Abstract
Background:
Left atrial appendage occlusion (LAAO) to prevent stroke in patients with atrial fibrillation has been evaluated in 2 randomized trials; post-approval clinical data are limited.
Objectives:
To describe the NCDR LAAO Registry and present patient, hospital and physician characteristics and in-hospital adverse event rates for Watchman procedures in the United States during its first 3 years.
Methods:
We described the LAAO Registry structure and governance, the outcome adjudication processes, and the data quality and collection processes. We characterize the patient population, performing hospitals, and in-hospital adverse event rates.
Results:
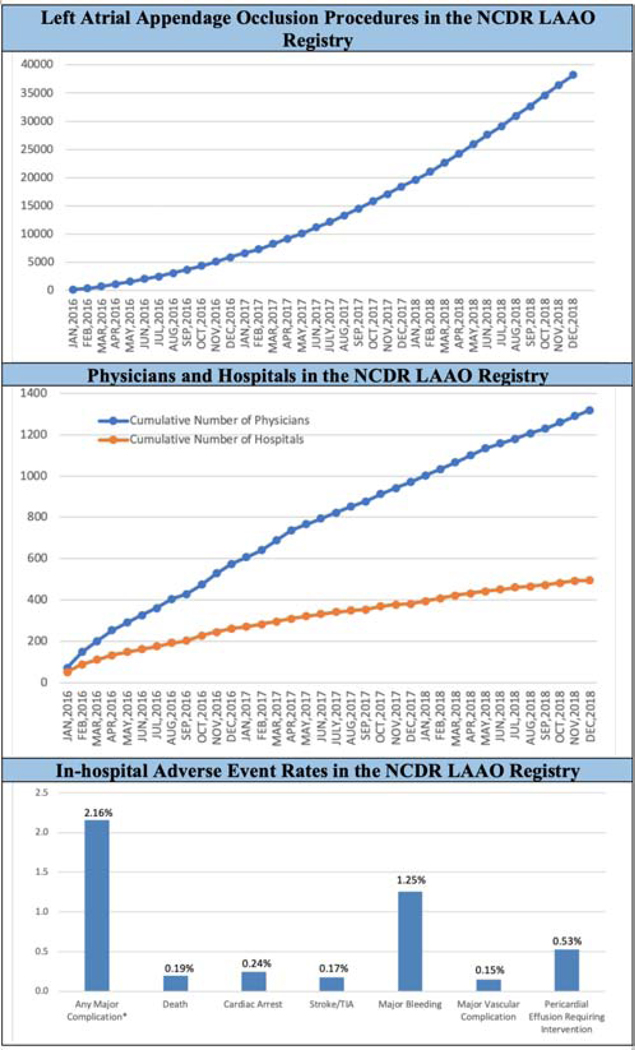
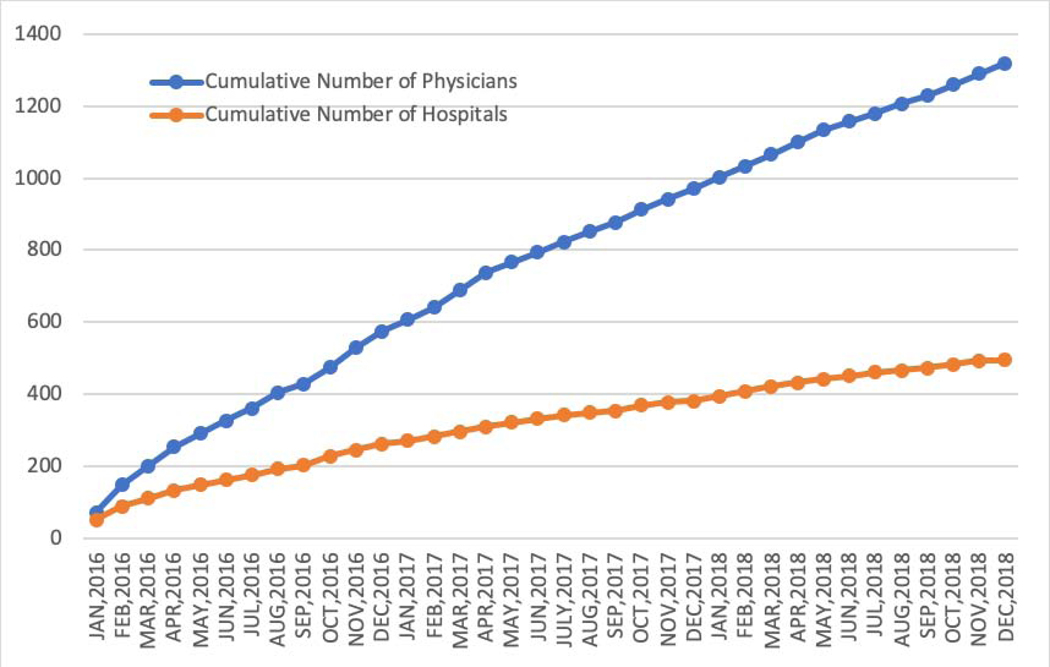
A total of 38,158 procedures from 495 hospitals performed by 1,318 physicians in the United States were included between January 2016 and December 2018. The mean patient age was 76.1±8.1 years, the mean CHA2DS2-VASC score was 4.6±1.5, and the mean HAS-BLED score was 3.0±1.1. The median annual number of LAAO procedures performed for hospitals was 30 (interquartile [IQR] range 26) and for physicians was 12 (IQR 12). Procedures were cancelled or aborted in 7% of cases; among cases in which a device was deployed, 98.1% were implanted with <5 mm leak. Major in-hospital adverse events occurred in 2.16% of patients; the most common complications were pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), while stroke (0.17%) and death (0.19%) were rare.
Conclusions:
The LAAO Registry has enrolled >38,000 patients implanted with the device. Patients who were generally older with more comorbidities than those enrolled in the pivotal trials; however, major in-hospital adverse event rates were lower than reported in those trials.
Keywords: atrial fibrillation, left atrial appendage occlusion, registry, stroke, bleeding, hospital volume
Condensed Abstract:
The LAAO Registry includes 38,158 Watchman implants, from 495 hospitals and 1,318 physicians in the United States in the first 3 years. Patients were older (mean 76.1 years) and had a higher mean CHA2DS2-VASC score (4.6) and HAS-BLED score (3) than the populations in previous trials and registries. The median annual number of LAAO procedures performed annually for hospitals was 28 and for physicians was 12 with wide variation. Major in-hospital adverse events occurred in 2.16% of patients; the most common complications were pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), while stroke (0.17%) and death (0.19%) were rare.
Introduction
Atrial fibrillation (AF) confers a 4-to-5-fold increased risk for ischemic stroke and accounts for approximately 15% of ischemic strokes in the United States each year (1–5). Longterm anticoagulation with warfarin or direct oral anticoagulants is the standard of care for stroke prevention for individuals with nonvalvular AF and a moderate or high stroke risk (6–11). LAAO lowers the risk of stroke by excluding the LAA from the systemic circulation and preventing thrombus formation and embolization;(12–18) it has emerged as a treatment option for AF patients at moderate to high risk of stroke who are poor candidates for long term anticoagulation (19). After two pivotal randomized trials with accompanying continued access protocol data, the Watchman LAAO device (Boston Scientific, Natick, Massachusetts) was approved by the U.S. Food and Drug Administration (FDA) in March 2015 for stroke prevention in AF.(20–23) Several other percutaneous LAAO devices are currently being developed and evaluated in clinical trials.(24,25) To better understand the utilization, safety and effectiveness of LAAO devices in “real world” clinical practice, the American College of Cardiology (ACC) and the Society for Coronary Angiography and Intervention (SCAI) collaborated with the FDA, the Centers for Medicare and Medicaid Services (CMS), and Boston Scientific to develop the National Cardiovascular Data Registry (NCDR) Left Atrial Appendage Occlusion Registry (LAAO Registry). In this paper, we present the patient, hospital, and physician characteristics as well as in-hospital adverse event rates for percutaneous LAAO procedures performed in the United States during the first 3 years of the LAAO Registry.
LAAO Registry Development and Structure
Registry Development
In anticipation of the expected FDA approval of the Watchman device, the NCDR considered developing a LAAO Registry in mid-2014, recognizing that comprehensive post-approval data collection and analysis would be essential for this potentially transformative new therapeutic modality. A team including cardiac electrophysiologists, interventional cardiologists with structural expertise, and registry experts was convened to develop a preliminary data collection form, which was presented for public comment during the summer of 2014. A multi-stakeholder team, including NCDR, the Society of Cardiovascular Angiography and Interventions (SCAI), FDA, CMS, and Boston Scientific refined the registry design.
