Nitrooleic acid, an endogenous product of nitrative stress, activates nociceptive sensory nerves via the direct activation of TRPA1. Mol Pharmacol 75, 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW Jr., Ghatta S, Carr MJ, and McAlexander MA (2008). Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol Pharmacol 73, 274–281. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, et al. (2007). 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 104, 13519–13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Chang RB, Waters HN, McKemy DD, and Liman ER (2008). The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem 283, 32691–32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer-Menkhoff I, and Lotsch J (2018). Human pharmacological approaches to TRP-ion-channel-based analgesic drug development. Drug Discov Today 23, 2003–2012. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, and Bautista DM (2011). TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, and Bautista DM (2013). The ion channel TRPA1 is required for chronic itch. J Neurosci 33, 9283–9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, and Hu H (2018). TRP Channels as Drug Targets to Relieve Itch. Pharmaceuticals (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Tu YH, Cooper AJ, Zhang Z, Katritch V, and Liman ER (2018). Activation Stoichiometry and Pore Architecture of TRPA1 Probed with Channel Concatemers. Sci Rep 8, 17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Le SC, Hsu AL, Borgnia MJ, Yang H, and Lee SY (2019). Structural basis of cooling agent and lipid sensing by the cold-activated TRPM8 channel. Science 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Wu M, Zubcevic L, Borschel WF, Lander GC, and Lee SY (2018). Structure of the cold- and menthol-sensing ion channel TRPM8. Science 359, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K (2016). Gctf: Real-time CTF determination and correction. J Struct Biol 193, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y, and Agard DA (2017). MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimova L, Sinica V, Kadkova A, Vyklicka L, Zima V, Barvik I, and Vlachova V (2018). Intracellular cavity of sensor domain controls allosteric gating of TRPA1 channel. Sci Signal 11. [DOI] [PubMed] [Google Scholar]
- Zivanov J, Nakane T, Forsberg B, Kimanius D, Hagen WJH, Lindahl E, and Scheres SHW (2018). RELION-3: new tools for automated high-resolution cryo-EM structure determination. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic L, Hsu AL, Borgnia MJ, and Lee SY (2019). Symmetry transitions during gating of the TRPV2 ion channel in lipid membranes. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic L, Le S, Yang H, and Lee SY (2018). Conformational plasticity in the selectivity filter of the TRPV2 ion channel. Nat Struct Mol Biol 25, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic L, and Lee SY (2019). The role of pi-helices in TRP channel gating. Curr Opin Struct Biol 58, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The coordinates are deposited in the Protein Data Bank with the PDB ID 6PQO (TRPA1WT-JT010), 6PQP (TRPA1WT-BITC) and 6PQQ (TRPA1C621S-Apo) the cryo-EM density maps have been deposited in EMDB with the ID EMD-20449 (TRPA1WT-JT010), EMD-20450 (TRPA1WT-BITC) and EMD-20451 (TRPA1C621S-Apo).


