Abstract
Background:
A sequential approach, synchronizing cell-cycle specific chemotherapy during VEGFR-TKI treatment breaks, may improve the therapeutic index of this combination therapy. In this study we investigate the safety/tolerability and pharmacodynamic effects of docetaxel used in sequential combination with the novel VEGFR-TKI X-82.
Methods:
Patients with advanced solid malignancies underwent 21-day treatment cycles with X-82 administered daily on days 1–14, a treatment break on days 15–20, and docetaxel administered on day 21. Randomization was 1:1 to either a low dose X-82 (200 mg) or high dose X-82 (400 mg) arm. Patients were scheduled to undergo four 3’-deoxy-3’−18F-fluorothymidine (FLT) PET/CT scans to assess changes in tumor cell proliferation. PET standardized uptake values (SUV) were summarized for tumors and changes were assessed using mixed effects models.
Results:
Fourteen patients were enrolled and treated with median 3.5 cycles (range 0–12). Three patients in the high dose cohort (50%) and three patients in the low dose cohort (38%) experienced at least one grade three adverse event during the study (infections, cytopenias, electrolyte abnormalities, and vascular complications). Four patients with thirteen metastatic tumors underwent FLT PET/CT scanning. During the cycle 1 X-82 exposure period, tumor SUVmax decreased by −11% (p=0.04). After administration of docetaxel and the cycle 2 X-82 exposure period, tumor SUVmax decreased −44% (p=0.03).
Conclusions:
The sequential combination of X-82 and docetaxel was safe and led to diminished tumor cell proliferation. Further, decrease in FLT uptake during cycle 2 (X-82 plus docetaxel) was greater than in cycle 1 (X-82 alone), suggesting sequential chemotherapy enhances the pharmacodynamic effect of therapy.
INTRODUCTION
Anti-angiogenic therapies target tumor vasculature, an essential component for tumor growth (1). However, clinical benefit derived from these agents has only been modest and all patients eventually progress on anti-angiogenic therapies. Development of novel therapeutic strategies to improve outcomes is critical. Bevacizumab, a monoclonal antibody targeting the VEGF ligand, was one of the first anti-angiogenic agents showing clinical success but only when combined with chemotherapy (2–8). This success was followed by development of a class of anti-angiogenic agents targeting the VEGF receptor family (VEGFR-TKIs) that showed improved single agent activity compared with bevacizumab. VEGFR-TKI monotherapies prolonged overall and progression free survival in multiple metastatic cancers (9–13). Given the success of VEGFR TKIs as monotherapy, a logical next step to improve efficacy was combination clinical trials with cytotoxic chemotherapy. Unfortunately, combination of VEGFR-TKIs with concurrent chemotherapy failed to show added benefit in many clinical trials (14–20). This prompted our group to pursue potential mechanisms that could explain the negative results.
Clinical work using 3’-deoxy-3’−18F-fluorothymidine (FLT) PET/CT imaging in patients treated with VEGFR-TKIs showed decreases in tumor proliferative and vascular parameters during VEGFR-TKI exposure, followed by a rapid vascular and proliferative rebound just days after a break in VEGFR-TKI dosing (21–23). This rebound, also known as VEGFR-TKI withdrawal flare, was evident with intermittent treatment cycles and independent of tumor type. This information, led us to hypothesize that a sequential rather than concurrent combination of VEGFR-TKI and chemotherapy would be most effective. We believe the withdrawal flare can be exploited by ‘synchronizing’ chemotherapy during VEGFR-TKI treatment breaks to maximize the therapeutic index of cell cycle-specific chemotherapy. This sequential treatment strategy, i.e. applying cell-cycle specific chemotherapy during VEGFR-TKI treatment breaks to specifically target the withdrawal flare, has not been studied clinically to our knowledge.
Thus, we investigated the sequential treatment approach using the novel VEGFR-TKI X-82 in combination with docetaxel. X-82 is a small molecule indolinone inhibitor of VEGFRs-1, −2, −3, platelet derived growth factor (PDGFR α and β), stem cell factor (c-kit), ligand for FLT-3, and receptor tyrosine kinase (RET). The aim of the study was to assess the safety, tolerability, and pharmacodynamic effects of this sequential treatment strategy. Serial FLT PET/CT scans and plasma VEGF measurements were performed in a subset of patients to assess differences between pharmacodynamic effects in cycle 1 (after X-82 exposure) and pharmacodynamic effects in cycle 2 (after docetaxel and X-82 exposure) (24–25).
METHODS
Study Population
Patients with histologically or cytologically confirmed solid malignancies that were metastatic or unresectable were enrolled on the study. Patients had to have measurable disease defined as at least one lesion that can be accurately measured in at least one dimension (longest diameter to be recorded) as greater than 20 mm with conventional techniques (CT, MRI, x-ray) or greater than 10 mm with spiral CT scan. All patients had ECOG performance status of 0–1 and normal organ and marrow function as defined by the protocol. Patients who had major surgery, chemotherapy, radiotherapy, or experimental therapy within 4 weeks prior to entering the study were excluded. Additionally, patients with poorly controlled hypertension were excluded due to potential complications associated with anti-VEGF therapy. Informed consent was obtained from all patients prior to entering the study and the study was approved by the Institutional Review Board at the University of Wisconsin.