The device was approved in March 2015,(26) and the LAAO Registry launched in late December 2015. CMS released a national coverage determination for patients undergoing percutaneous LAAO for non-valvular atrial fibrillation in February 2016, predicating reimbursement upon enrollment of patients in a prospective, national, audited registry with follow-up for at least four years following implantation.(27) With the guidance of the multi-stakeholder team and the support of FDA, CMS, and Boston Scientific Corporation, the LAAO Registry was designed to function as the formal post-market surveillance vehicle (Watchman New Enrollment PoST Approval Surveillance Analysis Plan [NESTed SAP]) required by FDA for the device, and it is currently the only registry approved by CMS to satisfy the coverage decision data submission requirements. Starting on April 1, 2016 U.S. hospitals were required to submit data for all Watchman procedures into the LAAO Registry in order to qualify for Medicare reimbursement (Supplemental Table 1). Hospitals are encouraged to submit data on all device recipients regardless of insurance status. While data are currently not available regarding whether this recommendation is universally followed, 90% of hospitals participating in the NCDR ICD Registry reported all procedures regardless of payer with a similar coverage with evidence requirement (28). Inclusion of patients undergoing LAAO with other devices not FDA approved for this indication is not required by any regulatory agency; however, these procedures are performed substantially less frequently in the US. As a result of the multi-stakeholder engagement process, the registry serves multiple purposes, including measurement of real-world quality of care for enrolling hospitals, fulfillment of FDA-mandated requirements, compliance with the CMS reimbursement mandate, and the creation of a unique data resource for ongoing clinical research.
Algorithmic Adjudication
The LAAO Registry developed and validated a novel process that is used to adjudicate adverse clinical events in follow-up based on that developed by the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry (STS/ACC TVT Registry™). Clinical trials and manufacturer-sponsored post-approval studies employ informed consent and centralized adjudication of events using a clinical events committee (CEC) model, while registries typically ask sites to assign outcomes locally without confirmation. The CEC process is systematic and accurate, but labor intensive and costly; comparatively, unadjudicated site-reporting of outcomes is less expensive but lacks standardization and is less accurate. Adjudicated adverse events include ischemic stroke, hemorrhagic stroke, undetermined stroke, TIA, intracranial hemorrhage, systemic embolism, major bleeding, and major vascular complication (Supplemental Table 2). A computer-based algorithm uses discrete combinations of registry data elements based on standard event definitions delineated by registry leadership and expert consultants to adjudicate adverse events. The automatic adjudicated process has been validated against formal CEC adjudication over the first two years of the registry. In cases where registry data elements are not adequately complete or conflicting, manual adjudication is used. The methods for the development of algorithmic adjudication in the LAAO Registry, and the results of the validation study evaluating the performance of the algorithmic adjudication processes compared with formal CEC adjudication will be presented in greater detail separately.
Governance of the NCDR LAAO Registry
The LAAO Registry Steering Committee oversees the development and revision of the data collection instruments, the adverse event adjudication system, and scientific research and publication procedures. As with all NCDR programs, a Research and Publications (R&P) Subcommittee, which reports to the Steering Committee, reviews research proposal applications for NCDR-funded research, abstracts, posters, and manuscripts prior to public presentation or publication. Proposals for NCDR-funded research using data from the LAAO Registry are reviewed for feasibility, scientific merit, and priority. The R&P Subcommittee also reviews abstracts, posters, and manuscripts resulting from industry and grant-funded research.
Data Collection, Data Quality and Feedback Reports
The LAAO Registry collects approximately 220 data elements from the implant hospitalization, 60 for each follow-up visit, and 15 data elements to support the adjudication of adverse events. A link to the full data collection forms for the index hospitalization and all follow-up visits is publicly available (https://cvquality.acc.org/docs/default-source/ncdr/datacollection/laao_v1–2_datacollectionform_2_2019.pdf?sfvrsn=70a181bf_2). Data include patient, provider and facility characteristics; procedure indications; pre-, intra-, and post-procedure medical and interventional details including LAA size, devices used, reasons for aborting or cancelling a procedure, residual leak size and imaging guidance methods used; and adverse event rates during the index procedure hospitalization. Data are collected at mandated follow-up visits at 45 days, 6 months, 1 year, and 2 years, including but not limited to stroke, intracranial hemorrhage, systemic embolism, major bleeding, major vascular complications, and death. Neurological assessment is performed at each follow-up visit and the Modified Rankin Scale is reported. At each of these follow-up visits, it is also reported if echocardiography, computed tomography, and magnetic resonance imaging has been performed and whether this demonstrates atrial thrombus or device margin residual leak. Adherence to mandated follow-up visits is reported as a process measure for each site. Linkage to Medicare administrative data will be performed to allow for ascertainment of adverse events in years 3 and 4 after implantation; for the minority of patients not billed through Medicare/Medicaid, adverse event rates cannot currently be captured through the LAAO Registry during years 3–4 of follow-up.
The NCDR utilizes a rigorous Data Quality Reporting (DQR) process to ensure that submissions are complete, valid, and accurate. An annual audit, in which submitted data are compared with source documentation and billing data to capture under-reported or mis-reported data. This audit process is conducted annually at randomly selected sites and included 20 sites during the last audit (approximately 5% of sites) with a 93.3% agreement rate between registry-reported data compared with source document review and 100% agreement between billing compared with registry-reported data.(29). The audit process includes feedback to participants and is a mechanism for the registry leadership to identify gaps in data collection and reporting that may be generalizable across sites.
Quality improvement reports are sent quarterly to sites enrolling in the LAAO Registry and include hospital enrollment volume, process measures, and outcomes data benchmarked against similar volume hospitals and national aggregates. Real-time feedback on measure performance is accessible to the hospital via the online registry dashboard. Thus, the registry serves an important role in quality improvement for participating hospitals.
Periodic Registry Updates
As with the other registry programs in the NCDR’s portfolio, the LAAO Registry will undergo periodic updates to the data collection form and processes, generally incorporating greater detail or improved clarity of existing data elements and adding new data elements when required by changes in clinical practice. The data collection form has been updated twice to date in July 2017 and October 2018.
Analytic Methods
Analyses for this manuscript were conducted using SAS, version 9.4 (Cary, N.C.). Cumulative numbers of patients enrolled, implanting physicians and implanting hospitals were calculated and plotted online graphs. Patient and hospital characteristics were compared using Pearson Chi Square or Wilcoxon rank-sum test as appropriate and reported as mean and standard deviation (SD), median and interquartile range (IQR), or number and percent with associated P values. We categorized participants as follows: 1) All procedures 2) successful procedures, 3) aborted procedures, and 4) cancelled procedures. Aborted procedures are defined as those in which venous access was performed, but in which a device was not ultimately deployed. A deployed device was defined as one that has been unsheathed and placed in the LAA but remains connected to a delivery catheter and can be re-sheathed and removed, while an implanted device was defined as one in which the device has been released from the delivery catheter and left in place in the LAA. Cancelled procedures were defined as those which were stopped prior to obtaining venous access.
We stratified participants by categories of CHA2DS2-VASC score and HAS-BLED scores.(30,31) Hospital and physician annual procedure volume were calculated (excluding cases that were cancelled but including cases that were started but aborted) and was divided into subgroups of volume and graphed in bar charts. Finally, we compared the number and percent of patients with major in-hospital adverse events overall and among those with a successful, aborted, or cancelled implant procedure.
Results
Patient Characteristics
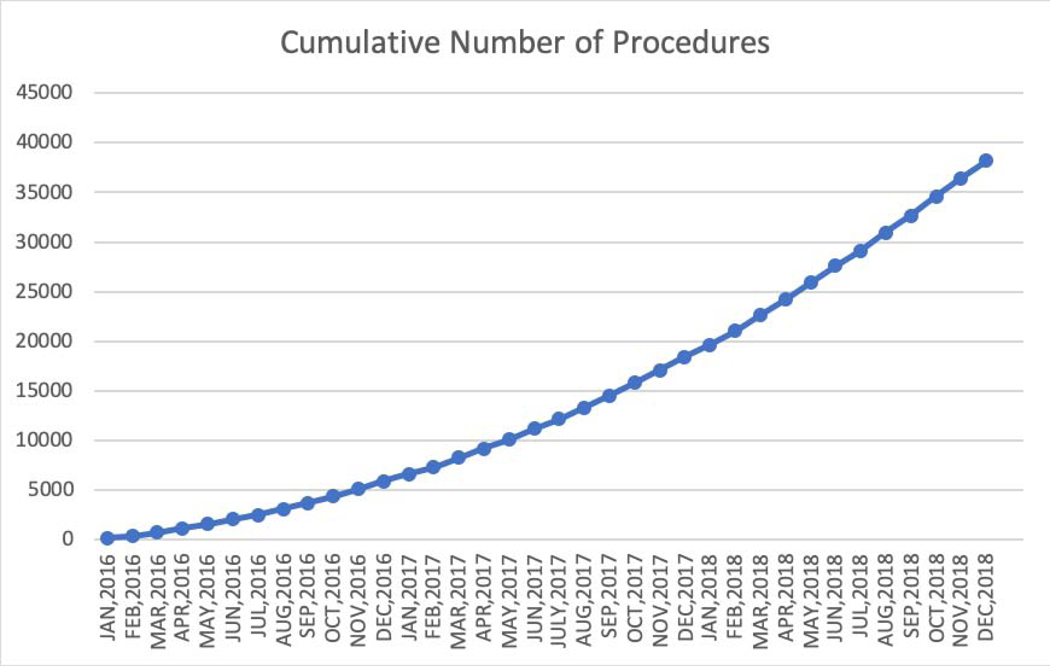
Between January 1, 2016 and December 31, 2018, data were collected for 38,158 Watchman procedures performed by 1,318 physicians in 495 hospitals in the United States (Figures 1A and 1B). Table 1 shows the sociodemographic characteristics and insurance status of the patients enrolled over this period. The mean age was 76.1 ± 8.1 years, 58.9% of the cohort was male, and most were white (92.6%). Minority representation was low relative to the national population, but the absolute numbers of non-white patients were much larger than in prior U.S. trial and registry populations (n=1768 [4.6%] for black patients and n=621 for Asian patients [1.6%]). CMS beneficiaries accounted for 86.9% of patients in the overall cohort.
Figure 1.
Procedure Volume (A), Implanting Physicians, and Implanting Hospitals (B) in the NCDR LAAO Registry. Between January 1, 2016 and December 31, 2018, data were collected for 38,158 left atrial appendage implant procedures performed by 1,318 physicians in 495 hospitals in the United States. NCDR= National Cardiovascular Data Registry, LAAO= Left Atrial Appendage Occlusion
Table 1.
Sociodemographic Characteristics and Insurance Providers for Watchman Patients Enrolled in the LAAO Registry between January 1, 2016 to December 31, 2018.
| Total | Successful | Aborted* | Cancelled* | ||
|---|---|---|---|---|---|
| Characteristic | Procedure | Procedure | Procedure | P value | |
| N (%) | N (%) | N (%) | N (%) | ||
| Overall | 38158 | 35417 | 1601 | 1140 | |
| Demographics | |||||
| Age, mean (SD), years | 76.1 (8.1) | 76.0 (8.1) | 77.2 (7.9) | 77.2 (7.9) | <0.0001 |
| Age categories | <0.0001 | ||||
| <55 | 496 (1.3) | 470 (1.3) | 17 (1.1) | 9 (0.8) | |
| 55 to 64 | 2303 (6.0) | 2176 (6.1) | 78 (4.9) | 49 (4.3) | |
| 65 to 74 | 12167 (31.9) | 11422 (32.3) | 419 (26.2) | 326 (28.6) | |
| 75 to 84 | 17799 (46.7) | 16438 (46.4) | 805 (50.3) | 556 (48.8) | |
| >=85 | 5393 (14.1) | 4911 (13.9) | 282 (17.6) | 200 (17.5) | |
| Sex | 0.3337 | ||||
| Male | 22468 (58.9) | 20844 (58.9) | 926 (57.8) | 698 (61.2) | |
| Female | 15672 (41.1) | 14556 (41.1) | 675 (42.2) | 441 (38.7) | |
| Race | 0.1715 | ||||
| White | 35345 (92.6) | 32805 (92.6) | 1498 (93.6) | 1042 (91.4) | |
| Black | 1768 (4.6) | 1653 (4.7) | 53 (3.3) | 62 (5.4) | |
| Hispanic | 138 (0.4) | 130 (0.4) | 5 (0.3) | 3 (0.3) | |
| Asian | 621 (1.6) | 565 (1.6) | 33 (2.1) | 23 (2.0) | |
| American Indian/Alaskan Native | 94 (0.3) | 90 (0.3) | 3 (0.2) | 1 (0.1) | |
| Native Hawaiian/Pacific Islander | 49 (0.1) | 46 (0.1) | 1 (0.1) | 2 (0.2) | |
| Other | 143 (0.4) | 128 (0.4) | 8 (0.5) | 7 (0.6) | |
| Primary insurance payer | 0.8359 | ||||
| Medicare/Medicaid | 33141 (86.9) | 30752 (86.8) | 1389 (86.8) | 1000 (87.7) | |
| Private health insurance | 4475 (11.7) | 4165 (11.8) | 188 (11.7) | 122 (10.7) | |
| Other | 542 (1.4) | 500 (1.4) | 24 (1.5) | 18 (1.6) |
SD= standard deviation
Aborted procedures are those which are concluded after central venous access is obtained. Cancelled procedures are those which are concluded prior to obtaining central venous access.
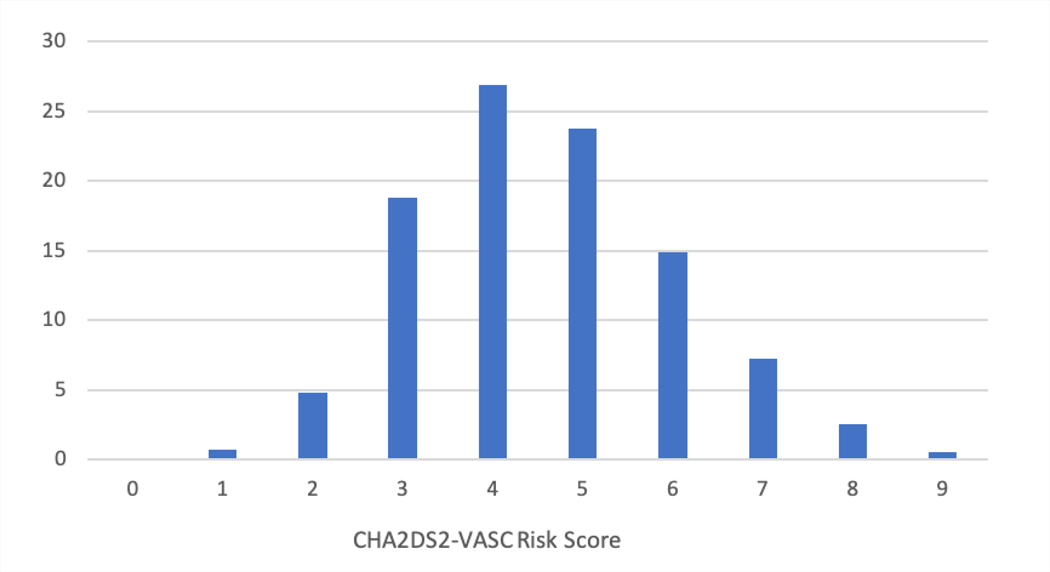
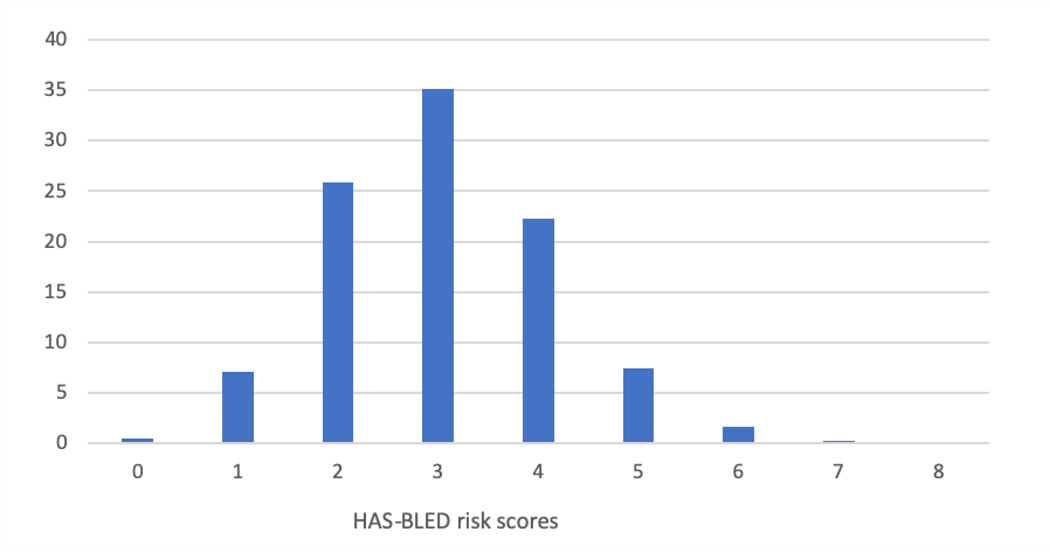
Table 2 shows the medical history of the patients enrolled in the first 3 years of the LAAO Registry. Patients had a mean CHA2DS2-VASC score of 4.6±1.5 (Figure 3A) and a mean HAS-BLED score of 3 ±1.1 (Figure 3B). A prior history of stroke was common (27.3%), and most (69.3%) had a history of prior bleeding. Among those with prior bleeding, the most common source was gastrointestinal (41.8%), followed by intracranial (11.9%) and epistaxis (6.4%). About half of patients had paroxysmal AF (51.9%), 30.7% had persistent or long-standing persistent AF, and 16.9% had permanent AF. Data were missing for <1% of patients for variables reported in Tables 1 and 2.