Drug Administration and Study Design
X-82 was provided to patients in 100 mg tablets. Patients underwent 1:1 randomization to two X-82 dose levels; in the high dose cohort, patients took 400 mg X-82 once daily and on the low dose cohort, patients took 200 mg X-82 once daily. Treatment cycles for all patients consisted of continuous X-82 dosing on days 1 to 14 followed by a break in X-82 dosing on days 15 to 21; docetaxel was administered intravenously (75 mg/m2) on day 21 of each treatment cycle (Figure 1). Patients continued on treatment until radiographic disease progression, clinical progression (based on physician discretion and or unacceptable toxicity), or patient withdrawal of consent. Patients were evaluated for response and radiographic progression every 3 cycles (9 weeks) using RECIST 1.1 guidelines (26). Objective response was defined as the best response measured by RECIST 1.1. Pharmacodynamic assessments, including FLT PET/CT imaging and plasma VEGF measurements, were performed at four timepoints: 1) baseline, 2) maximum X-82 exposure, 3) maximum X-82 washout and 4) maximum X-82 exposure post docetaxel.
Figure 1:
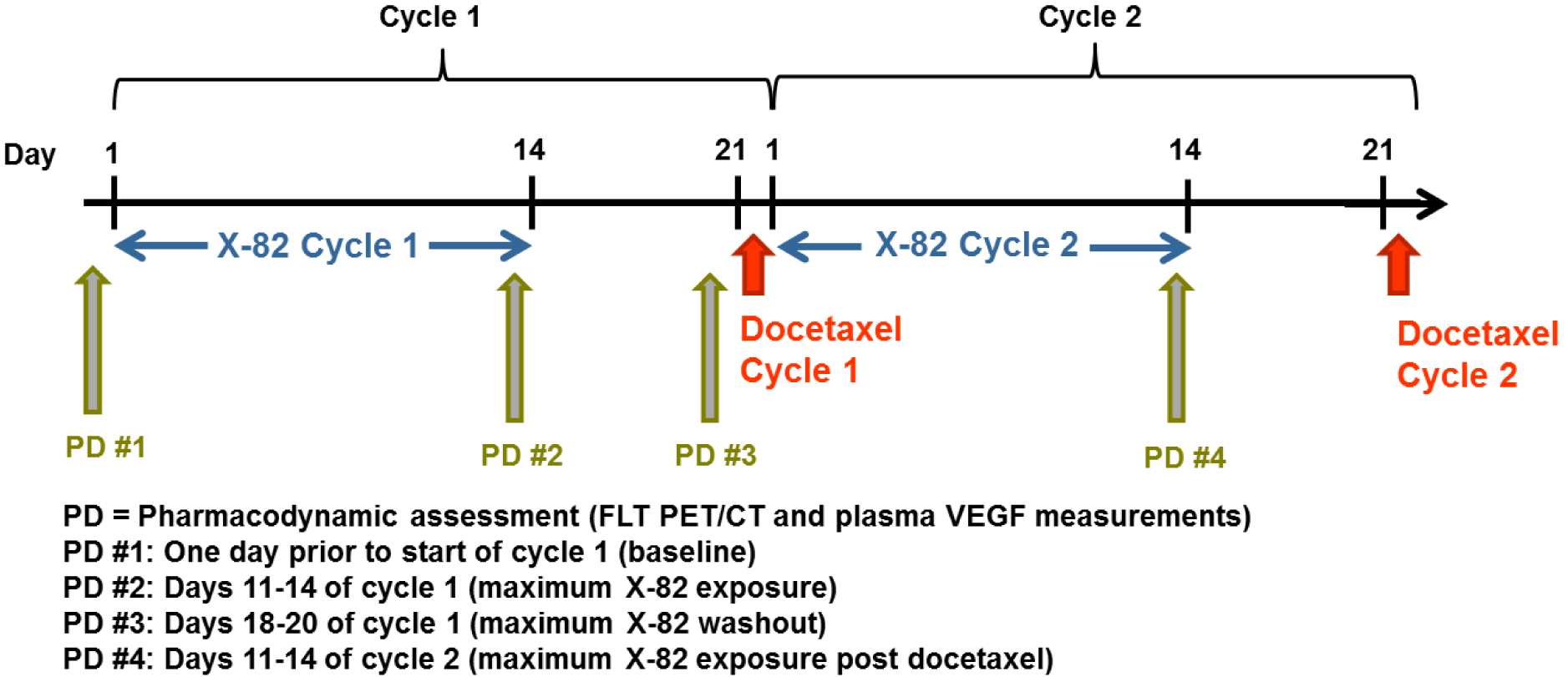
Study schema including drug administration and pharmacodynamic timepoints for the first two treatment cycles.
FLT PET/CT Imaging
FLT PET/CT scans were performed using a Discovery LS PET/CT scanner (General Electric, Waukesha WI). At the beginning of each imaging session patients were injected with FLT (mean injected dose 362 MBq, range 314–394 MBq). Sixty minutes post-injection patients underwent a CT scan followed by a whole-body PET scan (5 minutes per scanning position). The CT scan was used for PET attenuation correction and as an anatomic reference for identifying tumors. The PET scans were reconstructed with an iterative 3D ordered subsets expectation maximization algorithm with grid size 256×256, 2 iterations, 14 subsets, and 4 mm post-filter; the PET voxel size was 2.73×2.73×3.27 mm. Standardized uptake values (SUVs) normalized by patient weight were used to quantify cell proliferation in each PET voxel.