Table 2.
Medical History for Watchman Patients Enrolled in the LAAO Registry between January 1, 2016 to December 31, 2018.
| Total | Successful Procedure | Aborted* Procedure | Cancelled* Procedure | P value | |
|---|---|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) | N (%) | |
| Overall | 38158 | 35417 | 1601 | 1140 | |
| CHA2DS2- VASC score,mean (SD) | 4.6 (1.5) | 4.6 (1.5) | 4.6 (1.4) | 4.8 (1.5) | <0.0001 |
| Congestive heart failure | 14266 (37.4) | 13186 (37.2) | 582 (36.4) | 498 (43.7) | <0.0001 |
| Congestive heart failure class | 0.0007 | ||||
| NYHA class I | 3477 9.11 | 3218 9.09 | 147 9.18 | 112 9.82 | |
| NYHA class II | 6527 17.11 | 6051 17.09 | 266 16.61 | 210 18.42 | |
| NYHA class III | 3075 8.06 | 2835 8.00 | 116 7.25 | 124 10.88 | |
| NYHA class IV | 202 0.53 | 179 0.51 | 11 0.69 | 12 1.05 | |
| Hypertension | 35148 (92.1) | 32673 (92.3) | 1436 (89.7) | 1039 (91.1) | 0.0017 |
| Diabetes mellitus | 14396 (37.7) | 13456 (38.0) | 504 (31.5) | 436 (38.2) | <0.0001 |
| Prior stroke | 10433 (27.3) | 9584 (27.1) | 474 (29.6) | 375 (32.9) | <0.0001 |
| Prior transient ischemic attack | 5555 (14.6) | 5100 (14.4) | 247 (15.4) | 208 (18.3) | 0.0001 |
| Prior thromboembolic event | 7005 (18.4) | 6453 (18.2) | 316 (19.7) | 236 (20.7) | 0.0005 |
| Vascular disease | 16767 (43.9) | 15560 (43.9) | 689 (43.0) | 518 (45.4) | 0.0001 |
| Prior myocardial infarction | 7738 (20.3) | 7158 (20.2) | 331 (20.7) | 249 (21.8) | 0.5707 |
| Peripheral arterial disease | 5487 (14.4) | 5067 (14.3) | 243 (15.2) | 177 (15.5) | 0.4321 |
| Known aortic plaque | 1582 (4.2) | 1476 (4.2) | 56 (3.5) | 50 (4.4) | 0.3871 |
| HAS-BLED score, mean (SD) | 3.0 (1.1) | 3.0 (1.1) | 3.0 (1.1) | 3.1 (1.2) | 0.0118 |
| Uncontrolled hypertension | 10120 (26.5) | 9415 (26.6) | 406 (25.4) | 299 (26.2) | 0.7865 |
| Abnormal renal function | 5188 (13.6) | 4850 (13.7) | 170 (10.6) | 168 (14.7) | 0.0004 |
| Abnormal liver function | 1228 (3.2) | 1119 (3.2) | 52 (3.3) | 57 (5.0) | 0.0001 |
| Prior stroke | 10433 (27.3) | 9584 (27.1) | 474 (29.6) | 375 (32.9) | <0.0001 |
| Ischemic | 5834 (15.3) | 5325 (15.0) | 289 (18.1) | 220 (19.3) | <0.0001 |
| Hemorrhagic | 2798 (7.3) | 2564 (7.2) | 134 (8.4) | 100 (8.8) | 0.0002 |
| Undetermined | 2357 (6.2) | 2177 (6.2) | 100 (6.3) | 80 (7.0) | 0.0001 |
| Prior bleeding | 26762 (70.1) | 24819 (70.1) | 1151 (71.9) | 792 (69.5) | 0.1383 |
| Labile INR | 4458 (11.7) | 4135 (11.7) | 182 (11.4) | 141 (12.4) | 0.0175 |
| Alcohol use | 2114 (5.5) | 1973 (5.6) | 82 (5.1) | 59 (5.2) | 0.0007 |
| Antiplatelet medication use | 10585 (27.7) | 9795 (27.7) | 436 (27.2) | 354 (31.1) | 0.0334 |
| Non-steroidal inflammatory drug use | 11369 (29.8) | 10594 (29.9) | 473 (29.5) | 302 (26.5) | 0.0002 |
| Other history and risk factors | |||||
| Clinically relevant prior bleeding | 26466 (69.4) | 24515 (69.2) | 1149 (71.8) | 802 (70.4) | 0.0389 |
| Intracranial | 4550 (11.9) | 4185 (11.8) | 218 (13.6) | 147 (12.9) | 0.0482 |
| Epistaxis | 2425 (6.4) | 2245 (6.3) | 91 (5.7) | 89 (7.8) | 0.0159 |
| Gastrointestinal | 15965 (41.8) | 14782 (41.7) | 695 (43.4) | 488 (42.8) | 0.1802 |
| Other | 5686 (14.9) | 5300 (15.0) | 234 (14.6) | 152 (13.3) | 0.0354 |
| Fall risk | 15063 (39.5) | 13951 (39.4) | 656 (41.0) | 456 (40.0) | 0.0418 |
| Genetic coagulopathy | 333 (0.9) | 303 (0.9) | 21 (1.3) | 9 (0.8) | 0.0693 |
| Cardiomyopathy | 8098 (21.2) | 7459 (21.1) | 342 (21.4) | 297 (26.1) | 0.0018 |
| Ischemic | 4121 (10.8) | 3811 (10.8) | 158 (9.9) | 152 (13.3) | 0.0015 |
| Non-ischemic | 2828 (7.4) | 2592 (7.3) | 132 (8.2) | 104 (9.1) | 0.0036 |
| Chronic lung disease | 8101 (21.2) | 7525 (21.3) | 338 (21.1) | 238 (20.9) | 0.9544 |
| Coronary artery disease | 18126 (47.5) | 16824 (47.5) | 738 (46.1) | 564 (49.5) | <0.5294 |
| Sleep apnea | 9740 (25.5) | 9103 (25.7) | 366 (22.9) | 271 (23.8) | 0.0409 |
| Arrhythmia history | |||||
| Atrial fibrillation type | <0.0001 | ||||
| Paroxysmal | 19800 51.89 | 18566 52.42 | 806 50.34 | 428 37.54 | |
| Persistent (>7 days) | 8056 21.11 | 7489 21.15 | 334 20.86 | 233 20.44 | |
| Long-standing persistent (>1 year) | 3674 9.63 | 3366 9.50 | 151 9.43 | 157 13.77 | |
| Permanent | 6461 16.93 | 5848 16.51 | 300 18.74 | 313 27.46 | |
| Atrial flutter | 5201 13.63 | 4903 13.84 | 188 11.74 | 110 9.65 | 0.0001 |
SD= standard deviation, INR= international normalized ratio
Aborted procedures are those which are concluded after central venous access is obtained. Cancelled procedures are those which are concluded prior to obtaining central venous access
Figure 3.
Distribution of CHA2DS2-VASC (A) and HAS-BLED (B) Scores Among Patients Enrolled in the LAAO Registry between January 1, 2016 to December 31, 2018. Patients had a high risk of stroke and thromboembolism with a mean CHA2DS2-VASC score of 4.6±1.5 and a high risk of bleeding events with a mean HAS-BLED score of 3 ±1.1. LAAO= Left Atrial Appendage Occlusion.
Table 3 shows selected patient characteristics for patients enrolled in the LAAO Registry compared with patients enrolled in the PROTECT AF randomized trial and the EWOLUTION Registry. Patients in the LAAO Registry were substantially older (76.1±8.1) and had higher CHA2DS2- VASC scores (4.6±1.5), HAS BLED scores (3±1.1), and rates of clinically relevant bleeding (69.4%).
Table 3.
Selected Characteristics for Watchman Patients Enrolled in the LAAO Registry between January 1, 2016 to June 30, 2018 compared with Patients from the PROTECT AF Trial and the EWOLUTION Registry.
| Characteristics | PROTECT AF trial 2005-2008 (N=463 Intervention Arm) | PREVAIL trial 2011-2013 (N=269 Intervention Arm) | EWOLUTION Registry 2013-2015 (N=1025) | LAAO Registry 2016-2018 (N=38,158) |
|---|---|---|---|---|
| Demographics | ||||
| Age, mean (SD), year | 71.7 (8.8) | 74.0 (7.4) | 73.4 (8.9) | 76.1 (8.1) |
| Women, N (%) | 137 (29.6) | 87 (32.3) | 411 (40.1) | 15,672 (41.1) |
| Race, N (%) | ||||
| White/European | 425 (91.8) | 253 (94.1) | NA | 35,345 (92.6) |
| Black/African American | 6 (1.3) | 6 (2.2) | NA | 1768 (4.6) |
| Asian/Pacific Islander | 5 (1.1) | 1 (0.4) | NA | 670 (1.7) |
| Hispanic ethnicity, N (%) | 25 (5.4) | 6 (2.2) | NA | 138 (0.4) |
| Medical History | ||||
| CHA2DS2- VASC score, mean (SD) | 3.4 (1.5) | 3.8 (1.2) | 4.5 (1.6) | 4.6 (1.5) |
| Prior ischemic stroke/transient ischemic attack, N (%) | 82 (17.7) | 74 (27.5) | 312 (30.5) | 11,389 (29.9) |
| Prior congestive heart failure, N (%) | 124 (26.8) | 63 (23.4) | 350 (34.2) | 14,266 (37.4) |
| Prior diabetes mellitus, N (%) | 113 (24.4) | 91 (33.8) | 304 (29.7) | 14,396 (37.7) |
| Prior hypertension, N (%) | 413 (89.2) | 238 (88.5) | 885 (86.4) | 35,148 (92.1) |
| HAS BLED score, mean (SD) | NA | NA | 2.3 (1.2) | 3.0 (1.1) |
| Prior intracranial bleeding, N (%) | NA | NA | 155 (15.1) | 4550 (11.9) |
| Prior clinically relevant bleeding, N (%) | NA | NA | 396 (38.7) | 26,466 (69.4) |
SD= standard deviation
Hospital and Operator Characteristics
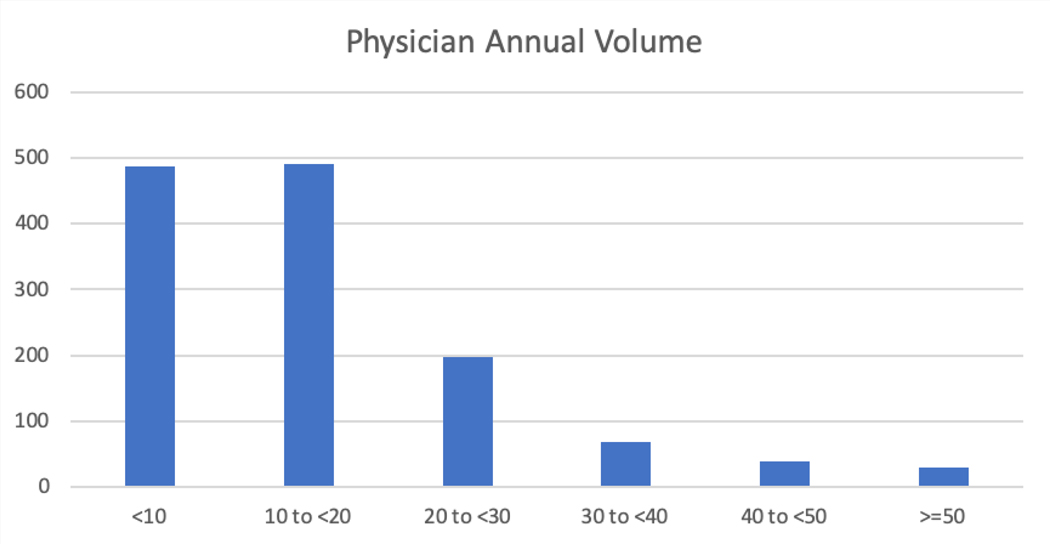
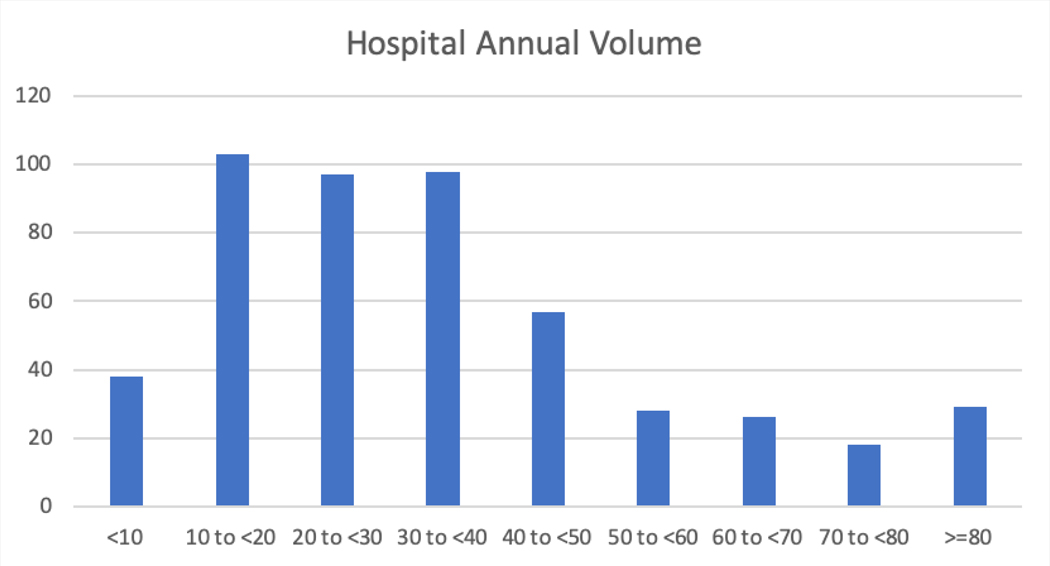
Table 4 shows the characteristics of the hospitals enrolling in the LAAO Registry. Most patients were enrolled in the South region (37.6%), followed by the West (22.7%), the Midwest (22.2%) and the Northeast (17.4%). Most hospitals were in urban areas (65.8%), were private or community hospitals (77.4%), and were teaching hospitals (64.7%). Enrolling hospitals were generally moderate to large with a median number of 504 beds. The median number of procedures performed annually for hospitals was 30 (interquartile range [IQR] 26) with most performing <40 procedures annually, although there was considerable variation in volume (Figure 4). Among physicians, the median number of LAAO procedures performed annually was 12 (IQR 12) with most performing <20 procedures annually, although there was considerable variation in annual volume (Figure 5).
Table 4.
Characteristics of the 495 Participating Hospitals in the LAAO Registry between January 1, 2016 to December 31, 2018.
| Characteristics | N (%) |
|---|---|
| Overall procedures | 38158 (100) |
| Hospital characteristics | |
| United States region | |
| Midwest | 8484 (22.2) |
| Northeast | 6649 (17.4) |
| South | 14363 (37.6) |
| West | 8654 (22.7) |
| Missing | 8 (0.0) |
| Hospital Location | |
| Rural | 2856 (7.5) |
| Suburban | 10193 (26.7) |
| Urban | 25109 (65.8) |
| Hospital Type | |
| Government | 407 (1.1) |
| Private/Community | 29546 (77.4) |
| University | 8205 (21.5) |
| Teaching Hospital | 24698 (64.7) |
| Hospital Number of Certified Beds, Median (IQR) | 504 (351) |
| Hospital Number of Certified Beds, Mean (SD) | 542 (276) |
| Hospital Annual LAAO Procedure Volume, Median (IQR) | 30 (26) |
| Hospital Annual LAAO Procedure Volume, Mean (SD) | 35.5 (25.4) |
SD= standard deviation, IQR= interquartile range
Figure 4.
Distribution of Hospital Annual Procedure Volume among 495 Participating Hospitals in the LAAO Registry between January 1, 2016 to December 31, 2018. The figure shows the number of hospitals in each annual volume category. The median number of LAAO procedures performed annually among enrolling hospitals was 30 (interquartile range [IQR] 26) with most hospitals performing <40 procedures annually, although there was considerable variation in annual volume. LAAO= Left Atrial Appendage Occlusion.