Using the resulting PET/CT scans and baseline diagnostic radiology reports, an experienced nuclear medicine physician identified tumors amenable for quantitative FLT PET analysis (i.e. solid tumors outside of regions with high background FLT uptake such as liver and bone marrow). The identified tumors were manually segmented using Amira software (ThermoFisher Scientific Inc., Waltham MA). Tumor cell proliferation was quantified by calculating the max (SUVmax), mean (SUVmean), and total (SUVtotal) SUV of tumor voxels. FLT PET/CT scans were not used for managing treatment of patients.
Plasma VEGF Measurements
Blood samples were drawn (4 mL) for analysis of VEGF levels by enzyme-linked immunosorbent assay (ELISA). For each sample, plasma was separated by centrifugation at approximately 1200g × 15 minutes, aliquoted into cryovials, and stored at −70°C until analysis. Each sample was analyzed using a commercially available 96-well plate quantitative sandwich immunoassay (Quantikine® human VEGF, R & D Systems, Minneapolis, MN) with a standard curve ranging from 31.2 to 500 pg/mL VEGF. At the time of assay, all samples and standards were brought to room temperature and prepared on the plate as recommended by the manufacturer. The plate was read at 450 nm using a Molecular Devices SpectraMax 190 plate reader.
Statistical Analysis
Changes in SUV metrics were evaluated using a linear mixed effects model with patient specific random effects and a compound symmetry correlation structure to account for multiple tumors within the same patient. A separate model was formulated for estimating the change in FLT uptake between each pair of time points. All SUV measurements were log-transformed before conducting the analyses to satisfy the normality assumption. Model estimated percentage changes and the corresponding 95% confidence intervals were back transformed and reported on the original scale. All P-values were two-sided and P<0.05 was used to define statistical significance. All model fitting was performed in R (v 3.2.00).
Percent changes in plasma VEGF measurements were calculated for each patient and summarized in terms of medians and ranges. Significant changes in VEGF levels across time points were assessed using Wilcoxon signed-rank tests. Significant differences in VEGF levels between the high and low dose X-82 cohorts were assessed using Wilcoxon rank-sum tests.
RESULTS
Patient Characteristics
From October 2014 to August 2016, 14 patients (8 patients in the low dose cohort; 6 patients in the high dose cohort) were enrolled at the University of Wisconsin Carbone Cancer Center (Table 1). The median patient age was 61 years (range 47 to 72) and 64% of patients were female. Patients had a variety of primary cancer histologies with the most common being lung carcinoma (n=3). The median number of RECIST identified tumors (target plus non-target tumors) at baseline was 5 (range 3 to 8). The median number of prior systemic therapy regimens was 2.5 (range 0–11). Eleven patients (79%) had been treated with prior chemotherapy. Two patients (14%) had been treated with a prior anti-VEGF agent.
Table 1:
Patient characteristics
| Patient | Age | Gender | Histology | Cohort | No. of prior systemic therapies | Prior VEGF therapy (Y/N) |
|---|---|---|---|---|---|---|
| 1 | 72 | Female | Ovarian carcinoma | High | 6 | N |
| 2 | 57 | Female | Lung carcinoma | High | 4 | Y |
| 3 | 57 | Female | Breast carcinoma | Low | 6 | N |
| 4 | 47 | Female | Thyroid carcinoma | High | 1 | Y |
| 5 | 64 | Female | Urothelial carcinoma | High | 2 | N |
| 6 | 71 | Male | Squamous cell carcinoma | Low | 4 | N |
| 7 | 65 | Female | Lung carcinoma | Low | 1 | N |
| 8 | 67 | Male | Lung carcinoma | Low | 2 | N |
| 9 | 54 | Female | Ovarian carcinoma | Low | 2 | N |
| 10 | 67 | Female | Endometrial carcinoma | High | 5 | N |
| 11 | 56 | Female | Breast carcinoma | Low | 11 | N |
| 12 | 65 | Male | Adenoid cystic carcinoma | Low | 0 | N |
| 13 | 53 | Male | Leiomyosarcoma | Low | 3 | N |
| 14 | 58 | Male | Unknown primary carcinoma | High | 0 | N |
Adverse Events
No patients experienced an adverse event greater than grade 3 that were possibly related to X-82. For the six patients in the high dose X-82 cohort, three patients (50%) experienced at least one grade 3 adverse event while on study (Table 2). For the eight patients in the low dose X-82 cohort, three patients (38%) experienced at least one grade 3 adverse event while on study. Of the 11 total grade 3 adverse events that were experienced, 7 (64%) occurred in cycle 5 or later. Two patients had docetaxel doses reduced to 60 mg/m2 after starting treatment due to persistent neutropenia.