Figure 5.
Distribution of Physician Annual Procedure Volume among 1147 Physicians in the LAAO Registry between January 1, 2016 to December 31, 2018. The figure shows the number of physicians in each annual volume category. Among implanting physicians, the median number of LAAO procedures performed annually was 12 (IQR 12) with most physicians performing <20 procedures annually, although there was considerable variation. LAAO= Left Atrial Appendage Occlusion.
Procedural Characteristics
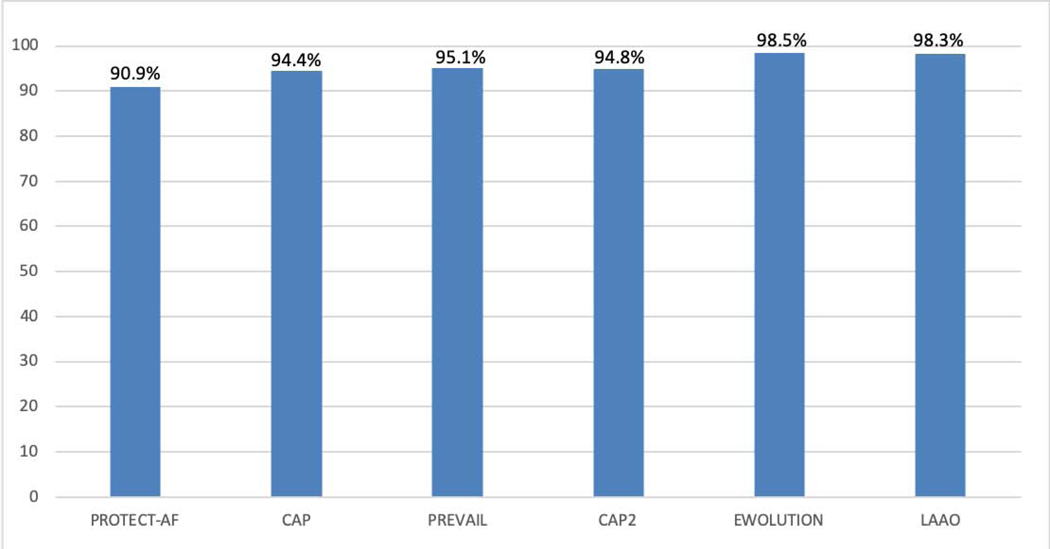
A device was deployed in 92.8% of cases (n=35,417). Procedures were cancelled prior to obtaining central venous access in 1,140 (3%), and procedures were aborted with at least venous access obtained but without deploying a device in 1,601 (4.2%). Among procedures in which a device was deployed, 98.3% were successfully implanted. This implant success rate was substantially higher than in the PROTECT AF and PREVAIL trials and the Continued Access Protocol registries but comparable to the more contemporary EWOLUTION registry (Figure 2). Among devices that were implanted, only 70 (0.2%) had a residual leak ≥5 mm.
Figure 2.
Implant success rates in the pivotal trials and registries compared with the LAAO Registry. Among procedures in the NCDR LAAO Registry in the first three years in which a device was deployed, 98.3% were successfully implanted, which was higher than in the pivotal trials and consistent with the more recent EWOLUTION Registry. LAAO= Left Atrial Appendage Occlusion.
Those who had a successful implant procedure were younger than those patients whose cases were cancelled or aborted (Table 1). Those who had their procedure cancelled were generally more ill and were more likely to have a history of cardiomyopathy, congestive heart failure, stroke, TIA, and to be taking antiplatelet agents; they were less likely to be taking nonsteroidal anti-inflammatory drugs (Table 2). While other differences were statistically significant due to the large sample size, the absolute differences were relatively modest.
Left atrial appendage or atrial thrombus was detected on the day of the procedure in 2.25% of patients in our overall cohort, 0.75% of those with successful implants, 2.25% of those with aborted procedures, and 48.8% of those with procedures cancelled prior to vascular access.
In-hospital Outcomes
Table 5 shows major in-hospital adverse events for the patients enrolled in the first 3 years of the LAAO Registry. Overall, death (0.19%) and cardiac arrest (0.24%) were uncommon. The most common major adverse events were pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), which were significantly more common among those whose procedure was aborted (8.0% pericardial effusion requiring intervention and 4.25% major bleeding). Ischemic stroke occurred in 0.12% of patients and transient ischemic attack occurred in 0.04% of patients overall and were more common among those whose procedures were aborted (0.37 and 0.06, respectively). All other forms of stroke or intracranial hemorrhage occurred rarely. Device embolization occurred in 0.07% of the overall cohort and in 0.87% of those whose procedures were aborted.
Table 5.
In-hospital Major Adverse Event Rates for Watchman Patients Enrolled in the LAAO Registry between January 1, 2016 to December 31, 2018.
| Adverse Event | Total | Successful Procedure | Aborted* Procedure | Cancelled* Procedure | P value |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| Overall | 38158 | 35417 | 1601 | 1140 | |
| Any In-hospital Major | 823 (2.16) | 663 (1.87) | 134 (8.37) | 26 (2.28) | <0.0001 |
| Adverse Event | |||||
| Death | 74 (0.19) | 61 (0.17) | 10 (0.62) | 3 (0.26) | 0.0003 |
| Cardiac Arrest | 93 (0.24) | 66 (0.19) | 22 (1.37) | 5 (0.44) | <0.0001 |
| Ischemic Stroke | 45 (0.12) | 39 (0.11) | 6 (0.37) | 0 (0.0) | <0.0001 |
| Hemorrhagic Stroke | 3 (0.01) | 3 (0.01) | 0 (0.0) | 0 (0.0) | <0.0001 |
| Undetermined Stroke | 2 (0.01) | 2 (0.01) | 0 (0.0) | 0 (0.0) | <0.0001 |
| Transient Ischemia Attack | 16 (0.04) | 14 (0.04) | 1 (0.06) | 1 (0.09) | <0.0001 |
| Intracranial Hemorrhage | 3 (0.01) | 3 (0.01) | 0 (0.0) | 0 (0.00) | <0.0001 |
| Systemic Arterial Embolism | 1 (0.0) | 0 (0.0) | 1 (0.06) | 0 (0.00) | <0.0001 |
| Major Bleeding | 478 (1.25) | 397 (1.12) | 68 (4.25) | 13 (1.14) | <0.0001 |
| Major Vascular Complication | 57 (0.15) | 47 (0.13) | 6 (0.37) | 4 (0.35) | <0.0001 |
| Myocardial Infarction | 14 (0.04) | 12 (0.03) | 1 (0.06) | 1 (0.09) | <0.0001 |
| Pericardial Effusion Requiring Intervention | 528 (1.39) | 383 (1.08) | 128 (8.0) | 17 (1.49) | <0.0001 |
| Pericardial Effusion with tamponade (percutaneous drainage) | 327 (0.86) | 250 (0.71) | 69 (4.31) | 8 (0.70) | <0.0001 |
| Pericardial Effusion without tamponade (percutaneous drainage) | 110 (0.29) | 93 (0.26) | 15 (0.94) | 2 (0.18) | <0.0001 |
| Pericardial Effusion (open cardiac surgery) | 91 (0.24) | 40 (0.11) | 44 (2.75) | 7 (0.61) | <0.0001 |
| Device Embolization | 30 (0.07) | 16 (0.05) | 14 (0.87) | 0 (0.0) | <0.0001 |
Among patients for whom a procedure was aborted, the rates of major in-hospital adverse events were substantially higher than in the overall cohort (death 0.6%, cardiac arrest 1.37%, ischemic stroke 0.37%, major bleeding 4.25%, pericardial effusion requiring intervention 8%, device embolization 0.87%); adverse event rates were also higher among patients in whom the procedure was cancelled (death 0.26%, cardiac arrest 0.44%, major bleeding 1.14%, pericardial effusion 1.49%, device embolization 0%).
Discussion
The LAAO Registry is a national program developed by the American College of Cardiology (ACC) in partnership with SCAI, FDA, CMS, and Boston Scientific. Over the first 3 years of the program, there has been robust growth in LAAO procedures, with over 38,000 performed at almost 500 hospitals and a median annual volume of 30 cases per hospital (Central Illustration). Individuals undergoing LAAO have substantially higher baseline thromboembolic and bleeding risk compared with those enrolled in the randomized clinical trials that led to regulatory approval. Finally, device implant success rates were higher than in the pivotal trial while rates of in-hospital major adverse events were lower. The LAAO Registry is the largest study of real-world LAAO procedures worldwide to date and will be an important data source to monitor LAAO safety and efficacy across a broad range of patient subgroups.