Table 2:
Adverse events of grade (Gr) 3 or greater that were possibly related to X-82.
| Adverse event | Number of pts low dose cohort |
|---|---|
| Gr 3 Hypertension | - |
| Gr 3 Low WBC | - |
| Gr 3 Infection | 1 |
| Gr 3 Anemia | - |
| Gr 3 Hyponatremia | 1 |
| Gr 3 Hypoalbumenia | - |
| Gr 3 Thrombosis | - |
| Gr 3 Rectal Hemorrhage | 1 |
Disease Response
For the six patients in the high dose X-82 cohort, the median time on treatment was 13 weeks (range 3 to 19). The objective responses for the high dose cohort were as follows: one patient (17%) with partial response, four patients (67%) with stable disease, and one patient who withdrew consent to participate in the study prior to any follow-up RECIST assessment making them unevaluable for objective response. For the eight patients in the low dose X-82 cohort, the median time on treatment was 8 weeks (range 1 to 36). The objective responses for the low dose cohort were as follows: three patients with stable disease (38%), three patients with progressive disease (38%), and two patients that were unevaluable for objective response.
FLT PET/CT Imaging
Four patients with fourteen metastatic tumors completed all four of the scheduled PET/CT scans and were included in the imaging pharmacodynamic assessment. Figure 2 shows a tumor with representative changes in SUV. Mixed effects modelling provided estimates of changes in tumor SUVs during therapy (Table 3). During the cycle 1 X-82 exposure period (X-82 alone), tumor SUVmax significantly decreased (mean change −11%; P=0.04). During the cycle 1 X-82 washout period, tumor SUVmax significantly increased (mean change +29%; P<0.01) consistent with the withdrawal flare phenomenon seen with other VEGFR-TKIs (23–25). During the cycle 2 X-82 exposure period (post docetaxel administration), tumor SUVmax significantly decreased (mean change −44%; P=0.03). When performing a paired test to compare the decrease in tumor SUVmax in the cycle 2 X-82 exposure period (post docetaxel) vs. that in the cycle 1 X-82 exposure period (X-82 alone), the cycle 2 X-82 exposure period was found to have a greater decrease in tumor SUVmax (P = 0.05). Percent changes relative to baseline for all analyzed tumors are shown in Figure 3.
Figure 2:
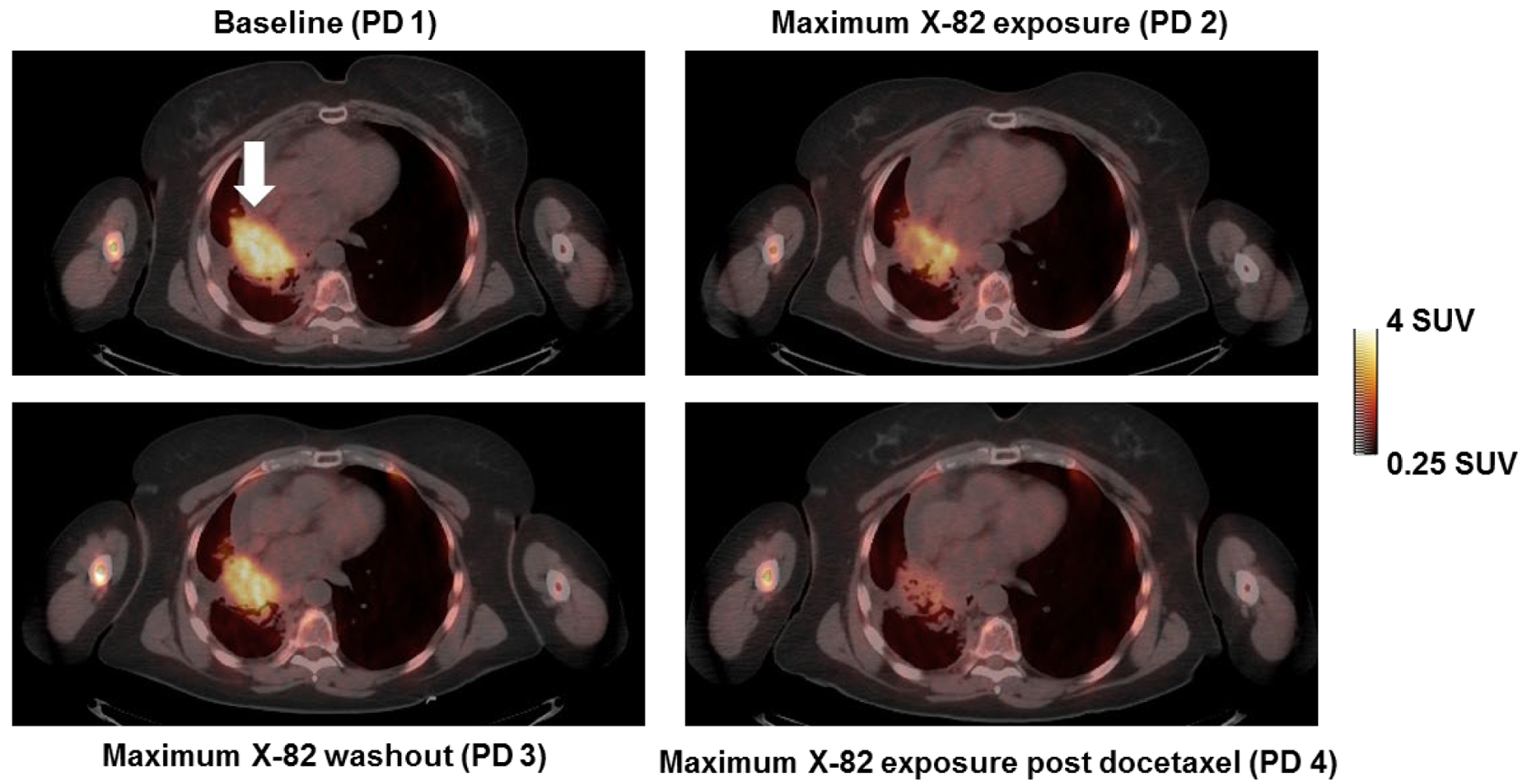
Axial FLT PET/CT slice of a lung tumor (Patient 2). The SUVmax for this tumor (indicated by arrow) was 4.2 g/mL at baseline, decreased to 3.7 g/mL at maximum X-82 exposure in cycle 1, then rebounded to 4.0 g/mL at maximum X-82 washout, and decreased to 2.0 g/mL at maximum X-82 exposure in cycle 2. This patient achieved a partial response as measured by RECIST but eventually progressed after 4 cycles due to development of new brain metastases.
Table 3:
Percent changes in tumor SUVs across timepoints
| SUV metric | Model Estimated Mean Change (%) | 95% Confidence Interval | P-Value | |
|---|---|---|---|---|
| Change PD1 to PD2 | ||||
| SUVmax | −11 | −20 to −2 | 0.04 | |
| SUVmean | −2 | −17 to +15 | 0.78 | |
| SUVtotal | −16 | −33 to +5 | 0.16 | |
| Change PD2 to PD3 | ||||
| SUVmax | +29 | +20 to +40 | <0.01 | |
| SUVmean | +19 | +10 to +28 | <0.01 | |
| SUVtotal | +71 | −9 to +220 | 0.13 | |
| Change PD3 to PD4 | ||||
| SUVmax | −44 | −63 to −14 | 0.03 | |
| SUVmean | −26 | −40 to −8 | 0.02 | |
| SUVtotal | −59 | −73 to −37 | <0.01 | |
Figure 3:
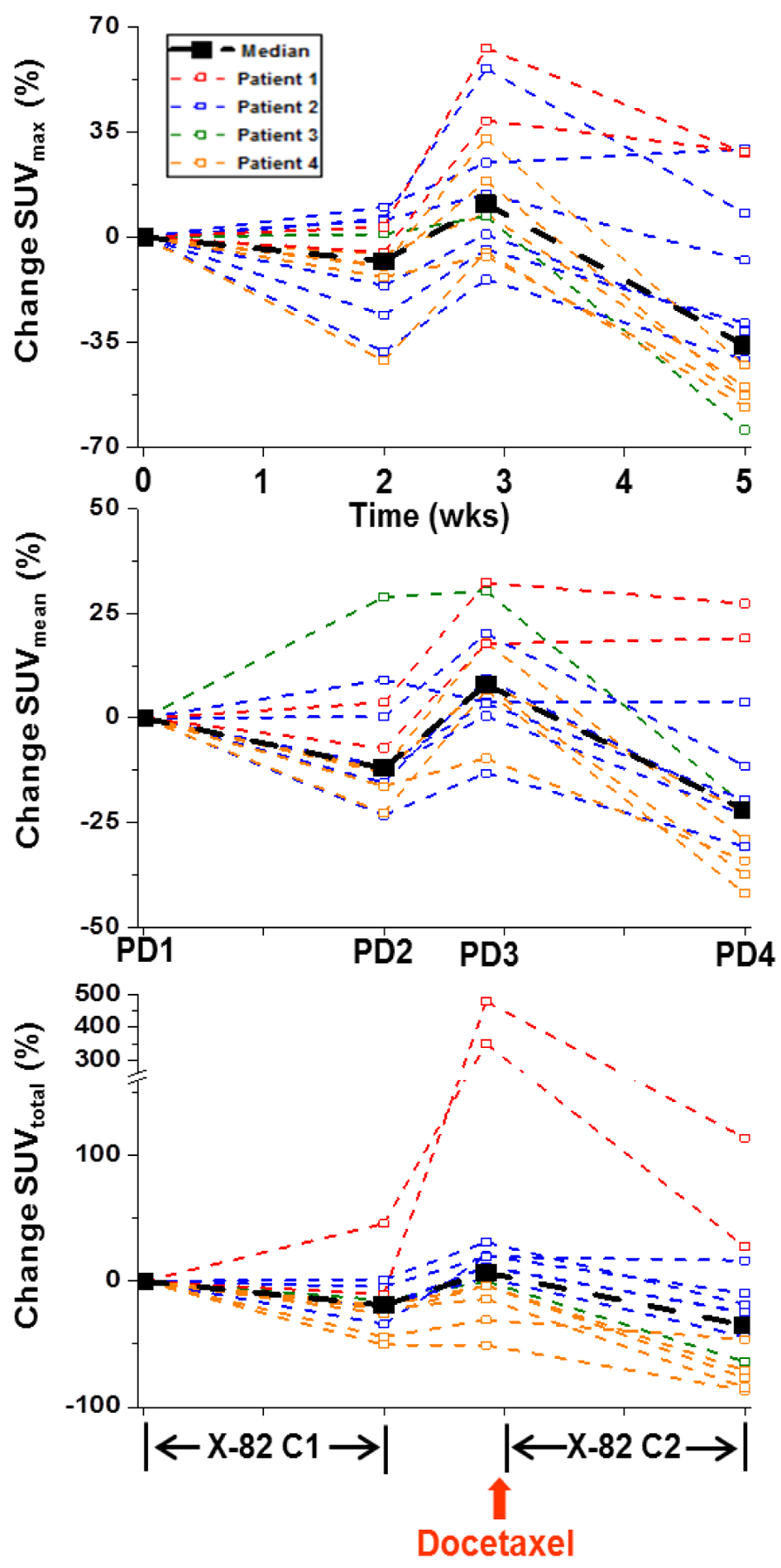
Percent change in tumor SUVs relative to baseline for SUVmax (top), SUVmean (middle), and SUVtotal (bottom). Tumors from the same patient are shown in the same color. Diminished SUVmax is evident for the majority of tumors during the cycle 1 (C1) and cycle 2 (C2) X-82 exposure periods. Increases in SUVmax are evident for the majority of tumors during the X-82 treatment break. Changes in SUVmean and SUVtotal had similar trends as SUVmax. However, SUVmean was less sensitive to therapy induced changes than SUVmax. SUVtotal was more sensitive than SUVmax to therapy induced changes but the SUVtotal changes demonstrated greater variability across patients than the SUVmax changes.
Plasma VEGF
Ten patients completed two or more plasma VEGF measurements and were included in the VEGF pharmacodynamic analysis (Table 4 and Figure 4). Changes in plasma VEGF were not significantly different between the low and the high dose X-82 cohorts. A combined analysis of both cohorts demonstrated a median increase in plasma VEGF of +13% during the cycle 1 X-82 dosing period (P = 0.57) and a median increase of +52% during the cycle 2 X-82 dosing period (P = 0.03).
Table 4:
Median percent changes in plasma VEGF for the low and high dose X-82 cohorts
| Change PD1 to PD2 | Low (n=4) | High (n=6) | Combined (n=10) |
| Median (%) | −13 | +17 | +13 |
| Range (%) | −53 to +37 | −55 to +195 | −55 to +195 |
| Change PD2 to PD3 | Low (n=4) | High (n=5) | Combined (n=9) |
| Median (%) | −19 | −32 | −29 |
| Range (%) | −45 to +174 | −76 to +137 | −76 to +174 |