Central Illustration. Procedure Volume, Implanting Physicians, Implanting Hospitals, and Major In-hospital Adverse Events.
Between January 1, 2016 and December 31, 2018 in the NCDR LAAO Registry, data were collected for 38,158 left atrial appendage implant procedures performed by 1,318 physicians in 495 hospitals in the United States. NCDR= National Cardiovascular Data Registry, LAAO= Left Atrial Appendage Occlusion
Prior studies have been informative but were limited to highly selected populations. The PROTECT-AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation, N=707) and PREVAIL (Prospective Randomized Evaluation of the Watchman LAA Closure Device In Patients with Atrial Fibrillation, N=407) pivotal trials were relatively small and designed as Bayesian non-inferiority trials comparing the device to warfarin anticoagulation in patients eligible for both over the long-term. In addition to the NCDR LAAO registry, prospective, observational studies of the device thus far include the CAP (Continued Access to PROTECT, N=566), CAP-2 (Continued Access to PREVAIL, N=579)(32), WASP (Left Atrial Appendage Closure with Watchman in Asian Patients)(33) and EWOLUTION (Registry on Watchman Outcomes in Real-Life Utilization, N=1025) (34) and a U.S. post-approval study (N=3,822) (35), which are modest in size, industry-funded, limited with regards to baseline data collection, and limited with regards to follow-up.
These data from the LAAO Registry demonstrate that patients undergoing commercial Watchman LAA closure in the United States are older and are at higher thromboembolic and bleeding risk than individuals participating in the pivotal trials and most earlier registries, with a mean CHA2DS2-VASC score of 4.6 and a mean HAS-BLED score of 3 (20,21,34,36). Most patients in the LAAO Registry had relative or absolute contraindications to long-term anticoagulation, including a 69% rate of prior bleeding and a 12% rate of intracranial bleeding. In comparison, only 13.3% of the patients enrolled in the PROTECT-AF and PREVAIL randomized clinical trials had a prior bleeding event.(20,21) These observed differences in patient characteristics likely arise from the differences between the inclusion criteria of the pivotal trials that led to FDA approval of the device and the requirements for CMS reimbursement. While the pivotal trials enrolled patients with CHA2DS2-VASC score of 1 or more who were candidates for long-term oral anticoagulation, CMS reimbursement requires patients with CHA2DS2-VASC score ≥ 3 who are suitable for short-term oral anticoagulation but deemed unable to take long-term oral anticoagulation. The LAAO Registry will provide an assessment of outcomes in this population that is markedly different than the trial populations overall and is also large enough to permit the study of important sub-groups, including women and under-represented populations that have not been studied extensively to date.
We found that a device was deployed in 93% of procedures attempted in the LAAO Registry, with 3% cancelled prior to obtaining venous access and 4% aborted after obtaining venous access but before deploying a device. The rate of cancelled and aborted procedures has not been previously reported among trials and registries; it is important to note that they were not uncommon outcomes in our study. Among procedures in which a device was deployed, 98.3% were implanted. Among devices that were implanted, only 70 (0.2%) had a residual leak ≥5 mm.
In PROTECT AF, a device was successfully implanted in 88% (408/463) of patients assigned to LAAO intervention and in 90.9% (408/449) of those in whom implantation was attempted (Figure 2).(20) In PREVAIL, implantation was performed in 95.1% of those in whom it was attempted suggesting improvement in procedural technique and operator experience overall.(21) In the EWOLUTION Registry a device was successfully deployed in 98.5% of patients, and 0.7% had a residual leak >5mm (20,21,34,36), which is comparable to our findings. The Munich consensus document on definitions, endpoints, and data collection requirements for LAAO clinical studies defined technical success as exclusion of the LAA, no device-related complications, and no leak >5 mm on color Doppler TEE. Procedural success was defined as technical success and no procedure-related complications, except for uncomplicated (minor) device embolization.(37) Because the reported success rates from the prior trials and registries used varying definitions and may not conform exactly to the Munich consensus definitions, we reported essentially all the separate elements of procedure technical success as defined by the Munich document to allow comparison with prior data. Our results show that implantation success rates in contemporary practice are higher than in the PROTECT AF trial, the PREVAIL trial and the Continued Access Protocol registries and comparable to the more recent EWOLUTION registry, reflecting possible improvement in patient selection, procedural protocols and operator technique over time.
We found that the median hospital annual procedure volume was moderate at 30, with most sites performing <40 procedures annually, but there was substantial variation and a substantial minority of sites performed relatively few procedures each year. The median physician annual procedure volume was lower at 12, with similarly wide variation. The extent to which procedural volume relates to outcomes in contemporary practice is unclear; early Watchman data outside the LAAO Registry has suggested a “learning curve” with lower complications with greater accumulated procedural volume (38). The LAAO Registry is accruing adverse events in follow-up out to 4 years which will allow for detailed investigation of the relationship between hospital or physician volume and outcomes in contemporary practice.
The pivotal Watchman trials reported 7-day procedure related adverse events while the LAAO Registry collects adverse events during the index hospitalization and some procedure related adverse events may not be captured until the 45 days follow-up time point. However, most major procedure-related adverse events occur acutely and will be detected during the index hospitalization, and event rates are broadly comparable to the 7-day event rates reported in the trials. We found that rates of in-hospital major adverse events were substantially lower than the 7- day procedure-related adverse events reported in the PROTECT AF trial (pericardial effusion requiring surgery or pericardiocentesis 4%, major bleeding 3.5%, procedure-related stroke 1.1%, device embolization 0.4%).(20) The rates of 7-day procedure related adverse events in PREVAIL were generally substantially lower than PROTECT AF, but still higher than those in the LAAO Registry (pericardial effusion requiring surgery or pericardiocentesis 1.9%, procedure-related stroke 0.7%, device embolization 0.7%). In EWOLUTION, the rate of 7-day procedure related adverse events was 2.8%. The 1-day procedure related adverse event rates reported in EWOLUTION were lower than the in-hospital adverse event rates we report from the LAAO Registry (major bleeding 0.7%, pericardial effusion 0.5%, device embolization 0.2%)
In-hospital major adverse events were more common among the 4% of patients for whom procedures were aborted or cancelled, which may explain why procedures were stopped in many cases. The group of patients who had cancelled procedures should include only those in whom the procedure was stopped prior to vascular access, but the rate of pericardial effusion was substantial suggesting that some of these were misclassified as cancelled rather than aborted. Nonetheless, the rates of adverse events were generally lower among those with cancelled procedures compared with those who had aborted case. Approximately half of those who had cancelled procedures had atrial thrombus detected on the day of the procedure, suggesting that this was a common cause for procedure cancellation.
The LAAO Registry will include active follow-up at 45 days, 6 months, 1 year, and 2 years and linkage to Medicare data to capture adverse events that occur during follow-up years 3 and 4 after implant. No other registries or large-scale observational studies include follow-up of this extent. The program has developed a computer-based algorithmic adjudication process to accurately categorize adverse events reported by sites to the registry. Given the relative size of the pivotal trials and the protracted FDA approval process of the device, questions remain regarding outcomes after LAAO procedures in contemporary practice. The LAAO Registry is potentially well-positioned to address many key issues. The registry includes a dedicated leadership team including staff at the ACC NCDR and a Steering Committee that continue to revise the data collection and reporting, so that the LAAO Registry is an evolving and iterative study capable of addressing the most pressing knowledge gaps.
Other percutaneous LAAO devices are being developed and evaluated in clinical trials within the U.S., including the LARIAT device (SentreHeart; Redwood City, California; clinicaltrials.gov identifier NCT02513797), the Amulet device (St. Jude Medical; Saint Paul, Minnesota; NCT02879448), the WaveCrest device (Biosense Webster, Diamond Bar, California; NCT03302494), and the next-generation Watchman FLX (Boston Scientific, Marlborough, Massachusetts; NCT02702271). If these devices are approved by the FDA, the LAAO Registry will be well-poised to evaluate the adoption, safety and effectiveness of these newer devices over time. Indeed, the registry has been designed to include any percutaneous device used to achieve left atrial appendage closure.
The LAAO Registry and the multi-stakeholder collaboration between professional societies, FDA, CMS, and industry represent an approach to that is likely to be increasingly employed in the United States. Success of the program will demonstrate that with a shared vision, a single registry model can be constructed to meet the needs of FDA, CMS and other healthcare payors, industry, quality/value experts, stakeholder societies and health outcomes researchers.