| Change PD3 to PD4 | Low (n=3) | High (n=4) | Combined (n=7) |
| Median (%) | +62 | +48 | +52 |
| Range (%) | +27 to +169 | −21 to +282 | −21 to +282 |
Figure 4:
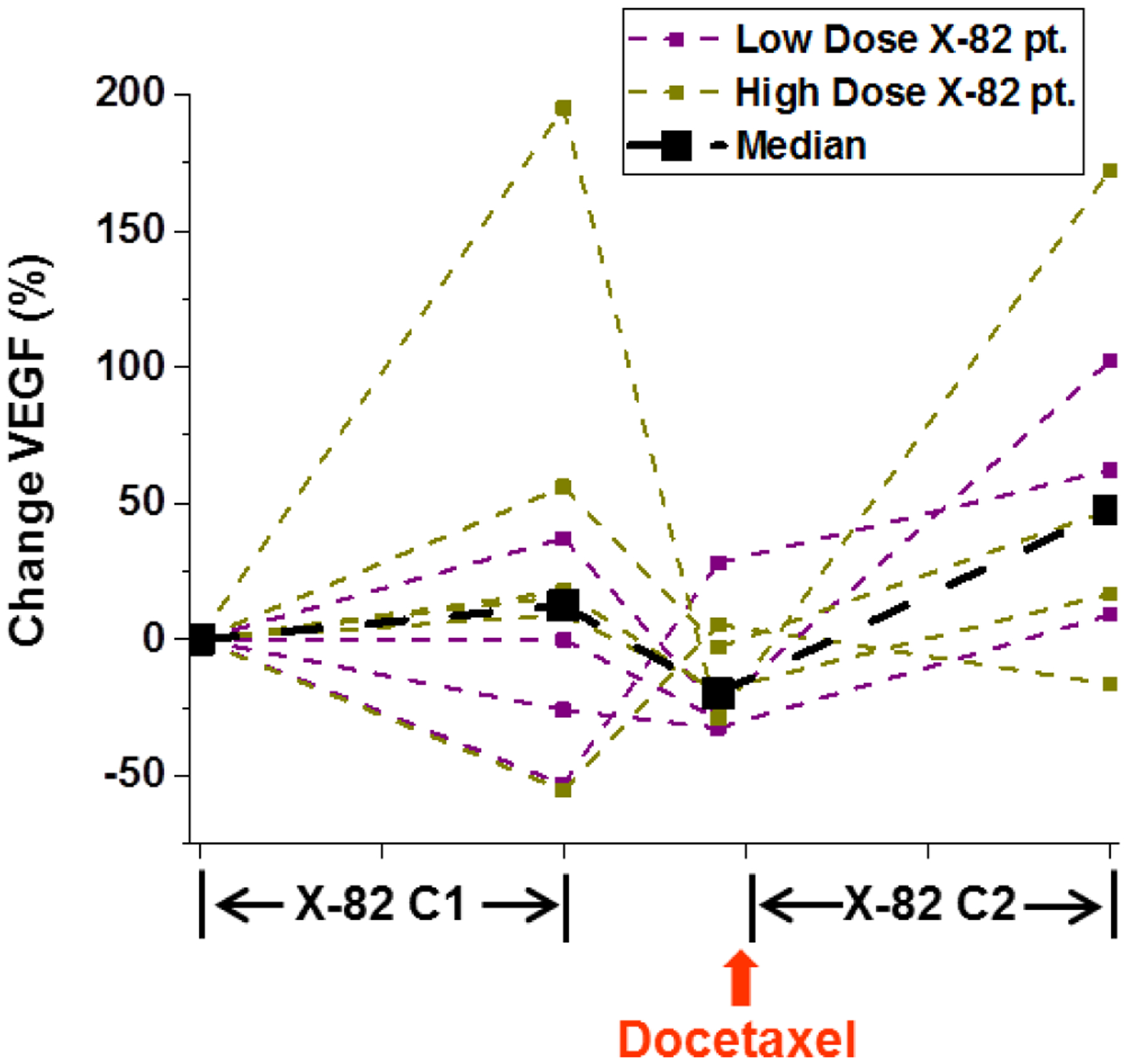
Percent changes in plasma VEGF relative to baseline. Median plasma VEGF levels increased for all patients during the cycle 1 (C1) and cycle 2 (C2) X-82 exposure periods; however, there was large amount of interpatient variability, particularly during the cycle 1 X-82 exposure period.
DISCUSSION
While combination of VEGF ligand targeting agents like bevacizumab with chemotherapy have shown added benefit, combining VEGFR-TKIs with chemotherapies has failed to achieve the same benefit in many studies (14–20). Although there have been exceptions, we hypothesized that the mostly negative results of VEGFR-TKIs with chemotherapy studies are due to suboptimal scheduling with a concurrent rather than sequential approach (27). This rationale formed the basis for this study where we investigated the effects of a novel VEGFR-TKI X-82 used in sequential combination with docetaxel applied during VEGFR-TKI treatment breaks. The primary goals of this study were to assess the safety/tolerability of treatment and assess pharmacodynamic changes during the sequential treatment regimen. None of the 14 patients in this study experienced an adverse event greater than grade three and 6 (43%) patients experienced a grade 3 adverse event (with the majority of these grade 3 events occurring after 5 cycles of therapy). The sequential combination of X-82 and docetaxel led to diminished tumor cell proliferation as measured by changes in FLT uptake. Further, a greater decrease in FLT uptake was evident during cycle 2 (X-82 plus docetaxel) than in cycle 1 (X-82 alone), suggesting sequential chemotherapy enhances the pharmacodynamic effect of therapy.
The effect of X-82 at two dose levels (400 mg daily vs. 200 mg daily) was assessed in this study. There were a greater number of patients with stable or partial response on 400 mg X-82 (5/6 patients; 84%) than those on 200 mg X-82 (3/8 patients; 34%). However, there were a greater number patients that experienced grade 3 adverse events on 400 mg X-82 (3/6 patients; 50%) than on 200 mg X-82 (3/8 patients; 38%).
Decrease in FLT PET parameters after two weeks of continuous X-82 exposure suggests an on-target effect of X-82 that is line with expected decreases in tumor vasculature and proliferation due to VEGFR-TKI exposure. Increases in FLT PET parameters during the X-82 washout period, indicates a tumor withdrawal flare that is consistent with increased tumor cell proliferation (14–16). After administration of docetaxel and two additional weeks of X-82 exposure, FLT PET parameters decreased again. A greater decrease in FLT PET parameters was evident in the second cycle of treatment suggesting greater decreases in tumor cell proliferation. These results support the hypothesis that ‘synchronizing’ cell-cycle chemotherapy with VEGFR-TKI treatment breaks will lead to greater anti-tumor effect than VEGR-TKI monotherapy; however, further clinical studies are warranted to confirm long-term clinical benefit. Although we did not assess the effects of docetaxel monotherapy, one prior study has shown that tumor FLT SUVmax had median change of −17.5% two weeks after administration of docetaxel to patients with breast cancer (28). This is less than the average change of −44% in FLT SUVmax reported in this study during the sequential combination of docetaxel and VEGFR-TKI, lending further support to the sequential treatment approach. It is important to note that three out of the four patients included in the FLT PET/CT analysis were in the high dose X-82 cohort.
This study was limited in that it accrued only 14 of the targeted 30 patients as the study was prematurely terminated due to a change in developmental strategy of the agent by the sponsor. However, this is one of few clinical studies assessing the effects of sequential chemotherapy applied during VEGFR-TKI treatment breaks. Further, X-82 is a novel VEGFR-TKI that has been studied little in clinical trials and in only one other clinical trial for treating cancer (29), indicating the potential value of these results for guiding future development of X-82 as well as development of improved therapeutic strategies with VEGFR-TKIs.