Limitations
The LAAO Registry relies on site-reported data, which may result in over- or underreporting of patient, physician or hospital data. As detailed above, unlike most registries, the NCDR program includes annual audits of site data collection; a novel and validated automatic event adjudication process to ensure data quality; and will include linkages to CMS claims data, which will reduce under-reporting bias for longer-term events.
Conclusions
The LAAO Registry is the largest registry of patients undergoing percutaneous LAAO procedures in the world. Hospital and physician procedural volumes vary substantially. To date, the 38,000 patients that have been enrolled in the LAAO Registry are at higher risk of both stroke and bleeding than those who participated in the clinical trials that led to FDA approval of the Watchman device. However, despite this more complex patient population, implant success rates in contemporary practice were higher and in-hospital major adverse event rates were lower compared with those reported in the pivotal randomized trials. The LAAO Registry will serve an important role in quality improvement for participating hospitals with real-time performance measure data available and quality improvement reports sent quarterly. Data collection, site reporting, and scientific inquiry will continue to iterate and evolve to address the questions and concerns of patients, hospitals, physicians, regulators, and the scientific community.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Systems-Based Practice:
In over 38,000 procedures captured during 3 years by the U.S. NCDR registry, patients with atrial fibrillation (AF) undergoing transcatheter left atrial appendage occlusion (LAAO) had a mean age 76.1 years with mean CHA2DS2-VASC score of 4.6 and mean HAS-BLED score of 3), all substantially higher than in previous trials and observational registries. Major in-hospital adverse events were less frequent than reported in pivotal trials, and stroke (0.17%) and death (0.19%) were rare.
Translational Outlook:
Future research should clarify the selection criteria for patients best suited to LAAO as opposed to treatment with target-specific oral anticoagulants (NOACs).
Acknowledgments
Funding: This study was funded by the American College of Cardiology (ACC) National Cardiovascular Data Registry (NCDR) and the National Heart, Lung and Blood Institute (NHLBI) grant R56HL142765 and R01HL142765.
Disclosures: Dr. Freeman reports salary support from the ACC NCDR and the NHLBI and consulting/advisory board fees from Boston Scientific, Medtronic, Janssen Pharmaceuticals, and Biosense Webster. Dr. Price reports consulting/advisory board fees and honoraria from Boston Scientific, Chiesi, USA, Medtronic, Abbott Vascular, W.L. Gore Medical, AstraZeneca, and Conformal Medical, and research grants (to institution) from Daiichi Sankyo. Dr. Rammohan reports consulting fees from Medtronic and Abbot Vascular. Dr. Turi has received lecturing honoraria from Abbott Vascular. Dr. Curtis has an institutional contract with the American College of Cardiology for his role as Senior Scientific Advisor of the NCDR. Dr. Masoudi has an institutional contract with the American College of Cardiology for his role as Chief Scientific Advisor of the NCDR. The other authors report no disclosures.
Abbreviations:
- AF
atrial fibrillation
- LAAO
left atrial appendage occlusion
- FDA
Food and Drug Administration
- ACC
American College of Cardiology
- NCDR
National Cardiovascular Data Registry
- SCAI
ciety for Coronary Angiography and Interventions
- CMS
Centers of Medicare and Medicaid Services
- CEC
clinical events committee
- STS
Society of Transthoracic Surgeons
- R&P
research and publications
- DQR
data quality reporting
- SD
standard deviation
- IQR
interquartile range
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 3.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med 1987;147:1561–4. [PubMed] [Google Scholar]
- 4.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin EJ, Virani SS, Callaway CW et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 6.Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet 1994;343:687–691. [PubMed] [Google Scholar]
- 7.Connolly SJ, Ezekowitz MD, Yusuf S et al. Dabigatran versus warfarin in patients with atrial fibrillation. The New England journal of medicine 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 8.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. The New England journal of medicine 2010;363:1875–6. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Mahaffey KW, Garg J et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. The New England journal of medicine 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 10.Granger CB, Alexander JH, McMurray JJ et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 11.Giugliano RP, Ruff CT, Braunwald E et al. Edoxaban versus warfarin in patients with atrial fibrillation. The New England journal of medicine 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 12.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996;61:755–9. [DOI] [PubMed] [Google Scholar]
- 13.Stoddard MF, Dawkins PR, Prince CR, Ammash NM. Left atrial appendage thrombus is not uncommon in patients with acute atrial fibrillation and a recent embolic event: a transesophageal echocardiographic study. J Am Coll Cardiol 1995;25:452–459. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Gallagher S. Percutaneous occlusion of the left atrial appendage in non-valvular atrial fibrillation for the prevention of thromboembolism: NICE guidance. Heart 2011;97:762–5. [DOI] [PubMed] [Google Scholar]
- 15.Bartus K, Bednarek J, Myc J et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm 2011;8:188–93. [DOI] [PubMed] [Google Scholar]
- 16.Fountain R, Holmes DR, Jr., Hodgson PK, Chandrasekaran K, Van Tassel R, Sick P. Potential applicability and utilization of left atrial appendage occlusion devices in patients with atrial fibrillation. Am Heart J 2006;152:720–3. [DOI] [PubMed] [Google Scholar]
- 17.Block PC, Burstein S, Casale PN et al. Percutaneous left atrial appendage occlusion for patients in atrial fibrillation suboptimal for warfarin therapy: 5-year results of the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) Study. JACC Cardiovasc Interv 2009;2:594–600. [DOI] [PubMed] [Google Scholar]
- 18.Bartus K, Han FT, Bednarek J et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108–18. [DOI] [PubMed] [Google Scholar]
- 19.January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 20.Holmes DR, Reddy VY, Turi ZG et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534–42. [DOI] [PubMed] [Google Scholar]
- 21.Holmes DR Jr., Kar S, Price MJ et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Reddy VY, Sievert H, Halperin J et al. Percutaneous Left Atrial Appendage Closure vs Warfarin for Atrial Fibrillation: A Randomized Clinical Trial. Jama 2014;312:1988–98. [DOI] [PubMed] [Google Scholar]
- 23.Reddy VY, Doshi SK, Kar S et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol 2017;70:2964–2975. [DOI] [PubMed] [Google Scholar]
- 24.Lee RJ, Lakkireddy D, Mittal S et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J 2015;170:1184–94. [DOI] [PubMed] [Google Scholar]
- 25.Lakkireddy D, Windecker S, Thaler D et al. Rationale and design for AMPLATZER Amulet Left Atrial Appendage Occluder IDE randomized controlled trial (Amulet IDE Trial). Am Heart J 2019;211:45–53. [DOI] [PubMed] [Google Scholar]
- 26.Waksman R, Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on Watchman left atrial appendage closure therapy. Am J Cardiol 2015;115:378–84. [DOI] [PubMed] [Google Scholar]
- 27.Decision Memo for Percutaneous Left Atrial Appendage (LAA) Closure Therapy (CAG-00445N). Centers for Medicare &Medicaid Services, 2016. [Google Scholar]
- 28.Masoudi FA, Ponirakis A, de Lemos JA et al. Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. Journal of the American College of Cardiology 2017;69:1427–1450. [DOI] [PubMed] [Google Scholar]
- 29.Messenger JC, Ho KK, Young CH et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484–8. [DOI] [PubMed] [Google Scholar]
- 30.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor based approach: the euro heart survey on atrial fibrillation. Chest 2010;137:263–72. [DOI] [PubMed] [Google Scholar]
- 31.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 32.Doshi SK, Kar S, Price MJ et al. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J Am Coll Cardiol 2015;65:2614–2623. [DOI] [PubMed] [Google Scholar]
- 33.Phillips KP, Santoso T, Sanders P et al. Left atrial appendage closure with Watchman in Asian patients: 2year outcomes from the WASP registry. Int J Cardiol Heart Vasc 2019;23:100358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boersma LV, Schmidt B, Betts TR et al. Implant success and safety of left atrial appendage closure with the Watchman device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J 2016;37:2465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy VY, Gibson DN, Kar S et al. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J Am Coll Cardiol 2017;69:253–261. [DOI] [PubMed] [Google Scholar]
- 36.Boersma LV, Ince H, Kische S et al. Efficacy and safety of left atrial appendage closure with Watchman in patients with or without contraindication to oral anticoagulation: 1Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017;14:13021308. [DOI] [PubMed] [Google Scholar]
- 37.Tzikas A, Holmes DR Jr., Gafoor S et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017;19:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledwoch J, Franke J, Gonzaga M et al. Left atrial appendage closure: First in man with the 4th generation Watchman device. Catheter Cardiovasc Interv 2016;87:787–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.