ACKNOWLEDGMENTS
The authors would like to thank the nurses and research specialists of the UWCCC Phase I Program for their efforts in managing this trial. The authors would also like to thank the WIMR PET imaging staff especially Chris Jaskowiak for her help with PET/CT scanning, the UW Cyclotron for preparing the radiotracer, and the patients for their participation in the study.
Acknowledgment of funding:
This clinical trial was funded by Tyrogenex.
REFERENCES
- 1.Folkman J Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971: 285:1182–1186. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. [DOI] [PubMed] [Google Scholar]
- 3.Giantonio BJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25(12):1539–1544. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham D, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14(11):1077–1085. [DOI] [PubMed] [Google Scholar]
- 6.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. [DOI] [PubMed] [Google Scholar]
- 7.Reck M, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL) Ann Oncol. 2010;21(9):1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reck M, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27(8):1227–1234. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27(20):3312–3318. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rini BI, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. [DOI] [PubMed] [Google Scholar]
- 13.Raymond E, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- 14.Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–30. [DOI] [PubMed] [Google Scholar]
- 15.Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–42 [DOI] [PubMed] [Google Scholar]
- 16.Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–62 [DOI] [PubMed] [Google Scholar]
- 17.Hecht JR, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol. 2011;29(15):1997–2003. [DOI] [PubMed] [Google Scholar]
- 18.Carrato A, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol. 2013;31(10):1341–1347. [DOI] [PubMed] [Google Scholar]
- 19.Crown JP, et al. Phase III trial of sunitinib in combination with capecitabine versus capecitabine monotherapy for the treatment of patients with pretreated metastatic breast cancer. J Clin Oncol. 2013;31(23):2870–2878. [DOI] [PubMed] [Google Scholar]
- 20.Rugo HS, Stopeck AT, Joy AA, et al. : Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol 29:2459–65, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Jeraj R, Vanderhoek M, Perlman S, Kolesar J, Harrison M, Simoncic U, Eickhoff J, Carmichael L, Chao B, Marnocha R, Ivy P, Wilding G (2011) Pharmacodynamic study using FLT PET/CT in patients with renal cell cancer and other solid malignancies treated with sunitinib malate. Clin Cancer Res 17:7634–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruce J, Scully PC, Carmichael LL, Eickhoff JC, Perlman SB, Kolesar JM, Heideman JL, Jeraj R, Liu G (2015) Pharmacodynamic study of axitnib in patients with advanced malignancies assessed with 18F-3’deoxy-3’fluoro-l-thymidine positron emission tomorgraphy/computed tomography. Cancer Chemotherapy and Pharmacology Volume 76, Issue 1, pp 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scarpelli Matthew, Justine Yang Bruce Lakeesha Carmichael, Eickhoff Jens, Kolesar Jill, Perlman Scott, Jeraj Robert, Liu Glenn. 18F-FLT PET/CT imaging in patients with advanced solid malignancies treated with axitinib on an intermittent dosing regimen. Cancer Chemotherapy and Pharmacology. 2016, 78:1245–1252. DOI: 10.1007/s00280-016-3183-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toyohara J, Waki A, Takamatsu S, Yonekura Y, Magata Y, Fujibayashi Y. Basis of FLT as a cell proliferation marker: comparative uptake studies with [3H]thymidine and [3H]arabinothymidine, and cell-analysis in 22 asynchronously growing tumor cell lines. Nucl Med Biol. 2002;29:281–7. [DOI] [PubMed] [Google Scholar]
- 25.Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, Luthra SK, Brady F, Price PM, Aboagye EO. 3′-deoxy-3′-[18F]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer research. 2003;63:3791–8. [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247 [DOI] [PubMed] [Google Scholar]
- 27.Reck Martin, Kaiser Rolf, Mellemgaard Anders, Douillard Jean-Yves, Orlov Sergey, Krzakowski Maciej, Joachim von Pawel Maya Gottfried, Bondarenko Igor, Liao Meilin, Gann Claudia-Nanette, Barrueco José, Birgit Gaschler-Markefski Silvia Novello. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. The Lancet Oncology 2014;15:143–155 [DOI] [PubMed] [Google Scholar]
- 28.Contractor Kaiyumars B., Kenny Laura M., Stebbing Justin, Rosso Lula, Ahmad Rizvana, Jacob Jimmy, Challapalli Amarnath, Turkheimer Federico, Adil Al-Nahhas Rohini Sharma, R. Charles Coombes and Eric O. Aboagye. [18F]-3′Deoxy-3′-Fluorothymidine Positron Emission Tomography and Breast Cancer Response to Docetaxel. Clin Cancer Res 2011;17:7664–7672 [DOI] [PubMed] [Google Scholar]
- 29.Tan J.Benjamin R., Picus Joel, Chan Emily, Lockhart Albert C., Roth Bruce J., Morton Ashley, Liang Chris, Wang-Gillam Andrea. Phase I study of X-82, an oral dual anti-VEGFR/PDGFR tyrosine kinase inhibitor, with everolimus in solid tumors. J Clin Oncol 34, 2016 (suppl; abstr 2588) [Google Scholar]


